Abstract
Background
Phytoestrogens are non-steroidal compounds possessing estrogenic activity present in significant amounts in soy-based pet foods. There is speculation that long-term consumption of phytoestrogen-rich diets could have biological effects but this has never been evaluated in dogs.
Hypothesis
Soy-based diets may affect general health, adrenocortical function, thyroid function, behavior and skin/coat quality in adult dogs.
Animals
Fifteen normal dogs were divided into two groups and fed either high-isoflavone (HID) or a low-isoflavone (LID) soy-based diet.
Methods
In this prospective controlled randomized trial end points of general health were assessed at baseline and up to one-year by evaluating body and dermatological condition, hematology, biochemistry profiles, urine-analysis (UA), serum concentrations of adrenal and thyroid hormones, and behavior. Student’s t-test (2 time points) analysis of variance with repeated measures (3 time points) was used to analyze differences in these parameters from baseline between diets.
Results
No differences were found in measures of skin/coat, body condition, or behavior, in either group. Results of hematology, biochemical profiles and urinalysis showed no differences between the two groups. Analysis of variance in repeated measures was used to analyze differences in hair follicles from baseline between diets when there were 2 time points. No differences were found in the evaluation of the skin, hair follicle and hair diameter size. Most adrenal and thyroid hormones did not change over time nor were they different by diet (P>.1). However, total T4 differences were higher in HI than LI group (15.3 vs −1.4 p=.07) and post-ACTH estradiol concentration differences were also increased in HI compared to LI groups (19 vs −5.6 pg/ml at 1 year, p=.0006).
Conclusions and Clinical Importance
These data suggest long-term ingestion of soy phytoestrogens may influence endocrine function in dogs and this could potentially impact results of studies evaluating endocrine function in dogs although larger studies are needed for confirmation.
Keywords: Dog, Endocrine, Isoflavones, Skin, Coat
Phytoestrogens are plant-derived, non-steroidal compounds possessing estrogenic activity. They are divided into three classes, one of which, the flavonoids, contains isoflavones. The principal dietary source of isoflavones is the soybean, which contains the compounds in four related chemical structures: the aglycones, the 7-O-glucosides, the 6-O-acetylglucosides and the 6′-O-malonylglucosides.1
Soy is a common dog food ingredient. A study of soy isoflavone content in commercially available canine diets found that many contain phytoestrogens.2
It has been speculated that consumption of phytoestrogen-rich diets could influence adrenal/gonadal steroidogenesis and thyroid function. Phytoestrogens may impair fertility and predispose to reproductive tract disorders in some animals and in man.3 Delayed puberty onset has been reported in rats fed a soy diet.4 Soy isoflavones such as daidzein and genistein accumulate in target tissues (liver, adrenals, etc) and are reported to inhibit 3β–hydroxysteroid dehydrogenase (HSD), aromatase and 17β-HSD, all enzymes involved in sex hormone steroidogenesis.5–6 Short-term administration of dietary soy has been reported to have a measurable, although modest, effect on thyroid hormone homeostasis in cats..7 Dietary isoflavones are associated with behavior changes such as decreased8 or increased9 anxiety in rats, and increased aggression and decreased social affiliative behavior in nonhuman primates.10
There has been only limited investigation of the effect of soy isoflavones on canine health and behavior. The hypothesis of our study is that canine diets containing soy isoflavone would influence the hormonal status (alteration of steroidogenesis and/or thyroid hormone production) that, in turn, could affect skin and coat quality by leading to hair loss and progressive thinning of the coat.
Materials and Methods
Animals
Thirty healthy dogs were recruited among staff and students of the Veterinary Hospital of the University of Pennsylvania. Pet owners were informed about procedures and written consent obtained. The protocol had undergone review and approval by the Hospital Protocol Review and the Institutional Animal Care and Use Committees. The number of dogs was derived from a power analysis based on what it was believed would be clinically relevant for our study. Dogs to be enrolled into the study had to be healthy based on medical history, physical and dermatological examination, and laboratory tests. The physical examination and blood work was also repeated at the end of the study. The inclusion criteria were: age 2–8 years, neuter status, (spayed females or castrated males) and coat type (long or short). Dogs with curly coat type (e.g. poodle) or with systemic or dermatological diseases were excluded.
Clinical evaluation & skin and coat quality
Physical and dermatological examination, including body weight and condition score, were done at the beginning of the study (0) and at 6 and 12 months. Owners also completed a questionnaire with a visual analog scale (0–10) grading the degree of dryness-greasiness of the coat and the skin, and the quality of the coat (shiny to dry) at 0 and 12 months.
Laboratory tests
Blood was collected by jugular venipuncture for routine hematological (CBC) and biochemical investigations. A coagulation profile and an ELISA test for heartworm, ehrlichiosis and Lyme disease were done. A fecal sample was analyzed to rule out intestinal parasites. Urine samples for standard urinalysis (UA) were collected by free catch. The CBC, biochemistry and UA were done at 0 and 12 months. Samples were collected during the morning (8:00–12:00) in fasted dogs (who were not fed after 8:00pm the previous day). Urine samples were collected either by the owner or by the investigators during the time spent at the hospital.
Three aliquots of serum were stored at −20°C for hormonal assays (a thyroid panel to Michigan State University (T4, T3, free T4 (fT4), free T3 (fT3), T4 and T3 autoantibody (T4AA, T3AA), thyroid stimulating hormone (TSH), and thyroglobulin autoantibody (TgAA) at 0 and 12 months and a basal sex hormone panel to the University of Tennessee. A second blood sample was collected one hour after intravenous administration of 5 μg/kg of tetracosactrin acetatea. The hormones measured in both samples included cortisol, 17-hydroxyprogesterone (17-OHP), androstenedione, estradiol, progesterone, testosterone and aldosterone. This test was done at 0, 6 and 12 months. The third serum sample together with a urine sample was sent to Tufts University to measure isoflavone content at 0, 6 and 12 months.
The source of reagents, assay procedures, and suitability for use in canine serum has been previously reported for all thyroid-related tests. Validation data for T4 and TSH11, T312, fT313, fT4 ED14, T4AA and T3AA15, and TgAA16 have been described.
The “sex-hormone panel” hormones were measured using human kits which have been previously validated for the dog.17–18 The sensitivity of the hormone assay is: testosterone (0.4 ng/ml), androstenedione (0.05 ng/ml), 17-OHP (0.08 ng/ml), progesterone (0.02 ng/ml), estradiol (7.2 pg/ml), cortisol (0.02 ug/dL).
Serum and urine samples were assayed for soy isoflavone (genistein, daidzein, and glycitein) concentrations in duplicate using a novel assay. Briefly, 500 uL urine or serum samples were mixed with 1.25 ug biochanin A (internal standard) and 500 uL 1 M phosphate buffer (pH 5) and enzymatically deconjugated with 25 uL β-glucuronidase/sulfatase (Sigma) at 37°C overnight. Samples were then neutralized with 500 uL 1 N NaOH, extracted into 5 mL of ethyl acetate and dried down. After reconstitution in 200 uL of HPLC mobile phase, samples were analyzed by HPLC with mass spectrometry detection and an atmospheric pressure chemical ionization sourceb. Initial mobile phase conditions consisted of 0.5% formic acid in water with 30% methanol at a flow rate of 300 uL/min with a linear gradient to 100% methanol over 9 minutes. Separation was achieved with a 150 × 2.0 mm 4 micron Synergi Fusion RP C-18 column (Phenomenex, Torrance, CA). Analytes were quantified by positive ion selective reaction monitoring with parent→product ion mass (m/z+) transitions of 271→215 for genistein, 255→199 for daidzein, and 285→270 for biochanin A. For quantitation purposes, standard curves were generated using pure standards dissolved in blank matrix. Regression coefficients for standard curves were consistently >0.98, while coefficients of variation of assay duplicates averaged less than 20%. The lower limit of quantitation (LOQ) of the assay was 50 nmoles/L. Urine isoflavone concentrations were expressed relative to creatinine concentration (nmoles/mg creatinine) to account for differences in urinary output.
Diets and study groups
Dogs were fed either a commercially available hydrolyzed soy isolate-basedc diet (high isoflavones diet; HID) or the same diet with an isolate in which isoflavones had been extracted (low isoflavones diet; LID) (Table 1). The method for processing soy isolates is proprietary. Preparation of a low isoflavone soy isolate involves alcohol extraction rather than the typical water extraction. Upon recruitment, dogs were assigned to one of two diets using a table of randomly generated numbers. Amounts fed were calculated using the equation 132 kcal metabolizable energy/kg0.75 body weight, adjusted to maintain body weight. The dry expanded diets were manufactured by Royal Canin, Rolla, MO, and were identified by a three letter code thereby masking all investigators to the diets’ isoflavone content. The caloric and nutrient content of each diet is presented in Table 1. Two batches of food were manufactured and supplied in four shipments during the yearlong study. The shelf life of each diet was at least 12 months. Owners were instructed to use only soy-free treats. A list of approved treats was provided to the participants. Owners also completed a questionnaire to evaluate dietary management prior to start the study and a monthly form in which they were to record daily, when necessary, any change in the dog’s appetite or fecal quality, and any extra food given apart from the diet and the allowed treats/biscuits.
Table 1.
Diet analysis and isoflavones composition.
| HIDa | LID | ||||
|---|---|---|---|---|---|
| Unit (as Fed) | Batch 1 | Batch 2 | Batch 1 | Batch 2 | |
| Moisture | % | 4.7 | 7.9 | 5.3 | 6.4 |
| Protein | % | 23.9 | 22.7 | 23.4 | 23.6 |
| Fat | % | 20.8 | 18.1 | 20.4 | 17.1 |
| Crude fiber | % | 2 | 2.1 | 2 | 2.1 |
| Ash | % | 6.8 | 7.5 | 6.9 | 7.9 |
| Genistein | mg/kg (DW) food | 81 | 55 | 6 | 2 |
| Daidzein | mg/kg (DW) food | 31 | 26 | 2 | 4 |
| Glycitein | mg/kg (DW) food | 17 | 11 | ND | ND |
Royal Canin Veterinary Diet™ Canine Hypoallergenic, Royal Canin, St charles, Mo
List of ingredients: ‘Rice, soy protein isolate hydrolysate, chicken fat, natural flavors, beet pulp, vegetable oil, sodium silico aluminate, dicalcium phosphate, calcium carbonate, fish oil, inulin, potassium chloride, monosodium phosphate, L-tyrosine, choline chloride, taurine*, borage oil, Vitamins [dl-alpha tocopherol (source of vitamin E), inositol, niacin, L-ascorbyl-2-polyphosphate (source of vitamin C*), d-calcium pantothenate, biotin, pyridoxine hydrochloride (vitamin B6), riboflavin (vitamin B2), thiamine mononitrate (vitamin B1), vitamin A acetate, folic acid, vitamin B12 supplement, vitamin D3 supplement], Trace Minerals [zinc amino acid chelate, zinc oxide, ferrous sulfate, manganese amino acid chelate, copper amino acid chelate, copper sulfate, manganous oxide, sodium selenite, calcium iodate], marigold extract, preserved with natural mixed tocopherols, rosemary extract, and citric acid.
ND: not detected; LOQ= 2mg/kg glycitein; 1 mg/kg genistein and daidzein
Batch 1: manufactured in August 2005; Batch 2: manufactured in April 2006
Soy isoflavone concentrations (aglycone) were measured in representative samples of the diets, collected at 0 and 6 months, by a validated method involving acid-methanol hydrolysis followed by HPLC with UV absorbance detection as previously described.19 The LOQ of the assay was 1 mg of genistein or daidzein/kg dry weight (DW) food, and 2 mg of glycitein/kg DW food.
Management
To standardize the management of the enrolled dogs, owners were provided a shampoo for normal coatd to be used as needed and flea/tick spot-on preventione to be used monthly.
Behavior
Behavioral assessment was accomplished through owner-report questionnaires. An extended questionnaire, distributed at 0, 6 and 12 months, included fear/anxiety screening, in which owners indicated on a visual analog scale (0–10) the degree of fear or anxiety noted in each of 13 situations. A difference of at least 3 points between months 0 and 12 was considered behaviorally meaningful. A brief, interim questionnaire was distributed on months 1–5 and 7–11, in which owners indicated whether there was an increase, decrease or no change in anxiety, aggression, and activity level compared to the dog’s behavior before the start of the study. Behavior results were not statistically analyzed because of small sample size.
Collection of skin biopsy specimens for histopathology
Two skin specimens from each dog were collected at the beginning of the study with a 6-mm punch biopsy under local anesthesia using 2% lidocaine from the chest and the flank and also one year later on the opposite side of the body.
Specimens were fixed in 10% formal saline and transferred to the laboratory for processing and examination. Forty, formalin-fixed, biopsy specimens from 20 dogs were evaluated. These were bisected vertically, along the line of the hair where possible, and one half of the specimen was horizontally trimmed. The sections were stained with haematoxylin and eosin using standard histological methods. All sections were initially examined “blind” i.e. without prior knowledge of the time or site of the skin sampled. Results were subsequently reviewed in relation to the time of sampling.
Statistical analyses
The study was powered to detect a 20% difference in hair follicles diameter with a 80% power and alpha set at .05. Fifteen dogs were needed in each group. To determine differences between diets with regard to the CBC, biochemistry data, UA results and the hormonal data including thyroid, adrenal hormones and the isoflavones content in the dogs serum and urine samples Student’s t-test on the differences between baseline and one year was used. In cases where the baseline values of the diet groups were significantly different, analysis of covariance was used, adjusting for baseline value. For biologic parameters for which there were 3 time points, (0, 6 and 12 months) analysis of variance in repeated measures on the differences from baseline was employed. Data are presented as means +/− standard error. Differences between time periods are presented as means with 95% CI. All analyses were performed using SAS statistical softwaref. A probability <.05 was considered statistically significant.
Results
Animals
Fourteen of the recruited dogs were in the HID group while 16 dogs were in the LID group. Twenty dogs completed the study (HID: 2 females and 8 males; LID: 4 females and 6 males). There was no difference in age between the two groups (HID: 4.1 +/− 1.7 vs LID: 5.3 +/− 2.6, p=.24). Ten dogs (four from the HID group and six from the LID group) were withdrawn: four (2 HID and 2 LID) for lack of owner compliance, three (LID) found the diet unpalatable, one (HID) developed a mast cell tumor, one (LID) developed retinal degeneration and behavior problems, and one (HID) developed aggressive behavior.
Clinical evaluation
General physical and dermatological examination revealed that all dogs were healthy at the beginning and end of the study.
Skin and coat quality
The owners of four (three HID, one LID) of the 20 dogs reported that the coat and skin quality of their dogs had improved during the one-year study. No significant differences were found between diet groups with regard to coat and skin quality (p>.05 for all). However, three owners of dogs in the HID group and only one owner of a dog in the LID group felt that the quality of the skin and coat had improved.
Body score, weight and food intake
There was no significant change in body condition score from baseline in either group and there was no difference in weight change over time between diets (mean weight change for HID=1.3 +/−.92 SEM vs LID 0.8 +/− .92 SEM, p=.7). Average food intake over the 12 months was 20.58 +/−2.4 in the HID group and 24.81 +/− 1.96g in the LID group.
Laboratory investigations
At the beginning of the study routine hematological and biochemical investigations, and UA gave values which were within normal limits in almost all dogs (data not shown). Coagulation profile was normal and fecal test was negative for endoparasite in all dogs. No antibodies were detected in all dogs for Dirofilaria immitis, Ehrlichia canis, but 7 dogs had antibodies for Borrelia burgdorferi. At 12 months six dogs (four HI, 2 LI) had slight increase of ALT (92–262 U/L, reference range: 16–91 U/L) and ALP (184-2,285, reference range: 20–155 U/L) but no statistical significance was found when the data between the two groups and in dogs within each group between the two time points were compared.
Hormonal assays - Thyroid
At the beginning of the study all dogs had values within the laboratory reference range. At 12 months the only abnormality detected was increased T4 concentration outside the normal reference range in one dog on the HID, but that dog also had slightly high T4AA (23%; reference range < 20%).
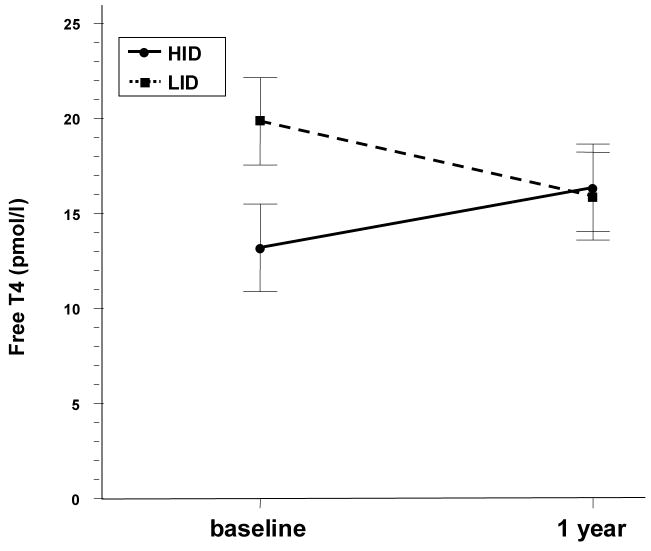
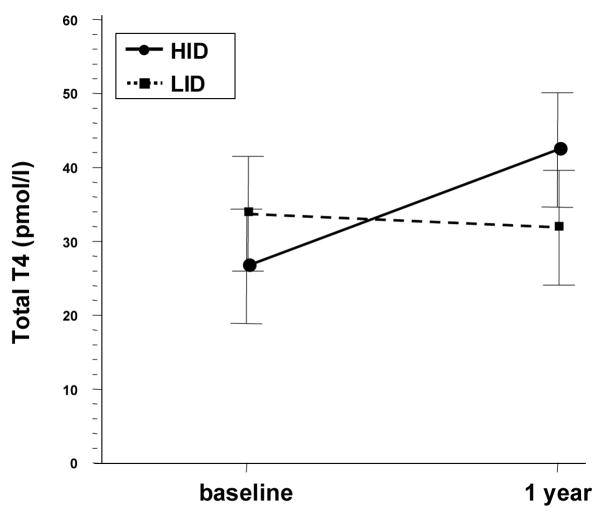
Free T4 and total T4 differences over time were significantly different between diets using the t-test (p=.005 and .04 respectively) (Table 2). However, for free T4, after adjusting for significant baseline differences (p=.02), no difference was noted between the diets over time (p=.18, Figure 1). In addition, after adjustment for non significant baseline differences in total T4 (p=.06), a difference persisted (T4 difference 15.3 vs −1.4) but was no longer statistically significant (p=.07, Figure 2).
Table 2.
Thyroid hormone panel showing the difference between diets over time.
| Parameter | Diet | Reference range | Baseline | 1 year | difference | Difference between diets | p-value* |
|---|---|---|---|---|---|---|---|
| Total T4 (pmol/l) | HID | 15–67 | 26.7 | 42.4 | 15.7 | 17.6 | 0.04 |
| LID | 33.8 | 31.9 | −1.9 | ||||
| Total T3 (pmol/l) | HID | 1.0–2.5 | 1.3 | 1.7 | 0.4 | 0.1 | 0.8 |
| LID | 1.3 | 1.6 | 0.3 | ||||
| Free T4 (pmol/l) | HID | 8–26 | 13.2 | 14.8 | 1.6 | 5.9 | 0.005 |
| LID | 19.9 | 15.6 | −4.3 | ||||
| Free T3 (pmol/l) | HID | 4.5–12.5 | 5.7 | 5.4 | −0.3 | −0.9 | 0.2 |
| LID | 5.1 | 5.7 | 0.6 | ||||
| T4AA (%) | HID | <20 | 3.3 | 2.9 | −0.4 | 1.8 | 0.5 |
| LID | 1.0 | 2.7 | −2.2 | ||||
| T3AA (%) | HID | <10 | 1.4 | 2.0 | 0.6 | 0.5 | 0.7 |
| LID | 1.0 | 1.1 | 0.1 | ||||
| CTSH (mU/l) | HID | <37 | 9.4 | 13.8 | 4.4 | −7.2 | 0.08 |
| LID | 5.8 | 17.4 | 11.6 | ||||
| CTSH (ng/ml) | HID | <0.68 | 0.1 | 0.2 | 0.071 | −0.114 | 0.1 |
| LID | 0.1 | 0.3 | 0.185 | ||||
| TGAA (%) | HID | <20 neg | 17.9 | 21.6 | 3.7 | −0.7 | 0.9 |
| LID | 13.4 | 17.8 | 4.4 |
p-value is for the differences between diets over time.
Fig 1.
Free T4 trends over time and between diets. Error bars represent Standard Error.
Fig 2.
Total T4 trends over time and between diets. Error bars represent Standard Error
Hormonal assays - Adrenal
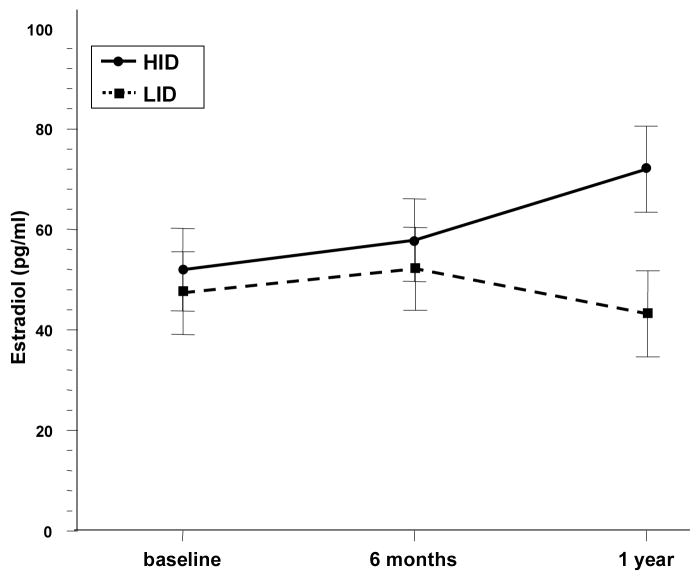
At the beginning of the study all dogs had values within the laboratory reference range. At 12 months the only abnormal values detected were slightly increased estradiol concentrations (baseline: mean 87.10, SD: 9.38; post-ACTH: 87.77, SD: 13.08) outside of the normal reference range in seven dogs: five (HID) and two (LID). No differences in hormone levels between diets over time were found except for estradiol (Table 3). Dogs fed the HID had an increase in estradiol levels over time whereas dogs fed the LID had a decrease (19 vs −5.6 pg/ml at 1 year, p=.0006, Figure 3). Adjustment for baseline differences did not alter this result.
Table 3.
Adrenal hormone panel - * p-values represent whether diets differed over time by ANOVA
| Pre ATCH stimulation | ||||||
|---|---|---|---|---|---|---|
| Parameter | Diet | Baseline | 6 months | 1 year | CM | SF |
| Cortisol (ng/ml) | HID LID |
28.3 19.41 |
26.18 32.01 |
32.219 33.773 |
2.0–56.5 | 2.1–58.8 |
| Androstenedione (ng/ml) | HID LID |
1.7 1.35 |
2.4 3.12 |
2.34 3.31 |
0.1–3.6 | 0.1–5.7 |
| Progesterone (ng/ml) | HID LID |
0.273 0.217 |
0.204 0.21 |
0.283 0.31 |
0.01–0.17 | 0.01–0.49 |
| 17 OH Progesterone (ng/ml) | HID LID |
0.211 0.247 |
0.345 0.384 |
0.253 0.318 |
0.01–0.22 | 0.01–0.07 |
| Testosterone (ng/ml) | HID LID |
0.032 0.037 |
0.024 0.02 |
0.028 0.031 |
0.01–0.24 | 0.01–0.32 |
| Estradiol (pg/ml) | HID LID |
53.95 45.04 |
59.95 54.69 |
66.56 43.07 |
23.1–65.1 | 30.8–69.9 |
| Aldosterone (g/ml) | HID LID |
ND ND |
35.7 34.95 |
25.94 44.24 |
3.5–139.9 | 3.5–139.9 |
| Post ATCH stimulation | ||||||
| Parameter | Diet | Baseline | 6 months | 1 year | CM** | SF** |
| Cortisol (ng/ml) | HID LID |
116.69 111.75 |
119.9111 109.33 |
121.96 110.5 |
70.6–151.2 | 65.0–174.6 |
| Androstenedione (ng/ml) | HID LID |
9.98 10.45 |
15.2 14.53 |
14.12 15.45 |
2.4–29.0 | 2.7–39.7 |
| Progesterone (ng/ml) | HID LID |
2.026 1.985 |
2.06 1.626 |
1.792 1.609 |
0.22–1.45 | 0.10–1.50 |
| 17 OH Progesterone (ng/ml) | HID LID |
1.796 2.009 |
2.538 2.765 |
2.075 1.806 |
0.25–2.63 | 0.40–1.62 |
| Testosterone (ng/ml) | HID LID |
0.036 0.046 |
0.028 0.024 |
0.034 0.034 |
0.02–0.42 | 0.02–0.45 |
| Estradiol* (pg/ml) | HID LID |
51.97 47.37 |
57.84 52.18 |
71 41.8 |
23.3–69.4 | 27.9–69.2 |
| Aldosterone (g/ml) | HID LID |
ND ND |
168.96 165.55 |
194.62 191.99 |
72.9–398.5 | 72.9–398.5 |
p-value: 0.01; ND: not done
reference range for castrated males (CM) and spayed females (SF)
Fig 3.
Estradiol post-ACTH trends over time and between diets. Error bars represent Standard Error. P values: at baseline (.97), at 6 months (.93), and at 1 year (.0006).
Diets
The HID had the following average isoflavone content: 28 mg daidzein/kg DW; 68 mg genistein/kg DW; and 14 mg glycitein/kg DW. The LID had the following average isoflavone content: 3 mg daidzein/kg DW; 4 mg genistein/kg DW; and <2 mg (LOQ) glycitein/kg DW (Table 1).
Based on average food consumption, the daily intake of isoflavones in each group of dogs was 2.12+/−0.25/kg bw and 0.16mg+/−0.11/kg bw in the HID and LID respectively.
The HID was more palatable than the LID. Three dogs found the LID less and less palatable throughout the study and to allow them to complete the study, two of the dogs were served the food moistened with warm water and one was served the food topped with a small amount of plain tomato sauce and grated parmesan cheese.
Isoflavone and metabolite levels in serum and urine
Daidzein, genistein, and glycitein concentrations were determined in serum and urine samples collected at 6 and 12 months to evaluate compliance and average isoflavone exposure. There was considerable variability in urine and serum isoflavone levels between dogs, probably reflecting the opportunistic timing of sample collection (i.e. collection without specific regard to when dogs consumed their last meal). Nevertheless several trends were (Table 4). Reflecting the dietary isoflavone content, genistein, daidzein and glycitein levels in serum and urine at 12 months were from 5 to 10 times higher in dogs on the HID compared with dogs on the LID. This difference in isoflavone content between diets was somewhat lower (up to 4-fold difference) in the 6 month samples compared with the 12 month samples.
Table 4.
Average serum and urine concentrations of phytoestrogens measured in dogs receiving either HID or LID measured at 6 and 12 months after the start of the study.
| Time (months) | Serum concentration (nmoles/L) | p-value | Urine concentration (nmoles/mg creatinine) | p-value | |||
|---|---|---|---|---|---|---|---|
| HID | LID | HID | LID | ||||
| Genistein | 6 | 166.4 | 147.4 | 0.23 | 0.744 | 0.193 | 0.013 |
| 12 | 222.7 | 75.7 | 1.185 | 0.164 | |||
| Daidzein | 6 | 246.7 | 68.3 | 0.008 | 0.739 | 0.220 | 0.012 |
| 12 | 254.4 | 7.9 | 1.000 | 0.170 | |||
| Glycitein | 6 | 91.7 | 0 | 0.02 | 0.570 | 0.174 | 0.008 |
| 12 | 76.5 | 0 | 0.754 | 0.084 | |||
| Total isoflavones | 6 | 413.8 | 215.7 | 0.03 | 1.935 | 0.413 | 0.004 |
| 12 | 476.9 | 83.6 | 2.255 | 0.333 | |||
Management
In seven dogs the shampoo was used monthly, in ten dogs the shampoo was used between 1–4 times per year, in one dog it was never used and a different product was used in two dogs. Four owners who had rarely shampooed their pet felt the dog’s coat had improved anyway. Advantix was used in 13 dogs, while a different product was used in seven dogs whose households included cats.
Behavior
LID group
There were generally few reported changes in fear or anxiety between 0 and 12 months. There appeared to be a decrease in anxiety or fear for 4 dogs in 1 (3 dogs) or 2 (1 dog) of the listed stimuli. One dog’s anxiety worsened with regard to only one stimulus. In all other situations for other dogs, there was either no change in score, or change was < 3 points. There were few overall changes in activity, anxiety or aggression noted over time. There appeared to be a decrease in anxiety in one dog which reversed itself (to “no change”) over the final 4 months. Another dog was reported as somewhat less aggressive for several months in the first half of the study. One dog was reported to be less active for a 5 month period, while another was more active.
HID group
There were no clear trends of change in fear or anxiety comparing responses at 0 and 12 months. There was improvement in anxiety for 2 dogs in 1 parameter each; two other dogs appeared to show increased anxiety. One dog showed a mixed response, with a decrease in anxiety for one stimulus and an increase for 3 others. There were more missing values for this group in the interim questionnaire data. Four dogs appeared to be more anxious in this group. One dog was more anxious, aggressive and active, with owners reporting “much more” anxiety over the final 4 months. In contrast, another dog in this group showed decreased anxiety (8 months) and aggression (7 months).
Histopathology
No differences were found on evaluation of the skin, the size of the hair follicles and the diameter of the hairs.
Discussion
To the best of our knowledge this is the first study to evaluate the effect of dietary phytoestrogens on canine health, endocrine status and their effect on skin and coat condition, and behavior. Our results show that feeding dogs for one year with a strictly soy-based diet does not seem to have either immediate implication on canine health, skin and coat quality, or behavior. The results of the endocrine tests to evaluate the thyroid and adrenal function even considering the small number of dogs and the short term study, show that changes in their endocrine function might be present, as reported in other species, but these need to be confirmed with long term studies.
The effect of phytoestrogens on thyroid function has been studied in laboratory animals, companion and farm animals and humans. In rats, cats and pigs fed a soy-based diet it has been reported an increase in fT4 and T4 concentrations without apparent change in T3 concentration.7,20 In another study in rats TSH and T4 but not T3 levels were decreased.21 In man it has been suggested that an association exists between soy formula feedings in infancy and autoimmune thyroid disease.22 In humans, soy is reported to either have no effect or result in reduced T3 concentrations but unchanged T4 concentrations.23–24 Exposure to soy protein in human studies was much lower than that in experimental animal studies, however. Increase in serum T4 concentrations relative to T3 concentrations could result from inhibition of the enzyme responsible for conversion of T4 to T3 (5′-iodothyronine deiodinase) as speculated previously.7 Although soy isoflavones have not yet been investigated as inhibitors of deiodinase, related flavonoid compounds found in plants such as biochanin A, rutin, and quercetin are potent inhibitors, with concentrations causing 50% decrease in enzyme activity in the low micromolar range.25,26 Deiodinase inhibition initially would be expected to decrease T3 concentrations. However, because T3 is the primary determinant of TSH release by the pituitary gland, homeostatic mechanisms should rapidly restore T3 concentrations nearly to reference range, but with a higher output of T4 from the thyroid gland, as required to overcome enzyme inhibition. Over the long term there may be goiter formation and associated local hypermetabolism which may result in genetic mutations and feedback dysregulation (autonomous nodules = toxic nodular goiter) with increased T4 beyond that needed leading to hyperthyroidism. A similar effect on serum thyroid hormone concentrations (higher T4 with unchanged T3 concentrations) could also be hypothesized to occur if soy resulted in enhanced clearance of T3 (but not of T4) through induction of the enzymes responsible for metabolism of T3. Inducers of T3 glucuronidation in rats (such as pregnenolone-16-alpha-carbonitrile) have been identified that result in thyroid hyperplasia.27
It has been shown that estradiol operates directly on thyroid tissue through estrogen receptors28 and the phytoestrogens can also bind to the estrogen receptors.29 Whether estrogen receptors are present in the canine thyroid glands that are capable of binding dietary phytoestrogens is currently unknown. In our study the results of the thyroid test suggest that small changes in total T4 may occur in dogs over a long period of time but further studies are necessary to support this theory.
It has been reported that adrenocortical function may be altered by phytoestrogen intake because genisteinand daidzein can inhibit the activity of 21-hydroxylase, which may lead to decreased cortisol synthesisand, as a consequence, increased (Dehydroepiandrosterone) DHEA/DHEA-sulphate synthesis by shunting metabolites away from the glucocorticoid synthetic pathway.30 These data were not confirmed by a more recent in vivo study in monkeys fed soy based diet where a lower adrenal weight and zona fasciculata thickness were found.31 It is unknown if phytoestrogens have an effect on the enzymes involved in canine steroidogenesis.
Phytoestrogens are structurally and functionally similar to estradiol and have the ability to selectively bind estrogen receptors (ER): ER-β greater than ER-α.32 Phytoestrogens are supposed to act as antiestrogens by competing with more potent endogenous estrogens for the binding to ER. In addition to the interaction with ER, dietary phytoestrogens might compete with endogenous estrogens for the active site of the estrogen-biosynthesizing and estrogen-metabolizing enzymes and thus reduce the concentration of biologically active endogenous estrogens. Coumestrol and genistein have been shown to inhibit the reduction of estrone to 17B-estradiol by acting on the 17,β-HSD type 1.33 Genistein and daidzein also inhibit the enzyme 3 β-HSD and 17 β-HSD in vitro in human placental microsomes5 and in the bovine adrenal glands.34
In our study no differences were seen between the two groups or in individual dogs between the three time points (0-6-12 months) for any steroids except estradiol. The differences between the two groups and in single dogs in the HI group were statistically significant suggesting phytoestrogens might have induced elevations of endogenous estrogens by blocking the estrogen receptors allowing more endogenous free estrogen to be measured. Clinical signs (e.g. hematological abnormalities, coat changes) were not detected in dogs with hyperestrogenism. One of the authors (JWO) has seen unusual signs of hyperestrogenism (polyuria and polydipsia, hepatomegaly, increased ALK and ALT and dilute urine) in dogs with estradiol concentration > 70 pg/ml. Seven of our dogs (6 males and 1 female) of which five were on HID and two on LID, had estradiol concentrations > 70 pg/ml. However, owners did not report PU/PD and only three dogs had slightly increased ALT and/or ALP at the end of the study. A vaginal smear to evaluate changes of the mucosal cell in the only female with elevated estradiol concentration was not performed.
Soy isoflavone concentrations were measured in serum and urine samples primarily to verify food intake compliance and also to evaluate phytoestrogen systemic uptake and excretion. As expected, average serum and urine isoflavone levels reflected the difference in content of these compounds present in the respective diets. However, we did observe a relatively high variability in levels between dogs. This is likely the result of the opportunistic sampling protocol we employed resulting in variation in the sample collection time relative to the previous meal. Such opportunistic (or convenience) urine sampling has been used extensively for evaluation of compliance in human soy diet studies, showing good correlation between soy intake and urinary isoflavone levels.35 Data are generally normalized to creatinine content to account for differences in urine output. Reported urinary genistein, daidzein and glycitein concentrations average 1.2, 1.9 and 0.6 nmoles/mg creatinine (respectively) for people receiving a soy-based diet35, which compare well to values determined for dogs in this study receiving the HID with average values of 1.2, 1.0 and 0.8 nmoles/mg creatinine at 12 months. Serum isoflavone concentrations in the dogs in this study also compare well to values reported for people. For instance, average maximum plasma genistein and daidzein concentrations of 298 and 654 nmoles/L (respectively) were recently reported for people that consumed a standardized soy beverage, compared with average values of 223 and 254 nmoles/L (respectively) for dogs receiving the HID. Consequently, the levels of exposure of the dogs in this study are comparable to studies in people evaluating the health effects of soy.
The only other report of isoflavone concentrations levels in dogs that could be identified is a toxicokinetic study of pure genistein administered orally to laboratory Beagles at doses from 50 to 500 mg/kg/day.36 However, since these dose levels are much higher than in our study, it is difficult to draw any direct comparisons. Indeed, the maximum reported genistein concentrations in serum ranged from 4 to 11 umoles/L depending on dose, which is more than 10 times higher than concentrations found in this study. Interestingly, all dogs in that study also received a standard canine laboratory animal diet, including 7.8% vegetable protein. Furthermore, the total genistein concentration was measured and found to be 77 mg/kg food DW, which is slightly higher than values we determined for the HID. Control dogs that received no additional genistein, apart from that contained in the food, were reported to have average maximal genistein concentrations ranging from 163 to 502 nmoles/L, which is comparable to average values for dogs receiving the HID of 166 nmoles/L (6 months) and 223 nmoles/L (12 months).
There were no clear behavioral differences between the HID and LID groups. More dogs in the HID group showed increases in either anxiety, aggression, or activity level. However, this was an inconsistent change (subsequent scores indicated “no change”) in several cases.
The long-term effect of soy on canine skin and hair follicles size and number are currently unknown. At the end of the one-year study no significant changes in hair follicle size and numbers were seen in our dogs thus suggesting that soy-based diet can be safely used. Further studies are necessary to evaluate the long-term effect of phytoestrogens in dogs and if the daily amount ingested could play a role over time as reported in rodents and human hair follicle cultures. Seiberg et al. have found that soymilk-treated mice experience delayed and reduced hair growth and their hairs are visibly thinner.37 Histologically, the hair follicles of the soymilk-treated mice and men were smaller in size with hair shaft diameter reduced by an average of 42% and hair bulb diameter by an average of 23%. Others have shown that isoflavones can reduce hair growth and genistein was shown to decrease hair growth by 60–80% when hair follicles isolated from scalp biopsy were treated in culture.38
Limitations in this study are:
the animal population. It would have been ideal to have dogs of same breed, age and sex but a mixed breed population of dogs was enrolled as it would be representative of our canine hospital population. Further studies in selected breeds should be carried out to evaluate the pharmacokinetic, bioavailability and metabolism of dietary phytoestrogens and to see if there are differences among breeds or within the same breed.
the required sample size of 15 dogs per each group was not reached but even if we did, it is unlikely we would have obtained results that are statistical different from the one we obtained.
lack of standardization of dogs on the same diet prior to starting the study. This would have stabilized them and allowed us to have baseline values which were more comparable than the one we had and to rule out biological differences caused by their diet prior to being enrolled in the study. This study was not designed to evaluate the effect of soy on canine metabolism but our aim was to maintain dogs’ weight adjusting their daily amount of food. It has been reported that consumption of soy isoflavones can increase metabolism inhibiting lipogenesis and stimulating lipolysis thus causing decreased body weight, adipose tissue deposition and leptin level.39
the number of suitable skin specimens available for histology to evaluate the hair follicle size and hair diameter.
Limitations regarding behavior were that, first, the sample size was small and was not powered to detect small differences between treatment groups. While individual dogs may have shown changed behavior, a larger sample size may have revealed consistent differences between groups. Second, in this yearlong study it was not possible to adequately control for changes in the management or social/physical environment for individual dogs. Third, this study used owner self-report questionnaires, introducing the risk of variability in opinion – for example, anxiety may be viewed differently by different owners.
Our study has shown that dogs do absorb and excrete phytoestrogens and these might have an effect on their hormonal status. As reported in other species some of the effects might be beneficial such as prevention of certain type of cancers, controlling obesity, ect.40, but there are also concerns about their estrogenic effect which, although weak, might have a biological effect when dogs are fed diets and treats with high concentrations of isoflavones on a long term. To assess the potential risks and benefits of soy isoflavones in puppies and adult dogs and the mechanisms by which health effects occur, it is essential to have a more complete understanding of isoflavone pharmacokinetics after consumption of soy foods. The impact of isoflavones on experiments carried out in animal to evaluate various health conditions should be considered when selecting a diet because some commercial available diets contain high isoflavones concentration which might have a biological effect.2,41 The selection of an appropriate diet would aid research by minimizing variables. For certain types of experiments in endocrinology or behavior, an isoflavone-free or isoflavone-low diet should be considered.
Acknowledgments
The study was supported by a grant from Royal Canin, SA, Aimargues, France.
The authors are grateful to The Solae Company, St Louis, MO for providing the isoflavone-extracted hydrolyzed soy isolate. We would also like to thank the owners of the dogs participating in the study, the dermatology technicians (Joe Rogosky, Colleen Pinney and Tara Miller) and the students who helped with the sampling. Virbac and Bayer Animal health kindly provided Allergroom shampoo and Advantix (respectively). Dr Court is supported by grants R01GM061834 and R21GM074369 from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, Maryland, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
The clinical work was done at the University of Pennsylvania.
tetracosactrin acetate, Synacthen, Alliance, UK
Deca XP Plus, Thermo Electron, Somerset, NJ
Royal Canin Veterinary Diettm Canine Hypoallergenic, Royal Canin, St Charles MO
Allergroom, Virbac, Forth Worth, TX, USA
Advantix, Bayer, Shawnee Mission, KS, USA
SAS statistical software version 9.1, SAS Institute, Cary, NC
References
- 1.Bu L, Setchell KD, Lephart ED. Influences of dietary soy isoflavones on metabolism but not nociception and stress hormone responses in ovariectomized female rats. Reprod Biol Endocrinol. 2005;3:1–8. doi: 10.1186/1477-7827-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casanova M, You L, Gaido KW, et al. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci. 1999;51:236–44. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 3.Cerundolo R, Court HM, Michel EK. Identification and concentration of soy isoflavones and lignans in commercial dog foods. Am J Vet Res. 2004;65:592–596. doi: 10.2460/ajvr.2004.65.592. [DOI] [PubMed] [Google Scholar]
- 4.Cerundolo R, Mauldin EA, Goldschmidt MH, et al. Adult-onset hair loss in Chesapeake Bay retrievers: a clinical and histological study. Vet Dermatol. 2005;16:39–46. doi: 10.1111/j.1365-3164.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen HJ, Walfish PG. Effects of estradiol benzoate on thyroid-pituitary function in female rats. Endocrin. 1978;103:1023–1030. doi: 10.1210/endo-103-4-1023. [DOI] [PubMed] [Google Scholar]
- 6.Court MH, Freeman LM. Identification and concentration of soy isoflavones in commercial cat foods. Am J Vet Res. 2002;63:181–185. doi: 10.2460/ajvr.2002.63.181. [DOI] [PubMed] [Google Scholar]
- 7.Daminet S, Paradis M, Refsal KR, et al. Short term influence of prednisone and phenobarbital on thyroid function in euthyroid dogs. Can Vet J. 1999;40:411–415. [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan AM, Underhill KE, Xu X, et al. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 9.Duncan AM, Merz BE, Xu X, et al. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999a;84:192–197. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira AC, Lisboa PC, Oliveira KJ, et al. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food Chem Toxicol. 2002;40:913–917. doi: 10.1016/s0278-6915(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 11.Fort P, Moses N, Fasano M, et al. Breast and soy-formula feedings in early infancy and the prevalence of autoimmune thyroid disease in children. J Am Coll Nutr. 1990;9:164–167. doi: 10.1080/07315724.1990.10720366. [DOI] [PubMed] [Google Scholar]
- 12.Frank LA, Hnilica KA, Rohrbach BW, et al. Retrospective evaluation of sex hormones and steroid hormone intermediates in dogs with alopecia. Vet Dermatol. 2003;4:91–97. doi: 10.1046/j.1365-3164.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 13.Frank LA, Rohrbach BW, Bailey EM, et al. Steroid hormone concentration profiles in healthy intact and neutered dogs before and after cosyntropin administration. Dom Anim Endocrin. 2003a;24:43–57. doi: 10.1016/s0739-7240(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 14.Hamann I, Seidlova-Wuttke D, Wuttke W, et al. Effects of isoflavonoids and other plant-derived compounds on the hypothalamus-pituitary-thyroid hormone axis. Maturitas. 2006;55 (Suppl 1):S14–25. [Google Scholar]
- 15.Hartley DE, Edwards JE, Spiller CE, et al. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology. 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MN, Ritskes-Hoitinga M. How isoflavone levels in common rodent diets can interfere with the value of animal models and with experimental results. Lab Anim. 2007;41:1–18. doi: 10.1258/002367707779399428. [DOI] [PubMed] [Google Scholar]
- 17.Krazeisen A, Breitling R, Moller G, et al. Human 17beta-hydroxysteroid dehydrogenase type 5 is inhibited by dietary flavonoids. Adv Exp Med Biol. 2002;505:151–61. doi: 10.1007/978-1-4757-5235-9_14. [DOI] [PubMed] [Google Scholar]
- 18.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrin. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 19.Le Bail JC, Champavier Y, Chulia AJ, et al. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000;66:1281–1291. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- 20.Lephart ED, West TW, Weber KS, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 21.Lephart ED, Porter JP, Lund TD, et al. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr Metab. 2004;1:1–14. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund TD, Rhees RW, Setchell KD, et al. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult Long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914:92–99. doi: 10.1016/s0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- 23.Makela S, Poutanen M, Lehtimaki J, et al. Estrogen-specific 17 beta-hydroxysteroid oxidoreductase type 1 (E.C.1.1.1.62) as a possible target for the action of phytoestrogens. Proc Soc Exp Biol Med. 1995;208:51–59. doi: 10.3181/00379727-208-43831. [DOI] [PubMed] [Google Scholar]
- 24.Martin JH, Crotty S, Nelson PN. Phytoestrogens: perpetrators or protectors? Future Oncol. 2007;3:307–318. doi: 10.2217/14796694.3.3.307. [DOI] [PubMed] [Google Scholar]
- 25.McClain RM, Wolz E, Davidovich A, et al. Subchronic and chronic safety studies with genistein in dogs. Food Chem Toxicol. 2005;43:1461–1482. doi: 10.1016/j.fct.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Nachreiner RF, Refsal KR, Graham PA, et al. Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism. Am J Vet Res. 2002;220:466–471. doi: 10.2460/javma.2002.220.466. [DOI] [PubMed] [Google Scholar]
- 27.Nachreiner RF, Refsal KR, Graham PA, et al. Prevalence of autoantibodies to thyroglobulin in dogs with nonthyroidal illness. Am J Vet Res. 1998;59:951–955. [PubMed] [Google Scholar]
- 28.Ohno S, Shinoda S, Toyoshima S, et al. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2002;80:355–363. doi: 10.1016/s0960-0760(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 29.Panciera DL, MacEwen EG, Atkins CE, et al. Thyroid function tests in euthyroid dogs treated with l-thyroxine. Am J Vet Res. 1990;51:22–26. [PubMed] [Google Scholar]
- 30.Paradis M, Sauve F, Charest J, et al. Effects of moderate to severe osteoarthritis on canine thyroid function. Can Vet J. 2003;44:407–412. [PMC free article] [PubMed] [Google Scholar]
- 31.Reinli K, Block G. Phytoestrogen content of foods - a compendium of literature values. Nutr Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 32.Setchell KD, Gosselin SJ, Welsh MB, et al. Dietary estrogens - a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- 33.Seow A, Shi CY, Franke AA, et al. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–140. [PubMed] [Google Scholar]
- 34.Simon NG, Kaplan JR, Hu S, et al. Increased aggressive behavior and decreased affiliative behavior in adult monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm Behav. 2004;45:278–284. doi: 10.1016/j.yhbeh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Spanka M, Hesch RD, Irmscher K, et al. 5′-Deiodination in rat hepatocytes: effects of specific flavonoid inhibitors. Endocrinology. 1990;126:1660–1667. doi: 10.1210/endo-126-3-1660. [DOI] [PubMed] [Google Scholar]
- 36.Vansell NR, Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of triiodothyronine (T3) and its metabolites. Toxicol Sci. 2002;65:184–191. doi: 10.1093/toxsci/65.2.184. [DOI] [PubMed] [Google Scholar]
- 37.Seiberg M, Liu JC, Babiarz L, Sharlow E, Shapiro S. Soymilk reduces hair growth and hair follicle dimensions. Exp Dermatol. 2000;10(6):405–13. doi: 10.1034/j.1600-0625.2001.100603.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann R, Eicheler W, Wenzel E, Happle R. Interleukin-1beta-induced inhibition of hair growth in vitro is mediated by cyclic AMP. J Invest Dermatol. 1997;108(1):40–2. doi: 10.1111/1523-1747.ep12285625. [DOI] [PubMed] [Google Scholar]
- 39.White HL, Freeman LM, Mahony O, et al. Effect of dietary soy on serum thyroid hormone concentrations in healthy adult cats. Am J Vet Res. 2004;65:586–591. doi: 10.2460/ajvr.2004.65.586. [DOI] [PubMed] [Google Scholar]
- 40.Wong CK, Keung WM. Bovine adrenal 3beta-hydroxysteroid dehydrogenase (E.C. 1.1.1. 145)/5-ene-4-ene isomerase (E.C. 5.3.3.1): characterization and its inhibition by isoflavones. J Steroid Biochem Mol Biol. 1999;71:191–202. doi: 10.1016/s0960-0760(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 41.Wood CE, Cline JM, Anthony MS, et al. Adrenocortical effects of oral estrogens and soy isoflavones in female monkeys. J Clin Endocrinol Metab. 2004;89:2319–2325. doi: 10.1210/jc.2003-031728. [DOI] [PubMed] [Google Scholar]