Abstract
Enhanced z-axis coverage with thin overlapping slices in breath-hold acquisitions with multidetector CT (MDCT) has considerably enhanced the quality of multiplanar 3D reconstruction. This pictorial essay describes the improvements in 3D reconstruction and technical aspects of 3D reconstruction and rendering techniques available for abdominal imaging. Clinical applications of 3D imaging in abdomen including liver, pancreaticobiliary system, urinary and gastrointestinal tracts and imaging before and after transplantation are discussed. In addition, this article briefly discusses the disadvantages of thin-slice acquisitions including increasing numbers of transverse images, which must be reviewed by the radiologist.
Keywords: Computed Tomography; Abdomen; Multidetector, 3D
State-of-the-art cross-sectional imaging techniques allow radiologists to visualize disease with greater certainty by subtracting the impact of overlying tissues, thus allowing separate evaluation of individual organs, which aids in the detection and characterization of pathology.
Radiologists discovered in the late 1970s that although diagnosis based on axial CT images alone was more sophisticated than with plain radiography, the lack of a third dimension (e.g. sagittal and coronal dimensions) was frequently frustrating. Many referring physicians with no basic training in cross-sectional imaging still encounter difficulties in appreciating normal anatomy and pathology on transverse CT images, being more familiar with anatomy depicted in the coronal plane.
Following the somewhat crude three-dimensional computer rendering algorithms initially developed in late 1970s, that allowed formation of images in the third dimension from data acquired in the axial plane, the development of single-slice helical CT and more recently multidetector CT (MDCT) scanners has opened new chapters in 3D imaging. These advances were made possible by the rapid acquisition of volumetric data in the lower case z-axis using thin slices and improved rendering algorithms, which facilitate exquisite 3D reformats, devoid of degradation by respiration and other physiological movements. The pace of progress is being hastened with the rapid developments in MDCT technology (1). We describe 3D rendering techniques available for abdominal imaging including multiplanar reformations, surface rendering, virtual endoscopy, volume rendering and maximum intensity projections. We also discuss clinical applications of 3D imaging in the abdomen including the liver, pancreaticobiliary system, urinary and gastrointestinal tracts.
This paper also discusses the steps required for introducing 3D reconstruction into a radiology department. The present article also discusses the disadvantage of reduced collimation MDCT scanning, which results in increasing numbers of images for review.
TECHNICAL IMPLEMENTATION OF 3D RECONSTRUCTION
Introduction of 3D Imaging
Having arrived at a point of recognition that 3D imaging techniques are valuable, the challenge facing an institution is to develop a practical basis for performing 3D imaging. At our institution, we have set up a 3D imaging laboratory for the purpose of developing special expertise that is easily accessible.
The process of 3D imaging began with image acquisition protocols, which were optimized for subsequent post-processing. We have taken the step of identifying certain CT protocols that would always require 3D analysis and modified these protocols so that 3D imaging is an integral part of these studies. Referring physicians ordering these examinations, now understand that they are simultaneously requesting additional 3D analysis. However, there are certain studies in which 3D imaging does not appear important prior to imaging the patient, but on reviewing axial images the radiologist may feel that 3D reconstruction may offer a problem solving option. In these situations, it is preferable to reconstruct the volumetric helical data in thinner sections and use this data for appropriate 3D reconstructions.
Based on the standardized protocols, the technologists in the 3D laboratory apply the appropriate 3D rendering software to create a data set of 3D images that is returned to the Picture Archiving Communications System (PACS) associated with the source images. Development of close communication between physicians and 3D technologists and close proximity of the 3D laboratory to the CT interpretation area allows radiologists to participate in 3D reconstruction with the technologists. In addition to 3D renderings there are a number of applications, which require quantitative measurements. These include planning prior to aortic stent graft placement, or volumetric analysis prior to liver resection (2) and living related organ donation (3, 4).
Protocol Design
Preparation for 3D CT should begin prior to the arrival of the patient in the CT scanner. It is important in most cases, to withhold positive oral and rectal contrast agents to obtain satisfactory 3D CT images. This is particularly important for CT angiogiography, and CT urography when 3D reconstruction is planned. For pancreatic protocol MDCT scanning, water can be used as a negative oral contrast agent to aid in imaging of the duodenum and ampullary region. Optimum bowel cleansing or labeling of fecal matter with agents, which facilitate digital post-processing image subtraction is critical for virtual endoscopy reconstruction.
Optimum contrast volume, rate of injection, and timing of the scan at the peak concentration are pre-requisites for most 3D angiography applications. Only good source axial image data can provide reasonable quality 3D reconstruction. It is therefore critical to obtain thin, overlapping sections of the region of interest with minimum motion artifacts.
3D RENDERING TECHNIQUES AVAILABLE FOR ABDOMINAL IMAGING
Multiplanar reconstruction (MPR) provides efficient computation of images that lie along the non-acquired orthogonal orientations of the scanned volume by readdressing the order of voxels in the scanned volume. It is a fast and interactive algorithm that can represent several arbitrary planes at once and create multiplanar display in real-time. Generally, it is helpful whenever pathology cannot be accurately assessed on axial plane images alone.
Surface rendering (shaded surface display, SSD) is a surface-fitting algorithm that creates triangle-based isosurfaces within 3D space. For surface rendering, the user specifies a threshold attenuation value close to the center of the signal difference between the brightest pixel found within the object of interest and the signal in the surrounding structures. Then, the surface-rendering algorithm loops on successive slices and determines whether its corner values straddle the threshold value. Non-straddling cells are discarded, while straddling pixel values are chosen to generate surface rendering datasets. With the surface created from the data, the remainder of the data is discarded.
Although this technique is associated with loss of density information, it can aid in preoperative planning for interventional endovascular procedures and visualization of complex anatomic situations such as pathology of the thoracic and abdominal aorta.
Virtual endoscopy (VE) processes scan datasets to provide simulated visualizations of specific organs similar to those produced by standard endoscopic procedures (5). With increasing availability of MDCT scanners and acquisition of isotropic data, virtual endoscopy can now be performed using SSD or volume rendering techniques to generate high-resolution VE images in three-dimensions and perform "fly-through displays" of the trachea, esophagus, and colon, which are comparable to standard endoscopic views (5). In addition, VE can define precise location, size, and shape of lesions, both within and outside of the region of interest, information which is not always easily available with conventional endoscopy. However, despite being comparable to conventional endoscopy, VE does not provide information on the "flat lesions" or color of the mucosa and suffers the obvious disadvantage of not providing immediate biopsy specimens of lesions seen.
Volume rendering (multiplanar volume rendering, MPVR) is the visualization and manipulation of objects represented as sampled data in three or more dimensions. The technique interpolates the entire data set rather than editing a single scan to generate 3D images directly from scanned volume data. Unlike other projection techniques such as SSD and MIP, MPVR does not distort objects in the reconstructed planes. It allows "quick view" of large MDCT scan data sets with comprehensive details of the anatomic orientation of lesion or structures of interest.
The maximum intensity projection (MIP) technique displays the pixels of greatest intensity along a predefined axis of the image. It is useful for the depiction of vascular anatomy when there is a large difference between attenuation values (Hounsfield value, HU) of vessels opacified by contrast agent, and the surrounding tissues. MIP is useful for all types of CT angiography and has also been used for CT Urography (6, 7).
Minimum intensity projection (MinIP) is the opposite of MIP technique (7). Whereas, the latter technique is used for displaying high attenuation structures, the former displays 3D images of structures with lower attenuation values such as the bile ducts, pancreatic duct or the unopacified renal collecting system or ureters. On MinIP, structures such as the hepatic biliary tree and dilated unopacified ureter are displayed in three-dimensional plane and the margins of such structures are also much more apparent (7).
CLINICAL APPLICATIONS
There are many situations in which 3D imaging simply provides a "pretty picture" and does not provide additional diagnostic information and therefore additional cost cannot be justified. As experience is gained with 3D reconstruction, the goal is no longer the acquirement of a "pretty picture" but the production of additional images, which will have additional diagnostic value. There are many conditions in which the classical radiologic description was made on plain radiographs or contrast examinations and which are not as easily depicted on transverse CT images (7). 3D can be helpful in the characterization of these conditions. These conditions will be discussed and illustrated with examples.
Liver
Increasingly the use of MDCT with CT angiography is replacing conventional catheter angiography for transplant work-up (3, 4). The routine utilization of 3D reconstruction as part of CT protocols in the evaluation of the recipient and donor prior to living-related transplantation allows complete work-up in a single imaging study. This eliminates the need for other invasive radiological procedures.
Liver transplantation
All organ recipients with end-stage liver failure are evaluated prior to liver transplantation with dual phase MDCT of the liver. The acquired image data in the arterial and portal venous phases allows state-of-the-art CT angiography, portal and hepatic venography to be performed (3, 4). Protocols for 3D CT angiography should not include oral contrast administration. 3D reformats can depict pre-operative and post-transplant vascular anatomy and anomalies particularly of the hepatic artery, portal and hepatic veins (Figs. 1-3) (3, 4).
Fig. 1.
Liver donor evaluation in a 50-year-old man with CT angiography. Maximum intensity projection image (A) demonstrates hepatic arterial anatomy with left hepatic (LH) artery originating from celiac trunk (arrow) and right hepatic (RH) artery arising from superior mesenteric (SM) artery. 3D surface rendering in the same patient depicts entire hepatic lobe volume (B) and left lobe volume (C) to determine liver volume in living-related liver donor.
Fig. 3.
3D reconstruction of axial source data of 42-year-old man, status post-liver transplant. Doppler examination did not definitively demonstrate hepatic arterial flow. Multiplanar reconstruction image shows patent donor (white arrowhead) and recipient hepatic artery (black arrowhead) and demonstrates the position of transjugular intrahepatic portrsystemic shunt (arrow).
To evaluate living related transplant donors, firstly, the liver parenchyma is assessed for focal liver lesions, and fatty infiltration, which can contraindicate transplantation and can negatively impact on the quality of the graft and result in the need for re-transplantation. Next, the arterial and hepatic venous anatomy needs to be thoroughly mapped and important vascular variant anatomy can be identified which is fundamental to surgical planning (3, 4). 3D reconstructions provide accurate information about normal vascular supply and some critical vascular anomalies that may contradict organ donation due to excessive risk either to the donor or recipient. 3D CT images can detect and identify all major variations of the hepatic vein confluences, portal vein trifurcations and all hepatic arterial variants. 3D reconstruction delineates portal and hepatic venous anatomy equally or better than conventional angiography and can identify the hepatic artery and its branches well enough to consider replacing conventional angiography, thus reducing cost and potential risk to the organ donor.
With paint brush technique, 3D reconstruction aids in estimation of liver volumes at either side of the proposed resection line that is crucial for determining whether remaining liver volume is sufficient to satisfy the metabolic needs of the donor (1). The volume of liver resected is important, as the proposed graft needs to be sufficient to maintain the needs of the recipient (3). A potential limitation of MDCT and 3D reconstruction, is their inability to provide a surgical roadmap of biliary anatomy and anomalous anatomy in both the donor and recipient. Failure to appreciate anomalous biliary anatomy can lead to graft failure and post-procedure bile leakage. Intraoperative cholangiography is currently advised to delineate biliary anatomy. Recently, MRCP and in particular, mangafodipir-enhanced T1 MRCP has been reported as a non-invasive means of evaluating biliary anatomy prior to transplantation (3).
Hepatic Resection
Prior to liver resection, all patients are evaluated with dual phase MDCT of the liver and image data are acquired in the arterial and portal venous phases which allows state-of-the-art CT angiography, portal and hepatic venography to be performed (3). This assesses the exact location of the liver tumor and its anatomic relationship to hepatic vasculature.
Virtual hepatectomy with 3D images and liver volume estimation before surgery is useful in planning the extent and nature of hepatic resection prior to liver resection. Estimation of liver volume using 3D techniques, when combined with clinical and laboratory evaluation of liver function, can facilitate the prediction of postoperative liver failure in patients undergoing resection, assist in volume-enhancing embolization procedures and the planning of staged hepatic resection for bilobar disease. Thus, 3D-reconstruction and pre-operative virtual hepatic resection has an impact on surgical planning and has obviated the need for conventional angiography in many centers.
Pancreaticobiliary Imaging
The significant improvements in 3D CT angiography as an adjunct to axial images have completely eliminated the need for catheter angiography in the diagnosis, staging and road mapping of peripancreatic vascular anatomy prior to surgery in many centers. Important factors in preoperative evaluation of patients with pancreatic cancer which can be addressed by 3D CT angiography include definition of peripancreatic vascular anatomy and its relationship to the pancreatic tumor as well as local invasion of structures such as duodenum, liver or tranverse colon (Fig. 4). These important factors can now be evaluated in a single pancreatic protocol CT scan. 3D reconstruction is now a routine standard component of most pancreatic protocol CT scans (8).
Fig. 4.
Contrast enhanced CT image (A) of a 72-year-old woman with large cystic mass (white arrow) in the pancreatic head. Coronal multiplanar volume rendering (B) shows relationship of the mass with the "C" loop of duodenum (black arrows).
Encasement of the superior mesenteric artery and/or vein renders the pancreatic tumor surgically unresectable. 3D reconstructions now provide exquisite anatomic detail of vascular structures in vicinity of focal pancreatic lesions (Fig. 5). 3D CT angiography has advantages over catheter angiography in its ability to illustrate anatomic structures outside the vascular lumen and therefore the relationship of the vessels to the tumor can be more clearly illustrated.
Fig. 5.
Multiplanar volume rendering image (A) of axial source data of a 60-year-old man with pancreatic adenocarcinoma (white arrows) encasing the portal vein and distal superior mesenteric vein (black arrows). Intraluminal filling defect suggestive of a thrombus is seen in the superior mesenteric vein. Note the exquisite display of 3D vascular anatomy of superior mesenteric artery (black arrowheads) in relation with the tumor (B).
3D Reconstructions can aid in answering several pertinent questions regarding resectability of a malignant pancreatic mass. Axial source images depict the involvement of superior mesenteric vein, but sagittal reformats are best for demonstrating superior mesenteric artery involvement. Coronal reformats help in demonstrating local extension to stomach and duodenum, which can aid in surgical planning and has major implications in planning the appropriate palliative treatment for patients with unresectable pancreatic tumors (8).
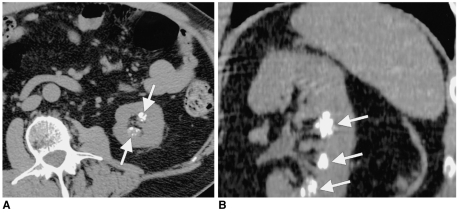
In addition, for detection of small pancreatic tumors, 3D reconstruction can aid in determining the level of dilated pancreatic and biliary ducts which is fundamental to localization of small or subtle pancreatic tumors, if viewed in conjunction with axial source images (9). Curved MPR can clearly demonstrate the dilated main pancreatic duct in patients with chronic pancreatitis, pancreatic cancer and mucin-producing pancreatic tumors (9, 10). Multiplanar CT pancreatography and distal cholangiography improves depiction of the pancreatic duct and bile ducts with image quality, which approaches that of ERCP. Park et al. (10) have reported the value of 3D CT cholangiography using MinIP projection to determine the level and cause of biliary obstruction (Figs. 6-8).
Fig. 6.
CT cholangiogram of dilated biliary ductal system in a 47-year-old woman with cholangiocarcinoma at porta hepatis (arrow). Axial CT image (A) shows dilated intrahepatic biliary system with hypodense mass at the porta hepatis. Minimum intensity projection (B) rendering provides a "cholangiographic" view of the dilated intrahepatic ductal system with mass in the porta hepatis region.
Fig. 8.
Virtual endoscopy of pancreatic duct in a 62-year-old woman.
Curved multiplanar reconstruction image shows a small hypodense mass in the pancreatic head (arrow) with dilated pancreatic duct (A). Virtual endoscopy (B) of pancreatic duct shows the attenuated lumen of the pancreatic duct due to extrinsic compression from periampullary carcinoma.
Urinary Tract
3D reconstruction has aided in convincing urologists of the advantages of CT urography over excretory urography in the evaluation of urinary tract pathology, as it allowed urologists to view images in the coronal plane, which were similar to excretory urography images (6). In addition, for radiologists, experience in the characterization of certain pathologies particularly those of the renal calices and papillae, such as renal tubular ectasia and papillary necrosis had been gained by evaluation of excretory urography images (Figs. 9, 10) (6). 3D reconstructions performed in the coronal plane closely resemble excretory urography images and review of these reconstructed images is very useful for characterizing these conditions. Indeed, Caoili et al. (6) have reported that 3D CT images are useful to radiologists and urologists as a "bridge" between excretory urography data and transverse CT data. 3D reconstruction also aids in evaluation of patients with anatomic variation of the urinary tract, contour abnormality of the renal outline and in confirmation of the site of ureteric obstruction (Figs. 11-13) (11). However, it should be emphasized that many 3D reformations suffer the same disadvantages as excretory urography if the transverse images are not reviewed in association. Many ureteral and bladder wall abnormalities are frequently detected on transverse images and can be missed on 3D reformats (6).
Fig. 9.
Coronal reformats aid with characterization of caliceal abnormalities.
Axial CT image (A) of a 31-year-old woman shows calcification in the renal papillae (arrows). Coronal multiplanar reconstruction (B) shows typical radiographic features of medullary nephrocalcinosis (arrows).
Fig. 10.
Axial CT image (A) of a 68-year-old man with diabetes mellitus and papillary necrosis shows a filling defect (sloughed papilla) in the renal calyces (black arrow). Maximum intensity projection image (B) shows fraying and blunting of the right renal calyces (white arrows), a classical radiographic feature of papillary necrosis first described with excretory urography.
Fig. 11.
Axial CT image (A) of a 32-year-old man with a hydronephrotic upper pole moiety (arrow) of a duplicated renal collecting system. Coronal multiplanar reconstruction (B) confirms hydronephrosis of upper pole moiety (arrow) and normal lower pole moiety.
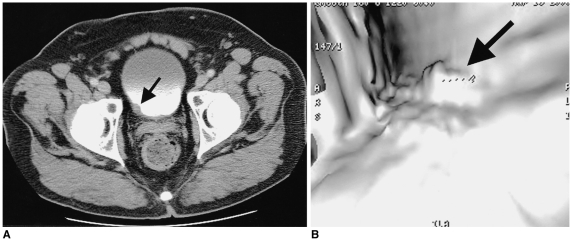
Fig. 13.
3D CT can combine advantages of intravenous urography and voiding cystourethrogram and retrograde urethrogrogram. Axial CT image (A) in 47-year-old man with recurrent urinary tract infection shows a dilated, ectopic left ureter (arrow) within the prostate. Sagittal multiplanar reconstruction (B) shows a dilated, ectopic ureter (arrow) opening into the posterior urethra. 3D surface rendering (C) demonstrates anatomic relationship of the ectopic ureter to the regional anatomy.
In management of renal masses, laparoscopic nephrectomy and partial nephrectomy are frequently technically more demanding procedures than conventional nephrectomy especially when performed using a retroperitoneal approach (12). These procedures are more difficult because visualization of renal hilar vascular anatomy is much more difficult intraoperatively than with conventional open nephrectomy. In addition, with retroperitoneal laparoscopic surgery, the plane in which the vessels are viewed is different (dorsocaudal) than with conventional surgery (12). Urologists have reported the important role of 3D CT angiography, which aids in planning laparoscopic nephrectomy, particularly in the detection of accessory renal arteries and veins. 3D CT angiography allows analysis of the extent of the renal mass and its relationship to renal vasculature and hilar structures in a variety of planes, which is fundamental to preoperative surgical planning.
Virtual CT cystoscopy evolved with CT colonography as a means of evaluating the bladder mucosa although it is much less widely utilized in clinical practice (Fig. 14). The additional information acquired at virtual CT cystoscopy can potentially aid in the planning of cytoscopy and cystoscopic resection of bladder tumors (13, 14). For polypoidal tumors, reports in the literature have suggested that virtual endoscopy of urinary bladder has equivalent accuracy to conventional cystoscopy, and may be very useful in the follow-up of patients following cytoscopic resection or other local treatments of bladder tumors thus reducing the costs and morbidity associated with conventional cytoscopy (13, 14). Virtual cystoscopy is particularly advantageous to patients in whom conventional cystoscopy is impossible due to urethral stricture.
Fig. 14.
Virtual cystoscopy is useful in evaluation of bladder tumors.
Axial (A) and virtual cystoscopy (B) images in a 67-year-old man demonstrate bladder wall thickening and irregularity (arrow). Virtual cystoscopy depicts the surface of the bladder mucosa and shows the size and site of the bladder neoplasm (arrow).
Gastrointestinal Tract
The utilization of 3D reconstruction is reserved for problem solving in gastrointestinal pathology. Coronal, sagittal and oblique reconstructions can aid in identifying precise anatomic location of stomach, small bowel and colonic lesions (Fig. 15).
Fig. 15.
3D CT of malignant colonic stricture in a 73-year-old man. Axial CT (A) and coronal multiplanar reconstruction (B) images show circumferential short segment thickening of the sigmoid colon (arrow) suggestive of colon cancer. 3D CT colonography (C) as a "double-contrast barium enema" simulating image, reveals short segment "apple-core" lesion (arrow) in the sigmoid colon.
CT colonoscopy
CT colonoscopy utilizes an array of multiplanar and endoluminal reconstructions to permit detailed evaluation of the entire large bowel (Fig. 16) (5). Data from our institution and others have demonstrated satisfactory sensitivity and specificity for detection of polyps greater than 1 cm, coincidentally, the lesions that are most likely to harbor malignancy (Fig. 16). One indisputable advantage of CT colonography has been in the completion of conventional colonography examinations when the endoscopist cannot reach the cecum.
Fig. 16.
Screening CT colonoscopy was performed in a 55-year-old man. Coronal reformat (A) and virtual colonoscopy (B) images demonstrate a polyp (arrow) in the ascending colon. 3D reconstruction in CT colonoscopy also helps in differentiating normal mucosal folds from intraluminal masses.
Colonic Stricture
The characterization of a colonic stricture can frequently be more thoroughly performed by 3D reconstruction of the area of narrowing. The stricture can then be examined in various planes for the features of malignant stricture initially described on single and double contrast barium enema. These imaging findings include mucosal destruction, 'shouldering' at the site of stricture, and length of the stricture can be more easily demonstrated with the aid of 3D reconstructions. We have found that 3D reconstruction can be useful in the diagnosis and confirmation of the presence of intussuseption (Fig. 17).
Fig. 17.
Axial image (A) shows a fat containing intraluminal mass (arrows) in a loop of ileum in a 49-year-old woman with intussuscepted Meckel's diverticulum. Coronal multiplanar reconstruction image (B) shows a tubular fat containing filling defect (arrows) in the ileal lumen that was contiguous with the mesenteric fat.
Bowel Ischemia
In the clinical setting of ischemic bowel, a CT angiogram can aid in thorough diagnostic evaluation. The use of dual phase acquisition CT scan coupled with 3D rendering allows the creation of SMA and SMV angiograms and can detect thrombosis or stenosis of these vessels (15). This data can be combined with enhancement characteristics of bowel to further question the possibility of acute or chronic bowel ischemia.
Inflammatory Bowel Disease
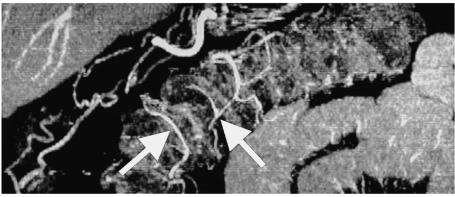
CT angiography maps of small and large bowel have been reported as having a potential role in the assessment of activity in inflammatory bowel disease. Enlarged distal branch vessels and hyperemia have been reported to be associated with increased activity (15) (Fig. 18).
Fig. 18.
Multiplanar volume rendering image of a 50-year-old man displays small vessels of the arterial plexus (arrows) of the large intestine. Depiction of vasa recti is showing promise in increasing the scope of CT evaluation of patients with bowel ischemia and assessment of disease activity in patients with inflammatory bowel disease.
16 SLICE MDCT AND BEYOND
The latest development of MDCT is the 16 slice CT scanner. MDCT can acquire sub-millimeter slices with the promise of equal image quality in the Z-axis (Fig. 19). The major impact of this rapidly evolving technology will be the refinement and optimization of 3D reformation with elimination of common artifacts such as "stair step" artifacts.
Fig. 19.
3D reconstruction with 16-slice CT scanner. Coronal multiplanar volume rendering (A) and minimum intensity projection (B) images reconstructed from sub-millimeter images (0.625 mm) of a 60-year-old woman show right renal mass. Note the exquisite demonstration of relation of the mass with right renal artery and an accessory renal artery (arrow) (General Medical Systems, Waukesha Wis, U.S.A.).
PROBLEMS ASSOCIATED WITH MDCT
From a practical standpoint, developments in MDCT scanning allow multiple thin slices to be acquired with increased z-axis coverage in a single breath-hold, which generates an extraordinary increase in the quantity of acquired data. Review of an enormous number of images poses significant constraints on radiologists' efficiency and may be simply impractical. Furthermore, the sheer number of images raises additional problems and expense for those departments, which have not yet converted to PACS and still use film for reading.
Rapid 3D reconstructions directly from the scanner console are currently being promoted by industry as a potential solution to these difficulties. This revolutionary thinking may alter the current practice of always reviewing axial 'source' images to using 3D reconstructions for primary interpretation. This would result in fewer images for review in most cases. However this approach will need thorough investigation and comparison to conventional CT practices before it can be widely embraced.
CONCLUSION
In certain circumstances, 3D reformatted images can demonstrate multiple abnormalities in a simple reconstructed image, which is ideal for consultation with referring physicians. A potential disadvantage of sophisticated 3D rendering techniques includes necessity of specialized personnel to generate quality images for evaluating specific clinical or imaging issues. However, when source images are equivocal, 3D reconstructions offer a problem-solving option by providing exquisite depiction of anatomy and pathology in multiple planes of choice.
Fig. 2.
Unexpected finding in a 35-year-old woman who underwent CT evaluation prior to liver donor surgery. Axial (A), multiplanar volume rendering (B) and maximum intensity projection (C) images show dissection of hepatic artery (arrow) with tortuous, dilated and featureless false lumen (arrowheads) without branching, which was not appreciated on conventional angiogram (D). The false lumen of common hepatic artery dissection is seen only on CT angiography. Distal communication of true and false lumens maintains the patency and perfusion of right and left hepatic arteries.
Fig. 7.
Double duct sign on 3D CT in a 65-year-old man with biopsy proven ampullary carcinoma (white arrowheads). Axial CT image (A) shows dilated common bile duct (black arrow) and pancreatic duct (white arrow). Curved multiplanar reconstruction (B) images show smooth dilatation of pancreatic duct (white arrow) and common bile duct (black arrow).
Fig. 12.
Coronal reformat accurately displays urinary anatomy. Maximum intensity projection shows a duplex non-dilated pelvicaliceal system on the left side.
References
- 1.Hu H, He HD, Foley WD, Fox SH. Four multidetector-row helical CT: image quality and volume coverage speed. Radiology. 2000;215:55–62. doi: 10.1148/radiology.215.1.r00ap3755. [DOI] [PubMed] [Google Scholar]
- 2.Wigmore SJ, Redhead DN, Yan XJ, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221–226. doi: 10.1097/00000658-200102000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamel IR, Kruskal JB, Keogan MT, Goldberg SN, Warmbrand G, Raptopoulos V. Multidetector CT of potential right-lobe liver donors. AJR Am J Roentgenol. 2001;177:645–651. doi: 10.2214/ajr.177.3.1770645. [DOI] [PubMed] [Google Scholar]
- 4.Bogetti JD, Herts BR, Sands MJ, Carroll JF, Vogt DP, Henderson JM. Accuracy and utility of 3-dimensional computed tomography in evaluating donors for adult living related liver transplants. Liver Transpl. 2001;7:687–692. doi: 10.1053/jlts.2001.26351. [DOI] [PubMed] [Google Scholar]
- 5.Rubin GD, Beaulieu CF, Argiro V, et al. Perspective volume rendering of CT and MR images: applications for endoscopic imaging. Radiology. 1996;199:321–323. doi: 10.1148/radiology.199.2.8668772. [DOI] [PubMed] [Google Scholar]
- 6.Caoili EM, Cohan RH, Korobkin M, et al. Urinary tract abnormalities: initial experience with multi-detector row CT urography. Radiology. 2002;222:353–360. doi: 10.1148/radiol.2222010667. [DOI] [PubMed] [Google Scholar]
- 7.Cody DD. AAPM/RSNA physics tutorial for residents: topics in CT. Image processing in CT. RadioGraphics. 2002;22:1255–1268. doi: 10.1148/radiographics.22.5.g02se041255. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513–1518. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita K, Furui S, Yamauchi T, et al. Minimum intensity projection image and curved reformation image of the main pancreatic duct obtained by helical CT in patients with main pancreatic duct dilation. Nippon Igaku Hoshasen Gakkai Zasshi. 1999;59:146–148. [PubMed] [Google Scholar]
- 10.Park SJ, Han JK, Kim TK, Choi BI. Three-dimensional spiral CT cholangiography with minimum intensity projection in patients with suspected obstructive biliary disease: comparison with percutaneous transhepatic cholangiography. Abdom Imaging. 2001;26:281–286. doi: 10.1007/s002610000140. [DOI] [PubMed] [Google Scholar]
- 11.Schreyer HH, Uggowitzer MM, Ruppert-Kohlmayr A. Helical CT of the urinary organs. Eur Radiol. 2002;12:575–591. doi: 10.1007/s003300101023. [DOI] [PubMed] [Google Scholar]
- 12.Marukawa K, Horiguchi J, Shigeta M, Nakamoto T, Usui T, Ito K. Three dimensional navigator for retroperitoneal laparoscopic nephrectomy using multidetector row computerized tomography. J Urol. 2002;168:1933–1936. doi: 10.1016/S0022-5347(05)64266-8. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Francis IR, Platt JF, et al. Bladder tumor detection at virtual cystoscopy. Radiology. 2001;218:95–100. doi: 10.1148/radiology.218.1.r01ja4995. [DOI] [PubMed] [Google Scholar]
- 14.Kim JK, Ahn JH, Park T, Ahn HJ, Kim CS, Cho KS. Virtual cystoscopy of the contrast material-filled bladder in patients with gross hematuria. AJR Am J Roentgenol. 2002;179:763–768. doi: 10.2214/ajr.179.3.1790763. [DOI] [PubMed] [Google Scholar]
- 15.Horton KM, Fishman EK. Volume-rendered 3D CT of the mesenteric vasculature: normal anatomy, anatomic variants, and pathologic conditions. RadioGraphics. 2002;22:161–172. doi: 10.1148/radiographics.22.1.g02ja30161. [DOI] [PubMed] [Google Scholar]