Abstract
Background
Some data suggest that increasing calcium intake may help prevent weight gain.
Objective
To test the hypothesis that calcium supplementation can prevent weight gain in people who are overweight or obese.
Design
Randomized placebo-controlled trial. Randomization was computer generated and allocation was assigned by pharmacy personnel who prepared intervention and placebo capsules. Participants, providers, and those who assessed outcomes were blinded to treatment assignment.
Setting
Single research center.
Participants
340 overweight (BMI between 25 and 30 kg/m2) and obese (BMI ≥ 30 kg/m2) adults, mean age 38.8±10.5 years.
Intervention
Calcium carbonate (1500mg/d elemental calcium) (n= 170) or placebo (n= 170), with meals for two years.
Measurements
Changes in body weight and fat mass (primary outcomes);
Results
Seventy-five percent of participants completed the trial (78% given calcium, 73% given placebo). There were no statistically or clinically significant between-group differences in change in body weight (difference between calcium and placebo groups +0.02kg, CI −1.64 to + 1.69, P=.98), body mass index (difference +0.32kg/m2 CI −0.41 to +1.02, P=.39) or body fat mass (difference +0.39kg CI −1.04 to +1.92, P=.55). Parathyroid hormone decreased in the calcium- compared to the placebo-treated group (−0.71pmol/L, 95% CI −1.28 to −0.13).
Limitations
The study took place at a research center, and its population was mostly women,
Conclusions
Dietary supplementation with 1500 mg/d of elemental calcium for 2 years had no statistically or clinically significant effects on weight in overweight and obese adults. Calcium supplementation is unlikely to have clinically significant efficacy as a weight gain preventive measure in such patients.
Keywords: obesity, adipose tissue, weight, weight gain, dietary factors, calcium
Introduction
The high prevalence of overweight and obesity in the United States (1) has stimulated great interest in identifying approaches that may help to prevent weight gain or improve the ability to lose weight. Increasing calcium intake is one potential means of weight management that has received much attention from both lay press (2–4) and the medical community (5–7). That interest has been stimulated by a series of cross-sectional studies (8–17) finding that children and adults with low reported consumption of dietary (primarily dairy) calcium have greater body weight, a higher degree of adiposity, and greater risk of having components of the metabolic syndrome than those who report they consume more calcium. Some (18–24), although not all (25–34), longitudinal investigations have also suggested that children and adults who consume a lower calcium diet tend to gain more weight than those with greater dietary or supplemental calcium intake. These data, together with some small clinical trials suggesting weight loss achieved during dieting may be augmented by supplemental (35) or dairy calcium (35–38), have led to expectations that substantially augmenting calcium intake might diminish body weight by 0.5kg or more each year.(5, 18, 22, 39)
Two possible mechanisms for an effect of dietary calcium on body weight have been suggested. First, calcium can combine with fatty acids in the intestine to form insoluble soaps that are therefore not absorbed (40, 41). Second, Zemel and colleagues (42–46) have proposed that low dietary calcium leads to increased adipocyte triglyceride deposition (47–49).
If calcium significantly affects fat accumulation, calcium supplementation could prevent additional weight and fat gain among those with high body weight. To test this hypothesis, we determined the effect of calcium carbonate supplementation for two years on the weight and body fat of overweight and obese adults.
Methods
Setting and Participants
Participants were recruited through advertisements posted in Bethesda, MD and through spontaneous reply to newspaper and radio advertising in the Washington DC metropolitan area seeking healthy adult volunteers for a project to study the “health effects of calcium supplementation.” Men and women 18 to 80y were eligible to enroll if they had a body-mass index ≥25kg/m2, and were free of cerebrovascular, cardiovascular, pulmonary, renal, hepatic, endocrinologic, or other significant medical disease. Women were ineligible if they were pregnant or breast-feeding or had received a recommendation from a health care professional to take calcium supplements for any condition. The regular use of medications known to affect body weight, a weight loss of 3% or greater in the preceding three months, reported total calcium intake in excess of 3.5g/day, use of supplemental calcium in excess of 300mg/d, use of vitamin D supplements in excess of 400IU/day, or a history of renal stones were exclusion criteria.
The research protocol was approved by the Institutional Review Board of the National Institute of Child Health and Human Development. Signed consent was obtained from each participant. Financial compensation was provided for subjects’ time and inconvenience. The trial was registered at www.clinicaltrials.gov (NCT00030238).
Design Overview
We conducted a single-center, randomized, double-blind, placebo-controlled trial from March 2002 to April 2006. Following an outpatient visit to determine eligibility and obtain initial assessments, participants entered a 2y double-blind treatment period.
Randomization and Interventions
Subjects were randomized, in a 1 to 1 ratio, to receive either 1500mg of elemental calcium (as calcium carbonate, purchased from Particle Dynamics, Inc., St. Louis, Mo) or placebo daily, administered as two divided doses with meals. Investigators assigned consecutive code numbers to subjects from pre-specified lists stratified by race/ethnicity, sex, and BMI (25–26.99, 27–29.99, 30 34.99, and BMI≥35). The allocations translating code numbers into study group assignments were generated using a pseudo-random number program by the National Institutes of Health Pharmaceutical Development Service using permuted blocks with stratification. Placebo and calcium capsules were prepared by the Pharmaceutical Development Service to appear identical. Study capsules were dispensed by pharmacy personnel not otherwise involved with the conduct of the study, with medication placed into identical appearing containers that differed only by the individual subject code number. No subject, investigator, or other medical or nursing staff interacting with subjects was aware of treatment assignments for the duration of the trial.
Initial Assessment
At their pre-randomization evaluation, subjects reported after an overnight fast and were weighed in hospital gowns using a digital scale (Life Measurement Instruments, Concord, CA) that was calibrated with a known weight before each subject’s measurement. Height was measured using a stadiometer calibrated before each measurement (Holtain Ltd., Crymych, UK). Abdominal and hip circumferences were assessed in triplicate to the nearest 0.1cm as recommended (50, 51) and triceps skinfold thickness was measured to the nearest 0.5 mm using Lange calipers (Cambridge Scientific Industries, Inc, Cambridge, MD) by research dietitians (authors B.I.D. and N.G.S.). Blood pressure, obtained after a 5-minute rest period, was measured three times at 5-minute intervals, from seated subjects using an automated sphygmomanometer (Dinamap-Plus, Critikon, Tampa, FL). Whole body dual energy x-ray absorptiometry (DEXA) was performed for estimation of total body fat mass (Delphi A, Hologic Inc., Bedford MA, software version 11.2). Intact parathyroid hormone concentrations were measured with 2-site immunochemiluminometric assays (52). Serum 25-hydroxy-vitamin D was measured using a competitive binding assay (Nichols Advantage, Nichols Diagnostic, San Clemente, CA) (53). Subjects with significant vitamin D deficiency (defined as serum 25-hydroxy-vitamin D<25.0nmol/L) were asked to take 400IU/d vitamin D (as ergocalciferol) in a multivitamin. Baseline dietary calcium intake was assessed using a 7-d food record that was reviewed by a registered dietitian with each subject to maximize accuracy and completeness and analyzed for dietary calcium intake using the Nutrition Data System for Research (NDS-R) software versions 4.04_32 and 4.05_33 (54). Calcium intake from multivitamins or calcium supplements was determined with a validated calcium questionnaire (55) that was reviewed for completeness through an interview with a registered dietitian. The calcium content as listed on the label of each multivitamin or calcium supplement was recorded. Dietary and supplemental vitamin D intakes were determined in the same fashion. Energy intake at baseline and at 2 years was estimated using a food frequency questionnaire that assessed total diet (55–57). Socioeconomic status was determined by Hollingshead score (58).
Outcomes and Follow-up
Primary efficacy end points were change in body weight and body fat mass at the end of 2 years of treatment. Secondary outcomes were fasting anthropometric measurements, body composition by DEXA, and change in blood pressure, assessed yearly along with questionnaire data on dietary and supplemental calcium intake (55).
In addition, subjects were contacted every 3 months to complete questionnaires regarding their adherence to the medication regimen, to assess their general health, and to obtain self-reports of mood, stress, physical activity, and hunger. At 6-month intervals, subjects returned to the clinic to exchange their unused study medication for a new supply. The tally of returned capsules was used to assess adherence. To examine adequacy of the masking procedure, at the conclusion of the study, subjects completed a questionnaire that requested they report their best guess regarding their study group assignment.
Statistical Analysis
All reported primary data analyses were pre-specified. Based on our own prior study of observed yearly changes in body weight over time (59), a total sample size of 256 subjects was needed to detect a 0.35kg/yr difference in weight change (i.e., 0.7kg over 2yr) between groups with 80% power. Subject accrual was set at 340 subjects to allow 25% loss to follow-up. Data were analyzed using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL). Efficacy was assessed in the intent-to-treat population, comprising all randomized participants. Two efficacy analyses were performed. The primary analysis used a multiple imputation model for missing data under a missing-at-random assumption (60). Using the computer program NORM (version 2.03) (61, 62), all available baseline, 1-year, and 2-year outcome measures were included in an imputation model along with age, sex, race, baseline serum 25-hydroxy-vitamin D concentration, baseline calcium intake, and treatment group. The coefficients from analyses of 20 imputed data sets were then combined into a single set of estimates according to Rubin’s rules (62, 63). To assess sensitivity of these results to the missing-at-random assumption, we conducted three additional analyses, assuming that all subjects who dropped out had major weight gain, defined as a gain ≥ 2.27 kg (5 lb) (59); that calcium-treated dropouts gained no weight while placebo-treated dropouts experienced a major weight gain; and that placebo-treated dropouts gained no weight while calcium-treated dropouts experienced a major weight gain. We used multiple imputations to impute the missing 2-year weight measurements, using the same imputation model used for the main analysis. For the three scenarios, fixed amounts were added to the imputed values, the results were reanalyzed by using analysis of covariance, and combined using Rubin’s rules. A second, confirmatory analysis used the last observation carried forward method for those who did not complete the study. Unadjusted analyses were also run both for the imputation and the last observation carried forward models. As all of these methods yielded similar results (i.e. finding no statistically significant impact of calcium supplementation on weight or fat change), only the imputation analyses are presented. No interim efficacy analyses were carried out. Baseline characteristics were examined by simple t-tests or, in the case of categorical data, with contingency table analysis.
Role of the funding source
The Intramural Research Program of the National Institutes of Health and the Office of Dietary Supplements, National Institutes of Health supported the study. Funding agencies played no part in study design or conduct; data collection, management, analysis, or interpretation; or manuscript development or approval.
Results
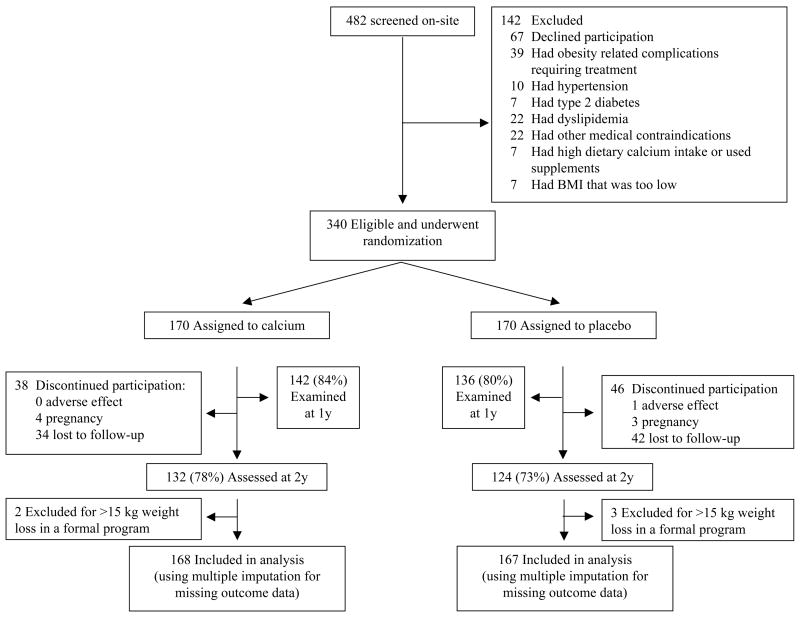
A total of 1904 persons responded to advertisements for the study; 1038 completed a telephone interview, appeared to meet eligibility requirements, and received a consent form by mail. A total of 482 subsequently agreed to attend a clinic visit during which eligibility was determined (Fig 1), among whom 67 elected not to participate and 75 were excluded because of medical conditions uncovered during evaluation. A total of 245 women and 95 men(mean age 38.8±10.5 years, range 18–71y) were randomized to take calcium carbonate or placebo between April, 2002 and January, 2004.
Figure 1.
Enrollment and Retention.
Baseline Characteristics
Among enrolled subjects, 39% were overweight (BMI≥25 but <30kg/m2) and 61% obese (BMI≥30kg/m2); 23% reported dietary calcium intake <600mg/d and 75% reported dietary calcium intake that was less than the US dietary reference intake for age 51–70y (1200mg/d) (64). At baseline, there were no statistically significant differences between treatment groups in age, sex, race/ethnicity, weight, BMI, body fat mass, indices of calcium intake, energy intake, or in serum 25-hydroxy-vitamin D or parathyroid hormone concentrations (Table 1). Thirty-six subjects were prescribed multivitamin supplements because of vitamin D deficiency at baseline assessment.
TABLE 1.
Baseline Participant Characteristics*
| Characteristic | Calcium (n=170) | Placebo (n=170) |
|---|---|---|
| Mean age (SD), y | 38.9 (10.5) | 38.7 (10.4) |
|
| ||
| Female sex, % | 72.9 | 72.4 |
|
| ||
| Race/Ethnicity, % + | ||
| Non Hispanic White | 60 | 62 |
| Non Hispanic Black | 30 | 32 |
| Hispanic White | 6 | 4 |
| Other | 4 | 2 |
|
| ||
| Mean weight (SD), kg | 94.5 (20.5) | 94.0 (20.5) |
|
| ||
| Mean body-mass index (SD), kg/m2 | 33.2 (6.8) | 33.6 (6.8) |
|
| ||
| Overweight/Obese, % | 38/62 | 40/60 |
|
| ||
| Mean fat mass (SD), kg | 35.5 (11.7) | 34.8 (11.8) |
|
| ||
| Median Hollingshead Scale of Socioeconomic Status (range) | 3(1–5) | 3 (1–5) |
|
| ||
| Mean energy intake (SD), kcal/d | 2110 (1238) | 2190 (1846) |
|
| ||
| Mean calcium intake (SD), mg/d | 878 (430) | 887 (350) |
|
| ||
| Calcium intake < 600 mg/d, % | 24.4 | 22.7 |
|
| ||
| Calcium intake < dietary reference intake (64), % | 75.5 | 74.4 |
|
| ||
| Mean serum 25-hydroxyvitamin D (SD), nmol/L | 58.9 (29.5) | 62.6 (33.7) |
|
| ||
| Mean serum parathyroid hormone (SD), pmol/L | 4.25 (1.94) | 4.42 (1.93) |
At baseline, there were no significant differences between treatment groups (all P>0.31).
Race and ethnicity were self-reported.
Overweight was defined as body-mass index between 25 and 29.99 kg/m2 and obesity as body-mass index ≥ 30 kg/m2.
Changes in Body Weight and Fat Mass
Eighty-two percent of subjects had body weight assessed after 1 year of randomization, and 75% completed the 2-year trial (78% given calcium and 73% given placebo, P=.38). There were no statistically significant differences in sociodemographic, anthropometric, or laboratory indices in subjects who did and did not complete the study (all P>.35). Data from 5 subjects who participated in formal weight reduction programs and lost more than 15kg were excluded because their weight loss was due to marked changes in their dietary and physical activity habits. These subjects’ weight changes deviated markedly from those of other members of the sample, and were identified as outliers (65).
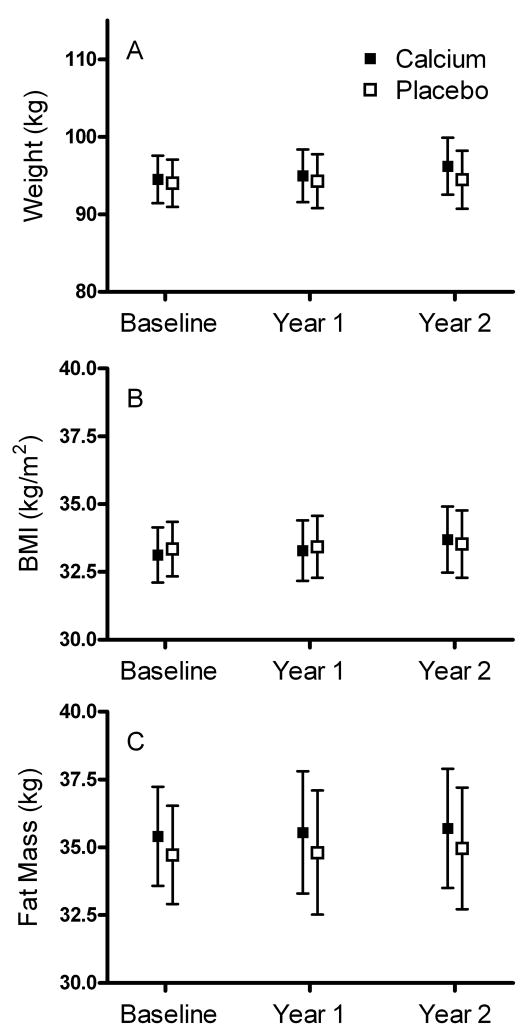
The measured change in body weight among all participants who completed the 2 year trial was +1.31±6.5kg (P<.001 vs. baseline weight); fat mass increased by +0.82±4.3kg (P=.004 vs. baseline fat mass), similar to expectations for an observational study (59). However, no statistically or clinically significant between-group differences in change in body weight (difference between calcium and placebo groups +0.02kg; 95% CI −1.64 to + 1.69kg, P=.98), body mass index (difference +0.32kg/m2; CI −0.41 to +1.02kg/m2, P=.39) or body fat mass (difference +0.39kg; CI −1.04 to +1.92kg, P=.55) over the 2-year study interval were found in analyses adjusted for age, race, and sex (Table 2) or in unadjusted analyses (all P>.40). There were also no statistically significant between-group differences in abdominal circumference, hip circumference, or triceps skinfold thickness (Table 2), or in body weight, fat mass, or other body composition variables using the last observation carried forward method with and without adjustment for covariates (all P>0.35).
TABLE 2.
Changes in variables at conclusion of the study.*
| Characteristic | Calcium (n=168) | Placebo (n=167) | Difference | P Value |
|---|---|---|---|---|
| Weight, kg | +0.54 (−0.70 to +1.79) | +0.52 (−0.82 to +1.86) | +0.02 (−1.64 to + 1.69) | .98 |
| Fat mass, kg | +0.40 (−0.61 to +1.41) | +0.01 (−1.15 to +1.16) | +0.39 (−1.04 to +1.92) | .55 |
| Body-mass index, kg/m2 | +0.18 (−0.33 to +0.69) | −0.14 (−0.73 to +0.45) | +0.32 (−0.41 to +1.02) | .39 |
| Abdominal circumference, cm | +0.95 (−1.43 to +3.34) | +1.01 (−1.51 to +3.53) | −0.06 (−2.34 to +2.23) | .90 |
| Hip circumference, cm | −0.80 (−3.21 to +1.62) | −1.87 (−4.41 to +0.68) | +1.07 (−1.24 to +3.39) | .36 |
| Triceps skinfold thickness, mm | −2.39 (−3.87 to −0.92) | −2.53 (−4.08 to −0.97) | +0.13 (−1.28 to +1.54) | .85 |
| Systolic blood pressure, mm Hg | −3.2 (−6.8 to +0.3) | −4.3 (−8.0 to −0.5) | +1.0 (−2.4 to +4.4) | .55 |
| Diastolic blood pressure, mm Hg | −1.8 (−4.0 to +0.3) | −2.5 (−4.8 to −0.2) | +0.7 (−1.4 to +2.7) | .76 |
Estimated marginal means (adjusted for covariates including age, race, and sex) with 95th percentile confidence intervals (in parentheses) reported from imputation analyses.
Post Hoc Analyses
Because prior studies (18, 36) have suggested calcium might alter weight gain to a greater extent in women or in Non-Hispanic Blacks, post-hoc analyses including interaction terms for group × sex and group × race were also run (Appendix Table 1). We found no significant effects on change in body weight or fat mass of calcium supplementation by sex or race. Analyses restricted to subjects with low baseline calcium intake (who might possibly garner greater benefit from supplementation) (22), subjects who were obese rather than overweight, and subjects who had more than 90% adherence by capsule counts throughout the study also found no significant effects of calcium supplementation for either weight change or fat mass change (Appendix Table 1). Calcium supplementation did not affect weight change after exclusion of all subjects who reported joining a commercial weight loss program (Appendix Table 1). Baseline 25-hydroxy-vitamin D concentration was unrelated to weight or fat mass change in all models (all P≥.21). An exploratory logistic regression model based on the analysis presented by Caan et al (22) to examine impact of calcium supplementation on likelihood for weight change among those with lower initial dietary calcium intake (<1200mg/d) also found no significant association between calcium supplementation and weight gain of 1–3kg (P>0.89) or greater weight gain (P>0.11).
APPENDIX TABLE 1.
Post-hoc Analyses.*
| Change in Body Weight (kg) | Change in Fat Mass (kg) | |||||
|---|---|---|---|---|---|---|
| Sample evaluated (n) | Calcium | Placebo | P Value | Calcium | Placebo | P Value |
| Model including group by race and group by sex interactions | ||||||
| All subjects (335) | +0.42 (−1.10 to +1.95) | −0.54 (−2.44 to +1.36) | .64 | +0.39 (−0.64 to +1.41) | −0.33 (−1.60 to +0.93) | .38 |
| Non-Hispanic black (101) | +0.73 (−1.99 to +3.44) | −0.12 (−2.41 to +2.22) | .53 | +0.22 (−2.15 to +2.58) | −0.27 (−1.97 to +1.43) | .53 |
| Women (244) | +0.55 (−1.12 to +2.22) | −0.37 (−2.46 to +1.73) | .94 | +0.51 (−0.60 to +1.63) | −0.07 (−1.46 to +1.33) | .78 |
| Subgroup Analyses: | ||||||
| Calcium intake <600 mg/d (80) | −0.79 (−3.87 to +2.27) | −0.64 (−3.94 to +2.65) | .89 | −0.10 (−2.42 to +2.21 | −0.40 (−2.87 to +2.07) | .75 |
| Calcium intake < 1200 mg/d (251) | +0.15 (−0.37 to +0.66) | −0.05 (−0.57 to +0.48) | .45 | −0.07 (−1.00 to +0.61) | +0.17 (−0.76 to +1.12) | .60 |
| Obesity, BMI ≥ 30 kg/m2 (203) | +0.13 (−1.86 to +2.11) | +0.46 (−1.62 to +2.53) | .72 | +0.11 (−1.23 to +1.46) | +0.35 (−1.05 to +1.75) | .71 |
| ≥ 90% adherent (152) | −1.21 (−3.39 to +0.97) | −1.16 (−3.74 to +1.15) | .96 | −0.68 (−2.10 to +1.75) | −0.55 (−2.06 to +0.96) | .86 |
| Did not participate in a commercial weight loss program (304) | +0.22 (−1.42 to +1.86) | +0.02 (−1.60 to +1.65) | .75 | +0.33 (−0.82 to +1.47) | +0.36 (−0.77 to +1.49) | .94 |
Estimated marginal means (95th percentile confidence intervals).
Missing Data Sensitivity Analysis
The analyses for assessing the sensitivity of the results to the missing-at-random assumption (Appendix Table 2) showed that the results were largely unchanged under the “Major Weight Gain” scenario, a model that assumes nonresponse bias is nondifferential (that is, the same in each treatment group). This result is to be expected because the proportion of patients with missing data was fairly similar in both study groups. When we assumed a differential nonresponse bias, the estimates of calcium versus placebo difference in weight change ranged from −0.79kg (CI, −2.18 to +0.59) to +.32kg (CI, −1.07 to +1.71). The “Major Weight Gain” scenario is considered much more plausible than either the best- or worst-case scenarios, since non-response (insufficient weight loss) is a common reason for dropping out of obesity studies (66).
APPENDIX TABLE 2.
Sensitivity to Missing Values of Estimated Intervention Effects on Change in Body Weight at 2 Years*
| Characteristic | Calcium (n=168) | Placebo (n=167) | Difference | P Value |
|---|---|---|---|---|
| “Major Weight Gain” Scenario: Model assuming subjects with major weight gain (2.27 kg) drop out | +0.50 (−0.71 to +1.70) | +0.77 (−0.37 to +1.91) | −0.27 (−1.66 to +1.12) | .70 |
| “Best Case for Calcium” scenario: Model assuming subjects taking calcium who drop out gain no weight, but subjects taking placebo who drop out have major (2.27 kg) weight gain | −0.01 (−1.22 to +1.19) | +0.78 (−0.36 to +1.92) | −0.79 (−2.18 to +0.59) | .26 |
| “Worst case for Calcium” scenario: Model assuming subjects taking placebo who drop out gain no weight, but subjects taking calcium who drop out have major (2.27 kg) weight gain | +0.46 (−0.75 to 1.67) | +0.14 (−1 to 1.29) | +0.32 (−1.07 to +1.71) | .65 |
Estimated marginal means for weight gain in kg (adjusted for covariates including age, race, and sex) with 95th percentile confidence intervals (in parentheses) reported from imputation analyses.
Questionnaire Data
As assessed by self report questionnaires completed at 3-month intervals, there were no between-group differences in general health, mood, stress, physical activity, or hunger (all P>.09). There were no statistically significant between-group differences in change in reported energy intake at 2-year follow-up (difference between calcium and placebo groups +28kcal, CI −244 to +300kcal, P=0.84) or in reported dietary + non-prescribed supplemental calcium intake (difference between calcium and placebo groups +18.6mg/d, CI −93 to +130mg/d, P=0.74). Reported vitamin D intake derived from the diet or supplements did not change significantly during the study (P’s>.40).
Laboratory Data
Parathyroid hormone concentrations decreased to a statistically significantly greater extent in the calcium-treated group compared to the placebo group (−0.71pmol/L, CI −1.28 to −0.13, P<0.001), suggesting subjects adhered to their prescribed regimen. Serum 25-hydroxy-vitamin D showed small decreases (difference from baseline for calcium −8.2nmol/L, CI −13.2 to +3.5; for placebo −4.5ng/mL, CI −10.0 to + 1.2) that were not significantly different between groups (difference −3.7ng/mL, CI −11.5 to +3.5, P=0.24).
Adverse Events and Adherence
Adverse events leading to study discontinuation were infrequent (Fig 1). The only clinically significant adverse event observed during the study was in a subject given placebo who required foot surgery. There were no significant between-group differences in reports of any adverse event during the study (all P>0.41). Adherence and change over time in adherence (measured by tallies of returned medication), did not differ between groups at any study interval from 6 months through 2 years (all P≥.78). Among all trial subjects, however, adherence decreased significantly during the trial from 92% of prescribed doses at 6 months to 82% at 2 years (P=.0014, 6 months vs. 2 year comparison).
Masking of assignment
We also assessed the success of masking in the assignment of subjects to the study groups. Almost two-thirds of subjects in both the calcium and placebo groups believed they were randomized to take placebo (64 vs. 65%, P=.89).
Discussion
On the basis of preliminary evidence suggesting that increasing calcium intake might lead to reduced weight gain, we tested the hypothesis that dietary calcium could alter the amount of weight and body fat gained in overweight and obese patients. Two years of calcium supplementation, taken as capsules at meals, did not greatly alter either body weight or body fat gain in overweight and obese adults. Overweight and obese adults were selected as subjects because, when heavier individuals are followed longitudinally, they tend to show greater weight gain over time than normal weight adults (59, 67) and are at greater risk for the development of complications from excessive body weight gain (68–71).
Previously published large trials of calcium supplementation were not designed with body weight as the primary endpoint; however, most have reported that additional calcium had insignificant effects on body weight (26, 28, 34, 72). Reid et al (26) reported no difference in body weight or fat mass among 1471 women randomized for the purpose of fracture prevention to take 1g elemental calcium as calcium citrate or placebo for 30 months. However, a tertiary analysis of the 36,282 women who took part in the Women’s Health Initiative (WHI) trial (22) found supplementation with 1000mg calcium plus 400IU cholecalciferol was associated with a small difference in body weight in the cohort (0.13kg) and lower risks for significant weight gain (1–3kg or >3kg) among those with dietary calcium intake <1200mg/d. The data for an effect of dairy or supplemental calcium on body weight from smaller clinical trials, many of which have also involved weight reduction programs, have been mixed (73), with a few finding a significant impact (18, 35–37) and others (30, 74–78) failing to show any differences. Similar heterogeneity in results has been found when the impact of supplemental calcium on blood pressure is examined (79). The current study is the first large randomized investigation specifically designed to examine if supplemental calcium could prevent weight gain. Given the relatively small impact of calcium on body weight found in the WHI and most other trials, we believe all of the data are consistent in suggesting limited efficacy for weight gain prevention among overweight or obese persons. The WHI result finding lower odds for gaining weight among those with low calcium intake who were given calcium and vitamin D supplements (22) was not replicated in the present study. One limitation that may explain why we did not reproduce the WHI findings is that this study was not powered to detect very small differences in body weight that could be attributable to calcium. However, it would seem to be of little clinical importance to determine if a supplement could change average body weight by a fraction of a pound over several years, as suggested by the WHI trial. Indeed, a difference attributable to calcium of −0.13kg is well within the confidence interval for weight change in the present trial. Another possibility why the WHI regimen, which included vitamin D supplementation as well as calcium provision, was associated with weight change is that adequate vitamin D is required for calcium to be well absorbed. However, we found no impact of baseline 25-hydroxy-vitamin D concentrations on weight change in this study. Significant changes in dietary or supplemental calcium and vitamin D intakes could therefore also conceivably affect study results. Total intakes were formally assessed by validated questionnaires once yearly, and changes in supplemental intakes were assessed every three months in the present study, but we cannot rule out changes in intakes not detected by these methods. A final possibility is that, since the WHI findings were tertiary re-analyses and not the primary or even secondary purposes of the study, the WHI’s positive results may have occurred by chance.
Some investigators have suggested that dairy products, rather than calcium alone, may have greater effects on body weight (35, 37), and lipid metabolism (80), a limitation of the present study is that it did not include subjects randomized to a high dairy calcium diet. It is also possible that some subjects’ dietary calcium intake was insufficiently low to demonstrate the impact of calcium supplementation. However, 75% of subjects consumed less than the daily recommended calcium intake, and one-quarter reported calcium intake <600mg/d; analyses restricted to either of these groups failed to show trends consistent with an impact of calcium on body weight gain. Another limitation is that adherence was measured only through counts of returned capsules and was not confirmed by other measurements. However, parathyroid hormone concentrations did decrease in the calcium-treated group, suggesting impact of the supplements. Finally, although this study’s participant demographic characteristics were similar to those reported for overweight individuals in the US, subjects were not recruited in a truly population-based fashion. Subjects who participate in research projects involving randomization may possibly be more health-conscious than the general population. Another sample-related limitation is that the groups studied consisted mostly of women. However, mean weight change in those who completed the study (+1.6kg over 2y) was quite similar to that reported in prior epidemiologic studies of adults measured longitudinally (81, 82).
In summary, supplementation with 1500mg per day of elemental calcium did not significantly alter weight or fat gain over a 2-year interval in overweight and obese adults. Thus, even though there may be other important reasons, such as fracture prevention (83), to recommend dietary calcium supplementation, we conclude that the extant data suggest that calcium supplementation is unlikely to have clinically significant efficacy as a weight gain preventive measure in people who are already overweight or obese.
Figure 2. Weight, BMI and Fat Mass in Study Subjects.
Measured mean (± 95% confidence intervals) for weight (A), BMI (B), and fat mass (C) in participants randomized to calcium (closed squares) or placebo (open squares). There were no significant differences between groups at any time point.
Acknowledgments
We thank the participants for their involvement in the trial. We also thank research assistants Marni Edelman, Amanda Spitalnik, and Carolyn Menzie for their excellent work as project coordinators for the study. Authors J Yanovski, N. Sebring, and B. Denkinger are Commissioned Officers in the United States Public Health Service.
Grant Support: By the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, grant Z01-HD-000641 and the Office of Dietary Supplements, National Institutes of Health, OD-2067.
Footnotes
ClinicalTrials.gov registration number: NCT00030238
Reproducible Research Statement: Study protocol and data set: Available upon request (email jy15i@nih.gov). Statistical code: Not available.
Requests for Single Reprints: Jack A. Yanovski, MD, PhD, National Institutes of Health, Hatfield CRC, 9000 Rockville Pike, Room 1-3330, Bethesda, MD 20892-1103, jy15i@nih.gov
Author Contributions: JAY was the Principal Investigator. He conceived and supervised the study, obtained funding, performed the analysis, interpreted the data, and produced the first draft of the manuscript. SJP assisted with study design obtaining funding, and study supervision and coordinated data acquisition. LBY coordinated data acquisition, assisted with analysis and interpretation of the data, and assisted with drafting of the manuscript. BID and NGS assisted with study design, data acquisition, and with analysis and interpretation of the data. KAC and JCR assisted with study design and data interpretation. TM assisted with acquisition of data. All authors contributed to critical revision of the manuscript for important intellectual content.
Potential Financial Conflicts of Interest: Dr. Yanovski has material transfer agreements or cooperative research agreements for research support with the pharmaceutical companies Roche and Obecure. Dr. Parikh is currently an employee of AstraZeneca, LP, but was an employee of the National Institutes of Health and had no conflicts of interest when he was involved with this study. As Principal Investigator, JAY had full access to all the data in the study, had responsibility for the integrity of the data and the accuracy of the data analysis, and had final responsibility for the decision to submit for publication.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Brody J. All That Calcium, and Maybe Weight Control Too. The New York Times. 2005 June 14 [Google Scholar]
- 3.Kalra S. Take calcium, lose weight. [November 29 Health Accessed December 12 2006];The Tribune On-Line edition. 2006 Wednesday; http://www.tribuneindia.com/2006/20061129/health.htm.
- 4.abc7chicago.com. Calcium could slow middle-age weight gain. 2006 Broadcast July 10, 2006. Accessed December 12,: http://abclocal.go.com/wls/story?section=health&id=4353931.
- 5.Parikh SJ, Yanovski JA. Calcium intake and adiposity. Am J Clin Nutr. 2003;77(2):281–7. doi: 10.1093/ajcn/77.2.281. [DOI] [PubMed] [Google Scholar]
- 6.Barba G, Russo P. Dairy foods, dietary calcium and obesity: a short review of the evidence. Nutr Metab Cardiovasc Dis. 2006;16(6):445–51. doi: 10.1016/j.numecd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Heaney RP. Normalizing calcium intake: projected population effects for body weight. J Nutr. 2003;133(1):268S–270S. doi: 10.1093/jn/133.1.268S. [DOI] [PubMed] [Google Scholar]
- 8.McCarron DA. Calcium and magnesium nutrition in human hypertension. Ann Intern Med. 1983;98(5 Pt 2):800–5. doi: 10.7326/0003-4819-98-5-800. [DOI] [PubMed] [Google Scholar]
- 9.Jacqmain M, Doucet E, Despres JP, Bouchard C, Tremblay A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 2003;77(6):1448–52. doi: 10.1093/ajcn/77.6.1448. [DOI] [PubMed] [Google Scholar]
- 10.Carruth BR, Skinner JD. The role of dietary calcium and other nutrients in moderating body fat in preschool children. Int J Obes Relat Metab Disord. 2001;25(4):559–66. doi: 10.1038/sj.ijo.0801562. [DOI] [PubMed] [Google Scholar]
- 11.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. Faseb J. 2000;14(9):1132–8. [PubMed] [Google Scholar]
- 12.Lovejoy JC, Champagne CM, Smith SR, de Jonge L, Xie H. Ethnic differences in dietary intakes, physical activity, and energy expenditure in middle-aged, premenopausal women: the Healthy Transitions Study. Am J Clin Nutr. 2001;74(1):90–5. doi: 10.1093/ajcn/74.1.90. [DOI] [PubMed] [Google Scholar]
- 13.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. Jama. 2002;287(16):2081–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 14.Loos RJ, Rankinen T, Leon AS, et al. Calcium intake is associated with adiposity in Black and White men and White women of the HERITAGE Family Study. J Nutr. 2004;134(7):1772–8. doi: 10.1093/jn/134.7.1772. [DOI] [PubMed] [Google Scholar]
- 15.Elwood PC, Strain JJ, Robson PJ, et al. Milk consumption, stroke, and heart attack risk: evidence from the Caerphilly cohort of older men. J Epidemiol Community Health. 2005;59(6):502–5. doi: 10.1136/jech.2004.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health. 2007;61(8):695–8. doi: 10.1136/jech.2006.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barba G, Troiano E, Russo P, Venezia A, Siani A. Inverse association between body mass and frequency of milk consumption in children. Br J Nutr. 2005;93(1):15–9. doi: 10.1079/bjn20041300. [DOI] [PubMed] [Google Scholar]
- 18.Davies KM, Heaney RP, Recker RR, et al. Calcium intake and body weight. J Clin Endocrinol Metab. 2000;85(12):4635–8. doi: 10.1210/jcem.85.12.7063. [DOI] [PubMed] [Google Scholar]
- 19.Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11(12):1961–6. doi: 10.1002/jbmr.5650111218. [DOI] [PubMed] [Google Scholar]
- 20.Drapeau V, Despres JP, Bouchard C, et al. Modifications in food-group consumption are related to long-term body-weight changes. Am J Clin Nutr. 2004;80(1):29–37. doi: 10.1093/ajcn/80.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez AJ, White E, Kristal A, Littman AJ. Calcium intake and 10-year weight change in middle-aged adults. J Am Diet Assoc. 2006;106(7):1066–73. doi: 10.1016/j.jada.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Caan B, Neuhouser M, Aragaki A, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007;167(9):893–902. doi: 10.1001/archinte.167.9.893. [DOI] [PubMed] [Google Scholar]
- 23.Lin YC, Lyle RM, McCabe LD, McCabe GP, Weaver CM, Teegarden D. Dairy calcium is related to changes in body composition during a two-year exercise intervention in young women. J Am Coll Nutr. 2000;19(6):754–60. doi: 10.1080/07315724.2000.10718075. [DOI] [PubMed] [Google Scholar]
- 24.Moore LL, Bradlee ML, Gao D, Singer MR. Low dairy intake in early childhood predicts excess body fat gain. Obesity (Silver Spring) 2006;14(6):1010–8. doi: 10.1038/oby.2006.116. [DOI] [PubMed] [Google Scholar]
- 25.Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159(6):543–50. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- 26.Reid IR, Horne A, Mason B, Ames R, Bava U, Gamble GD. Effects of calcium supplementation on body weight and blood pressure in normal older women: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90(7):3824–9. doi: 10.1210/jc.2004-2205. [DOI] [PubMed] [Google Scholar]
- 27.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83(3):559–566. doi: 10.1093/ajcn.83.3.559. [DOI] [PubMed] [Google Scholar]
- 28.Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J Bone Miner Res. 2006;21(3):397–405. doi: 10.1359/JBMR.051206. [DOI] [PubMed] [Google Scholar]
- 29.Boon N, Koppes LL, Saris WH, Van Mechelen W. The relation between calcium intake and body composition in a Dutch population: The Amsterdam Growth and Health Longitudinal Study. Am J Epidemiol. 2005;162(1):27–32. doi: 10.1093/aje/kwi161. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen JK, Molgaard C, Michaelsen KF, Astrup A. Calcium supplementation for 1 y does not reduce body weight or fat mass in young girls. Am J Clin Nutr. 2006;83(1):18–23. doi: 10.1093/ajcn/83.1.18. [DOI] [PubMed] [Google Scholar]
- 31.DeJongh ED, Binkley TL, Specker BL. Fat mass gain is lower in calcium-supplemented than in unsupplemented preschool children with low dietary calcium intakes. Am J Clin Nutr. 2006;84(5):1123–7. doi: 10.1093/ajcn/84.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Beverage consumption is not associated with changes in weight and body mass index among low-income preschool children in North Dakota. J Am Diet Assoc. 2004;104(7):1086–94. doi: 10.1016/j.jada.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Phillips SM, Bandini LG, Cyr H, Colclough-Douglas S, Naumova E, Must A. Dairy food consumption and body weight and fatness studied longitudinally over the adolescent period. Int J Obes Relat Metab Disord. 2003;27(9):1106–13. doi: 10.1038/sj.ijo.0802370. [DOI] [PubMed] [Google Scholar]
- 34.Trowman R, Dumville JC, Hahn S, Torgerson DJ. A systematic review of the effects of calcium supplementation on body weight. Br J Nutr. 2006;95(6):1033–8. doi: 10.1079/bjn20051727. [DOI] [PubMed] [Google Scholar]
- 35.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. 2004;12(4):582–90. doi: 10.1038/oby.2004.67. [DOI] [PubMed] [Google Scholar]
- 36.Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13(7):1218–25. doi: 10.1038/oby.2005.144. [DOI] [PubMed] [Google Scholar]
- 37.Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes (Lond) 2005;29(4):391–7. doi: 10.1038/sj.ijo.0802880. [DOI] [PubMed] [Google Scholar]
- 38.Shahar DR, Abel R, Elhayany A, Vardi H, Fraser D. Does dairy calcium intake enhance weight loss among overweight diabetic patients? Diabetes Care. 2007;30(3):485–9. doi: 10.2337/dc06-1564. [DOI] [PubMed] [Google Scholar]
- 39.Harris RB. Dairy protein, calcium and body weight--the need for a mechanism. Int J Obes (Lond) 2005;29(4):388–90. doi: 10.1038/sj.ijo.0802878. [DOI] [PubMed] [Google Scholar]
- 40.Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr. 1993;123(6):1047–53. doi: 10.1093/jn/123.6.1047. [DOI] [PubMed] [Google Scholar]
- 41.Shahkhalili Y, Murset C, Meirim I, et al. Calcium supplementation of chocolate: effect on cocoa butter digestibility and blood lipids in humans. Am J Clin Nutr. 2001;73(2):246–52. doi: 10.1093/ajcn/73.2.246. [DOI] [PubMed] [Google Scholar]
- 42.Zemel MB. Role of calcium and dairy products in energy partitioning and weight management. Am J Clin Nutr. 2004;79(5):907S–912S. doi: 10.1093/ajcn/79.5.907S. [DOI] [PubMed] [Google Scholar]
- 43.Shi H, Halvorsen YD, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000;3(2):75–82. doi: 10.1152/physiolgenomics.2000.3.2.75. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. Faseb J. 2001;15(14):2751–3. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- 45.Shi H, Dirienzo D, Zemel MB. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. Faseb J. 2001;15(2):291–3. doi: 10.1096/fj.00-0584fje. [DOI] [PubMed] [Google Scholar]
- 46.Zemel MB, Miller SL. Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr Rev. 2004;62(4):125–31. doi: 10.1111/j.1753-4887.2004.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 47.Cummings NK, James AP, Soares MJ. The acute effects of different sources of dietary calcium on postprandial energy metabolism. Br J Nutr. 2006;96(1):138–44. doi: 10.1079/bjn20061803. [DOI] [PubMed] [Google Scholar]
- 48.Melanson EL, Sharp TA, Schneider J, Donahoo WT, Grunwald GK, Hill JO. Relation between calcium intake and fat oxidation in adult humans. Int J Obes Relat Metab Disord. 2003;27(2):196–203. doi: 10.1038/sj.ijo.802202. [DOI] [PubMed] [Google Scholar]
- 49.Melanson EL, Donahoo WT, Dong F, Ida T, Zemel MB. Effect of low- and high-calcium dairy-based diets on macronutrient oxidation in humans. Obes Res. 2005;13(12):2102–12. doi: 10.1038/oby.2005.261. [DOI] [PubMed] [Google Scholar]
- 50.Anon. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 51.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Manual. Champaign IL: Human Kinetics Publishers, Inc; 1988. [Google Scholar]
- 52.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006;64(5):523–9. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth HJ, Zahn I, Alkier R, Schmidt H. Validation of the first automated chemiluminescence protein-binding assay for the detection of 25-hydroxycalciferol. Clin Lab. 2001;47(7–8):357–65. [PubMed] [Google Scholar]
- 54.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 55.Sebring NG, Denkinger BI, Menzie CM, Yanoff LB, Parikh SJ, Yanovski JA. Validation of three food frequency questionnaires to assess dietary calcium intake in adults. J Am Diet Assoc. 2007;107(5):752–9. doi: 10.1016/j.jada.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 57.Thompson FE, Subar AF, Brown CC, et al. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102(2):212–25. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 58.Hollingshead AB. Hollingshead two factor index of social position. In: Miller DC, editor. Handbook of research design and social measurement. 5. Newbury Park, CA: Sage Publications; 1957. pp. 351–359. 1991. [Google Scholar]
- 59.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000;342(12):861–7. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4(3):175–84. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 61. [Accessed July 24, 2007 program];];Software for multiple imputation. 1997 [Google Scholar]
- 62.Shafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- 63.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 64.Medicine Io. Anonymous. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 65.Barnett V, Lewis T. In: Outliers in Statistical Data. 2. Shewhart WA, Wilks SS, editors. New York: John Wiley & Sons; 1984. Wiley Series in Probability and Mathematics. [Google Scholar]
- 66.Davis MJ, Addis ME. Predictors of attrition from behavioral medicine treatments. Ann Behav Med. 1999;21(4):339–49. doi: 10.1007/BF02895967. [DOI] [PubMed] [Google Scholar]
- 67.Williamson DF, Kahn HS, Byers T. The 10-y incidence of obesity and major weight gain in black and white US women aged 30–55 y. Am J Clin Nutr. 1991;53(6 Suppl):1515S–1518S. doi: 10.1093/ajcn/53.6.1515S. [DOI] [PubMed] [Google Scholar]
- 68.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132(3):501–13. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 69.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322(13):882–9. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 70.Ballard-Barbash R, Schatzkin A, Taylor PR, Kahle LL. Association of change in body mass with breast cancer. Cancer Res. 1990;50(7):2152–5. [PubMed] [Google Scholar]
- 71.Sahyoun NR, Hochberg MC, Helmick CG, Harris T, Pamuk ER. Body mass index, weight change, and incidence of self-reported physician-diagnosed arthritis among women. Am J Public Health. 1999;89(3):391–4. doi: 10.2105/ajph.89.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winzenberg T, Shaw K, Fryer J, Jones G. Calcium supplements in healthy children do not affect weight gain, height, or body composition. Obesity (Silver Spring) 2007;15(7):1789–98. doi: 10.1038/oby.2007.213. [DOI] [PubMed] [Google Scholar]
- 73.Lanou AJ, Barnard ND. Dairy and weight loss hypothesis: an evaluation of the clinical trials. Nutr Rev. 2008;66(5):272–9. doi: 10.1111/j.1753-4887.2008.00032.x. [DOI] [PubMed] [Google Scholar]
- 74.Bowen J, Noakes M, Clifton PM. Effect of calcium and dairy foods in high protein, energy-restricted diets on weight loss and metabolic parameters in overweight adults. Int J Obes (Lond) 2005;29(8):957–65. doi: 10.1038/sj.ijo.0802895. [DOI] [PubMed] [Google Scholar]
- 75.Harvey-Berino J, Gold BC, Lauber R, Starinski A. The impact of calcium and dairy product consumption on weight loss. Obes Res. 2005;13(10):1720–6. doi: 10.1038/oby.2005.210. [DOI] [PubMed] [Google Scholar]
- 76.Shapses SA, Heshka S, Heymsfield SB. Effect of calcium supplementation on weight and fat loss in women. J Clin Endocrinol Metab. 2004;89(2):632–7. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lappe JM, Rafferty KA, Davies KM, Lypaczewski G. Girls on a high-calcium diet gain weight at the same rate as girls on a normal diet: a pilot study. J Am Diet Assoc. 2004;104(9):1361–7. doi: 10.1016/j.jada.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13(8):1344–53. doi: 10.1038/oby.2005.163. [DOI] [PubMed] [Google Scholar]
- 79.Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated metaanalysis of randomized controlled trials. Am J Hypertens. 1999;12(1 Pt 1):84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 80.Lorenzen JK, Nielsen S, Holst JJ, Tetens I, Rehfeld JF, Astrup A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am J Clin Nutr. 2007;85(3):678–87. doi: 10.1093/ajcn/85.3.678. [DOI] [PubMed] [Google Scholar]
- 81.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150(3):665–72. [PubMed] [Google Scholar]
- 82.Williamson DF. Descriptive epidemiology of body weight and weight change in U.S. adults. Ann Intern Med. 1993;119(7 Pt 2):646–9. doi: 10.7326/0003-4819-119-7_part_2-199310011-00004. [DOI] [PubMed] [Google Scholar]
- 83.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]