Abstract
Background. In Fabry disease, progressive glycolipid accumulation leads to organ damage and early demise, but the incidence of renal, cardiac and cerebrovascular events has not been well characterized.
Methods. We conducted a retrospective chart review of 279 affected males and 168 females from 27 sites (USA, Canada, Europe). The pre-defined study endpoints included progression of renal, cardiac and cerebrovascular involvement and/or death before the initiation of enzyme replacement therapy.
Results. The mean rate of estimated glomerular filtration rate (eGFR) decline for patients was −2.93 for males, and −1.02 ml/min/1.73 m2/year for females. Prevalence and severity of proteinuria, baseline eGFR <60 ml/min/1.73 m2 and hypertension were associated with more rapid loss of eGFR. Advanced Fabry nephropathy was more prevalent and occurred earlier among males than females. Cardiac events (mainly arrhythmias), strokes and transient ischaemic attacks occurred in 49, 11, 6% of males, and in 35, 8, 4% of females, respectively. The mean age at death for 20 male patients was 49.9 years.
Conclusions. Baseline proteinuria, reduced baseline eGFR, hypertension and male gender were associated with more rapid progression of Fabry nephropathy. The eGFR progression rate may increase with advancing nephropathy, and may differ between subgroups of patients with Fabry disease.
Keywords: albuminuria, arrhythmia, Fabry disease, nephropathy, proteinuria, stroke
Introduction
Fabry disease is an X-linked lysosomal storage disorder due to alpha-galactosidase A (α-GalA) deficiency. This deficiency causes the progressive accumulation of globotriaosylceramide (GL-3) and related glycosphingolipids, particularly in vascular endothelial cells, renal cells and cardiomyocytes [1].
Nephropathy is one of the major complications of Fabry disease. Biopsies reveal GL-3 accumulation in tubular epithelial cells, glomerular and endothelial cells, with focal and global glomerulosclerosis as early as in the second decade of life [2–5]. The major signs of Fabry nephropathy include reduced glomerular filtration rate (GFR), isosthenuria and proteinuria [1,3,6], and affected males typically progress to kidney failure by the fourth decade of life [7]. Cardiovascular and cerebrovascular events also contribute to morbidity and mortality. Heterozygous females may be asymptomatic or develop overt disease, presumably due to skewing of X-chromosomal inactivation [1,8–12].
The natural history of Fabry disease in patients prior to receiving enzyme replacement therapy (ERT) may provide insights to the underlying pathophysiology, and a context for assessing outcomes once ERT is initiated. Therefore, we conducted a chart review of 447 patients to document the severity and progression of their nephropathy, as well as cardiovascular and cerebrovascular events and death before ERT.
Materials and methods
Study design
We obtained a collection of retrospective data from patients with Fabry disease followed between 1944 (first record across all datasets) and 2002. The clinical records of 447 patients from 27 participating expert sites in five countries (USA 19 sites, Canada 5 sites and 1 site in Czech Republic, Denmark and the Netherlands) were reviewed.
Once patients, guardians or next of kin consented and medical records’ release had been obtained after local Institutional Review Board approval, if applicable, all available pre-defined clinical data were abstracted onto case report forms by an independent contract research organization [Abt Associates Clinical Trials (AACT), Cambridge, MA, USA]. The abstracted information included patient and disease characteristics, key laboratory values over time and history of renal, cardiac and cerebral vascular diseases. Patient names remained blinded. Duplicate patient data obtained at more than one site were identified, and patients’ records were merged. Statistical services were provided by Genzyme Corporation, Cambridge, MA, USA.
Patients
Eligible patients included those diagnosed with Fabry disease during life or at the time of death. Based on review of the clinical findings, α-GalA activities and α-GalA genotypes, most (>95%) of the affected males and heterozygous females had or were from families with the classic phenotype [1,13,14]. Patients were excluded if they had confounding renal or other diseases (e.g. diabetic nephropathy, cancer). Charts were abstracted only up to the time of initiation of ERT.
Data collection and analysis
Fabry nephropathy
Data collected included chronic renal insufficiency or failure, and proteinuria or ratio of urinary protein to urinary creatinine (>0.3 g protein per day or >0.3 g/g). End-stage renal disease (ESRD) was defined by a requirement for chronic dialysis or transplantation. A renal event was defined as kidney transplantation, >40 days of chronic dialysis, an increase in serum creatinine by 50% from baseline (i.e. at first entry in the abstracted medical records) to a value >1.4 mg/dl or serum creatinine ≥6 mg/dl.
Available serum creatinine values for 243 males and 152 females were used to calculate eGFR [15]. These patients were stratified by their baseline eGFR ≥60 or <60 ml/min/1.73 m2. Chronic kidney disease (CKD) staging was not used because the creatinine measurements were from multiple centres, but those with eGFR <60 ml/min/1.73 m2 were classified as having CKD [15]. For blood pressure, body weight, body mass index (BMI) and urinary protein, the data recorded within ± 1 year and closest to the baseline eGFR were used for computations. To be certain that the initial values extracted from the medical records were a valid representation of the baseline status of the patients, repeated measures of weight, systolic and diastolic blood pressures and urinary protein excretion within 6 months of the initial assessment showed no significant changes, except for diastolic blood pressure for which there was a 3.9 mmHg decrease. The second assessment of eGFR did not occur until 2.6 years after the initial assessment, and revealed a decline in kidney function of −2.38 ml/min/1.73 m2/year, which is consistent with the overall decline in eGFR reported in this paper. The median number of serum creatinine determinations available for the patients was 6 (range 3–33) samples, and the median follow-up period for the patients was 5.6 years (range 0.1–28.2 years). The median date for the initial creatinine assessment for the cohort was July 1994 (range June 1963–September 2001). Patients with three or more eGFR assessments were included in eGFR slope calculations; data from patients after they had reached ESRD were excluded. The eGFR slopes were computed for patients stratified by baseline eGFR ≥60 or <60 ml/min/1.73 m2 (CKD), as well as by baseline urinary protein groups (<0.1, 0.1 to <1 and ≥1 g/24 h).
Cardiac events
Events included myocardial infarction (MI) determined by electrocardiographic (ECG) changes, probable ECG change with symptoms and abnormal enzymes or MI noted in the medical records; percutaneous transluminal coronary angioplasty; intra-aortic balloon pump; coronary artery bypass graft; valve replacement; any type of arrhythmia [i.e. bradyarrhythmias, ventricular arrhythmias, supraventricular arrhythmias, premature (extra) beats] and presence of related symptoms, anti-arrhythmic medication, cardioversion, pacemaker, defibrillator; angina (new, rest or increasing angina, ECG with angina) and heart failure (physical findings, dyspnoea, imaging and intravenous medications).
Cerebrovascular events
These included stroke or transient ischaemic attacks (TIA) classified by vascular territory or by amaurosis fugax. Stroke was classified as due to haemorrhage or infarction, and the territory was noted.
Other clinical data
These included demographics, α-GalA mutation, onset of symptoms, weight, blood pressure, use of angiotensin-converting enzyme (ACE) inhibitors and diabetes. The dates were recorded for first symptom attributed to Fabry disease and for death.
Mutational analyses
Genomic DNA was isolated from blood collected in EDTA, and the α-GalA exons and adjacent intronic and promoter regions were sequenced using standard techniques [14,16].
Statistical analyses and calculations
Means with standard deviations and/or medians with ranges were used to summarize continuous variables. Mixed models with random intercepts and slopes for each patient, and fixed effects for gender and eGFR and proteinuria subgroups were used for eGFR slopes. Baseline eGFR was used as a covariate in the mixed models. Time to first renal, cardiac or stroke event, or death was determined using Kaplan–Meier curves by gender.
Results
Patient population
Characteristics of the 447 patients are presented in Table 1. The mean age at first entry in the abstracted medical records (baseline) was 38.6 and 44.9 years for males and females, respectively; data were available for a median of 12 years per patient. Symptom onset for males was usually before the age of 15 years (mean 10.5 years), whereas age at first symptom was later for females (mean 17.4 years). Females were also diagnosed later than males (mean age 29.7 versus 23.9 years, respectively). Patients with a known family history had earlier diagnoses [24.8 ± 14.7 years (n = 352) versus 34.2 ± 16.8 years (n = 52)]. The diagnosis of Fabry disease was confirmed by α-GalA activity and/or mutation analyses in 96% of the patients.
Table 1.
Patient characteristics
| Male (n = 279) | Female (n = 168) | Total (n = 447) | |
|---|---|---|---|
| Mean (range) age, years | |||
| At data abstraction | 38.6 (5.0–73.0) | 44.9 (10.3–77.1) | 41.0 (5.0–77.1) |
| At first symptom (n = 263, 185 M, 78 F) | 10.5 (0.3–56.0) | 17.4 (2.6–56.1) | 12.6 (0.3–56.1) |
| At diagnosis (n = 404, 258 M, 146 F) | 23.9 (0–66.5) | 29.7 (0–76.2) | 26.0 (0–76.2) |
| At death | 49.9 (34.5–59.4) | 52.6 (36.3–70.1) | 50.3 (34.5–70.1) |
| Number of patients deceased, n (%) | 20 (7) | 3 (2) | 23 (5) |
| Ethnicity, n (%) | |||
| White | 238 (85) | 144 (86) | 382 (85) |
| Hispanic | 20 (7) | 5 (3) | 25 (6) |
| African American | 6 (2) | 2 (1) | 8 (2) |
| Asian | 1 (0) | 0 (0) | 1 (0) |
| Not reported | 10 (4) | 16 (10) | 26 (6) |
| Diagnostic confirmation, n (%) | |||
| α-GalA activity and mutation | 212 (76) | 128 (76) | 340 (76) |
| α-GalA activity, only | 29 (10) | 18 (11) | 47 (11) |
| α-GalA mutation, only | 23 (8) | 17 (10) | 40 (9) |
| α-GalA activity/mutation not reporteda | 15 (5) | 5 (3) | 20 (4) |
| α-GalA activity | |||
| Plasma (nmol/h/ml) | |||
| n | 120 | 64 | NA |
| Mean (±SD) | 1.0 (1.85) | 6.2 (6.11) | NA |
| Leukocytes (nmol/h/mg) | |||
| n | 121 | 82 | NA |
| Mean (±SD) | 2.1 (3.67) | 28.3 (29.30) | NA |
| Fabry genotypeb, n | 238 | 145 | 383 |
| Missense, n (%) | 117 (49) | 74 (51) | 191 (50) |
| Nonsense, n (%) | 49 (21) | 29 (20) | 78 (20) |
| Deletion, n (%) | 42 (18) | 22 (15) | 64 (17) |
| Insertion, n (%) | 9 (4) | 4 (3) | 13 (3) |
| Splice site, n (%) | 10 (4) | 7 (5) | 17 (4) |
| Complex, n (%) | 5 (2) | 3 (2) | 8 (2) |
| Residual activity variant, n (%) | 6 (3) | 6 (4) | 12 (3) |
NA, not applicable; SD, standard deviation; α-GalA, alpha-galactosidase A.
aOf the 20 patients with α-GalA activity/genotype not reported, 14 (11 males and 3 females) have reported a family history of Fabry disease and 3 others had α-GalA activity values, but the units were not specified.
bPercentages are based on the number of patients with genotype data in each column.
Fabry nephropathy
Estimated glomerular filtration rate
Baseline serum creatinine values were obtained for 243 males and 152 females and used to calculate eGFR with the MDRD equation [15]. Table 2 presents patient characteristics stratified by gender and baseline eGFR ranges. The average age of males with baseline eGFR ≥60 (n = 189) or CKD [eGFR <60 ml/min/1.73 m2 (n = 54)] was 27.3 and 41.8 years, respectively. A similar pattern was seen in females although the baseline eGFR values were obtained about 10 years later: 38.0 (n = 129) and 51.9 (n = 23) years, respectively.
Table 2.
Clinical values in Fabry males and females stratified by baseline estimated glomerular filtration rate (eGFR; ml/min/1.73 m2)
| Males | Females | |||
|---|---|---|---|---|
| eGFR ≥60 (n = 189) | eGFR <60a (n = 54) | eGFR ≥60 (n = 129) | eGFR <60a (n = 23) | |
| Age at baseline eGFR | ||||
| Mean (SD) | 27.3 (11.9) | 41.8 (9.9) | 38.0 (14.0) | 51.9 (12.3) |
| Urinary protein (g/24 h), n | 52 | 25 | 40 | 8 |
| Mean (SD) | 0.6 (1.0) | 2.6 (2.3) | 0.3 (0.4) | 1.1 (1.5) |
| Median | 0.2 | 1.7 | 0.2 | 0.3 |
| Range | 0–5.7 | 0.3–8.7 | 0–1.7 | 0–4.3 |
| Overt proteinuriab, n (%) | 19 (37) | 24 (96) | 8 (20) | 4 (50) |
| ACE inhibitor use, n (%) | 26 (14) | 22 (41) | 13 (10) | 5 (22) |
| Blood pressurec, n | 151 | 46 | 98 | 15 |
| Systolic, mean (SD) | 125 (15) | 128 (17) | 123 (18) | 135 (18) |
| Diastolic, mean (SD) | 74 (12) | 79 (12) | 75 (12) | 82 (7) |
| Hypertensived, n (%) | 98 (52) | 43 (80) | 62 (48) | 18 (78) |
SD, standard deviation; eGFR, estimated glomerular filtration rate.
.aCKD defined as eGFR <60 ml/min/1.73 m2.
bOvert proteinuria defined as >0.3 g protein in 24-h urine collection. Urinary protein assessment is the closest available reading within ± 1 year of baseline eGFR assessment.
cBlood pressures are the closest reading within ± 1 year of baseline eGFR assessment.
dHypertension is defined as systolic ≥130 or diastolic ≥80 mmHg, or patient is indicated on case report form as hypertensive on medications.
Urinary protein values were available for 125 (32%) patients at the time that their baseline serum creatinine values were recorded. Overt proteinuria (>0.3 g/24 h) was present in 96% of males and 50% of females with baseline CKD. Patients with CKD more commonly received ACE inhibitor therapy, but only a minority of males and females with CKD were treated with these drugs.
Hypertension
The mean systolic and diastolic blood pressures were normal: 124 mmHg (range 70–186) systolic and 71 mmHg (range 42–111) for 142 males ≤30 years of age, and 119 mmHg (range 90–172) systolic and 72 mmHg (range 43–110) for 50 females ≤30 years of age; and 130 mmHg (range 86–174) systolic and 76 mmHg (range 43–110) for 188 males >30 years of age, and 127 mmHg (range 85–185) systolic and 77 mmHg (range 49–110) for 126 females >30 years of age. Hypertension (systolic or diastolic blood pressure >130 or >90 mmHg, respectively) was present in 14% of 142 and 27% of 188 males, and 6% of 50 and 22% of 126 females ≤30 and >30 years, respectively. While ∼80% of both males and females with CKD at baseline were hypertensive, only ∼50% of those with baseline eGFR ≥60 ml/min/1.73 m2 were hypertensive (Table 2).
Progression of chronic kidney disease
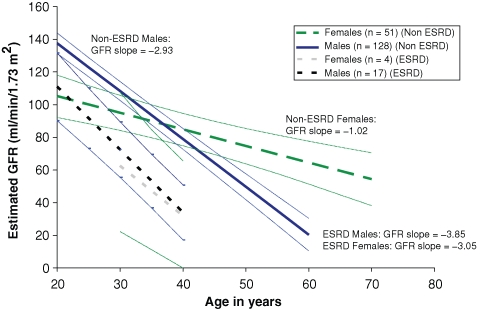
The rate of eGFR decline (progression) was stratified by gender and ultimate progression to ESRD (Figure 1). Of males who had at least three serum creatinine determinations, mean progression rates for those who developed (n = 17) or did not develop ESRD (n = 128) were −3.85 and −2.93 ml/min/ 1.73 m2/year, respectively (difference statistically significant, P = 0.0354). For females who developed (n = 4) or did not develop ESRD (n = 51), mean progression rates were −3.05 and −1.02 ml/min/1.73 m2/year, respectively (P = 0.0653). When stratified by the CKD status (Table 3), the progression rates were 2-fold greater for both males and females who had baseline CKD compared to those who had higher baseline eGFR values. The progression rates for males with eGFR ≥60 ml/min/1.73 m2 and CKD were −3.0 and −6.8 ml/min/1.73 m2/year, respectively. Progression rates for females with baseline eGFR ≥60 ml/min/1.73 m2 and CKD were −0.9 and −2.1 ml/min/1.73 m2/year, respectively. These progression rates were significantly higher for males than for females in each baseline eGFR strata; P-values were 0.043 and 0.037, respectively (Table 3). The baseline ages for each group, as well as the duration of follow-up after the baseline, are also shown in Table 3.
Fig. 1.
eGFR regression slopes with 95% CI for male and female patients who progressed to ESRD during the observation period, compared to those who did not progress to ESRD, based on medical record review. ESRD was defined by institution of renal replacement therapy (dialysis or transplant) or achieving a serum creatinine >6 mg/dl. The eGFR (ml/min/1.73 m2) and progression rates (ml/min/1.73 m2/year) were averaged for all patients who had at least three serum creatinine determinations available from the medical record review. Modelling lines for patients who progressed to ESRD were extended back to start at the minimum age for baseline eGFR, i.e. ∼20 years for males and ∼30 years for females (see Table 5). The lines end on average at about the age of ESRD for these patients, i.e. ∼40 years. Modelling lines for patients without ESRD were extended to cover the ages where the data ended, i.e. ∼70 years for some females and ∼60 years for some males.
Table 3.
Progression rates for males and females stratified by baseline estimated glomerular filtration rate (eGFR; ml/min/1.73 m2)
| Males | Females | |||
|---|---|---|---|---|
| Parameter | eGFR ≥60 (n = 117) | eGFR <60a (n = 28) | eGFR ≥60 (n = 42) | eGFR <60a (n = 13) |
| eGFR slope (ml/min/1.73 m2/year)b | ||||
| Mean (SEM) | −3.0 (0.1) | −6.8 (1.5) | −0.9 (0.9) | −2.1 (1.6) |
| Time from baseline eGFR to last eGFR (years) | ||||
| Mean (SD) | 8.5 (6.8) | 2.4 (2.5) | 7.8 (7.2) | 4.3 (2.5) |
| Median | 6.7 | 1.3 | 5.6 | 4.4 |
| Baseline age (years) | ||||
| Mean (SD) | 27.8 (12.0) | 41.9 (10.5) | 38.7 (13.1) | 52.1 (13.6) |
| Median | 27.0 | 42.0 | 38.7 | 50.7 |
SEM, standard error of the mean; SD, standard deviation; eGFR, estimated glomerular filtration rate.
P-values for differences males versus females; eGFR ≥60: P = 0.043; eGFR <60: P = 0.037. eGFR assessments after the start of dialysis or transplant or serum creatinine >6 mg/dl (530 μmol/l) are not used in computation of eGFR slopes. Based on a mixed model with fixed effects for intercept, slopes and covariate adjustment for baseline eGFR values along with random effects for individual patient intercepts and slopes.
aCKD defined as eGFR <60 ml/min/1.73 m2.
bPatients with three or more eGFR assessments were included in the eGFR slope calculations.
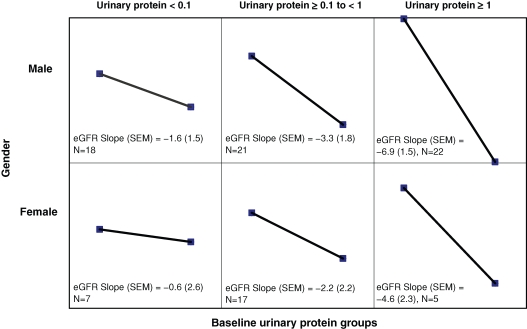
Progression rates were stratified by baseline urinary protein excretion (Figure 2). Higher baseline proteinuria levels were associated with more rapid progression rates. The rates were −1.6, −3.3 and −6.9 ml/min/1.73 m2/year in males with baseline proteinuria <0.1 g/24 h, 0.1–1 g/24 h and ≥1 g/24 h, respectively (Table 4). A similar finding was seen in females stratified for baseline proteinuria: the rates were −0.66, −2.2 and −4.6 ml/min/1.73 m2/year in females with baseline proteinuria <0.1 g/24 h, 0.1–1 g/ 24 h and ≥1 g/24 h, respectively (Table 4). Males and females with higher baseline levels of proteinuria were older and had lower baseline eGFR values; they also had greater progression rates than those with less proteinuria (Table 4).
Fig. 2.
eGFR progression slopes (ml/min/1.73 m2/year) for male and female patients stratified by baseline 24-h urinary protein excretion (g/24 h). The y-axis represents eGFR (ml/min/1.73 m2) and the x-axis in each panel represents a 12-month span. SEM = standard error of the mean.
Table 4.
Characteristics of Fabry males and females stratified by baseline urinary protein
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Baseline proteinuria (g/24 h) | <0.1 | 0.1–1.0 | >1.0 | <0.1 | 0.1–1.0 | >1.0 |
| Number of patients | 18 | 21 | 22 | 7 | 17 | 5 |
| Mean age, years (SD) | 22.8 (12.8) | 36.0 (12.1) | 38.9 (10.3) | 39.0 (22.0) | 42.3 (12.1) | 47.2 (11.7) |
| Progression rate, ml/min/1.73 m2/year (SEM) | −1.6 (1.5) | −3.3 (1.8) | −6.9 (1.5) | −0.6 (2.6) | −2.2 (2.2) | −4.6 (2.3) |
| Mean baseline eGFR, ml/min/1.73 m2 (SD) | 138 (56.5) | 84.6 (37.1) | 58.5 (25.6) | 91.9 (41.2) | 89.6 (38.5) | 63.4 (18.9) |
| Mean follow-up time, years (SD) | 5.2 (5.4) | 2.5 (3.0) | 2.4 (2.1) | 3.3 (2.0) | 1.7 (1.6) | 5.6 (1.6) |
SD, standard deviation; SEM, standard error of the mean; eGFR, estimated glomerular filtration rate.
End-stage renal disease
A total of 49 males and 8 females progressed to ESRD, at a median age of 39.5 and 42.4 years, respectively (Table 5). They had advanced Fabry nephropathy at the baseline evaluation, with average eGFR for males (n = 32) and females (n = 6) of 40.9 and 22.3 ml/min/ 1.73 m2, respectively. The mean ages at baseline evaluations were 37.8 (males) and 42.0 years (females). For those with documented proteinuria, 90% of males and all females who progressed to ESRD had overt proteinuria (>0.3 g/24 h). Average values were 3.0 ± 2.7 g/24 h for males and 2.1 ± 2.0 g/24 h for females (Table 5). Fifty-seven patients reached ESRD, and of these, 28 males and 5 females had kidney transplants.
Table 5.
Summary information for patients with Fabry nephropathy who developed end-stage renal diseasea
| Males | Females | |
|---|---|---|
| ESRD patients (n) | 49 | 8 |
| Age at ESRD (years) | ||
| Mean (SD) | 39.5 (9.6) | 42.4 (11.7) |
| Median | 39.5 | 41.3 |
| Range | 18.0–58.0 | 29.5–65.9 |
| Baseline eGFR (ml/min/1.73 m2), n | 32 | 6 |
| Mean (SD) | 40.9 (30.8) | 22.3 (22.8) |
| Median | 34.1 | 12.9 |
| Range | 38–110 | 1.5–53.5 |
| Age at baseline eGFR (years) | ||
| Mean (SD) | 37.8 (8.9) | 42.0 (11.3) |
| Median | 39.6 | 41.9 |
| Range | 19.9–52.6 | 29.5–61.7 |
| Urinary proteinb (g/24 h), n | 10 | 3 |
| Mean (SD) | 3.0 (2.7) | 2.1 (2.0) |
| Median | 2.3 | 1.4 |
| Range | 0.1–8.7 | 0.5–4.3 |
| Overt proteinuriac, n (%) | 9 (90) | 3 (100) |
| Age at baseline urinary protein (years) | ||
| Mean (SD) | 34.3 (10.0) | 46.2 (14.5) |
| Median | 32.6 | 43.2 |
| Range | 19.9–48.8 | 33.4–61.9 |
| Genotypes | ||
| Patients with genotypesd, n | 36 | 5 |
| Missense, n (%) | 19 (52.8) | 3 (60.0) |
| Non-sense, n (%) | 8 (22.2) | 1 (20.0) |
| Splicing defect, n (%) | 3 (8.3) | 1 (20.0) |
| Complex, deletion, insertion, or frameshift, n (%) | 6 (16.7) | 0 |
SD, standard deviation; eGFR, estimated glomerural filtration rate; ESRD, end-stage renal disease.
aSerum creatinine ≥6 mg/dl (530 μmol/l) or chronic dialysis or transplantation.
bUrinary protein assessment is the closest reading within ± 1 year of baseline eGFR assessment.
cOvert proteinuria defined as >0.3 g protein in 24-h urine collection.
dOf the 16 patients without genotypes, 3 males had leukocyte α-GalA activity of 2, 7 and 16.4 nmol/h/mg, 2 (1 male and 1 female, respectively) had plasma α-GalA activity of 0.47 and 3.8 nmol/h/ml, and 8 other patients (7 males and 1 female) had reported a family history of Fabry disease.
Cardiac and cerebrovascular events
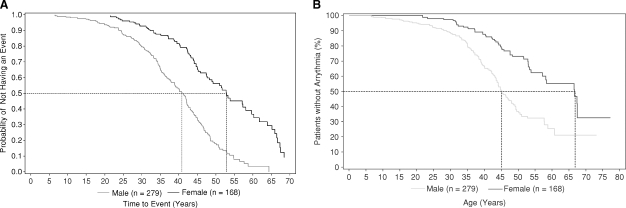
There was an age difference between males and females at the time of the first renal, cardiac or stroke event or death (Figure 3A). The initial events occurred in males before 10 years of age and continued for decades. Females did not have events until after 20 years of age. Half of the males and females had an event by 41 and 53 years, respectively.
Fig. 3.
(A) Kaplan–Meier estimate of time to first renal, cardiac, stroke event or death. Events were defined as detailed in the section ‘Data collection and analysis’. (B) Kaplan–Meier estimate of time to first cardiac arrhythmia. Male and female patients are shown as separate lines in each panel. Cardiac arrhythmias were defined as detailed in the section ‘Data collection and analysis’.
Cardiac events and interventions are summarized in Table 6; 137 males (49%) and 59 (35%) females had an event by a mean age of 36.2 and 44.4 years, respectively. Forty-six patients (11% of males, 9% of females) with available data were reported to have definite ECG changes. Angina was reported in 13% of males and 14% of females. MIs were reported for 7 (3%) males and 3 (2%) females. Only 3% of patients (11 males, 2 females) had findings of cardiac failure. Arrhythmias [including bradyarrhythmias, ventricular arrhythmias, supraventricular arrhythmias, premature (extra) beats] were by far the most common cardiac event, and were reported for 116 (42%) of the males and 46 (27%) of females. Approximately 1/3 of patients with an arrhythmia reported symptoms with a higher percentage of females (46%) than males (23%). Kaplan–Meier estimates of time to first arrhythmia are shown in Figure 3B. In males, arrhythmias first appeared in adolescence whereas in females they appeared in the early 20s. By the age of 45 years, 50% of males had a documented rhythm disturbance.
Table 6.
Summary of cardiac events in Fabry males and females
| Males | Females | All patients | |
|---|---|---|---|
| Cardiac event | (n = 279) | (n = 168) | (n = 447) |
| Any cardiac event, n (%) | 137 (49) | 59 (35) | 196 (44) |
| MI, n (%) | 7 (3) | 3 (2) | 10 (2) |
| Definite ECG change, n (%) | 31 (11) | 15 (9) | 46 (10) |
| Probable ECG change with symptoms and abnormal enzymes, n (%) | 0 | 0 | 0 |
| Death from MI, n (%) | 0 | 0 | 0 |
| Cardiac procedures, n (%) | 20 (7) | 9 (5) | 29 (6) |
| PTCA | 6 (2) | 2 (1) | 8 (2) |
| IABP | 1 (0) | 0 | 1 (0) |
| CABG | 5 (2) | 1 (1) | 6 (1) |
| Valve replacement | 3 (1) | 0 | 3 (1) |
| Cardiac hospitalization | 15 (5) | 11 (7) | 26 (6) |
| Angina, n (%) | 37 (13) | 23 (14) | 60 (13) |
| Rest | 12 (4) | 10 (6) | 22 (5) |
| Increasing | 13 (5) | 10 (6) | 23 (5) |
| Change in resting ECG | 3 (1) | 3 (2) | 6 (1) |
| New onset | 15 (5) | 16 (10) | 31 (7) |
| Positive stress test | 15 (5) | 7 (4) | 22 (5) |
| Cardiac failure, n (%) | 11 (4) | 2 (1) | 13 (3) |
| Physical findings | 7 (3) | 1 (1) | 8 (2) |
| Exercise intolerance | 8 (3) | 1 (1) | 9 (2) |
| Cardiac imaging | 8 (3) | 1 (1) | 9 (2) |
| IV medications | 3 (1) | 0 | 3 (1) |
| Arrhythmiaa, n (%) | 116 (42) | 46 (27) | 162 (36) |
| Arrhythmia symptoms | 27 (10) | 21 (13) | 48 (11) |
| Arrhythmia interventions | |||
| Anti-arrhythmic medication | 3 (1) | 4 (2) | 7 (2) |
| DC-cardioversion | 6 (2) | 2 (1) | 8 (2) |
| Pacemaker | 9 (3) | 1 (1) | 10 (2) |
| Defibrillator | 1 (0) | 1 (1) | 2 (0) |
MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; IABP, intra-aortic balloon pump; CABG, coronary artery bypass graft.
aIncludes bradyarrhythmias, ventricular arrhythmias, supraventricular arrhythmias, premature (extra) beats.
Cerebrovascular events (Table 7) included TIAs [17 (6%) males, 6 (4%) females] and strokes [30 (11%) males, 14 (8%) females]. The mean age for first TIA was 40.9 and 43.1 years, for males and females, respectively.
Table 7.
Cerebrovascular events in Fabry males and females
| Males | Females | All patients | |
|---|---|---|---|
| Cerebrovascular event | (n = 279) | (n = 168) | (n = 447) |
| TIAs, n (%) | 17 (6) | 6 (4) | 23 (5) |
| Mean age at first TIA in years (SD) | 40.9 (13.2) | 43.1 (15.5) | 41.5 (13.5) |
| Strokes | 30 (11) | 14 (8) | 44 (10) |
| Ischaemia | 26 (9) | 14 (8) | 40 (9) |
| Large vessel infarcts | 6 (2) | 5 (3) | 11 (2) |
| Small vessel infarcts | 13 (5) | 6 (4) | 19 (4) |
| Unknown | 7 (3) | 3 (2) | 10 (2) |
| Haemorrhagic | 3 (1) | 1 (0.5) | 4 (1) |
| Mean age at first stroke in years (SD) | 41.7 (12.2) | 44.9 (14.0) | 42.7 (12.7) |
TIA, transient ischaemic attack; SD, standard deviation.
Ischaemic stroke was considerably more common than haemorrhagic stroke (9% of males, 8% of females versus 1% of males, 0.5% of females) and was more often characterized as small vessel infarcts. Ischaemic stroke was reported for 16 (6%) males and 6 (4%) females, and middle cerebral was the most common location identified. The mean age for first strokes was 41.7 and 44.9 years, and for large vessel strokes 33.6 and 45.0 years for males and females, respectively.
Events in young males
Twelve males <18 years had cardiac or cerebrovascular events, consisting of arrhythmias in 11 and a stroke in 1 at age 6.7 years. One male with arrhythmia documented at age 17.5 years had an episode of angina 4 years later.
Death
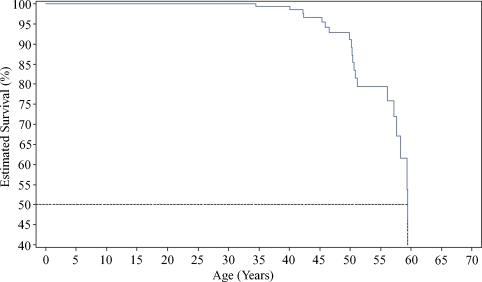
The mean age at death of 20 males was 49.9 years (Table 1). Three females died of unknown causes at the age of 36, 51 and 71 years, respectively. Kaplan–Meier analysis (Figure 4) showed that male Fabry patients had a 10% risk of death by 50 years of age, and the likelihood of death increased 5-fold to 50% by the age of 60 years.
Fig. 4.
Kaplan–Meier-estimated survival rates for male Fabry patients (n = 279).
Discussion
This study defines the natural history of the renal, cardiac and cerebrovascular complications in patients with Fabry disease before institution of ERT. Retrospective chart reviews, for a median of 12 years for each patient, described the progression of Fabry nephropathy, and the frequency and nature of life-threatening complications.
Males developed symptoms and were diagnosed earlier than females, and the majority of women were symptomatic with moderate to severe manifestations of Fabry disease. This may reflect an ascertainment bias inherent in the study design, but recent studies evaluating larger cohorts of females [8–12] have found that they range from asymptomatic to as severe as males with Fabry disease. An important cause of phenotypic variation in females is presumably due to random X-inactivation [8,11,12].
Rapid progression of the Fabry nephropathy was more prevalent among males than females, and older patients were more likely to have severe Fabry nephropathy at their first evaluation. Patients who developed ESRD had more rapid rates of progression than those who did not develop ESRD. Patients with advanced Fabry nephropathy (e.g. CKD) progressed more rapidly than those with baseline eGFR ≥60 ml/min/1.73 m2.
Branton et al. reported a mean progression rate of −12.2 ml/min/year in 14 untreated male Fabry patients who had stage 3 CKD at baseline and progressed to ESRD [17]. The progression rates ranged from −3.3 to −33.7 ml/ min/year, suggesting heterogeneity of the underlying processes. Progression may accelerate as nephropathy progresses, as the kidney function drops below a certain threshold. The progression rate should be monitored as an important outcome measure in Fabry nephropathy.
Overt proteinuria was an important finding in our study, and in a recent Fabry Registry study [10], and was more prominent in males and females with CKD. Overt proteinuria was noted in 90% of males and all females who progressed to ESRD. Consistent with recent studies [18–22], proteinuria was a risk factor for progression of nephropathy. Females had a similar association between baseline proteinuria and progression, but the slopes were less steep than those in males with similar levels of proteinuria.
Studies with ACE inhibitors showed that in type 1 diabetic [23] and nondiabetic patients with proteinuric CKD [24,25], reduction of proteinuria was associated with slowing of progression and lower risk of ESRD. Similar findings have been obtained with type 2 diabetic patients treated with angiotensin receptor blockers [26,27]. Data from the Fabry patients in this study were mostly from the era before the frequent use of these agents; only about a third of patients with CKD received anti-proteinuric agents.
The progression rate of Fabry nephropathy for females with eGFR values ≥60 ml/min/1.73 m2 was −0.9 ml/ min/1.73 m2/year, similar to that of healthy women. In contrast, females with CKD progressed more rapidly (−2.1 ml/ min/1.73 m2/year). When stratified by baseline proteinuria, females with overt proteinuria had substantial progression rates. These results show that proteinuria significantly increases the rate of progression of Fabry nephropathy in females.
Placebo-controlled clinical trials and open-label extension studies in patients with Fabry disease have evaluated the effects of ERT with recombinant agalsidase alfa at 0.2 mg/kg every other week (EOW) [22,28] and agalsidase beta at 1.0 mg/kg EOW [18,20,29]. ERT stabilizes GFR in patients with early Fabry nephropathy (relatively normal GFR, minimal proteinuria) [20,22] and clears GL-3 from renal cells [4]. Moreover, slowing of disease progression also has been shown for patients with moderate renal disease [18,21]. Early diagnosis, comprehensive evaluation of kidney function, aggressive management of the proteinuria and early initiation of ERT should optimize efforts to prevent or slow the progression of Fabry nephropathy [18,20–22,30].
The present study confirms the high prevalence of cardiac events [8,12,31], particularly of arrhythmias in Fabry patients. Only 2% of the patients had MIs while 13% had angina events. Comparison with non-matched data from the general adult US population (MIs, 5.5% of males, 3.4% of females; angina/coronary heart disease, 5.5% of males, 3.4% of females) [32] suggests that angina events may occur more frequently in Fabry patients. While no paediatric females had a known arrhythmia event, arrhythmias were relatively common among males aged <18 years. Thus, cardiac evaluation should be routinely performed in teenage and older Fabry patients [33].
The prevalence of TIAs and strokes was similar to other reports [8,31]. Most strokes were ischaemic, and most were small vessel infarcts. The most common documented location was the middle cerebral distribution. Cardiac complications and hypertension, associated with CKD, are likely contributing factors in the aetiology of TIAs and stroke in Fabry patients [34]. The ages at which males and females experienced cardiac events or strokes differed. On average, females had these events in their third or later decades of life, while males experienced them as early as in adolescence. Hypertension was more prevalent in males than in females. Whether hypertension is a risk factor for the occurrence of TIAs and stroke remains to be determined.
The mean age at death of 49.9 years for male patients was similar to that reported by others [17], and represents a reduction of approximately 25 years compared to the US general population [35]. For female patients, a median cumulative survival of 70 years has been previously reported approximating a reduction of 10 years as compared to the general population [35,36]. Early recognition of childhood symptoms [37,38], and timely therapeutic intervention offer the best hope for Fabry patients.
Although this study was carefully conducted with pre-defined analysis of the medical records of patients seen at expert centres, there are several evident limitations to this study that have to be acknowledged: (a) incomplete, inconsistent or erroneous documentation would decrease the power of subgroup analyses; (b) the fact that data were extracted from primary medical records over a 60-year span raises questions about validity and choice of endpoints. There have been changes in laboratory methods for measuring serum creatinine, and a central laboratory was not used for uniform analyses. Renal events were defined as an increase in measured serum creatinine by 50% to a value >1.4 mg/dl. Reference values have decreased over the years by 0.2–0.4 mg/dl, so the absolute change in kidney function cannot be over-interpreted other than to say that the 50% change in measured creatinine was an indication of significant decline in function; (c) similar concerns can be raised about using the extracted serum data to calculate eGFR precluding more detailed analysis of the eGFR data other than the dichotomous distinction between CKD (eGFR <60) and eGFR ≥60 ml/min/1.73 m2. However, the source of variability is lessened by using the regression slopes of the change in eGFR over time; (d) despite the fairly robust size of the cohort, the numbers of patients in some categories (e.g. the patients who progressed to ESRD in Figure 1) is too small to make meaningful comparisons between the categories; (e) a number of symptomatic complaints [e.g. premature (extra) beats] were retrospectively extracted from the medical records; (f) selection bias may well have influenced the inclusion of more severely affected individuals, and could have delayed the inclusion of female patients who were previously thought to be ‘carriers’ without important manifestations of Fabry disease and (g) the possibility that the regression slope of change in MDRD eGFR with time may underestimate the true rate of progressive loss of kidney function, especially if the GFR is relatively normal, is well described [39]. As a consequence, Fabry patients with low-grade proteinuria and relatively normal GFR, such as those presented in Figure 2, may have greater rates of progression than would be estimated by the current methodology.
Within these considerations, this study extends the existing knowledge of the natural history of Fabry disease prior to the initiation of ERT and, in particular, describes the progression rates of the Fabry nephropathy for patients stratified by gender and baseline proteinuria.
In conclusion, male patients with Fabry disease typically progressed more rapidly than females, but a considerable number of female patients in fact do have progressive Fabry nephropathy. Higher baseline proteinuria, systemic hypertension and lower baseline eGFR were associated with progression of eGFR loss and are important in evaluating therapeutic expectations and the response to ERT and adjunctive therapies in Fabry disease.
Acknowledgments
The authors are indebted to the patients whose data were included in this study, and to the physicians, nursing staffs and study coordinators who provided the patients’ care at all the investigational sites. The authors would like to acknowledge the efforts of Hans Ebels (Genzyme Corporation) in assisting with the preparation of the manuscript for submission. In particular, the authors would like to acknowledge the principal investigators at the study sites for their contributions: United States of America—J. Barranger, Pittsburgh, PA; K. Brandspiegel, Elizabeth City, NC; D. Brennan, St Louis, MO; J. Charrow, Chicago, IL; C.M. Eng, Houston, TX; R.W. Erbe, Buffalo, NY; P. Fernhoff, Atlanta, GA; R. Finkel, Philadelphia, PA; R. Hopkin, Cincinnati, OH; M.C. Leary, Boston, MA; C. Schmitt, Everett, WA; K.B. Sims, Boston, MA; R. Steiner, Portland, OR; J. Thomas, Denver CO; N. Weinreb, Coral Springs, FL; Czech Republic—S. Magage, Prague; Canada—D.G. Bichet, Montreal, QC; A. Chan, Edmonton, Alberta; J. Clarke, Toronto, Ontario; S. Dyack, Halifax, Novo Scotia; P. Wyatt, Toronto, Ontario. Furthermore, the authors wish to thank the nursing staffs of the general clinical research centres at our institutions for their assistance with this study. Genzyme Corporation supported data collection by Abt Associates Clinical Trials. This work was funded in part by grants from the National Center for Research Resources of the National Institutes of Health (NIH) grants to the General Clinical Research Centers at the Mount Sinai School of Medicine (5 M01 RR00071), Cedars-Sinai Medical Center (5 M01 RR00032), University of California, San Francisco (5 M01 RR01271), and University of Alabama at Birmingham (5 M01 RR00032). These studies also were supported in part by the research program of the National Institute of Neurological Disorders and Stroke, and an NIH research grant (MERIT Award, 5 R37 DK34045) to R.J.D. WW was supported by the Winnick Family Clinical Development Scholar Award. The authors wish to thank Fanny O’Brien, PhD (senior director of Biostatistics, BioMedical Operations, Genzyme Corporation) for statistical services.
Conflict of interest statement. R.S was partly funded by Shire Human Genetic Therapies and Amicus Therapeutics for research. M.B. is a member of the Board of Advisors of the Fabry Registry and is compensated for the services by Genzyme Corporation, and has received speaking fees from Genzyme related to lysosomal storage disorders. J.B. has nothing to disclose. G.E.L received reimbursement of expenses and small honoraria for lectures on the management of Fabry disease, from Genzyme Corporation, Shire Human Genetic Therapies and Actelion. All honoraria are donated to the ‘Gaucher Stichting’, a national foundation that supports research in the field of lysosomal storage disorders. S.P received research, educational and programmatic support from Genzyme Corporation. S.A.S. has been in receipt of honoraria, from Genzyme Corporation, for lectures on Fabry disease. W.R.W. is a paid consultant for Genzyme Corporation and Amicus Therapeutics; has been in receipt of speaker fees from Genzyme Corporation, an unrestricted educational grant from Amicus Therapeutics, and research support from Genzyme Corporation and Amicus Therapeutics. D.G.W. is a paid consultant for Genzyme Corporation, and has been in receipt of speaker fees and research support. R.J.D. is consultant, research grantee and inventor of patents licensed to Genzyme Corporation, and consultant and founding stockholder of Amicus Therapeutics.
References
- 1.Desnick R, Ioannou Y, Eng C. Alpha-galactosidase a deficiency: fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 2.Gubler MC, Lenoir G, Grunfeld JP, et al. Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978;13:223–235. doi: 10.1038/ki.1978.32. [DOI] [PubMed] [Google Scholar]
- 3.Sessa A, Meroni M, Battini G, et al. Renal pathological changes in Fabry disease. J Inherit Metab Dis. 2001;24:66–70. doi: 10.1023/a:1012423924648. [DOI] [PubMed] [Google Scholar]
- 4.Thurberg BL, Rennke H, Colvin RB, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Tøndel C, Bostad L, Hirth A, et al. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–776. doi: 10.1053/j.ajkd.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld J, Lidove O, Joly D, et al. Renal disease in Fabry patients. J Inherit Metab Dis. 2001;24:71–74. doi: 10.1023/a:1012475908718. [DOI] [PubMed] [Google Scholar]
- 7.Thadhani R, Wolf M, West ML, et al. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61:249–255. doi: 10.1046/j.1523-1755.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43:347–352. doi: 10.1136/jmg.2005.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampmann C, Baehner F, Whybra C, et al. Cardiac manifestations of Anderson–Fabry disease in heterozygous females. J Am Coll Cardiol. 2002;40:1668–1674. doi: 10.1016/s0735-1097(02)02380-x. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz A, Oliveira JP, Waldek S, et al. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant. 2008;23:1600–1607. doi: 10.1093/ndt/gfm848. [DOI] [PubMed] [Google Scholar]
- 11.Wang RY, Lelis A, Mirocha J, et al. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. 2007;9:34–45. doi: 10.1097/gim.0b013e31802d8321. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 14.Shabbeer J, Yasuda M, Benson SD, et al. Fabry disease: identification of 50 novel alpha-galactosidase A mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum Genomics. 2006;2:297–309. doi: 10.1186/1479-7364-2-5-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 16.Shabbeer J, Robinson M, Desnick RJ. Detection of alpha-galactosidase A mutations causing Fabry disease by denaturing high performance liquid chromatography. Hum Mutat. 2005;25:299–305. doi: 10.1002/humu.20144. [DOI] [PubMed] [Google Scholar]
- 17.Branton M, Schiffmann R, Kopp JB. Natural history and treatment of renal involvement in Fabry disease. J Am Soc Nephrol. 2002;13:139–143. [PubMed] [Google Scholar]
- 18.Banikazemi M, Bultas J, Waldek S, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- 19.Breunig F, Weidemann F, Strotmann J, et al. Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int. 2006;69:1216–1221. doi: 10.1038/sj.ki.5000208. [DOI] [PubMed] [Google Scholar]
- 20.Germain DP, Waldek S, Banikazemi M, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007;18:1547–1557. doi: 10.1681/ASN.2006080816. [DOI] [PubMed] [Google Scholar]
- 21.Schiffmann R, Askari H, Timmons M, et al. Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol. 2007;18:1576–1583. doi: 10.1681/ASN.2006111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffmann R, Ries M, Timmons M, et al. Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant. 2006;21:345–354. doi: 10.1093/ndt/gfi152. [DOI] [PubMed] [Google Scholar]
- 23.Lewis EJ, Hunsicker LG, Bain RP, et al. The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 24.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 25.Peterson JC, Adler S, Burkart JM, et al. The Modification of Diet in Renal Disease Study. Blood pressure control, proteinuria, and the progression of renal disease. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 27.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 28.Schiffmann R, Kopp JB, Austin HA, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 29.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 30.Tahir H, Jackson LL, Warnock DG. Antiproteinuric therapy and Fabry nephropathy: sustained reduction of proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol. 2007;18:2609–2617. doi: 10.1681/ASN.2006121400. [DOI] [PubMed] [Google Scholar]
- 31.Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 32.National Center of Health Statistics Data Analyses 2005. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5606a2.htm .
- 33.Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8:539–548. doi: 10.1097/01.gim.0000237866.70357.c6. [DOI] [PubMed] [Google Scholar]
- 34.Fellgiebel A, Müller MJ, Ginsberg L, et al. CNS manifestations of Fabry's disease. Lancet Neurol. 2006;5:791–795. doi: 10.1016/S1474-4422(06)70548-8. [DOI] [PubMed] [Google Scholar]
- 35.National Center of Health Statistics Data Analyses 2005. http://www.cdc.gov/nchs/data/hus/hus07.pdf#027 .
- 36.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr Res. 2008;64:550–555. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- 38.Ramaswami U, Whybra C, Parini R, et al. Clinical manifestations of Fabry disease in children: data from the Fabry Outcome Survey. Acta Paediatr. 2006;95:86–92. doi: 10.1080/08035250500275022. [DOI] [PubMed] [Google Scholar]
- 39.Rule AD. Understanding estimated glomerular filtration rate: implications for identifying chronic kidney disease. Curr Opin Nephrol Hypertens. 2007;16:242–249. doi: 10.1097/MNH.0b013e328057de8b. [DOI] [PubMed] [Google Scholar]