Abstract
Objective
To determine, by means of long-term follow-up evaluation, the outcome and accuracy of stereotactic core-needle biopsy (SCNB) of non-mass calcifications observed at mammography, and to analyze the factors contributing to false-negative findings.
Materials and Methods
Using a 14-gauge needle, SCNB was performed in cases involving 271 non-mass calcified lesions observed at mammography in 267 patients aged 23-72 (mean, 47) years. We compared the SCNB results with those of long-term follow-up which included surgery, mammography performed for at least six months, and reference to Korean Cancer Registry listings. We investigated the retrieval rate for calcifications observed at specimen mammography and histologic evaluation, and determined the incidence rate of cancer, sensitivity, and the underestimation rate for SCNB. False-negative cases were evaluated in terms of their mammographic findings, the effect of the operators' experience, and the retrieval rate for calcifications.
Results
For specimen mammography and histologic evaluation of SCNB, the retrieval rate for calcifications was, respectively, 84% and 77%. At SCNB, 54 of 271 lesions (19.9%) were malignant [carcinoma in situ, 45/54 (83%)], 16 were borderline, and 201 were benign. SCNB showed that the incidence of cancer was 5.0% (6/120) in the benign mammographic category and 31.8% (48/151) in the malignant category. The findings revealed by immediate surgery and by long-term follow-up showed, respectively, that the sensitivity of SCNB was 90% and 82%. For borderline lesions, the underestimation rate was 10%. For false-negative cases, which were more frequent among the first ten cases we studied (p = 0.01), the most frequent mammographic finding was clustered amorphous calcifications. For true-negative and false-negative cases, the retrieval rate for calcifications was similar at specimen mammography (83% and 67%, respectively; p = 0.14) and histologic evaluation (79% and 75%, respectively; p = 0.47).
Conclusion
In this study group, most diagnosed cancers were in-situ lesions, and long-term follow-up showed that the sensitivity of SCNB was 82%. False-negative findings were frequent during the operators' learning period.
Keywords: Breast, biopsy; Breast neoplasms, diagnosis; Stereotaxis
Since stereotactic breast biopsy was first described in 1990 (1), the procedure - though less common in Korea - has been widely used in western countries as an adjunct to breast radiology. The continually increasing number of women undergoing annual mammographic screening has led to an increase in the number of (mostly calcified) nonpalpable abnormalities identified (2). Calcification has been shown to be a component in up to 50% of malignant lesions (3), and in 84% of ductal carcinomas in situ (DCIS), the presence of calcification has been demonstrated by mammography (4). Although morphological analysis can provide guidelines for the management of these patients, many calcifications are indeterminate, and without the use of core biopsy, unnecessary surgical biopsy for benign lesions or the delayed diagnosis of malignant lesions is therefore likely.
In Korea, because of the relatively high expense involved and a poor understanding of the need for stereotactic equipment, ultrasonography (US)-guided core biopsy is more popular. It should be borne in mind, we believe, that for this procedure, the proper indication is a sonographically visible mass, and the use of US-guided biopsy for calcifications is limited. According to most published reports, however, the targets for core biopsy are all types of nonpalpable mammographic abnormalities, including a mass, calcifications, or a calcified mass (5-7). The reported concordance rate for surgical and stereotactic biopsy is 87-96% (5).
In this article, we describe our findings, including those determined during long-term follow-up, regarding the outcome and accuracy of stereotactic core-needle biopsy (SCNB) of non-mass calcifications observed at mammography. We also analyze the factors contributing to false-negative findings.
MATERIALS AND METHODS
Between April 1997 and March 2002, 284 SCNBs of nonpalpable mammographic calcifications were attempted at our institution. Thirteen of these (5%) were aborted [calcifications too faint or diffuse (n=8); breast too thin (n=3); calcifications too posterior (n=2)], and 267 consecutive patients aged 23-72 (mean, 47) years thus underwent 271 SCNBs. Our preference was to use US-guided core biopsy for all nonpalpable breast lesions (including calcifications) requiring biopsy, and SCNB only where US-guidance was regarded as too difficult after evaluating the imaging findings. Thus, during the above period, SCNB was used in only 20 cases involving a suspicious density or mass. Unless calcifications were within a mammographically fatty area, the usual purpose of pre-SCNB US was to locate a calcified mass.
Prior to commencing the procedure, the radiologists used the Breast Imaging Reporting and Data System (BI-RADS) to determine a mammographic assessment category of between 2 and 5.
SCNB was performed using a prone table stereotactic mammographic unit (StereoGuide DSM; LoRad Medical Systems, Danbury, Conn., U.S.A.), an automated gun (Biopty Gun; Bard, Covington, Ga., U.S.A.), and a 14-gauge needle (Biopty-Cut; Bard, Covington, Ga., U.S.A.). The median number of core specimens obtained was seven (range, five to 20). A long-excursion (2.3-cm throw) needle was used in the majority of cases, but in eight, the short-excursion type (1.2-cm throw) was used because of limited breast thickness. After adequate core tissues were obtained, the specimens were placed on a saline-moistured filter paper and radiographed without compression on a digital receptor of stereotactic equipment at 24-28 kVp and 8 mAs. If no calcified particles were visible, additional specimens were obtained until calcifications were identified or until further sampling was difficult due to either recurrent failure to acquire adequate tissue, or a patient's intolerance (8). All studies involved one of two radiologists (B-K.H. or Y.H.C.).
If SCNB detected malignancy, a borderline lesion, or a nonspecific benign lesion with definite radiologic-pathologic discordance, needle localization-excision was performed. Borderline lesions were those in which the pathologic findings indicated a high possibility of carcinoma, and included atypical ductal hyperplasia (ADH) and mucocele-like lesions (MLL). Where calcifications were mammographically assesed as benign (B1-RADS 2 or 3) and the histologic specimen was benign, close follow-up was recommended, and rebiopsy was not routinely performed even though the histologic specimen did not contain calcifications. A patient with a benign SCNB diagnosis - even where this showed radiologic-pathologic concordance - was advised to undergo mammographic follow-up, but needle localizationexcision was performed if the patient wanted the lesions removed, or they had progressed.
The pathologic results of SCNB and surgical biopsy were analyzed and compared with the mammographic category. Surgical biopsy was performed in 101 cases, and follow-up mammograms obtained 6-48 (mean, 16) months later were available in 98. The Korean Cancer Registry provided additional data regarding the development of cancer among our study population. The incidence rate of cancer, sensitivity, and underestimation rate for SCNB were investigated. The retrieval rates for calcification observed at specimen mammography and histologic evaluation were examined, and the respective numbers of true-negative and false-negative cases were compared. The latter were evaluated with regard to their mammographic findings, the effect of the operators' experience, and the retrieval rate for calcifications observed at specimen mammography, and histologic evaluation. Statistical significance was determined using Fisher's exact test.
An SCNB diagnosis of a borderline lesion with a surgical diagnosis of in-situ or invasive carcinoma, or an SCNB diagnosis of in-situ carcinoma with a surgical diagnosis of invasive carcinoma was regarded as a "histologic underestimate." An SCNB finding of benignancy or insufficiency was regarded as "false-negative" if the pathologic findings at surgery indicated malignancy, irrespective of the interval between surgical biopsy and SCNB. An SCNB diagnosis of carcinoma was considered "true-positive," with or without confirmation by means of excisional biopsy.
RESULTS
Calcifications were observed in 228 of 271 cases (84%) at specimen mammography and 210 of 271 cases (77%) at histologic evaluation of core tissue.
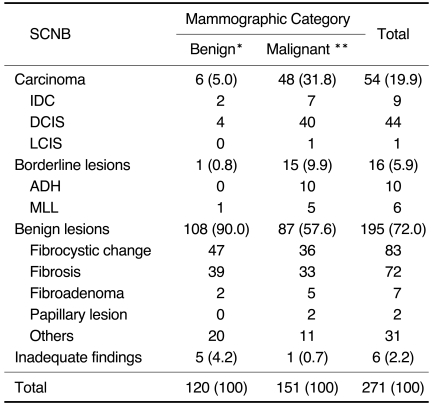
Table 1 compares the pathologic results of SCNB with mammographic category. At SCNB, 54 of 271 lesions (19.9%) were malignant, 16 (5.9%) were borderline (ADH, 10; MLL, 6), and 195 (72.0%) were benign; in six cases (2.2%), adequate pathologic diagnosis was impossible. Of 54 malignant lesions, 45 (83%) were carcinomas in-situ.
Table 1.
Comparison of the Pathologic Results of SCNB and the Mammographic Category of 271 Non-mass Calcified Lesions
Note.-Numbers in parentheses represent percentages.
IDC = invasive ductal cancer, DCIS = ductal carcinoma in-situ, LCIS=lobular carcinoma in-situ, ADH=atypical ductal hyperplasia, MLL=mucocele-like lesion
*BI-RADS category 2 or 3, **BI-RADS category 4 or 5
Table 2 shows the incidence rate of cancer as a function of mammographic category, comparing the findings of SCNB with the final diagnosis in 101 surgical biopsies and 170 cases involving follow-up. (In 98 of these, mammographic follow-up was longer than 6 months; in 72, the patients were lost to follow-up but were matched with data stored at the Korean Cancer Registry.) In the group in which mammographic assessment was benign (BI-RADS 2 or 3), the incidence of cancer was 5.0% (6/120) for SCNB and 8.3% (10/120) for surgical biopsy, while for the BIRADS 3 group, the corresponding figures were 5.5% (6/109) and 9.2% (10/109). In the BI-RADS 4 or 5 group (malignant assessment), the respective incidences were 31.8% (48/151) and 37.7% (57/151).
Table 2.
The Incidence Rate of Cancer as a Function of Mammographic Category: Comparison between SCNB Findings and Final Diagnosis, Including the Surgical and Follow-up Results
*BI-RADS category 2 or 3, **BI-RADS category 4 or 5
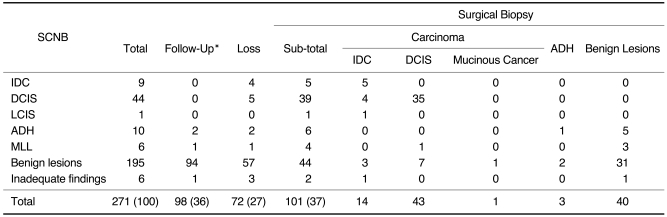
Table 3 displays the results of long-term follow-up, including a comparison of the pathologic results obtained at SCNB and surgical biopsy. Twelve additional cancers (false-negative cases) were found at immediate (n=6) or delayed (n=6) surgical biopsy among 211 cases with a benign or inadequate histologic result at SCNB, and one more cancer (a histologically underestimated case) was found among 16 cases in which SCNB revealed borderline histologic findings. Thus, among the 271 lesions, 67 cancers (24.7%) were finally diagnosed. Six false-negative cases, all of which were shown to be DCIS, were disclosed at immediate surgical biopsy performed due to radiologic-SCNB pathologic discrepancy. In one such case, although the mammographic category was BI-RADS 3, needle localization-surgical biopsy and SCNB were both performed on the same day, since this was our first SCNB case. The immediate surgical biopsy result indicated an SCNB sensitivity of 90% (55/61). Between six and 48 (mean, 19.8; median, 15.5) months after SCNBs, six further false-negative cases were disclosed at delayed surgical biopsy and by examining the listings maintained at the Korean Cancer Registry. On the basis of the long-term follow-up result, the final sensitivity of SCNB was thus found to be 82% (55/67). At the time of final diagnosis, four of six lesions were nonpalpable and the axillary lymph node was not involved, but two were palpable and associated with axillary lymph node metastasis. The pathologic diagnosis was invasive ductal cancer in four cases, mucinous cancer with DCIS in one, and DCIS in one. One false-negative case in which the final diagnosis was still DCIS was diagnosed 48 months after SCNB. Two others were diagnosed as cancers at other hospitals 18 and 25 months later, respectively, and information regarding the pathologic diagnosis, radiologic findings, and location of the cancers was obtained from those hospitals and the Korean Cancer Registry.
Table 3.
Long-Term Follow-Up Data, Including a Comparison of the Pathologic Results Obtained at SCNB and Surgical Biopsy
Note.-Numbers in parentheses represent percentages.
IDC=invasive ductal cancer, DCIS=ductal carcinoma in situ, LCIS=lobular carcinoma in situ, ADH=atypical ductal hyperplasia, MLL=mucocele-like lesion
*The follow-up interval was 6-48 (mean, 16; median, 12) months.
The underestimation rate of surgically excised borderline lesions and carcinomas in-situ was 10.0% (1/10) and 12.5% (5/40), respectively. One MLL was upgraded to a DCIS; no ADH was upgraded to a carcinoma, but five of six were downgraded at excision to usual ductal hyperplasia or fibrocystic change. Two ADH patients were lost to follow-up and their names did not appear in the records at the Korean Cancer Registry.
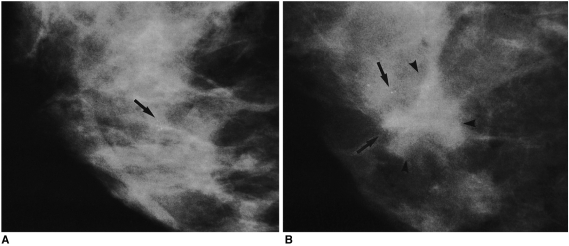
Mammographically, false-negative cases appeared as clustered, multiple, faint, amorphous calcifications (Figs. 1A, B). False-negative cases accounted for 12 of the total of 67 cancers (18%) [4 of 8 (50%) of those diagnosed during the first year, and 3 of 4 (75%) of those among the first ten cases (a proportion significantly higher than among the remaining 261 cases) (p = 0.01)]. If the period during which the first ten cases were diagnosed is excluded, being regarded as a learning period, the false-negative rate decreases from 18% (12/67) to 14% (9/54) and the sensitivity of SCNB increases from 82% (55/67) to 86% (54/63). For true-negative and false-negative cases, the retrieval rate for calcifications was not significantly different between specimen mammography [170/204 (83%) versus 8/12 (67%) (p = 0.14)] and histologic evaluation [162/204 (79%) versus 9/12 (75%) (p = 0.47)].
Fig. 1.
A 46-year-old woman with calcifications detected at screening mammography.
A. Routine mammogram obtained at the time of SCNB depicts multiple amorphous round calcifications in a 7-mm cluster, but no mass (arrow). Ultrasonography also failed to identify a mass associated with these calcifications (not shown here). Mammography of the SCNB core tissue specimen revealed a calcified particle, and the histologic diagnosis was fibrocystic change, with calcifications.
B. Mammogram obtained 13 months after SCNB, at which time the patient reported the presence of a lump, reveals a 2-cm-sized, irregular-shaped mass (arrowheads) at the same site, where a similar number of calcifications were present (arrows). Ultrasonography visualized two 2-cm sized masses above and below the nipple (not shown here). A modified radical mastectomy revealed the presence of a 4-cm-sized invasive ductal carcinoma, and single axillary lymph node metastasis.
DISCUSSION
In the past, needle localization-excision was considered the best technique for the accurate diagnosis of nonpalpable breast lesions detected during screening. The problems associated with diagnostic surgical biopsy include, however, a large scar, the general risks associated with open surgery (particularly where general anesthesia and hospitalization are involved), and a low positive biopsy rate (9).
In Korea, US-guided core needle biopsy has an established role in the diagnosis of nonpalpable breast lesions, but for SCNB, this is not so. Many nodules or masses observed at US have, therefore, been biopsied under US-guidance, but those not seen at US, such as non-mass calcifications, have been neglected, only followed-up, or operated on in two steps, diagnostic and therapeutic. If calcifications are identified at US, US-guided core biopsy may, of course, be possible; its advantages over SCNB include a shorter procedure time and less patient discomfort, particularly in Asian women with small breasts, in whom positioning and compression during stereotactic biopsy are difficult. However, the reported success rate of pre-SCNB US in identifying calcifications is 35.8% (10), and although the success rate of US-guided core biopsy of calcifications identifiable at US has been reported to be up to 100% (11), the difficulty of accurately targeting the tiny specular reflectors, as well as interference in the multipass technique by introduced air, is well recognized. Accordingly, the biopsy of non-mass calcifications requires the use of stereotactic equipment.
The incidence rate of cancer (i.e. the positive biopsy rate) determined using an image-guided core needle, for nonpalpable lesions, varies according to the target population, whether or not probably-benign lesions are included. Most reports suggest, however, that for nonpalpable lesions, the rate is 13 to 37% (12-14). According to the findings of a multi-institutional study undertaken by Parker et al. (12), the incidence rate of cancer for stereotactic and US-guided biopsy was similar (15% vs 17%), but for DCIS alone, the rate was much higher for stereotactic-guided biopsy (31.4% vs 6.9%). A domestic report on US-guided core needle biopsy showed that DCIS accounted for 10.9% of all cancers (13). In Korea, where stereotactic equipment is not generally used, a diagnosis of DCIS still depends on needle localization-excision or close observation until a DCIS becomes larger or progresses to an invasive cancer. DCIS is a precursor of invasive breast carcinoma, and generally becomes invasive within three to five years, particularly at high grades, though a low-grade DCIS can take up to 20 years to progress to an invasive cancer (15). The natural course of a DCIS is still unknown, but because the purpose of screening is to detect and remove a cancer during its early stage, effort has focused on increasing the detection rate during the DCIS phase. We suggest, therefore, that the use of SCNB in Korea should be further encouraged. In this study, the histologic findings of SCNB showed that DCIS accounted for 83% of all cancers.
Our results showed that among lesions whose mammographic category was benign, cancer was diagnosed at SCNB in 5.0% of cases (6/120). If these lesions had been neglected, six cases of cancer [DCIS (n=4) and invasive cancer (n=2)] could have been missed. In this group, follow-up revealed four more cancers, of which three (including two that progressively became invasive) were detected at least nine months after SCNB. Sickles (16) suggested that among category- 3 lesions ("probably benign") requiring follow-up every six months for two or three years, the incidence of cancer is ≤2%. Numerous articles have shown that the positive core biopsy rate of BI-RADS 3 was 3-13% (9, 17); the 5% detection rate for BI-RADS 3 noted in this study does not reflect the cancer rate for all BI-RADS 3 lesions, for which mammographic follow-up was routinely recommended. Some patients, however, such as those with a past history of breast cancer, a personal preference, or about whom a clinician was concerned, underwent core biopsy. It is not intended that the findings of this study should encourage tissue sampling of all BI-RADS 3 lesions, but the inclusion of many such cases reflects the recent trend of histologically confirming an apparently benign lesion in order to dispel a patient's anxiety and that of their clinicians (and perhaps radiologists). Another concern relating to the follow-up of BI-RADS 3 lesions is that a patient might not follow the instructions received. In fact, 62/157 (39.5%) of our patients whose SCNB results were benign and three with borderline results (ADH or MLL), and who were asked to return six months later, were lost to follow-up. Among this former group, two additional invasive cancers were discovered at other hospitals. In patients with BI-RADS 3 lesions, follow-up mammography should, therefore, be strictly enforced.
Some surgeons have suggested that BI-RADS 4 lesions should be surgically excised, though in our study group, the positive biopsy rate for calcifications of this grade was 31.8% (48/151). Excluding the 39 surgically excised lesions for which discordance was strong, the use of SCNB meant that for 64 of 151 BI-RADS 4 lesions, an unnecessary surgical biopsy was unequivocally avoided.
In this study, the sensitivity of SCNB, based on the results of immediate surgical biopsy and long-term follow-up was, respectively, 90% (55/61) and 82% (55/67). As compared to reported false-negative rates of 0.3-8.2% in a large core-needle-biopsy series (18), the false-negative rate of 18% in our series was, we thought, too high. Since, however, SCNB showed that the cancers in this study group were mostly DCIS (45/54, 83%), and the target mammographic findings were non-mass calcifications, this level of accuracy is not lower than that noted in other reports (12, 19). Brenner et al. (19) reported that the sensitivity of core biopsy was 99% for invasive cancer and 67% for DCIS. In addition, its diagnostic accuracy was 98-99% for masses and 87-91% for calcifications. In our study, sensitivity was 82% overall, 79% for invasive cancer cases, and 85% for DCIS. The histologic results for the five missed invasive cancers were, however, obtained at surgical biopsy performed between 6 and 25 months after SCNB. In these cases, the histologic diagnosis at the time of SCNB could have been ADH or DCIS.
Although several published reports have suggested that the diagnostic accuracy of SCNB and surgical excision is similar (1, 7, 20, 21), many variables may determine that of the former. These include the mammographic appearance of the lesions (mass, calcified mass, or calcifications), the number and volume of core biopsy samples, and progressive improvement in the performance of the radiologists involved. In eight cases, due to the limitation imposed by the compressed thickness of the breast, we were obliged to use short excursion needles, and in one of the patients involved, immediate surgery indicated a false-negative finding. A recent development, namely vacuum-assisted percutaneous biopsy, which is able to obtain a larger volume of suspicious tissue after only one puncture, holds much promise, particularly for determining the nature of nonpalpable, US-nonvisible microcalcified lesions (9).
One of the most important factors contributing to the accuracy of the procedures is improvement which occurs as operators gain experience (19, 22). Earlier investigators have suggested that for stereotactic breast biopsy, a learning curve exists, and have reported that significantly higher technical success rates and lower false-negative rates were achieved after the first five to 20 cases involving stereotactic 14-gauge automated core biopsy (22).
The reported calcification retrieval rate at specimen mammography has generally been 85-100% (22-24), but, in this study, the rate was 84%. This slightly lower figure might be due to the larger population of probably benign lesions, which were generally small clusters of indistinct calcifications. An earlier study (24) noted that amorphous calcifications portended higher failure rates at stereotactic biopsy, even with a vacuum-assisted device. Although the usual radiographic settings for the imaging of core specimens are 22-23 kVp and 7-20 mAs (23, 24), we performed specimen mammography using the monitor of a digital stereotactic machine set at 24-28 kVp and 8 mAs: at these settings, most calcifications were visible. Because of the amorphous morphology encountered encountered, we were not always convinced of the presence of calcifications, and under such circumstances and in order to verify the accuracy of targeting, searched for these in the histologic specimens obtained. However, study of the histologic correlation of core biopsy specimens suggested that if calcification is not visible on a core biopsy radiograph, any microscopic calcification observed in histologic sections would not represent radiologic calcification (because microscopic tissue calcification would be smaller than that resolved at radiography) (25). Among 16 cases in which we ensured targeting accuracy by confirming the presence of calcification in a histologic specimen, two were found to be false-negative. Our histologic retrieval rate for calcifications was lower than the radiographic retrieval rate, and radiographically visible calcium was not evident at pathologic analysis in 25 of 228 cases (11%). For this, there are at least three possible explanations: calcium may be lost during tissue preparation, or can be dislodged from the tissue by the blade; calcifications made up of calcium oxalate crystals are difficult to visualize in histologic sections; tissue may be incompletely sectioned (23). Thus, if radiographically visible calcifications in core tissues are not identified in histologic sections, and the histologic diagnosis is benign, it is essential to establish the presence of calcium oxalate crystals by using a polarized microscope or to search for histologically visible calcifications by obtaining deeper sections. In this way, a false-negative diagnosis can be avoided. In a paraffin block, a histologically diagnostic area may be deeper (25). Although we did not obtain additional deeper sections in false-negative cases, calcification was was observed in one such case on a core specimen radiograph but not histologically, and invasive ductal cancer was diagnosed 18 months later. In our study, both mammographic and histologic retrieval rates for calcifications showed no significant difference between true-negative and false-negative cases, a finding which may reflect the inadequate amount of specimen obtained at spring-loaded automated gun biopsy. This group consisted predominantly of lesions seen as non-mass calcifications at mammography; histologic examination showed that most diagnosed cancers were DCIS, but this may reflect the difficulty of diagnosing ADH using only a limited amount of core tissue. The low incidence rate of ADH (3.6%) and the absence of histologic underestimation of ADH diagnosed at SCNB suggest this possibility.
Since false-negative findings were concentrated among the first ten cases investigated, we suggest that cases diagnosed during the learning period should be more carefully followed up or further investigated by means of surgical biopsy.
In conclusion, although stereotactic biopsy can be reliably employed for the evaluation of calcifications observed at mammography, long-term follow-up showed that when the predominant mammographic finding was non-mass calcifications and the most commonly diagnosed cancer was DCIS, the sensitivity of SCNB was 82%. False-negative findings were frequent during the operators' learning period.
References
- 1.Parker SH, Lovin JD, Jobe WE, et al. Stereotactic breast biopsy with a biopsy gun. Radiology. 1990;176:741–747. doi: 10.1148/radiology.176.3.2167501. [DOI] [PubMed] [Google Scholar]
- 2.Fine RE, Boyd BA. Stereotactic breast biopsy: a practical approach. Am Surg. 1996;62:96–102. [PubMed] [Google Scholar]
- 3.Kopans DB. Breast imaging. 2nd ed. Philadelphia: Lippincott-Raven; 1998. p. 317. [Google Scholar]
- 4.Stomper PC, Connolly JL, Meyer JE, Harris JR. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology. 1989;172:235–241. doi: 10.1148/radiology.172.1.2544922. [DOI] [PubMed] [Google Scholar]
- 5.Liberman L, Dershaw DD, Glassman JR, et al. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151–157. doi: 10.1148/radiology.203.1.9122384. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblatt R, Fineberg SA, Sparano JA, Kaleya RN. Stereotactic core needle biopsy of multiple sites in the breast: efficacy and effect on patient care. Radiology. 1996;201:67–70. doi: 10.1148/radiology.201.1.8816522. [DOI] [PubMed] [Google Scholar]
- 7.Jackman RJ, Nowels KW, Shepard MG, Finkelstein SI, Marzoni FA. Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994;193:91–95. doi: 10.1148/radiology.193.1.8090927. [DOI] [PubMed] [Google Scholar]
- 8.Liberman L, Dershaw DD, Glassman JR, et al. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151–157. doi: 10.1148/radiology.203.1.9122384. [DOI] [PubMed] [Google Scholar]
- 9.Apesteguia L, Mellado M, Saenz J, Cordero JL, Reparaz B, Miguel CD. Vacuum-assisted breast biopsy on digital stereotaxic table of nonpalpable lesions non-recognizable by ultrasonography. Eur Radiol. 2002;12:638–645. doi: 10.1007/s00330-001-1168-2. [DOI] [PubMed] [Google Scholar]
- 10.Cheung YC, Wan YL, Chen SC, et al. Sonographic evaluation of mammographically detected microcalcifications without mass prior to stereotactic core needle biopsy. J Clin Ultrasound. 2002;30:323–331. doi: 10.1002/jcu.10074. [DOI] [PubMed] [Google Scholar]
- 11.Soo MS, Baker JA, Rosen EL, Vo TT. Sonographically guided biopsy of suspicious microcalcifications of the breast: a pilot study. AJR Am J Roentgenol. 2002;178:1007–1015. doi: 10.2214/ajr.178.4.1781007. [DOI] [PubMed] [Google Scholar]
- 12.Parker SH, Burbank F, Jackman RH, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193:359–364. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 13.You JK, Kim EK, Kim MH, et al. The usefulness of ultrasound-guided core needle biopsy for non-palpable breast lesion. J Korean Radiol Soc. 2002;46:601–606. [Google Scholar]
- 14.Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717–723. doi: 10.1148/radiology.208.3.9722851. [DOI] [PubMed] [Google Scholar]
- 15.Page DL, Rogers LW, Schuyler PA, Dupont WD, Jensen RA. The natural history of ductal carcinoma in situ of the breast. In: Silverstein MJ, editor. Ductal carcinoma in situ of the breast. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2002. pp. 17–21. [Google Scholar]
- 16.Sickles EA. Management of probably benign breast lesions. Radiol Clin North Am. 1995;33:1123–1130. [PubMed] [Google Scholar]
- 17.Gisvold JJ, Goellner JR, Grant CS, et al. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994;162:815–820. doi: 10.2214/ajr.162.4.8140997. [DOI] [PubMed] [Google Scholar]
- 18.Jackman RJ, Nowels KW, Rodriquez-Soto J, Marzoni FA, Finkelstein SI, Shepard MJ. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210:799–805. doi: 10.1148/radiology.210.3.r99mr19799. [DOI] [PubMed] [Google Scholar]
- 19.Brenner RJ, Fajardo L, Fisher PR, et al. Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol. 1996;166:341–346. doi: 10.2214/ajr.166.2.8553943. [DOI] [PubMed] [Google Scholar]
- 20.Parker SH, Lovin JD, Jobe WE, Burke BJ, Hopper KD, Yakes WF. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology. 1991;180:403–407. doi: 10.1148/radiology.180.2.1648757. [DOI] [PubMed] [Google Scholar]
- 21.Elvecrog EL, Lechner MC, Nelson MT. Nonpalpable breast lesions: correlation of stereotaxic large-core needle biopsy and surgical biopsy results. Radiology. 1993;188:453–455. doi: 10.1148/radiology.188.2.8327696. [DOI] [PubMed] [Google Scholar]
- 22.Liberman L, Benton CL, Dershaw DD, Abramson AF, La Trenta LR, Morris EA. Learning curve for stereotactic breast biopsy: how many cases are enough? AJR Am J Roentgenol. 2001;176:721–727. doi: 10.2214/ajr.176.3.1760721. [DOI] [PubMed] [Google Scholar]
- 23.Liberman L, Evans WP, III, Dershaw DD, et al. Radiography of microcalcifications in stereotaxic mammary core biopsy specimens. Radiology. 1994;190:223–225. doi: 10.1148/radiology.190.1.8259409. [DOI] [PubMed] [Google Scholar]
- 24.Berg WA, Arnoldus CL, Teferra E, Bhargavan M. Biopsy of amorphous breast calcifications: pathologic outcome and yield at stereotactic biopsy. Radiology. 2001;221:495–503. doi: 10.1148/radiol.2212010164. [DOI] [PubMed] [Google Scholar]
- 25.Dahostrom JE, Sutton S, Jain S. Histologic-radiologic correlation of mammographically detected microcalcification in stereotactic core biopsies. Am J Surg Pathol. 1998;22:256–259. doi: 10.1097/00000478-199802000-00016. [DOI] [PubMed] [Google Scholar]