Abstract
Objective
Neurocognitive disorders are devastating consequences of HIV infection. While antiretroviral regimens have been efficacious in both improving life expectancy and decreasing dementia, there has not been an effect on the overall prevalence of HIV-associated neurocognitive disorders. Whether early institution of treatment, or treatment with drugs that effectively penetrate the blood brain barrier, would help protect from such conditions is not known. Using the SIV/macaque model, we investigated the hypothesis that early introduction of antiretroviral treatment can protect the brain.
Design and Methods
Animals were inoculated with SIV, and upon resolution of the acute infection period divided into two groups and treated, or not, with combination antiretoviral therapy. Viral, immune, and physiological parameters were measured during the course of infection, followed by assessment of viral, immune, and molecular parameters in the brain.
Results
We observed that even with agents that show poor penetration into the CNS, early antiretroviral treatment prevented characteristic neurophysiological and locomotor alterations arising after infection, and resulted in a significant decrease in brain viral load. Although the number of infiltrating immune cells in the brain did not change with treatment, their phenotype did, favoring an enrichment of effector T cells. Early treatment also significantly lowered brain levels of IFNα, a cytokine that can lead to neurocognitive and behavioral alterations.
Conclusion
Early antiretroviral treatment prevents CNS dysfunction by decreasing brain viral load and IFNα levels, which can have a profound impact over the course of infection.
Keywords: SIV, brain, neuroAIDS, antiretroviral
INTRODUCTION
A high proportion of HIV-1-infected individuals develop alterations in neurocognition (referred to as HIV-associated neurocognitive disorders, HAND), which are most prominent at later phases of infection. In the current era of HAART, the incidence of the severe form, HIV-1 associated dementia (HAD) has decreased [1], however other, less severe, conditions persist. With the treatment-induced longer life expectancy, the prevalence of CNS complications has risen in comparison to other AIDS-related complications, and as a whole, is either unchanged or possibility increasing [2-6]. There are many proposed reasons for this, such as viral mutation, failure of therapy, CNS immunopathology due to the virus-host interaction, and/or lack of blood brain barrier (BBB) penetration of therapy. While the role of brain-penetrating antiretroviral agents in general HIV therapy is not clear, it has been found that the use of drugs thought to have higher CNS penetration or activity are associated with lower cerebrospinal fluid (CSF) viral loads [7, 8].
While the exact mechanism of HAND is not known, HIV infection of the CNS clearly initiates the disease. Macrophages and microglia are the only productively infected cells in the CNS, and thus, an indirect neurotoxic effect has been suggested [9]. Viral proteins, products of infected and/or activated microglia and macrophages, intrathecal immunoactivation, accumulation and persistency of immune cells in the brain parenchyma, are probably also part of the pathogenesis and neurodegenerative process.
Simian immunodeficiency virus (SIV) infection in rhesus macaques reproduces all aspects of human infection with HIV, including the development of neurocognitive disorders [10, 11]. Neurophysiological testing has revealed that changes in CNS function are detectable early after infection [12, 13]. This is attributed to the early presence of the virus in the CNS, triggering events that can lead to neuronal damage. Among the alterations in the brain tissue in the SIV model, infiltrating immune cells, predominantly CD8+ T cells, are detectable early after infection and persist within the CNS [13]. These accumulating CD8 cells are in a highly activated state, based on the expression of surface activation markers and transcription of cytolytic and pro-inflammatory cytokines, and a high proportion of them are specific to the virus [14].
In addition to neurophysiological changes, we have found that SIV infection results in changes in the regulation of temperature and movement as well as alterations in cognitive and motor function [15-17]. To examine the role of treatment in the reversal of early identifiable changes in CNS function, we had previously examined the effects of antiretroviral therapy, using monotherapy with 9−2[R-phosphonomethoxy propyl]adenine (PMPA, tenofovir). This treatment (which was instituted following the identification of such abnormalities) was indeed able to reverse neurophysiological abnormalities, and following cessation of treatment, they returned [12]. However the general decrease in locomotor activity was not responsive to this regimen.
Yet little is known about the HIV or SIV infected brain in the setting of antiretroviral therapy. Indeed where infected individuals have access to such treatment, this is the most common state of the CNS in HIV infection. In order to address the effects of antiretroviral therapy on the brain, we report here studies on two groups of SIV-infected animals – one receiving combination antiretroviral therapy (the reverse transcriptase inhibitor tenofovir plus the protease inhibitor nelfinavir), and the other receiving placebo as a control. Neither of these agents are clinically considered efficacious in the CNS [7, 8, 18]. While our earlier study examined the brains after cessation of monotherapy [12], here, animals were sacrificed while on active combination therapy (or placebo) to assess changes in the brain environment during treatment.
METHODS
Animals
Rhesus monkeys, free from SIV, type D simian retrovirus, and herpes B virus were obtained from Covance (Alice, TX) and Charles River (Key Lois, FL). All animal experiments were performed with approval from the Scripps Institutional Animal Care and Use Committee and followed NIH guidelines. Blood samples were drawn from the femoral vein from animals under ketamine anesthesia, and plasma was separated by centrifugation from cells in EDTA-anticoagulated blood. CSF was obtained from the cisterna magna. CSF samples containing >0.1% blood contamination (determined by RBC counts) were discarded. Necropsy was performed after terminal anesthesia. During necropsy, animals were perfused intracardially with sterile PBS containing 1 U/ml heparin before tissue samples were taken for cell isolation, virus quantification, and formalin fixation for histology.
Radiotelemetry
Body temperature and gross locomotor activity were measured by means of radiotelemetry implants, placed while the animals were under isoflurane anesthesia, with postoperative analgesia with buprenorphine. Signals from individually implanted telemetry transmitters (Physiotel TA10TA-D70; Data Sciences International, St. Paul, MN) were received by individual receivers (model RMC-1, Data Sciences International), which were connected to the data acquisition matrix, and the data were processed using Dataquest ART 4.0 software (Data Sciences International). Temperature and activity were continuously monitored before and after infection. The values used for this study correspond to the average of 12 hr daytime recording periods.
Viral Infection
A cell-free stock of SIV (derived from SIVmac251) obtained after serial passage of SIV-infected microglia was the inoculum used [19]. Animals received 0.25 ml of the stock, diluted into RPMI-1640 for intravenous injection, containing 5 ng/ml of p27 (gag) antigen.
Antiviral treatment
Antiretroviral-treated animals received one daily intramuscular injection of tenofovir (Gilead, Foster City, CA), 30 mg/kg in phosphate buffered saline, at 11:30 AM, and nelfinavir mesylate (Pfizer, New York, NY) was administered orally at 20 mg/kg, in banana-flavored primate tablets (Bio-Serve, Frenchtown, NJ), twice a day, at 6 AM and at 4 PM. The treatment was initiated at 8 weeks after SIV inoculation and lasted until termination. Animals # 422, 424, 492 and 495 were treated with placebo (placebo injections and pills), whereas animals # 425, 431, 433 and 498 received the active agents. All animals were sacrificed between 15 and 16 weeks after SIV inoculation while still receiving the treatment, per experimental protocol.
Viral Quantitation
To measure viral load, SIV RNA in plasma, CSF, and brain tissue, quantitative branched DNA signal amplification assay was performed by Siemens Reference Testing Laboratory (Emeryville, CA). Each sample was measured in duplicate and reported as average value.
Electrophysiology
Electrophysiological analysis of brainstem auditory evoked potential (BSAEP) was performed on ketamine (20 mg/kg)-anesthetized animals as described previously [20]. Averaged peak latencies were calculated and compared between group means.
Peripheral mononuclear cells
Buffy coats obtained from centrifugation of EDTA-anti-coagulated blood, and cell suspensions from spleen, deep cervical and inguinal lymph nodes, passed through a nylon mesh, were submitted to a Ficoll-Isopaque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation for isolation of the mononuclear fraction. After washing, cells were enumerated in a Coulter Z2 (Coulter Co., Miami, FL) and resuspended in complete RPMI-1640, containing 10% fetal calf serum (FCS) at a concentration of 107/ml, for labeling or cryopreservation.
Brain cells
The meninges were carefully removed, and the brain mechanically and enzymatically disassociated, and immune cells purified on a Percoll gradient as previous described [13], and quantified in a Coulter Z2.
Flow cytometry
Cells isolated as described (2 × 105 to 10 × 105) were stained with 50 μl mixtures of antibodies diluted according to a previous titration in staining buffer (HBSS with 2% FCS and 0.01% NaN3). The antibodies used for the staining were anti-monkey CD3-biotin (clone FN-18, Biosource) followed by Streptavidin-APC (Pharmingen, San Diego, CA), anti-human CD8-PE (clone DK25, Dako), anti-CD11a-FITC (clone 25.3.1, Immunotech), anti-CD45RA (clone 5H9, BD Pharmingen, San Diego, CA), anti-Mac-1-PE (clone M1/70(9), BMB), anti-CD95 (clone DX2, BD Pharmingen) and anti-CD4-PE (clone OKT4, hybridoma obtained from the ATCC, with the secreted antibody purified and PE-conjugated in the laboratory). Isotype controls (BD Pharmingen) were also applied. The cells were acquired through a FacsCalibur before analysis of data in CellQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, CA).
Real Time PCR
For RNA extraction total RNA was purified from samples using TRIzol Reagent (Invitrogen, Carlsbad, CA) following the protocol of the manufacturer, with an additional centrifugation step to remove cellular debris. RNA was further purified (RNeasy mini kit; Qiagen, Valencia, CA), and quantified by 260 nm UV absorption. Specific RNA transcripts were quantified using real-time PCR. The primers and probe sequences for β2M, CCL5, G1P3, HLA-DRα, IL15, IL1β, TNFα, and the control genes 18S, GAPDH, and TBP were designed for rhesus macaque sequences, and were previously reported [14, 21, 22]. The primers and probe sequences for IFNα were homologous to rhesus □□□α2, and were: (forward primer) GCCTGAAGGACAGACATGACTTT, (reverse primer) GGATGGTTTGAGCCTTTTGG, (FAM/TAMRA-labeled probe) CCCCAGGAGGAGTTTGGCAACCA. Relative amounts of each cytokine mRNA in the samples was obtained upon normalization of the average cycle threshold (Ct) against the average of the three control genes. Reactions were performed in a Stratagene MX30000 (La Jolla, CA).
Statistical analysis
Statistical analysis was done using Student's t test or ANOVA, followed by a post-test when appropriate. Alpha was set at 0.05 for determination of significance. The tests were performed using Microsoft Excel X for Mac, or Prism version 4.0 for Mac (GraphPad Software, La Jolla, CA).
RESULTS
Antiretroviral treatment ameliorates sensory evoked potential abnormality and decreases brain viral load
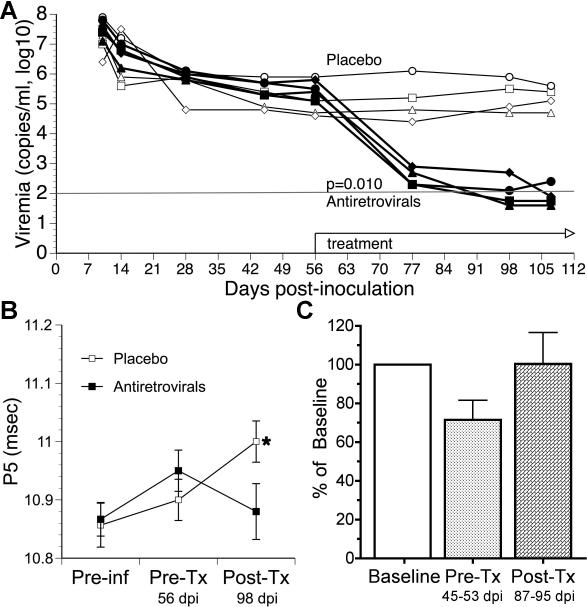
Plasma viral load dropped as a result of the treatment in all 4 treated animals, as compared to both pre-treatment levels of viremia and to those found in the control animals that received placebo, throughout the treatment, and until termination (Figure 1A). In three of the treated animals the plasma viral load dropped to below the limit of detection, whereas the fourth reached levels near the limit of detection. Although CSF was not sampled as frequently as blood, in all four of the antiretroviral-treated animals the CSF viral load dropped below the limit of detection following treatment, compared to just one of the four control animals.
Figure 1.
Effect of antiretrovirals on viral load, sensory evoked potentials, and locomotor activity (A). Plasma viral load over time, with the period of treatment indicated. Two-way ANOVA revealed a significant effect of treatment. (B) BSAEP P5 latency before infection (Preinf), after infection but before treatment (Pre-Tx), and after treatment antiretrovirals or placebo (Post-Tx). One-way ANOVA with Dunnett's post-test revealed that the placebo animals had significantly delayed latencies at the Post-Tx time point versus pre-infection (p=0.022 for the ANOVA, p<0.05 for the post-test), whereas the antiretroviral treated animals did not show a significant change. (C) Average day time locomotor activity (LCA), normalized to each animal's pre-inoculation mean, with values represent the average values during pre-infection period (Preinf), days 45 to 55 pi before treatment (Pre-Tx) and from day 87 to 95 pi after treatment (Post-Tx). One-way ANOVA did not reveal a significant change.
Changes in viral load did not correlate with changes on the relative or absolute numbers of either CD4 or CD8 T cells (data not shown) in the blood, and such T cell numbers did not differ significantly between the groups. The treatment regimen did prevent the neurophysiological changes induced by infection, measured as a delay in late wave (P5) of the BSAEP (Figure 1B). After infection the motor activity began to decrease, but returned to baseline following treatment (Figure 1C). While technical problems prevented the analysis of motor activity in the control group, we consistently observe a significant decline in motor activity following infection [12, 17].
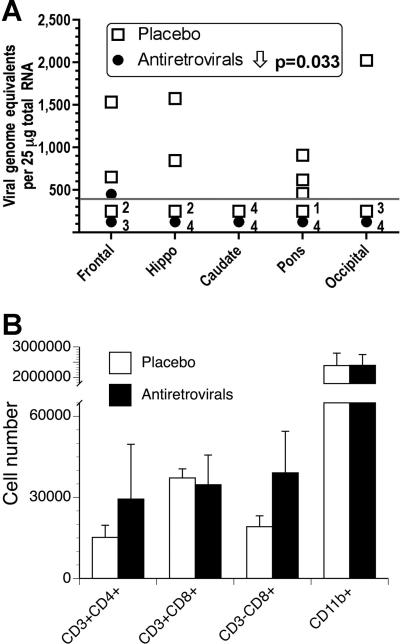
Following sacrifice, viral and immune parameters were analyzed in the brain. Treatment was successful in lowering the viral load in the brain (Figure 2A). Although amount of virus was reduced, the number of brain infiltrating CD8+ T cells, which were close to 4-fold higher than in uninfected animals, did not differ between groups (13,482 ± 4,822 CD8+ cells/uninfected brain; 37,196 ± 3,361 CD8+ cells/placebo-treated brain; and 34,683 ± 10,990 CD8+ cells/antiretroviral-treated brain) (Figure 2B). Similarly, the numbers of CD4 T cells, CD3-CD8+ NK cells or CD11b+ cells (microglia and macrophages) in infected brains, were similar to the numbers found in uninfected animals for these populations (not shown), and there was not difference in placebo versus anti-retroviral-treated animals.
Figure 2.
Viral and immune parameters in the brain. (A) Brain viral load in the indicated regions of the brain. The limit of detection of the assay is indicated by the dotted line; the number of samples from each group that were below that limit is indicated. Two-way ANOVA revealed a significant effect of treatment. (B) Distribution of cell subpopulations in the brain parenchyma of the two groups of SIV-infected monkeys. There were no significant differences between the groups.
Antiretroviral treatment alters memory T cells in the brain of infected monkeys
In order to assess whether treatment led to differences in immune cell phenotypes, several surface markers that can be altered by cellular activation were evaluated on CD4 and CD8 T cells from the brain, CSF, blood, spleen and liver. The percentage of CD11a-expressing T cells in infected animals was consistently higher than in uninfected controls (not shown), did not distinguish between infected groups subjected or not to antiretroviral treatment, either in blood, spleen, liver or in the brain (not shown). However, in the CSF, CD8 cells expressing high levels of CD11a were more abundant in placebo treated animals (84.8% ± 18.4 CD8 T cells) than in anti-retroviral treated animals (48.6% ± 8.2) (p=0.016, Student's t test). Neither CCR5, CD25 (IL2R), nor CD122 (IL15R) were significantly altered by treatment in any of these sites (not shown).
In addition to these T cell activation markers, we also investigated whether the antiretroviral treatment alters the memory phenotype or the distribution of memory cells. Overall, we observed that surface molecules that correlate with the memory status were able to distinguish groups, especially within the CD4 compartment. CD95 expression identifies memory T cells in monkeys [23, 24]. We further divided CD95+ cells for the expression of CCR7. The down-modulation of this marker within the CD95+ fraction correlates with the development of an effector memory phenotype (TEM), while cells that remain CCR7+ play a central memory role (TCM). The expression of CCR7, within the CD95+ fraction, was significantly different among CD4 cells in the two non-lymphoid organs, the liver and the brain, between the groups (Table 1). In the animals that received antiretrovirals, there is an increase of TEM (cells with lower CCR7 expression) at the expense of TCM (high CCR7 expression) phenotype compared to placebo-treated animals (p=0.016 and p=0.005, for brain and liver, respectively, Student's t test). In the CD8 compartment, the proportion of CCR7-expressing cells within CD95+ cells also changed in the brain with the antiretroviral treatment, with again a lower percentage of CCR7+ cells indicating a higher proportion of TEM (and lower TCM) CD8+ cells (Table 1). The spleen had more CCR7+ cells within the CD95+ compartment, indicating a higher percentage of TCM (and thus lower TEM) CD8+ cells upon treatment in that organ.
Table 1.
T cell memory (CD95+) subsets assessed for CCR7 expression.
| % CCR7+ cells within CD4+CD95+ | % CCR7+ cells within CD8+CD95+ | |||
|---|---|---|---|---|
| Placebo | Antiretrovirals | Placebo | Placebo | |
| Blood | 76.9 ± 8.3 | 77.4 ± 12.6 | 58.7 ± 9.7 | 55.3 ± 7.9 |
| Spleen | 63.2 ± 3.4 | 66.9 ± 3.6 | 39.7 ± 5.7 | 62.6 ± 7.6* |
| Liver | 82.3 ± 6.9 | 56.4 ± 9.9* | 44.1 ± 6.0 | 35.6 ± 12.7 |
| Brain | 64.2 ± 7.1 | 44.6 ± 9.6* | 19.9 ± 4.4 | 11.6 ± 3.1* |
Within the memory (CD95+) CD4 and CD8 T cell population, CCR7 expression was determined to delineate TCM (CCR7+) and TEM (CCR7−).
p<0.05 between groups, Student's t-test.
Thus the T cell proportions in are altered in the organs SIV infected antiretroviral treated animals compared to those animals that are infected but not treated. Specifically, in the brains of SIV-infected animals, the antiretrovial treatment resulted in a change both in the CD4 and CD8 T cell compartments, characterized by an increase in TEM.
Antiretroviral therapy affects host-response gene expression in the infected brain
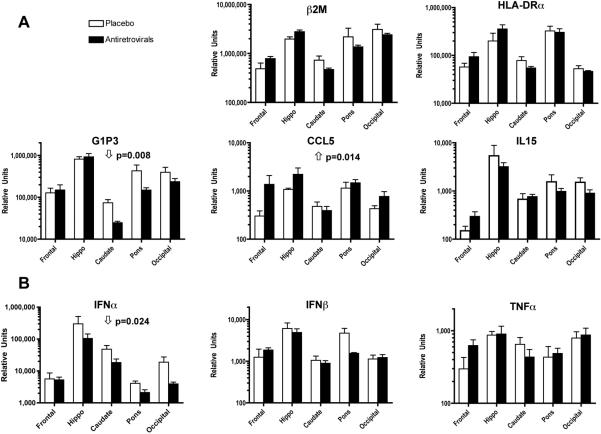
The changes in the brain viral load and infiltrating immune cell phenotypes found in treated animals reveals a significant change in the virus/host interaction within the CNS. We have previously found that this can be reflected in the gene expression pattern in the infected brains. In the chronic, stable phase of infection, we identified a number of genes whose expression differed between the brains of uninfected and SIV-infected animals, including the those involved in class I and class II antigen presentation (β2-microglobulin: β2M, and HLA-DRα), interferon-induced genes, including the anti-viral, anti-apoptotic molecule G1P3, and the chemokine CCL5 (also known as RANTES) [21]. In addition, in chronically infected animals, we have identified the cytokine IL15 as elevated in the brain [14]. We therefore examined the expression of these genes in the brains of the infected, treated animals as compared to the levels found in the infected, placebo-treated animals’ brains.
The expression of two of these genes was modified by the treatment regimen. G1P3 was reduced by treatment, whereas in contrast CCL5 was elevated; the others were not significantly altered (Figure 3A). Since both of these genes can be responsive to interferon (IFN) and other inflammatory molecules, we next assessed the expression of IFNβ and β, as well as TNFα. While the levels of IFNβ and TNFα in the brains were not altered by antiretroviral treatment, the expression of IFNα was significantly lower in the brains of treated animals (Figure 3B). Interestingly, expression of these genes differed across brain regions. In both the control and antiretroviral treated groups, the mean expression of these molecules was significantly higher in the hippocampus than the other brain regions (two-way ANOVA p<0.0001, Bonferroni's post-tests p<0.05 for both control and treated groups for hippocampus vs. frontal, caudate, pons, and occipital).
Figure 3.
Transcriptional quantification within the brain. Molecules previously found to be altered in the stable phase of SIV infection in the brain (A), and selected proinflammatory molecules (B), were evaluated in RNA preparations obtained from different segments of SIV-infected monkey's brain. The relative quantification was performed using quantitative real time-PCR. Normalized values correspond to the average ± SEM of 4 animals that received combination antiretroviral therapy and 4 animals that received placebo. Statistical analysis was performed using two-way ANOVA, and p values given when there was a significant effect of treatment.
DISCUSSION
Using an antiretroviral treatment regimen considered clinically to have low CNS efficacy, instituted in SIV-infected animals following the resolution of the acute viremic stage, we confirmed and expanded upon our prior results that a reduction of plasma viral load induced by the therapy is beneficial for preventing/treating CNS dysfunction. Here, modeling an early commencement of treatment, for example after an individual is exposed, develops an acute viremic syndrome, is recognized as infected and begins therapy, we find that treatment leads to a significant reduction in the viral load in the brain. Although the magnitude of the infiltrating immune cells into the brain is unchanged, treatment induces an alteration in the memory phenotypes in the brain as well as a peripheral organ, the liver. Finally, alterations of the host transcriptional response to infection occur, with a decrease in IFNα and the IFN-responsive G1P3, and an increase in CCL5.
Our studies and those of others reveal that virus enters the brain early, and is easily detectable by the second week after inoculation [25-27]. The RNA viral load then drops in the brain as it does outside of the brain, likely due to immune control. The decrease in viral load in the brain that we observed following treatment is likely either to a lower level of continued infection of the brain from peripheral virus, which itself is markedly diminished, and/or more effective immune control of the virus within the brain. The overall number of infiltrating immune cells is unchanged by antiretroviral treatment in the infected brain. However there was an overall enrichment of effector CD4 cells, and decline in CD8 central memory T cells. Such a shift in phenotype argues for increased active immune control of virus in the brain or for less killing of target cells. Although we did not quantify the percentage of virus-specific cells, the shifting to an effector phenotype, correlating with a successful decrease of viral load, may contribute to the generation of a more focused response, with less bystander activity, which may be rather beneficial both to control the virus and avoid damage to neuronal components.
Other studies have found that monkeys with chronic SIV infection and treated with antiretrovirals showed changes in the distribution of the memory phenotype within SIV-specific cytotoxic T lymphocytes in the blood, from TEM to TCM [28]. We did not see such a shift in blood; possibly due to the earlier institution of treatment as well as our examination of bulk CD8 T cells as opposed to viral epitope-specific CTL. In any regard, in the brain we found the opposite in both CD4 and CD8 T cells, with a shift from TCM to TEM. This shift may represent changes in the brain CD8 population that favor more effective immune control of virus, resulting in the lowered brain viral load. However, the antiretroviral drugs may reduce the brain viral load by controlling virus peripherally. This can result in lowered infection and killing of active TEM cells, this increasing their frequency. Regardless the mechanism, the enrichment of effector memory caused by early antiretroviral treatment resembles the phenotype observed in live attenuated vaccination protocols [29], suggesting the potential of development of a long lasting protective environment and priming by reducing the initial set point viral load.
Treatment, and the subsequent lowering of brain and blood virus, resulted in a significant decrease of IFNα in the infected brain. This finding is a potential key for the amelioration of CNS dysfunction, manifested in HIV infection as cognitive and motor symptoms, and in our experiments as abnormal delay in sensory evoked potentials. Although we cannot exclude a direct effect from the drugs themselves, this is correlated with the decrease of infected cells in the CNS. Increased IFNα in the basal ganglia in patients has been shown to contribute to neurological and behavioral changes [30-35]. Patients with HIV dementia have high levels of IFNα in the CSF [36, 37]. Also, in a mouse model of neuroAIDS, in which HIV-infected macrophages are intracerebrally injected in SCID mice, cognitive dysfunction correlates with the expression of IFNα on neurons and glial cells [38, 39]. Interestingly IFNα treatment is used therapeutically in some viral infections and cancer, but such treatment often results in neurological deficits and behavioral alterations [40-44], strongly suggesting a direct correlation between levels of this cytokine and CNS dysfunction.
IFNα can also influence the local T cell response. It supports an effector phenotype on T cells, but by transiently increasing apoptosis also contributes to the elimination of activated cells [45]. In addition, similar to IL15, IFNα can support the division of memory cells in an antigen independent way, facilitating the enrichment of bystander cells [46, 47]. However whether there is a linkage between the reduction in IFNα and the changes found in T cell phenotypes in the brain is not known.
In contrast to the decrease in IFNα, CCL5 is increased in the brains of treated animals. Whether this increase found with treatment may result from, or possibly contribute to, the difference in T cell memory phenotype is not known. In addition to its chemokine properties, CCL5 is among the soluble inhibitory factors with the ability to inhibit HIV-1 infection and spread [48] and thus may contribute to the reduction in brain viral load. Furthermore, CCL5 treatment of microglia was found to reduce their expression of the proinflammatory molecules, thus may act in protecting neurons from their deleterious effects [49].
Interestingly, the inflammatory mediator profiling revealed that, irrespective of treatment, higher levels of their mRNAs could be found in the hippocampus as opposed to other brain regions. Certainly region-specific gene expression occurs in the primate brain[50], but whether this contributes to these region-specific differences found in the infected brain is unknown. However it has been found that hippocampal microglia, compared to microglia from other regions of the brain, express higher levels of some inflammatory mediators [51]. Our findings may reflect such increased proclivity of hippocampal microglia to express inflammatory molecules.
Overall, our results reveal the significance of early treatment, even with antiretroviral agents that do not penetrate the BBB. Early administration of antiretrovirals is effective in lowering virus in the brain as well as preventing early signs of CNS dysfunction. Whether such early treatment will be effective in preventing the development of HAND is not known. However a later introduction of antiretrovirals may not have such a beneficial effect, given the chronicity of virus-host interaction and its resulting effects in the brain. The effect of early treatment on the development of CNS disorders is worthy of study, since both early as well as chronic changes in the brain, including levels of virus and cytokines, can have a profound impact over the course of infection and overall patient prognosis.
ACKNOWLEDGEMENTS
We thank Mrs. Nancy Delaney for administrative assistance, and Dr. Kent Osborn for his indispensable role during necropsies. We thank Dr. Norbert Bishofberger (Gilead) for generously supplying the tenofovir. This work was funded by NIH grants MH073490, MH062261, and MH072477. This is the manuscript # 19889 of The Scripps Research Institute, and # 3 from the UNMC CITN.
Funding for this work was received from the NIH. Tenofovir was received from Gilead.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 3.Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- 4.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 2003;17:1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 5.Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 10.Fox HS. Virus-host interaction in the simian immunodeficiency virus-infected brain. J Neurovirol. 2008;14:286–291. doi: 10.1080/13550280802132824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burudi EM, Fox HS. Simian immunodeficiency virus model of HIV-induced central nervous system dysfunction. Adv Virus Res. 2001;56:435–468. doi: 10.1016/s0065-3527(01)56035-2. [DOI] [PubMed] [Google Scholar]
- 12.Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, et al. Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, et al. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 14.Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, et al. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol. 2007;178:5812–5819. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- 15.Huitron-Resendiz S, Marcondes MC, Flynn CT, Lanigan CM, Fox HS. Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc Natl Acad Sci U S A. 2007;104:15138–15143. doi: 10.1073/pnas.0707171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold LH, Fox HS, Henriksen SJ, Buchmeier MJ, Weed MR, Taffe MA, et al. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 17.Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: Summary of current knowledge and recommendations for further research. Antiviral Res. 2009 doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watry D, Lane TE, Streb M, Fox HS. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146:914–923. [PMC free article] [PubMed] [Google Scholar]
- 20.Prospero-Garcia O, Gold LH, Fox HS, Polis I, Koob GF, Bloom FE, Henriksen SJ. Microglia-passaged simian immunodeficiency virus induces neurophysiological abnormalities in monkeys. Proc Natl Acad Sci U S A. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts ES, Huitron-Resendiz S, Taffe MA, Marcondes MC, Flynn CT, Lanigan CM, et al. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J Neurosci. 2006;26:4577–4585. doi: 10.1523/JNEUROSCI.4504-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burudi EM, Marcondes MC, Watry DD, Zandonatti M, Taffe MA, Fox HS. Regulation of indoleamine 2,3-dioxygenase expression in simian immunodeficiency virus-infected monkey brains. J Virol. 2002;76:12233–12241. doi: 10.1128/JVI.76.23.12233-12241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Marcondes MC, Penedo MC, Lanigan C, Hall D, Watry DD, Zandonatti M, Fox HS. Simian immunodeficiency virus-induced CD4+ T cell deficits in cytokine secretion profile are dependent on monkey origin. Viral Immunol. 2006;19:679–689. doi: 10.1089/vim.2006.19.679. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti L, Hurtrel M, Maire MA, Vazeux R, Dormont D, Montagnier L, Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 26.Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- 27.Roberts ES, Burudi EM, Flynn C, Madden LJ, Roinick KL, Watry DD, et al. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. J Neuroimmunol. 2004;157:81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Mueller YM, Petrovas C, Do DH, Altork SR, Fischer-Smith T, Rappaport J, et al. Early establishment and antigen dependence of simian immunodeficiency virus-specific CD8+ T-cell defects. J Virol. 2007;81:10861–10868. doi: 10.1128/JVI.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollman E, Smith MZ, Brooks AG, Purcell DF, Zuber B, Ramshaw IA, Kent SJ. Killing kinetics of simian immunodeficiency virus-specific CD8+ T cells: implications for HIV vaccine strategies. J Immunol. 2007;179:4571–4579. doi: 10.4049/jimmunol.179.7.4571. [DOI] [PubMed] [Google Scholar]
- 30.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, et al. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida K, Alagbe O, Wang X, Woolwine B, Thornbury M, Raison CL, Miller AH. Promoter polymorphisms of the interferon-alpha receptor gene and development of Interferon-induced depressive symptoms in patients with chronic hepatitis C: preliminary findings. Neuropsychobiology. 2005;52:55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]
- 36.Rho MB, Wesselingh S, Glass JD, McArthur JC, Choi S, Griffin J, Tyor WR. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 37.Perrella O, Carreiri PB, Perrella A, Sbreglia C, Gorga F, Guarnaccia D, Tarantino G. Transforming growth factor beta-1 and interferon-alpha in the AIDS dementia complex (ADC): possible relationship with cerebral viral load? Eur Cytokine Netw. 2001;12:51–55. [PubMed] [Google Scholar]
- 38.Sas A, Jones R, Tyor W. Intra-peritoneal injection of polyclonal anti-interferon alpha antibodies cross the blood brain barrier and neutralize interferon alpha. Neurochem Res. 2008;33:2281–2287. doi: 10.1007/s11064-008-9715-8. [DOI] [PubMed] [Google Scholar]
- 39.Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-alpha in the brain. Aids. 2007;21:2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- 40.Pavol MA, Meyers CA, Rexer JL, Valentine AD, Mattis PJ, Talpaz M. Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology. 1995;45:947–950. doi: 10.1212/wnl.45.5.947. [DOI] [PubMed] [Google Scholar]
- 41.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 42.Hauser P, Soler R, Reed S, Kane R, Gulati M, Khosla J, et al. Prophylactic treatment of depression induced by interferon-alpha. Psychosomatics. 2000;41:439–441. doi: 10.1176/appi.psy.41.5.439. [DOI] [PubMed] [Google Scholar]
- 43.Scheibel RS, Valentine AD, O'Brien S, Meyers CA. Cognitive dysfunction and depression during treatment with interferon-alpha and chemotherapy. J Neuropsychiatry Clin Neurosci. 2004;16:185–191. doi: 10.1176/jnp.16.2.185. [DOI] [PubMed] [Google Scholar]
- 44.Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25:39–47. [PubMed] [Google Scholar]
- 45.Kaser A, Enrich B, Ludwiczek O, Vogel W, Tilg H. Interferon-alpha (IFN-alpha) enhances cytotoxicity in healthy volunteers and chronic hepatitis C infection mainly by the perforin pathway. Clin Exp Immunol. 1999;118:71–77. doi: 10.1046/j.1365-2249.1999.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, et al. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- 47.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- 49.Gamo K, Kiryu-Seo S, Konishi H, Aoki S, Matsushima K, Wada K, Kiyama H. G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity. J Neurosci. 2008;28:11980–11988. doi: 10.1523/JNEUROSCI.2920-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khaitovich P, Muetzel B, She X, Lachmann M, Hellmann I, Dietzsch J, et al. Regional patterns of gene expression in human and chimpanzee brains. Genome Res. 2004;14:1462–1473. doi: 10.1101/gr.2538704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren L, Lubrich B, Biber K, Gebicke-Haerter PJ. Differential expression of inflammatory mediators in rat microglia cultured from different brain regions. Brain Res Mol Brain Res. 1999;65:198–205. doi: 10.1016/s0169-328x(99)00016-9. [DOI] [PubMed] [Google Scholar]