Abstract
Background. Regional citrate anticoagulation is a very effective anticoagulation method for haemodialysis. However, it is not widely used, primarily due to the risk of hypocalcaemia. We studied citrate and calcium kinetics to better understand safety aspects of this anticoagulation method.
Methods. During 15 haemodialysis treatments with a calcium-free dialysis solution, citrate was infused pre-dialyser and calcium was substituted post-dialyser. Systemic and extracorporeal citrate and calcium concentrations were repeatedly measured to calculate citrate and calcium pharmacokinetics.
Results. Removal by dialysis constituted the major elimination pathway of citrate (83 ± 5%). Systemic citrate load and concentrations were low (17 ± 7 mmol/4 h, 0.3 ± 0.15 mmol/l). Combined use of calcium-free dialysate and citrate infusion increased diffusible calcium to 80% of total calcium and induced substantial dialytic loss of calcium (43 ± 4 mmol/4 h). Since calcium was substituted, systemic calcium balances were positive (∼+5 mmol) and concentrations stable. Calcium supplementation correlated with calcium dialytic losses, which in turn were dependent on total calcium and haematocrit.
Conclusions. When using calcium-free dialysate during citrate anticoagulation, hypocalcaemia is very likely unless calcium is re-infused, because large amounts of calcium are lost in the dialysate. However, an accumulation of citrate in the patient's systemic circulation is an unlikely cause of hypocalcaemia since most of the citrate is removed by dialysis. Calcium substitution and monitoring are the most important safety measures. We propose a rational approach based on haematocrit and total calcium for the choice of the starting calcium supplementation rate.
Keywords: calcium, citrate, dialysis, pharmacokinetics, regional anticoagulation
Introduction
Systemic heparinization is the anticoagulation of choice in stable patients on chronic haemodialysis. However, if a patient is at increased risk of bleeding, e.g. after surgery, in the presence of coagulopathy, in pericarditis or in patients actively bleeding, systemic anticoagulation with heparin can cause fatal haemorrhage [1]. In these cases regional citrate anticoagulation (RCA) has long been advocated as an alternative [2]. The anticoagulation effect of citrate is due to formation of complexes with calcium ions, which are essential cofactors of the coagulation process. The benefit of this method is that anticoagulation is limited to the dialysis circuit. The most dangerous complication is systemic hypocalcaemia, which may be even life threatening. Cardiac collapse has previously been described after an application of citrate anticoagulation during dialysis [3]. Systemic calcium levels may be decreased during regional citrate anticoagulation by two mechanisms. The first possible mechanism of hypocalcaemia is citrate infusion. Citrate infused into the arterial line of the dialysis circuit may reach the patient's systemic circulation although it is partially removed by dialysis. Citrate in the systemic circulation binds ionized calcium and may induce hypocalcaemia. This effect may be more pronounced in the case of liver dysfunction with impaired citrate metabolism or with limited dialysate elimination during continuous renal replacement therapies (CRRT) [4]. The second mechanism of hypocalcaemia is the dialytic removal of calcium due to the use of calcium-free dialysate. Many RCA protocols for haemodialysis utilize calcium-free dialysis fluid, because this prevents calcium uptake from the dialysate and the reduction of the anticoagulation effect. Exact quantities of citrate and calcium removed by dialysis are usually unknown since they are not routinely measured. The prediction of the amounts removed is even more complex as they depend on many parameters e.g. the characteristics of the dialyser used, blood and dialysate flow rates or the dialysable concentration of calcium inside the dialyser.
Apart from liver failure, little is known about other factors that may contribute to the development of hypocalcaemia during RCA in stable dialysis patients. For this reason we studied citrate pharmacokinetics and its effect on calcium levels during RCA in stable dialysis patients without signs of liver failure. The goal was to identify parameters contributing to the risk of hypocalcaemia and thus to provide more precise guidelines for the safe application of this anticoagulation method.
Subjects and methods
Patients
Patients (n = 10) with renal failure requiring dialysis and increased risk of bleeding participated in the study after giving written informed consent. The clinical characteristics of the patients are summarized in Table 1. All patients were in stable clinical condition and were treated in the dialysis unit of the Munich University Hospital. The majority of patients were at risk of bleeding due to recent surgery. None of the patients received blood or blood products or were treated with plasma exchange in the 24 h prior to the study. Patients were dialysed with citrate anticoagulation as long as an increased bleeding risk existed (1–3 dialysis treatments per patient). In total 15 haemodialysis treatments were investigated. The study was carried out according to the Declaration of Helsinki and the protocol was approved by the local ethics committee.
Table 1.
Clinical characteristics of patients
| Patient | Age/sex | Diagnoses | Indication for citrate anticoagulation | No. of treatments/vascular access |
|---|---|---|---|---|
| 1. | 70/F | CRF, M. Wegener | Haemoptysis | 2/catheter |
| 2. | 72/F | CRF, M. Wegener | After surgery | 2/a-v fistula |
| 3. | 53/M | CRF; hypertension | After surgery | 1/a-v fistula |
| 4. | 52/M | ARF, M. Wegener | Haemoptysis | 3/catheter |
| 5. | 79/F | CRF; hypertension, diabetes mellitus | After surgery | 1/a-v fistula |
| 6. | 55/M | CRF, polycystic kidney disease | After surgery | 1/a-v fistula |
| 7. | 64/M | CRF, M. Wegener | History of heparin-induced bleeding | 1/a-v fistula |
| 8. | 68/M | CRF, hypertension, chronic pyelonephritis | After thyroid biopsy | 1/a-v fistula |
| 9. | 69/M | ARF after heart surgery | After surgery | 2/catheter |
| 10. | 51/M | CRF, chronic glomerulonephritis | Immediately before surgery | 1/a-v fistula |
Haemodialysis
Dialysis was carried out using a modified 4008 dialysis machine with citrate module (Prometheus®, Fresenius Medical Care, Bad Homburg, Germany) and high-flux dialysers (Fresenius Medical Care, Bad Homburg, Germany, FX50: 5 treatments, F60S: 10 treatments, both dialysers have similar in vitro clearances for urea, 189 and 185 ml/min for QB 200 ml/min, respectively).
Blood pump speed during citrate anticoagulation was limited by the Prometheus device to 200 ml/min to reduce citrate load and accumulation. This resulted in the mean effective blood flow rate of 183 ± 2 ml/min. Effective blood flow rate was automatically estimated by one of the modules of the Prometheus device. We recorded effective blood flow rate as displayed by the dialysis machine at the beginning of dialysis, during each blood sampling and shortly before the end of dialysis, i.e. at least seven times during every treatment. The variations were minimal (± 1 ml/min per session). Ultrafiltration rate (492 ± 76 ml/h) was adjusted according to the patients’ needs. The flow and temperature of the calcium-free dialysis solution (SK-F 419/1, magnesium = 0.5 mmol/l, Fresenius Medical Care, Bad Homburg, Germany) was 500 ml/min and 37°, respectively. The initial bicarbonate concentration of 32 mmol/l was adjusted depending on the measured systemic pH and was on average reduced to 28 mmol/l. Vascular access was either via an arterio-venous fistula (eight treatments) or a double lumen Shaldon catheter (seven treatments) (see Table 1). The majority of haemodialysis sessions lasted 4 h. Two treatments were successfully carried out over 5 and 6 h, since two patients needed a longer time to achieve the required ultrafiltration volume.
Anticoagulation
The citrate module of the Prometheus® device, consisting of citrate and calcium infusion pumps, was used for anticoagulation. The tri-sodium citrate solution (500 mmol/l) was infused into the arterial line prior to the blood pump at a dose of 3.3 mmol/l of blood flow. The calcium chloride solution (500 mmol/l) was infused into the venous line at an initial dose of 1.1 mmol/le of blood flow. Ionized calcium was measured repeatedly and calcium infusion rate was adapted to maintain initial calcium concentration. If ionized calcium was out of the normal range in the pre-dialysis measurement, then calcium infusion was aimed to reach the normal range (1.1–1.3 mmol/l). At the end of the treatment, we examined citrate and calcium containers. The infused volumes of solutions were always consistent with the infusion pump settings and the duration of treatment confirming the accuracy of the citrate and calcium pumps.
Laboratory tests
Blood samples were taken from the arterial line of the extracorporeal circuit prior to the citrate infusion site (‘arterial samples’) at time points 0 min, +15 min, +45 min, +105 min, +165 min, +225 min. Three post-filter samples were taken from the venous return line during HD (+45 min, +165 min, +225 min). Four post-HD samples (‘post0’min, +post15 min, +post30 min, +post240 min) were taken in addition.
All samples were analysed for citrate concentration, electrolytes (total calcium, ionized calcium, magnesium, sodium and chloride) and acid–base status. Additionally, in the pre-treatment samples haematocrit and protein concentration were measured.
The samples for blood gas analysis and ionized calcium were collected in lithium–heparin syringes and immediately processed in the dialysis unit using RapidlabTM 348, Bayer Diagnostics, Munich, Germany. Total calcium was measured photometrically. Other electrolytes, haematocrit and protein concentrations were measured using standard laboratory techniques. Citrate concentrations were determined using an enzymatic assay (R-Biopharm, Darmstadt, Germany). All laboratory tests were carried out by the same person using identical procedures and equipment.
Calculations
As citrate does not enter erythrocytes [5], we calculated plasma water clearance of citrate, KCit, using the formula [6]
where Cin and Cout are plasma water concentrations of citrate at the inlet and the outlet of the dialyser, respectively. The inlet concentration was the measured ‘arterial’ concentration (Cart) corrected for the infused amount of citrate [CSol*QCit/QP + QCit] and water. The outlet concentration was the measured post-filter concentration. All measured serum concentrations were converted to plasma water concentration.
QP: plasma water flow rate at the dialyzer inlet [QB * (1−haematocrit)*(1−protein conc./100) + QCit].
CSol: concentration of citrate solution
QCit: citrate solution flow rate
UF: ultrafiltration rate
In addition, we calculated the removal of citrate mass by dialysis (extraction ratio) from an a-v difference of the dialyser including the effect of ultrafiltration 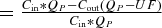 . The amount of citrate returning to the patient equal to net citrate dose was assessed as the difference between the infused and the removed citrate.
. The amount of citrate returning to the patient equal to net citrate dose was assessed as the difference between the infused and the removed citrate.
Citrate half-life was calculated using citrate concentrations from the first three post-treatment samples according to first-order kinetics. The apparent distribution volume of citrate was assessed using software for the modelling and simulation of dynamic systems (software VisSim 4.5, Visual Solutions Inc., Westford, MA, USA). This software was also used to validate the value of citrate half-life.
The flux (loss) of calcium across the dialyser membrane was calculated from the concentration gradient of total calcium at the inlet and the outlet of the dialyser (arterial and post-filter samples, respectively), which was corrected for ultrafiltration. The net balance was the difference between the infused and the removed calcium.
In addition, diffusible calcium concentration was calculated. Calcium in the blood is partially bound to proteins and this calcium cannot be removed by dialysis. Thus, diffusible calcium represents calcium that is not bound to proteins and can pass through the dialyser membrane. During dialysis with citrate anticoagulation, diffusible calcium consists mainly of citrate–calcium complexes. Since citrate in the dialyser also consists mainly of citrate–calcium complexes, the measured clearance of citrate is a good estimate of the clearance of citrate-calcium complexes. Therefore, the concentration of diffusible calcium in the dialyser was estimated from the flux of calcium and the clearance of citrate [Caflux/KCit]. Diffusible calcium was 80 ± 3% of total calcium in the blood inside the dialyser capillaries.
Calcium loss is very important to guide the substitution rate of calcium, but its direct measurement is not practicable in routine dialysis. Therefore, we developed a method to estimate calcium loss using clinically available parameters: total calcium concentration, haematocrit, protein concentration, blood flow rate, dialysate flow rate and mass transfer area coefficient of citrate (F60S: KoA = 337 ml/min, own unpublished data).
Calcium removal was calculated from standard equation of a flux (flux = diffusible solute conc. * clearance) using KoA of citrate for the calculation of the clearance and diffusible calcium equal to 80% of total calcium, according to the results of this study:
where K is the clearance of citrate (or citrate–calcium complexes) calculated according to equation proposed by Sargent and Gotch [7]:
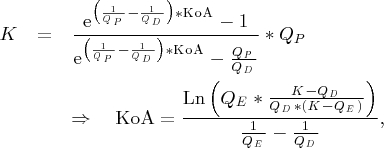 |
where QD is the dialysate flow rate and QP is the plasma water flow rate.
The losses calculated according to this equation were compared with the measurements in the first treatment hour (+45 min samples).
Statistical analysis
Data are presented as mean ± standard deviation. The t-test for paired samples or Wilcoxon's signed-rank test was used as appropriate to compare differences between pre- and post-HD values. Independent data samples were evaluated with a t-test or Wilcoxon's rank-sum test. The normality of sample distribution was checked by the Kolmogorov–Smirnov test with Lilliefors’ correction. Correlations were measured with the use of Pearson's and/or Spearman's correlation coefficient. A value of P < 0.05 was considered statistically significant (two-tailed tests). Statistical analysis was performed using SigmaStat software version 2.03.
Results
Anticoagulation
All treatments were carried out without adverse events. The extracorporeal circuit was effectively anticoagulated during all treatments. No complete occlusion of the system occurred. Visual inspection revealed minimal thrombus formation in 5 out of 15 haemodialysis sessions. All clots observed were located in the ‘arterial’ part of the extracorporeal circuit (arterial chamber: 4, arterial sampling site: 1 and filter inlet: 1). During one dialysis two clots were formed simultaneously (in the arterial chamber and in the filter). In the treatments affected, post-filter ionized calcium was always below the detection limit (<0.2 mmol/l).
Systemic citrate concentration
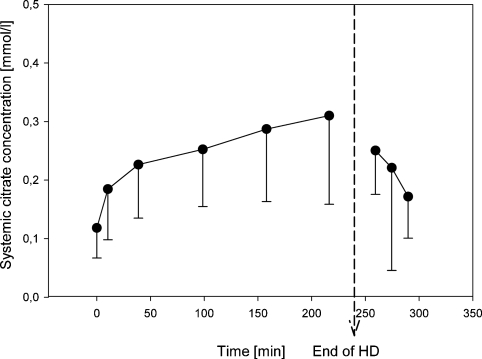
Systemic citrate concentration increased steadily during dialysis from 0.12 ± 0.05 mmol/l (pre-HD) to 0.31 ± 0.15 mmol/l (+225 min) but most rapidly in the first 40 min of HD (Figure 1, Table 2). The highest measured value was 0.72 mmol/l. Concentrations measured 4 h after the treatment were comparable to baseline (0.10 ± 0.04 mmol/l) indicating that citrate did not accumulate between HD sessions.
Fig. 1.
Systemic citrate concentration (mmol/l) during and after haemodialysis.
Table 2.
Laboratory parameters: haematocrit, total protein, citrate, electrolytes, pH and bicarbonate (pH and bicarbonate shown separately for patients with different vascular access: a-v fistula or catheter)
| Pre-dialysis | Post-dialysis | |
|---|---|---|
| Haematocrit | 0.31 ± 0.06 | – |
| Total protein (g/dl) | 5.9 ± 1.0 | – |
| Albumin (g/dl) | 3.5 ± 0.6 | – |
| Citrate (mmol/l) | 0.12 ± 0.06 | 0.25 ± 0.07* |
| Ionized calcium (mmol/l) | 1.10 ± 0.1 | 1.19 ± 0.09* |
| Total calcium (mmol/l) | 2.17 ± 0.16 | 2.20 ± 0.17 |
| Sodium (mmol/l) | 142 ± 4 | 139 ± 2 |
| Magnesium (mmol/l) | 0.9 ± 0.16 | 0.8 ± 0.09 |
| Chloride (mmol/l) | 100 ± 5 | 101 ± 2 |
| pH (fistula) | 7.41 ± 0.03 | 7.46 ± 0.02 |
| pH (catheter) | 7.41 ± 0.03 | 7.43 ± 0.04 |
| Actual bicarbonate (mmol/l) (fistula) | 21 ± 2.4 | 24± 1.7 |
| Actual bicarbonate (mmol/l) (catheter) | 25 ± 2.8 | 26 ± 2.0 |
*P < 0.05 for the difference between pre- and post-dialysis values.
Citrate removal by the dialyser and net citrate dose
The removal of citrate into the dialysate was very efficient amounting to 83 ± 5% of the citrate entering the dialyser (i.e. the externally infused citrate and the citrate present in the systemic circulation). The plasma water clearance of citrate was 98 ± 10 ml/min. During 4 h of HD, a net citrate dose delivered to the systemic circulation of the patient was 17 ± 7 mmol (Table 3).
Table 3.
Pharmacokinetic data
| Plasma water flow rate (ml/min) | 118 ± 11 |
| Citrate plasma water clearance (ml/min) | 98 ± 10 |
| Net citrate dose per 4 h of HD (mmol) | 17 ± 7 |
| Citrate mass removal (%) | 83 ± 5 |
| Citrate half-life (min) | 60 ± 29 |
| Citrate distribution volume, litres (% of body weight) | 41 ± 22 (54 ± 18) |
| Calcium removal rate, mmol/min (mg/min) | 0.18 ± 0.02 (7.2 ± 0.8) |
| Calcium loss per 4 h of HD, mmol (mg) | 43 ± 4 (1720 ± 160) |
| Calcium substitution per 4 h of HD, mmol (mg) | 48 ± 1 (1920 ± 40) |
Distribution volume and metabolism of citrate
After 4 h of treatment, the apparent distribution volume of citrate was 41 ± 22 l, which corresponded to 54 ± 18% of the body weight. The measured systemic citrate concentrations best fitted with a two-compartmental model suggesting that in the first 2 h citrate distribution was not yet in a steady state. In all patients citrate was readily metabolized with a mean half-life of 60 ± 29 min. Citrate half-life did not correlate with body weight (Pearson: r = 0.175, P = 0.568).
Unusual case of citrate pharmacokinetics
In one patient with normal liver function, abnormal citrate pharmacokinetics were observed and therefore this patient was evaluated separately. In this patient, the citrate concentration at baseline was ∼5-fold higher than the average of the remaining patients (0.63 mmol/l versus 0.12 mmol/l). The blood samples of this patient were analysed three times with high reproducibility excluding possible lab errors. Citrate concentrations during haemodialysis remained relatively constant in this patient, probably due to very efficient removal of citrate into the dialysate (90% of the amount entering the dialyser). Citrate anticoagulation was performed without side effects in this patient. The reason for this high baseline citrate concentration remains elusive. Increased citrate levels were reported so far in bone diseases associated with osteolysis [8]. However, at the time of the study there was no clinical evidence of bone disease in this patient.
Systemic ionized and total calcium concentrations
Clinical symptoms of hypo- or hypercalcaemia were not observed. Calcium values were stable during all treatments. Only 7 out of 78 measured ionized calcium values during HD were out of the range: 1.0–1.3 mmol/l (min: 0.9 mmol/l, max: 1.38 mmol/l).
Diffusible calcium and protein-bound calcium
In the systemic blood under normal conditions, ∼40% of calcium is bound to proteins and 60% of calcium can cross the dialyser membrane (diffusible calcium). A shift of calcium ions from protein binding to citrate complexes, changing the proportion of bound to diffusible calcium, is possible in the extracorporeal circuit during RCA. Our results demonstrate that an important shift of calcium ions from proteins actually occurs in the extracorporeal circuit during RCA, which decreases protein-bound calcium to 20 ± 3%, increasing diffusible calcium to 80 ± 3% of total calcium.
Measured calcium removal
Of the 80 ± 3% of calcium that could cross the dialyser membrane, 64 ± 5% moved to the dialysis solution leading to average calcium removal rate of 0.18 ± 0.02 mmol/min. This rate varied among the patients by almost 50%, ranging from 0.13 to 0.22 mmol/min. Calcium loss correlated strongly with the product of total calcium concentration and the measured citrate clearance, r = 0.942, P < 0.001. Linear regression yielded the equation Caloss = −0.00546 + 0.875*Catot*KCit, r2 = 0.887, P < 0.001. Interestingly, ionized calcium concentration was less predictive of calcium loss than total calcium (r = 0.633, r2 = 0.401, P = 0.001, Caloss = 0.0705 + 0.982*Caion*KCit).
Calcium removal calculated from KoA
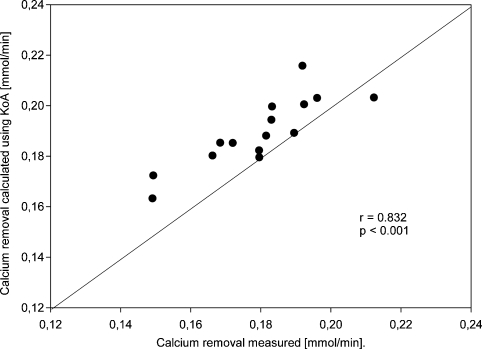
Calcium loss calculated using KoA correlated strongly with the measured value: r = 0.832; r2 = 0.692, P < 0.001, Caloss-calc. = 0.0696 + 0.667*Caloss-meas. (Figure 2) and was slightly higher than the measured one on average by 0.01 mmol/min.
Fig. 2.
Correlation between measured calcium removal and removal calculated from KoA (mmol/min).
Only in one patient was calculated calcium removal was slightly below the measured value (by 0.009 mmol/min). This patient had the lowest protein concentration (4.3 g/dl versus all other patients ≥ 5.5 g/dl) resulting in low protein binding of calcium and in a higher proportion of diffusible calcium (84% versus average value 80%). Therefore, calcium removal was higher than expected.
Post-filter calcium concentrations
Post-filter total calcium concentrations were decreased to 0.8 ± 0.1 mmol/l (arterial concentration: 2.2 ± 0.2 mmol/l). All measured post-filter ionized calcium concentrations were at or below detection limit (≤0.2 mmol/l).
Calcium supplementation
Calcium supplementation was initially proportional to the effective blood flow (0.205 ± 0.005 mmol/min). During the majority of treatments, supplementation rate was slightly decreased to keep the pre-dialysis ionized calcium value. In the fourth treatment hour, it was on average 0.195 ± 0.005 mmol/min. Total amount of calcium supplemented (mmol) correlated significantly with total amount of calcium removed into the dialysate (mmol), r = 0.797, P < 0.001.
Net balance of calcium
In 13 out of 15 treatments, the initial calcium supplementation rate was higher than the calcium removal rate and these sessions ended with a positive calcium balance (mean: 5 mmol/per HD, max: 17 mmol/per HD). Positive calcium balances did not increase systemic ionized or total calcium concentrations accordingly. No correlation between positive net balance and calcium concentrations was observed (total calcium: r = 0.397, P = 0.143, ionized calcium: r = −0.114, P = 0.685). In contrast, the modest negative calcium balance observed in two patients (up to −1 mmol/per HD) was associated with small post-dialytic decreases in the total calcium level and in one patient also in ionized calcium.
Concentrations of other electrolytes and acid–base status
Systemic levels of other electrolytes (sodium, magnesium, chloride) were stable during all treatments (Table 2). Low dialysate magnesium (0.5 mmol/l) reduced average systemic magnesium concentrations. Despite this, all measured magnesium values were within the normal range. The bicarbonate concentration in the dialysis solution was adjusted during the treatments according to the monitored pH. Using this approach, no alkalosis or acidosis was observed. The changes of acid–base status during the treatment were typical for bicarbonate-based dialysis (Table 2). Also after the treatment, acid–base values remained within normal range. Despite metabolism of citrate increasing bicarbonate concentration alkalosis did not develop in any of the patients.
Discussion
Anticoagulation for chronic dialysis patients with contraindications to heparin administration (risk of bleeding or heparin-induced thrombocytopaenia type 2, HIT-2) remains challenging. The regimens used alternatively, e.g. regional heparinization with protamine, direct thrombin inhibitors, prostacyclin or anticoagulation-free dialysis, encounter considerable limitations. Whether because of expense, lack of effectiveness or complexity, neither of them has gained widespread acceptance. Regional citrate anticoagulation offers advantages, which could make it a first-choice regimen for alternative anticoagulation. Citrate anticoagulation not only reduces bleeding risk [9,10] but also can be more effective than heparin in preventing thrombus formation in the dialysis circuit [11]. Furthermore, a depletion of calcium in the extracorporeal circuit during RCA is known to attenuate neutrophil degranulation, and thus may reduce oxidative stress [12]. Despite many recognized advantages, only few dialysis centres apply citrate anticoagulation on a routine basis, primarily because the relationships between citrate infusion, calcium levels and dialysis parameters are complex. Although numerous protocols have been published so far, a generally accepted standard prescription of RCA for haemodialysis has not yet evolved. In addition, the application of RCA is cumbersome because it usually requires additional pumps for citrate and calcium infusions, which are not provided by standard dialysis machines. These issues not only increase the complexity of the RCA procedure but also the likelihood of complications, which may occur more frequently if the method is rarely used [13]. Of the possible complications, the most dangerous is systemic hypocalcaemia, since it can cause life-threatening arrhythmias.
This study presents citrate and calcium pharmacokinetics during high-flux dialysis, which to our knowledge, has not yet been investigated in detail during this type of treatment. The results of this investigation may help to better understand possible mechanisms disturbing calcium homeostasis and shows a rationale approach for calcium substitution during high-flux dialysis.
Most frequently, an increase in systemic citrate concentration is suspected as a cause of hypocalcaemia during citrate anticoagulation. We observed that during high-flux dialysis, systemic citrate concentrations had a high margin of safety. All measured citrate values were well below the level of 2.5 mmol/l known to be toxic for heart function in case no calcium supplementation is carried out [14]. Moreover, citrate concentrations were also lower than reported during aphaeresis procedures where in contrast to dialysis calcium is not routinely supplemented [15–17]. Earlier studies in dialysis, in which comparable doses of citrate were used, reported higher systemic citrate concentrations as well [18]. Lower citrate concentrations in our study are most likely due to the use of high-flux dialysers removing 83% of the infused citrate. Using these dialysers, clearance of citrate constituted ∼60% of total urea clearance for a blood flow of 180–200 ml/min, a ratio similar to published data [2].
As other investigators of citrate pharmacokinetics, we assumed citrate metabolism to follow first-order kinetics [19–21]. It can be argued that estimation of citrate half-life using a two-compartment model may be more appropriate than using post-treatment citrate concentrations. If a two-compartment model is used, then citrate half-life differs by <10% (55 ± 25 min versus 60 ± 29 min). Independently from the method used, citrate half-life in our patients was longer than those reported by Apsner et al. and Kramer et al. in the control groups with normal kidney function (33 and 36 min) [19,20]. Similar results in renal failure patients have already been reported by Bauer et al. (49 min) [21]. Thus, it would be tempting to say that renal failure might be associated with impaired citrate metabolism, which is, however, in contrast to the conclusion made by Bauer et al. In this study, citrate half-life in the end-stage renal failure patients did not differ significantly from the results of the control group without renal failure (59 min). The clinical characteristics of this control group were similar to that of the study by Apsner et al. (patients with autoimmune or lipid disturbances undergoing immune- or lipidapheresis) [19]. It remains at present unclear why citrate half-lives differ so much in similar patient groups. Also the question whether haemodialysis patients have impaired citrate metabolism requires further clarification in future studies. Nevertheless, during high-flux dialysis, citrate metabolism is of minor importance as the dialysis procedure is effectively removing citrate and only little needs to be metabolized. This amount was successfully metabolized in our patients and did not accumulate between treatments. However, the role of citrate metabolism could be more important during continuous dialysis modalities, because of a larger total load of citrate, which is delivered over a longer treatment time compared to intermittent HD [4].
Another parameter that may influence systemic citrate levels is the distribution volume of citrate. A lower volume of distribution will result in higher plasma levels of citrate. According to our data in stable dialysis patients during 4 h of dialysis, citrate is easily distributed within a large volume (∼40 l). Thus, in adult patients the effect of distribution volume on citrate concentration is limited. However, we observed that in the first 2 h of dialysis the distribution of citrate was not yet in a steady state, which probably contributed to the more rapid increase in systemic citrate concentration over this time. The apparent volume of distribution after 4 h of treatment was very reminiscent to the estimate of the total body water known for healthy people (54% of the body weight). Nevertheless, an interpretation of distribution in terms of body water compartments requires caution, as dialysis patients may have varied hydration status and water compartments compared to healthy people. Other authors reported smaller distribution volumes of citrate [19,20]. However, in previous studies, citrate was administered over a shorter time (120 min versus 240 min in our study). This may potentially lead to a lower distribution volume, as a disposition of a substance is a time-dependent process. In addition, it should be emphasized that distribution patterns may differ significantly depending on the clinical characteristics of the studied population, e.g. critically ill patients as studied by Kramer et al. [20] may suffer from numerous conditions potentially affecting distribution patterns such as impaired microcirculation, varying degree of oedema or even dehydration. Thus, our results of citrate distribution should not be extrapolated to other patient populations, e.g. critically ill patients.
In summary, if high-flux dialysers are used, elimination of citrate by dialysis constitutes the major elimination pathway of citrate, whereas metabolic degradation of citrate is of minor importance. Small amounts of citrate reaching systemic circulation are easily distributed within a large volume resulting in only low increases in systemic citrate concentrations. These small increases are probably well buffered by shifts of calcium ions from proteins and therefore no measurable decreases of ionized calcium are detected. Aphaeresis studies provide additional evidence that systemic citrate concentrations <1.0 mmol/l do not measurably decrease ionized calcium and are well tolerated without calcium supplementation [15,17]. In line with these observations, in our study calcium supplementation correlated strongly with calcium removal (r = 0.797, P < 0.001) indicating that the risk of hypocalcaemia during citrate anticoagulation is mainly associated with calcium dialytic loss. Thus, identifying factors influencing calcium removal is important, in order to improve the accuracy of calcium supplementation. Two major parameters determined calcium loss: (1) total serum calcium concentration prior to dialysis and (2) clearance of the citrate–calcium complexes.
The influence of total serum calcium is due to the fact that calcium removal is determined by diffusible calcium that in turn depends on total calcium concentration. Diffusible calcium inside the dialyser was a constant fraction of total calcium (80 ± 3%). Because of the constant relation of diffusible to total calcium, total calcium is an important determinator of calcium removal.
Clearance of the citrate–calcium complexes is the second key parameter influencing calcium removal and substitution. The majority of citrate anions were bound to calcium; thus the measured citrate clearance was rather the clearance of citrate–calcium complexes. Obviously, as with all dialysis procedures, dialyser surface area, permeability of the dialyser as well as blood and dialysate flow rates, all effect the clearance of a solute, in this case of citrate–calcium complexes. For the purpose of this study, these dialysis parameters were kept similar during all dialysis sessions (dialysers with the same clearance characteristics, a fixed dialysate flow rate of 500 ml/min and the blood pump speed of 200 ml/min in all patients). Despite similar blood flow and other dialysis parameters, patients experienced different citrate clearances and different calcium removal. The relevant patient parameter influencing clearance of citrate–calcium complexes was haematocrit. In contrast to urea, citrate is not enter red blood cells [22]. Therefore, citrate clearance correlated more closely with plasma flow than with blood flow. At a given blood flow rate (183 ml/min), patients with a lower haematocrit had a higher plasma flow rate, thus a higher clearance of citrate–calcium complexes and a higher removal of calcium. For this reason, patients with a lower haematocrit required a higher calcium substitution rate during citrate anticoagulation.
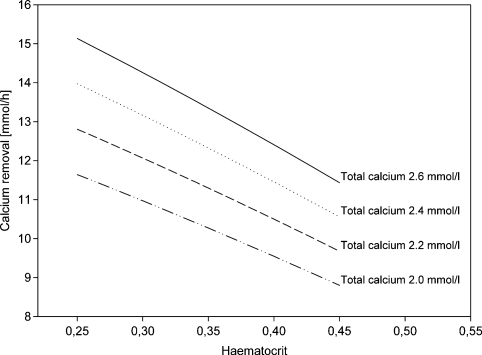
Our data indicate that the risk of hypocalcaemia during citrate anticoagulation can be reduced substantially, if negative calcium balance is avoided by supplementing calcium at a rate not lower than calcium removal. Since calcium removal is so important in guiding calcium substitution and its direct measurement is not practicable in routine dialysis, we developed a nomogram (Figure 3) that allows estimation of calcium removal based on clinically available parameters, total calcium concentration and haematocrit, provided that other dialysis parameters are kept constant (type of dialyser, blood and dialysate flow rate). Using this nomogram (Figure 3), it can be predicted that if haematocrit is low (<0.3) and total calcium is in the normal range (2.2– 2.6 mmol/l), a calcium substitution rate of 12 mmol/h will be insufficient to replace calcium losses. This was confirmed in two patients with low haematocrit in our study. In contrast, patients with higher haematocrit (≥0.45) require less calcium substitution than 12 mmol/h if they have normal total calcium.
Fig. 3.
Nomogram of calcium removal (mmol/h) calculated for different haematocrit and total calcium concentrations using dialyser F60S (KoA for citrate: 337 ml/min), blood pump speed: 200 ml/min (effective blood flow rate: 180 ml/min), dialysate flow rate: 500 ml/min, protein concentration = 6.5 g/dl.
The presented nomogram on calcium removal can be used to estimate a required starting dose of calcium supplementation. At present, it is a frequent practice to use the same initial calcium supplementation rate and then to adjust it to the measured ionized calcium level. We propose to consider calcium dialytic loss for the choice of the initial calcium supplementation rate in order to avoid hypocalcaemia due to negative calcium balance or calcium overdosing due to highly positive calcium balances. This approach can help to estimate the initial calcium supplementation but does not replace ionized calcium measurements to confirm the accuracy of calcium dosing since metabolic conditions of patients may change calcium supplementation requirements in addition to calcium dialytic loss, e.g. due to the development of alkalosis or acidosis. In addition, ionized calcium measurements are sometimes the only safety measures to detect equipment malfunctions or handling errors that may also lead to hypocalcaemia such as reversing of citrate and calcium infusions [13]. Thus, an adequate calcium substitution and calcium monitoring are the most important safety measures during citrate anticoagulation.
The strength of the proposed calcium substitution approach is that in contrast to fixed dosing schemes it also considers patient characteristics and helps to avoid hypocalcaemia from the beginning of dialysis, particularly in patients with a low haematocrit and/or high total calcium level. Calcium substitution rates based on the nomogram will result in a small positive calcium balance. This is due to the use of an in vitro value of KoA for citrate in our equation for calcium removal and substitution. In vitro KoA values are typically higher than values reached in vivo. Positive calcium balance provides an additional safety margin to avoid hypocalcaemia and is comparable to conventional dialysis. During conventional haemodialysis, patients also receive a calcium load since calcium concentrations in standard dialysis solutions are higher than ionized calcium in the blood [18]. Positive calcium balance was well tolerated in this study. There is ongoing concern, however, whether a positive calcium balance during dialysis may have detrimental effects, e.g. calcification of soft tissues and blood vessels. If desired, both anticoagulation methods can easily achieve a neutral or negative calcium balance using a lower calcium content of the dialysate or a lower calcium substitution rate, respectively.
The limitation of the presented calcium nomogram (Figure 3) is that it is valid only for certain fixed dialysis settings (dialyser F60S, blood pump speed of 200 ml/min and dialysate flow rate of 500 ml/min). Many variations of a dialysis technique are possible. If different dialysers, blood or dialysate flow rates are used, then different removal of calcium and thus substitution rate have to be expected and the nomogram needs to be modified by the user. We used a blood pump speed of 200 ml/min, which had already been successfully applied during citrate anticoagulation by other authors [23–25]. Using this blood flow rate, particularly in large patients, an adequate weekly dialysis dose will be provided either with the longer treatment time, e.g. 6 h per session or with daily dialysis, which is common in the intensive care setting. In the nomogram, we also did not include the effect of serum protein concentration on calcium losses. At a given haematocrit and total serum calcium concentration, a low protein level (<5 g/dl) may result in a higher proportion of diffusible calcium inside the dialyser (84% instead of 80%) and thus in a higher calcium removal. Therefore, we suggest increasing the calcium supplementation rate given by the nomogram by 4% in patients with a protein level of <5 g/dl.
It is important to note that this model of calcium removal and substitution is not applicable to continuous renal replacement therapies in intensive care patients. In this setting, citrate anticoagulation is carried out over a longer period of time leading to a higher total citrate load. Lower clearance rates typical for continues treatments will reduce citrate removal increasing systemic citrate levels compared to intermittent HD. Furthermore, critically ill patients may have difficulties to metabolize this high citrate load due to reduced liver function or impaired distribution of citrate. Therefore, during continuous treatment modalities, other approaches to estimate calcium dosing during citrate anticoagulation are required.
In conclusion, during high-flux dialysis systemic citrate load and citrate plasma concentrations are very low. Thus, in theory, citrate anticoagulation during intermittent dialysis should also be safe in patients with impaired liver function [26], but this requires further studies. In contrast, calcium losses due to the use of calcium-free dialysis solution are substantial. Hypocalcaemia is likely to develop if calcium substitution is less than dialytic removal. Matching calcium supplementation to dialyser removal is the best way to prevent both calcium overload and hypocalcaemia, respectively. A nomogram is provided to estimate calcium removal based on total calcium concentration and haematocrit assuming the use of a typical high flux dialyzer (Fresenius F60S), a blood pump speed of 200 ml/min and a dialysate flow of 500 ml/min. Calcium removal based on the nomogram can be used as a surrogate for the choice of the initial calcium substitution rate (Figure 3). Use of this nomogram will, in addition to calcium monitoring, reduce the risk of hypocalcaemia during citrate anticoagulation. Since an individual variability in metabolism and disposition of citrate and calcium as well as malfunctions of the used equipment cannot be excluded, monitoring of ionized calcium during citrate anticoagulation remains indispensable.
Our data clarify some important aspects of citrate and calcium kinetics e.g. the dependence on haematocrit and total serum calcium concentration of the patient. The knowledge of citrate and calcium pharmacokinetics and the use of modern dialysis machines with integrated citrate and calcium pumps may make the application of this valuable anticoagulation method easier and thus available to a broader number of patients.
Acknowledgments
This work was supported by the European Commission Marie Curie Fellowship grant (J. Kozik-Jaromin). The cooperation of the medical, nursing and laboratory staff from the Nephrology Department of Munich University Hospital is gratefully acknowledged.
Conflict of interest statement. J.K.-J. and V.N. are employed by Fresenius Medical Care. J.B. has served as consultant for Fresenius Medical Care.
References
- 1.Hirsh J, Raschke R, Warkentin TE, et al. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1995;108:258S–275S. doi: 10.1378/chest.108.4_supplement.258s. [DOI] [PubMed] [Google Scholar]
- 2.Pinnick RV, Wiegmann TB, Diederich DA. Regional citrate anticoagulation for hemodialysis in the patient at high risk for bleeding. N Engl J Med. 1983;308:258–261. doi: 10.1056/NEJM198302033080506. [DOI] [PubMed] [Google Scholar]
- 3.Charney D, Salmond R. Cardiac arrest after hypertonic citrate anticoagulation for chronic hemodialysis. ASAIO Trans. 1990;36:M217–M219. [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Finkel KW, Gitomer JJ, et al. Unexpected severe hypocalcemia during continuous venovenous hemodialysis with regional citrate anticoagulation. Am J Kidney Dis. 1999;33:e8. doi: 10.1016/s0272-6386(99)70249-0. [DOI] [PubMed] [Google Scholar]
- 5.Whitfield LR, Levy G. Permeability of human and rat red blood cells to citrate. Thromb Res. 1981;21:681–684. doi: 10.1016/0049-3848(81)90270-x. [DOI] [PubMed] [Google Scholar]
- 6.Werynski A. Evaluation of the impact of ultrafiltration on dialyzer clearance. Artif Organs. 1979;3:140–142. doi: 10.1111/j.1525-1594.1979.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 7.Sargent J, Gotch F. Principles and Biophysics of Dialysis. In: Jacobs C, Kjellstrand C, Koch K, Winchester J, editors. Replacement of Renal Function by Dialysis. Dordrecht: Kluwer; 1996. pp. 34–102. [Google Scholar]
- 8.Nordmann J, Nordmann R. Organic acids in blood and urine. Adv Clin Chem. 1961;4:53–120. doi: 10.1016/s0065-2423(08)60035-9. [DOI] [PubMed] [Google Scholar]
- 9.Flanigan MJ, Von Brecht J, Freeman RM, et al. Reducing the hemorrhagic complications of hemodialysis: a controlled comparison of low-dose heparin and citrate anticoagulation. Am J Kidney Dis. 1987;9:147–153. doi: 10.1016/s0272-6386(87)80092-6. [DOI] [PubMed] [Google Scholar]
- 10.Monchi M, Berghmans D, Ledoux D, et al. Citrate versus heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med. 2004;30:260–265. doi: 10.1007/s00134-003-2047-x. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer R, Moser D, Frass M, et al. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney Int. 1999;56:1578–1583. doi: 10.1046/j.1523-1755.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Bohler J, Schollmeyer P, Dressel B, et al. Reduction of granulocyte activation during hemodialysis with regional citrate anticoagulation: dissociation of complement activation and neutropenia from neutrophil degranulation. J Am Soc Nephrol. 1996;7:234–241. doi: 10.1681/ASN.V72234. [DOI] [PubMed] [Google Scholar]
- 13.Clark JA, Schulman G, Golper TA. Safety and efficacy of regional citrate anticoagulation during 8-h sustained low-efficiency dialysis. Clin J Am Soc Nephrol. 2008;3:736–742. doi: 10.2215/CJN.03460807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunker JP, Bendixen HH, Murphy AJ. Hemodynamic effects of intravenously administered sodium citrate. N Engl J Med. 1962;266:372–377. doi: 10.1056/NEJM196202222660802. [DOI] [PubMed] [Google Scholar]
- 15.Hester JP, McCullough J, Mishler JM, et al. Dosage regimens for citrate anticoagulants. J Clin Apheresis. 1983;1:149–157. doi: 10.1002/jca.2920010306. [DOI] [PubMed] [Google Scholar]
- 16.Bolan CD, Cecco SA, Wesley RA, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42:935–946. doi: 10.1046/j.1537-2995.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 17.Bolan CD, Greer SE, Cecco SA, et al. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–1171. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 18.Janssen MJFM, Huijgens PC, Bouman AA, et al. Citrate versus heparin anticoagulation in chronic haemodialysis patients. Nephrol Dial Transplant. 1993;8:1228–1233. [PubMed] [Google Scholar]
- 19.Apsner R, Schwarzenhofer M, Derfler K, et al. Impairment of citrate metabolism in acute hepatic failure. Wien Klin Wochenschr. 1997;109:123–127. [PubMed] [Google Scholar]
- 20.Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450–2455. doi: 10.1097/01.CCM.0000084871.76568.E6. [DOI] [PubMed] [Google Scholar]
- 21.Bauer E, Derfler K, Joukhadar C, et al. Citrate kinetics in patients receiving long-term hemodialysis therapy. Am J Kidney Dis. 2005;46:903–907. doi: 10.1053/j.ajkd.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Marx J, Bourdeau J. Calcium metabolism. In: Maxwell M, Kleeman C, Narins R, editors. Clinical Disorders of Fluid and Electrolyte Metabolism. New York: Mcgraw-Hill; 1987. pp. 207–317. [Google Scholar]
- 23.Hocken AG, Hurst PL. Citrate regional anticoagulation in haemodialysis. Nephron. 1987;46:7–10. doi: 10.1159/000184287. [DOI] [PubMed] [Google Scholar]
- 24.Faber LM, de Vries PM, Oe PL, et al. Citrate haemodialysis. Neth J Med. 1990;37:219–224. [PubMed] [Google Scholar]
- 25.Dhondt A, Vanholder R, Waterloos MA, et al. Citrate anticoagulation does not correct cuprophane bioincompatibility as evaluated by the expression of leukocyte surface molecules. Nephrol Dial Transplant. 1998;13:1752–1758. doi: 10.1093/ndt/13.7.1752. [DOI] [PubMed] [Google Scholar]
- 26.Morath C, Miftari N, Dikow R, et al. Sodium citrate anticoagulation during sustained low efficiency dialysis (SLED) in patients with acute renal failure and severely impaired liver function. Nephrol Dial Transplant. 2008;23:421–422. doi: 10.1093/ndt/gfm629. [DOI] [PubMed] [Google Scholar]