Abstract
Background. Microalbuminuria (MA) is a known predictor of cardiovascular disease (CVD) in European origin populations, but such data are lacking in native Indo-Asian populations, where CVD risks are high. Major electrocardiographic (ECG) changes are predictive of cardiovascular mortality. We determined the association of MA with major ECG changes in the general population of Pakistan.
Methods. A total of 3143 subjects aged ≥40 years from 12 randomly selected communities in Karachi participated. MA was defined as the urine albumin to creatinine (ACR) ratio of < 300 mg/g creatinine and ≥17 mg/g in men and ≥25 mg/g in women from a single-spot morning urine sample. Major changes on ECG were coded in duplicate using Minnesota classification.
Results. The mean age of subjects was 51.5 (10.7) years. The median (25–75 percentile) ACR was 4.2 (2.9–7.9) mg/g in men and 6.0 (3.9–10.8) mg/g in women (P < 0.001). The overall prevalence (95% CI) of MA was 12.3% (11.1–13.5%), and 20.3% in those with major ECG changes. In a multivariable model, major ECG changes (OR, 95% CI) (1.50, 1.10–2.00), diabetes (3.57, 2.93–4.35), hypertension (2.30, 1.85–2.86), female sex (0.61, 0.53–0.69), age (1.09, 1.05–1.13, for each 5-year increase) and eGFR (0.80, 0.78–0.81, for each 10 mg/g increase) were independently associated with MA.
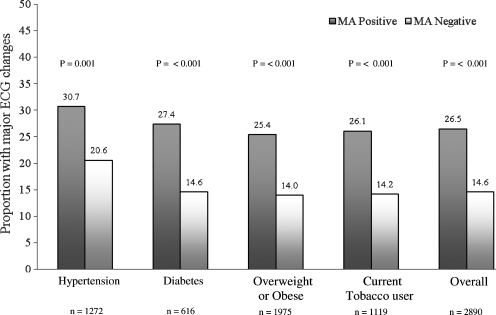
The presence of MA increased the prevalence of major ECG changes from 21 to 31% in those with hypertension (44.9%), 15 to 28% among those with diabetes (21.4%), 14 to 26% among those with overweight or obesity (68.4%) and 14 to 26% among current users of tobacco (38.7%) (P < 0.001) each.
Conclusions. The strong association between MA and major ECG changes underscores the importance of screening Indo-Asian subjects for MA for unmasking underlying CVD, especially those with hypertension, diabetes, obesity, and tobacco users.
Keywords: cardiovascular disease, ethnicity, hypertension, microalbuminuria
Introduction
Albuminuria is a known predictor of cardiovascular disease (CVD) in the Western population [1]. Reduction in proteinuria may reduce some of this risk [2]. Recent data suggest that the risk of cardiovascular disease (CVD) due to proteinuria may be graded, continuous and present at levels of urine albumin excretion that are even lower than the current operational definition of microalbuminuria (MA) [2,3]. The National Kidney Foundation recommends a gender-specific cut-off of the albumin to creatinine ratio for screening for MA [4]. Routine screening for MA is recommended for all patients with diabetes, but not necessarily in other high-risk groups such as those with hypertension [5].
Epidemiological studies have shown that major ECG changes are predictive of cardiovascular morbidity and mortality in men and women.
People of Indo-Asian origin have one of the highest susceptibilities to coronary artery disease (CAD) in the world [6,7]. Reports also suggest that compared to European-origin populations, people of Indo-Asian descent might be at enhanced risk of MA [8]. However, data on the association of MA with CVD in the Indo-Asian population are scarce and have been inconclusive, in part due to limited enrolment of Indo-Asian subjects in trials conducted in the West [9].
The estimation of burden of MA and its strength of association with CVD along the determinants of this relationship would guide the development of public health programs for prevention and management of chronic diseases. Such targeted screening strategies could be particularly useful in populations shown to be at high risk of CVD, diabetes and MA. We therefore conducted these analyses to determine the prevalence of MA and its association with major ECG changes in adult representatives of the general population in Karachi, Pakistan.
Methods
Ethical approval was obtained from Ethics Review Committee at Aga Khan University, Karachi.
Study design
A cross-sectional study as the first stage of a prospective study of cluster randomization trial of strategies to control hypertension. Trial registration number: NCT00327574.
Inclusion criteria
All subjects aged 40 years or above and able to consent.
Exclusion criteria
Poorly mobile patients, those mentally incompetent and unable to give consent, known advanced liver or kidney failure and pregnancy.
Sampling details
The Federal Bureau of Statistics has divided the city of Karachi into 5000 clusters each of about 250 households. On average, each household contains seven individuals, 20% of whom are aged 40 years or over. About 85% of all clusters (4200) are in the low-income group neighbourhoods (average household income less than US $120 per month) and were mapped into 12 geographically contiguous areas of 350 clusters each. One cluster was randomly selected (computer generated) from each area for inclusion in the study. A census was done and a listing of all individuals from all households in the selected areas was made.
Trained community health workers paid home visits to the selected individuals with an invitation to participate in the study. All subjects were evaluated after obtaining informed consent. The evaluation included the following: (a) history and physical examination; (b) questionnaire that details smoking status, food frequency questionnaire (FFQ) and other lifestyle factors, known status and World Health Organization (WHO) Rose questionnaire [10] for angina and international physical activity questionnaire (IPAQ); (c) assessments of BP with a calibrated automated device (‘Omron HEM-737™ Blood Pressure Monitor’) in the sitting position after 5 min of rest; (d) anthropometry (height, weight, waist and hip circumferences); (e) laboratory tests for risk factors for CVD including fasting blood glucose (Synchron Cx-7/Delta, Beckman, USA), lipid profile (HITACHI-912, Roche Japan), serum creatinine (calibrated at the Cleveland Clinic Laboratories), spot urine albumin (measured using nephelometry using the Array Systems method on Beckman Coulter) and urine creatinine (Synchron Cx-7/Delta, Beckman, USA); and (f) a 12-lead electrocardiogram (ECG). Those with elevated blood pressure, and not on antihypertensive medications, were visited again for re-measurement of blood pressure on a subsequent visit. All assessments were performed to a standard protocol that conformed to the international standards for definitions and measurements, and external quality control of albumin measurements were performed by the Bio-Rad Laboratories Inc., Irvine, CA, USA. The study was conducted over 1 year (2005–06).
Variable definitions
MA was defined as the urine albumin to creatinine (ACR) ratio of < 300 mg/g (34 mg/mmol) creatinine, ≥17 mg/g (1.9 mg/mmol) in men and ≥25 mg/g (2.8 mg/mmol) in women, from a single-spot morning urine sample [11].
Major ECG changes were defined as ECG abnormalities coded in duplicate according to the Minnesota classification: 4–1 or 4–2 or 5–1 or 5–2 or 6–1 or 6–2 or 7–1 or 7–2 or 8–1 or 8–3 [12,13].
Hypertension was defined as mean systolic BP ≥ 140 mmHg or mean diastolic BP ≥ 90 mmHg measured 20 min apart, on two separate occasions, or while taking antihypertensive medications.
Diabetes was defined as fasting blood glucose 126 mg/dl (7.0 mmol/l) or greater or being on antidiabetic medication [14].
Estimated glomerular filtration rate (eGFR) was calculated using the MDRD study equation [15].
Statistical analysis
All data analyses were done in SAS version 9.1.2 (SAS Institute Inc., Cary, NC, USA). The prevalence of outcomes (95% CI) was calculated for the screened population, accounting for the clustered design. The distributions of variables were compared between the population with and without the outcome using the t-test for continuous variables and the chi-squared test for discrete variables.
Multivariable models were built for the primary outcome of MA using forward stepwise selection with criteria for P = 0.2. Logistic regression analyses were performed using proc survey logistic in SAS. Candidate predictors included sociodemographic variables such as age, gender, level of education, economic status physical activity, smoking status, fasting blood lipid levels (LDL cholesterol, HDL cholesterol, triglycerides), body mass index, waist circumference, major ECG changes, hypertension and diabetes. Factors associated with MA with P < 0.05 were retained in the final model. We also searched for interactions between factors associated with MA and major ECG changes on MA.
Results
A target sample population of 3546 subjects aged 40 years or over, stratified by sex were invited to enrol in the study. A total of 3143 (88.6%) of the invited subjects consented to enrol in the study, and 2891 (92%) gave urine samples. The mean age of the subjects was 51.5 (10.7) years and 48% were males. The characteristics of the subjects are shown in Table 1.
Table 1.
Baseline population characteristics
| Individuals with MA | Unadjusted odds | Adjusted odds ratio | Adjusted odds ratio | ||
|---|---|---|---|---|---|
| Variables | Total (2891) | (n = 355) | ratio | model I | model II |
| Age (years), mean (SD) | 51.5 (10.7) | 56.3 (11.7) | 1.23 (1.21–1.26) (each 5 year increase) | 1.22 (1.20–1.25) (each 5 year increase) | 1.09 (1.05–1.13) (each 5 year increase) |
| Gender, n (%) | |||||
| Men | 1379 (47.7) | 187 (13.5) | 1 | 1 | 1 |
| Women | 1513 (52.3) | 168 (11.1) | 0.80 (0.67–0.96) | 0.78 (0.65–0.93) | 0.61 (0.53–0.69) |
| Educational attainmenta, n (%) | |||||
| Illiterate | 1000 (34.6) | 137 (13.7) | 1.22 (0.95–1.57) | NS | NS |
| Literate | 1892 (65.4) | 218 (11.5) | 1 | ||
| SESbn (%) | |||||
| Low | 2002 (69.2) | 234 (11.7) | 1 | NS | NS |
| Middle | 890 (30.8) | 121 (13.6) | 1.19 (0.83–1.70) | ||
| Current tobacco userc, n (%) | |||||
| No | 1771 (61.3) | 213 (12.0) | 1 | NS | NS |
| Yes | 1119 (38.7) | 142 (12.7) | 1.06 (0.85–1.33) | ||
| Physical activity METd (min/week) | |||||
| <840 | 1769 (61.2) | 244 (13.8) | 1.45 (1.14–1.85) | 1.35 (1.05–1.74) | NS |
| ≥840 | 1123 (38.8) | 111 (9.9) | 1 | 1 | |
| Waist circumference (cm) (SD) | 89.6 (12.4) | 92.0 (12.4) | 1.19 (1.13–1.25) | – | NS |
| Central obesitye, n (%) | 1936 (66.9) | 256 (13.2) | 1.32 (1.03–1.69) | – | NS |
| Body mass index, mean (SD) | 25.7 (5.7) | 25.8 (5.3) | 1.00 (0.98–1.03) | – | NS |
| Overweight/obesityf, n (%) | 1976 (68.4) | 244 (12.4) | 1.04 (0.80–1.36) | – | NS |
| Serum cholesterol (mg/dl), mean (SD) | 187.9 (39.3) | 193.2 (47.5) | 1.00 (1.00–1.01) | – | NS |
| HDL, mean (SD) | 40.2 (10.2) mg/dl 1.03 (0.26) mmol/l | 39.7 (10.3) 1.02 (0.26).mmol/l | 0.94 (0.90–0.99), each 10 mg/dl increase | – | NS |
| LDL, mean (SD) | 115.5 (30.8) mg/dl 2.96 (0.79).mmol/l | 118.0 (36.7) mg/dl 3.03 (0.94).mmol/l | 1.03 (1.01–1.05) each 10 mg/dl increase | – | NS |
| Triglycerides, mean (SD) | 159.3 (95.5) mg/dl 1.79 (1.07) mmol/l | 184.0 (111.8) mg/dl 2.07 (1.26) mmol/l | 1.02 (1.01–1.04) (each 10 mg/dl increase) | – | 1.01 (1.01–1.02) (each 10 mg/dl increase) |
| Hyperlipidaemiag, n (%) | 1007 (34.9) | 136 (13.5) | 1.20 (0.93–1.54) | – | NS |
| Diabetesh, n (%) | 616 (21.4) | 164 (26.6) | 3.97 (3.40–4.64) | – | 3.57 (2.93–4.35) |
| Hypertensivesi, n (%) | 1279 (45.0) | 241 (18.9) | 3.22 (2.60–3.97) | – | 2.30 (1.85–2.86) |
| CADj, n (%) | 150 (5.2) | 27 (18.2) | 1.64 (0.94–2.86) | – | NS |
| Positive Rose angina questionnaire, n (%) | 372 (12.9) | 40 (10.8) | 0.85 (0.58–1.25) | – | NS |
| Major ECG abnormalitiesk, n (%) | 462 (16.0) | 94 (20.3) | 2.11 (1.56–2.87) | – | 1.50 (1.10–2.00) |
| Composite CHD (CHD + Rose)l, n (%) | 956 (33.1) | 145 (15.2) | 1.47 (1.16–1.86) | – | NS |
| Q Waves changesm, n (%) | 65 (2.2) | 11 (16.9) | 1.47 (0.86–2.51) | – | NS |
| eGFRn (ml/min/ 1.73 m2) mean (SD) | 85.1 (20.0) | 75.0 (23.8) | 0.73 (0.72–0.75) (each 10 ml/min/ 1.73 m2 increase) | – | 0.80 (0.78–0.81) (each 10 ml/min/ 1.73 m2 increase) |
aEducational attainment: illiterate: no formal education and cannot read or write at all; literate: can read and write with understanding or completed at least V grade of formal education.
bSES was extracted from Pakistan Social and Living Standards Measurement Survey (PSLM) 2006–07 Federal Bureau of Statistics.
cCurrent tobacco users were defined as having tobacco in the form of smoking or chewing for at least 100 times in their lifetime or currently using.
dPhysical activity MET (Metabolic Equivalent) Score was defined as follows:
Total MET-minutes/week = Walk (METs − min*days) + Moderate (METs*min*days) + Vigorous (METs*min*days).
eCentral obesity was defined as having a waist circumference of ≥80 cm in women and ≥90 cm in men.
fOverweight/obesity was defined as Asian specific criterion of ≥23 kg/m2.
gHyperlipidaemia was defined as fasting serum cholesterol of ≥5.18 mmol/l (≥200 mg/dl).
hDiabetes was defined as patients taking antidiabetic drugs or fasting glucose level ≥126 mg/dl.
iHypertension defined as patients taking antihypertensive drugs or mean systolic blood pressure ≥140 mmHg or mean diastolic blood pressure ≥90 mmHg.
jCAD defined as presence of major Q-waves on ECG or history of heart attack diagnosed by a doctor.
kMajor ECG abnormalities defined as the following codes on ECG: Minnesota codes 4–1 or 4–2 or 5–1 or 5–2 or 6–1 or 6–2 or 7–1 or 7–2 or 8–1 or 8–3.
lComposite CHD: coronary artery disease was defined as the outcome of ischaemia on ECG (Minnesota codes 1–3 or 4–1 or 4–2 or 4–3 or 5–1 or 5–2 or 5–3 or 7–1) or past history of heart attack or Q-waves changes (Minnesota codes 1–1 to 1–2) or Positive Rose angina questionnaire.
mQ-wave changes: Minnesota codes 1–1 to 1–2.
neGFR: GFR is estimated for.
Male: [186 × ((sc)−1.154] × [(age in years)−0.203)].
Female: [(186 × ((sc)−1.154] [(age in years)−0.203)) × 0.742].
LDL or HDL: to convert mg/dl to mmol/l, divide by 39.
Triglycrides: to convert mg/dl to mmol/l, divide by 89.
The median urine albumin to creatinine ratio (25–75th percentile) was 5.2 (3.3–9.5) mg/g: 4.2 (2.9–7.9) mg/g in men and 6.0 (3.9–10.8) mg/g in women (P < 0.001)). The overall prevalence of MA was 12.3%: 13.5% in men and 11.1% in women (P = 0.05).
The unadjusted and adjusted odds for factors associated with MA are shown in Table 1. Increase in age, decrease in estimated GFR, and presence of diabetes, hypertension and major changes on ECG each were independently and positively associated with MA. None of the interactions were statistically significant.
Figure 1 illustrates the increase in the prevalence of major ECG changes in the presence of MA in the overall study population, as well as in subgroups with hypertension, diabetes, overweight or obesity and with tobacco use.
Fig. 1.
Association of major ECG changes and microalbuminuria (MA).
Discussion
The Indo-Asian population accounts for one-sixth of the world's inhabitants. This is the first report on the prevalence of MA and its relationship with CVD from a representative general population of an urban Indo-Asian city. We found that MA was independently associated with major ECG changes in men and women. Subjects with hypertension, diabetes, overweight or obesity and tobacco users exhibited a significant increase in the prevalence of ECG abnormalities in the presence of MA. These findings underscore the importance of screening Indo-Asians for MA, especially the individuals at high risk of CVD.
Reports in migrant Indo-Asian populations with MA suggest a faster progression of kidney disease compared to native Europeans [16]. However, data on prevalence, determinants and clinical conditions associated with MA in the native Indo-Asian populations are somewhat limited. These populations are relatively unique in terms of experiencing a double burden of undernutrition as well as rising rates of overweight and obesity, both enhancing susceptibility to chronic diseases including hypertension, diabetes, cardiovascular and kidney diseases [17].
Prevalence of MA
We found a high prevalence of MA in the order of 12.3% in the overall population aged 40 years or above. This burden was substantially greater among those with hypertension (18.9%) and diabetes (26.6%). While comparable population-based representative data on MA are not available from the Asia–Pacific region, the Microalbuminuria Prevalence Study (MAPS) reported a prevalence of 39.8% in a total of 6800 hypertensive diabetic adult patients from 10 Asian countries [18]. These findings and others from the region are consistent with those observed in the subjects with diabetes in our study [19,20].
Factors associated with MA
We found that the conventional predictors of MA (which also predict CVD) such as hypertension, diabetes and low eGFR were also independently associated with MA (Table 1). However, the association of MA with major ECG changes persisted even after accounting for these risk factors, indicative of incremental contribution of MA per se or its role as a marker of end organ damage. Consistent with this, the presence of MA was significantly associated with the concomitant presence of major ECG changes by 1.5-fold in those with hypertension and by 2-fold in those with diabetes, obesity or in tobacco users (Figure 1).
Implications of findings
Although the precise mechanism of the association between MA and CVD remains unknown, a common underlying endothelial pathology seems a plausible common link. It is critical to underscore that this association was present in those with and without other risk factors for CVD (diabetes, hypertension, obesity). If confirmed, this relationship may call for the modification of the approach to evaluation and management of several high-risk conditions. For instance, the existing JNC-7 guidelines for cardiovascular risk assessment in non-diabetic subjects with hypertension include the assessment of dipstick proteinuria or clinical albuminuria only [5] and the measurement of MA is recommended only among those with diabetes. Our findings would suggest that all hypertensive subjects should be screened for MA regardless of diabetes status, as its presence would indicate existing end organ damage. The same would apply to obese subjects and smokers. In fact, post hoc analysis of data from Losartan Intervention For Endpoint Reduction (LIFE) in the hypertension study suggests a potential role of MA as a modifiable risk factor on CVD as benefit of lowering albuminuria with Losartan on left ventricular hypertrophy [21]. Randomized controlled trials are needed to determine whether more aggressive management such as intensive control of blood pressure or lipids or use of blockers of RAAS is warranted for prevention of CVD in subjects with MA.
Limitations
Our study has limitations; first, we tested only a single urine sample, while confirmed diagnosis of MA requires persistence on at least two out of three consecutive tests. Moreover, concomitant haematuria and pyuria were not ruled out as their presence may indicate transient albuminuria. However, a single urine test is considered appropriate for epidemiological studies [22]. Second, ECG findings have not been validated in the Indo-Asian population. However, MA has been associated with CVD mortality in other populations, and our findings of the link between MA and major ECG changes suggest that the same is likely to apply to Indo-Asians as well [23]. Third, MDRD Study equations may be suboptimal for use in Indo-Asians, requiring a correction factor or new equation for precise estimation [24,25]. However, our use of estimated GFR as a continuous term in the multivariable model is likely to account for the relationship between unit changes in level kidney function in albuminuria independent of major ECG changes.
Finally, ours was a cross-sectional study, and the direction of causality cannot be established. Nevertheless, we demonstrate a strong association between MA and major abnormalities on ECG.
Strengths and conclusion
The major strengths of our study include a large representative sample from the general population and use of a validated screening tool for assessing MA [26].
In conclusion, ours is the first study on the prevalence of MA based on a representative adult population of an urban city in Indo-Asia, and the first to show the association between MA and major abnormalities on ECG in this population. We found a high burden of MA in the native urban Indo-Asian population, and an independent association of MA with major ECG changes. These findings highlight the potential role of MA as a risk factor or a marker of CVD in this population. Thus, screening for MA may help identify and risk stratify Indo-Asians in need of intensive cardiovascular risk management. These findings have substantial implications for native and migrant Indo-Asian populations globally. Future studies are needed to elucidate whether aggressive management for CVD risk reduction including lowering albumin excretion levels in Indo-Asian subjects with MA confers an advantage from CVD morbidity and mortality [27].
Acknowledgments
This study was supported by an award from the Wellcome Trust, UK (Dr Jafar). We would like to thank other members of the Hypertension Research Group (HRG). Members of the HRG other than the authors include Drs J. Hatcher, S. Badruddin, A. Hameed, F. Jafary, A. Khan, M. Karim, A. Gilani, S. Jessani, Mr R. Bux, M. Saleem, P. Cosgrove and Ms A. Khan (Aga Khan University, Karachi, Pakistan) and Drs N. Chaturvedi and N. Poulter (Imperial College, UK).
Conflict of interest statement. None declared.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 3.Diercks GF, Hillege HL, van Boven AJ, et al. Relation between albumin in the urine and electrocardiographic markers of myocardial ischemia in patients without diabetes mellitus. Am J Cardiol. 2001;88:771–774. doi: 10.1016/s0002-9149(01)01849-5. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Available at: http://www.kidney.org/professionals/KDOQI/guidelines_ckd/p7_risk_g14.htm. (accessed on 1 June 2006)
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924–929. doi: 10.1161/CIRCULATIONAHA.105.583815. [DOI] [PubMed] [Google Scholar]
- 7.Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA. 2007;297:286–294. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 8.Fischbacher CM, Bhopal R, Rutter MK, et al. Microalbuminuria is more frequent in South Asian than in European origin populations: a comparative study in Newcastle, UK. Diabet Med. 2003;20:31–36. doi: 10.1046/j.1464-5491.2003.00822.x. [DOI] [PubMed] [Google Scholar]
- 9.Tillin T, Forouhi N, McKeigue P, et al. Microalbuminuria and coronary heart disease risk in an ethnically diverse UK population: a prospective cohort study. J Am Soc Nephrol. 2005;16:3702–3710. doi: 10.1681/ASN.2005060584. [DOI] [PubMed] [Google Scholar]
- 10.Cook DG, Shaper AG, MacFarlane PW. Using the WHO (Rose) angina questionnaire in cardiovascular epidemiology. Int J Epidemiol. 1989;18:607–613. doi: 10.1093/ije/18.3.607. [DOI] [PubMed] [Google Scholar]
- 11.Mattix HJ, Hsu CY, Shaykevich S, et al. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 12.De Bacquer D, De Backer G, Kornitzer M. Prevalences of ECG findings in large population based samples of men and women. Heart. 2000;84:625–633. doi: 10.1136/heart.84.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bacquer D, De Backer G, Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80:570–577. doi: 10.1136/hrt.80.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, et al. Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Chandie Shaw PK, Baboe F, van Es LA, et al. South-Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch-European diabetic patients. Diabetes Care. 2006;29:1383–1385. doi: 10.2337/dc06-0003. [DOI] [PubMed] [Google Scholar]
- 17.Jafar TH, Qadri Z, Islam M, et al. Rise in childhood obesity with persistently high rates of undernutrition among urban school-aged Indo-Asian children. Arch Dis Child. 2008;93:373–378. doi: 10.1136/adc.2007.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weir MR. Albuminuria predicting outcome in diabetes: incidence of microalbuminuria in Asia-Pacific Rim. Kidney Int Suppl. 2004:S38–39. doi: 10.1111/j.1523-1755.2004.09209.x. [DOI] [PubMed] [Google Scholar]
- 19.Abo-Zenah H, El-Benayan A, El Nahas AM. Prevalence of increased albumin excretion rate in young Saudi adults. Nephron Clin Pract. 2008;108:c155–162. doi: 10.1159/000115328. [DOI] [PubMed] [Google Scholar]
- 20.Pan CY, Ho LT, Soegondo S, et al. Prevalence of albuminuria and cardiovascular risk profile in a referred cohort of patients with type 2 diabetes: an Asian perspective. Diabetes Technol Ther. 2008;10:397–403. doi: 10.1089/dia.2007.0296. [DOI] [PubMed] [Google Scholar]
- 21.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: iosartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 23.Yuyun MF, Khaw KT, Luben R, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33:189–198. doi: 10.1093/ije/dyh008. [DOI] [PubMed] [Google Scholar]
- 24.Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- 25.Srinivas S, Annigeri RA, Mani MK, et al. Estimation of glomerular filtration rate in South Asian healthy adult kidney donors. Nephrology (Carlton) 2008;13:440–446. doi: 10.1111/j.1440-1797.2008.00967.x. [DOI] [PubMed] [Google Scholar]
- 26.Jafar TH, Chaturvedi N, Hatcher J, et al. Use of albumin creatinine ratio and urine albumin concentration as a screening test for albuminuria in an Indo-Asian population. Nephrol Dial Transplant. 2007;22:2194–2200. doi: 10.1093/ndt/gfm114. [DOI] [PubMed] [Google Scholar]
- 27.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]