Abstract
Objective
To evaluate the high resolution computed tomography (HRCT) findings of bronchiolitis obliterans (BO) after bone marrow transplantation (BMT).
Materials and Methods
During the past three years, 11 patients were diagnosed as having BO after BMT when they developed irreversible air flow obstruction, with an FEV1 value of less than 80% of the baseline value, without any clinical evidence of infection. All 11 patients underwent HRCT, of whom eight also underwent follow-up HRCT. The HRCT images were assessed retrospectively for the presence of decreased lung attenuation, segmental or subsegmental bronchial dilatation, diminution of peripheral vascularity, centrilobular nodules, and branching linear structure on the inspiratory images. The lobar distribution of the decreased lung attenuation and bronchial dilatation was also examined. The presence of air trapping was investigated on the expiratory images. The interval changes of the HRCT findings were evaluated in those patients who had follow-up images.
Results
Abnormal HRCT findings were present in all cases; the most common abnormalities were decreased lung attenuation (n=11), subsegmental bronchial dilatation (n=6), diminution of peripheral vascularity (n=6), centrilobular nodules or branching linear structure (n=3), and segmental bronchial dilatation (n=3). Expiratory air trapping was noted in all patients. The decreased lung attenuation and bronchial dilatations were more frequent or extensive in the lower lobes. Interval changes were found in all patients with follow-up HRCT: increased extent of decreased lung attenuation (n=7); newly developed or progressed bronchial dilatation (n=4); and increased lung volume (n=3).
Conclusion
HRCT scans are abnormal in patients with BO, with the most commonly observed finding being areas of decreased lung attenuation. While the HRCT findings are not specific, it is believed that their common features can assist in the diagnosis of BO in BMT recipients.
Keywords: Bone marrow, transplantation; Bronchiolitis obliterans; Computed tomography (CT), high-resolution
Bronchiolitis obliterans (BO), a relatively infrequent disease, is characterized clinically by persistent progressive airflow obstruction unresponsive to bronchodilator therapy. The pathologic findings of BO are constrictive bronchiolitis and submucosal and peribronchiolar fibrosis, which result in extrinsic narrowing and obliteration of the lumen of the small bronchi and bronchioles. The diverse contributing etiologies include toxic-fume exposure, viral and mycoplasma infection, collagen vascular disease, drug reaction and organ transplantation (1, 2). BO has received considerable attention as a major cause of obstructive lung disease along with active chronic graft-versus-host disease (GVHD) after bone marrow transplantation (BMT) (3-5). The incidence of BO after BMT in adults has been evaluated as being less than 3% (6, 7), although Schultz et al. (8) reported a relatively high (19.4%) frequency in children. The disease can often be diagnosed on the basis of the patient's medical history, physical examination, plain chest radiography, arterial blood gas analysis, lung function test, and ventilation/perfusion lung scan, although in severely ill patients any uncertainty should be resolved by conducting a histological examination of an open-lung biopsy specimen. Owing to the difficulties involved in obtaining a histologic specimen and the limitations associated with the functional diagnosis of BO, high resolution computed tomography (HRCT) has emerged as a useful adjunct to lung function tests for diagnosing BO after organ transplantation (9). There have been many reports about the HRCT features of BO in patients after lung transplantation (10-17). However, reports about the HRCT findings of BO in patients after BMT are rare (9, 18). Also, there are few reports about the interval changes on HRCT of BO after BMT (9). We evaluated the HRCT findings in patients who developed BO after allogenic BMT, as well as the interval changes of HRCT in those patients who had follow-up images.
MATERIALS AND METHODS
Patients
During the past three years, 362 patients underwent allogenic BMT at our institution. Of these, 11 patients were diagnosed as having BO after BMT (five men and six women). Their ages ranged from 19 to 42 years (mean: 30 years). BMT was performed in cases of chronic myelocytic leukemia (n=7), acute lymphocytic leukemia (n=2), acute myelocytic leukemia (n=1), and severe aplastic anemia (n=1).
Ten of the 11 patients with BO experienced chronic graft versus host disease (GVHD) before the diagnosis of BO. The remaining patient experienced chronic GVHD concurrent with the onset of BO. The most commonly affected sites were the skin (n=11) and liver (n=8). BO was diagnosed 4-42 months (mean, 15 months) after BMT. During the follow-up period, two patients died of respiratory failure (4 and 15 months after the diagnosis of BO). Fungal pneumonia was present in one of these two patients.
Assessment of BO
All patients routinely had lung function tests performed the month preceding BMT and at 3-12 months after BMT. Additional pulmonary function testing was done if the patients had symptoms suspicious for BO. BO was diagnosed according to the criteria of BO established by the International Society for Heart and Lung Transplantation (19), namely irreversible airflow obstruction with an FEV1 value of less than 80% of the baseline value, without clinical evidence of infection. Three patients underwent bronchoalveolar lavage and one patient underwent transbronchial lung biopsy. For these three patients, no organisms were found in the bronchoalveolar lavage fluid. Transbronchial biopsy showed the presence of chronic bronchitis and bronchiolitis obliterans. One patient underwent open lung biopsy and the diagnosis of BO was obtained histologically.
HRCT
HRCT scans were performed on all patients at the time of their initial diagnosis (Somatom Plus, Siemens, Erlangen, Germany). HRCT scans during deep inspiration were obtained throughout the entire thorax with 1.5-2 mm thick axial sections at 1cm intervals; they were reconstructed using a high spatial frequency algorithm. The tube current was 240 mA at a voltage of 120 kV. Expiratory scans were obtained at the levels of the aortic arch, midway between the aortic arch and the trachea carina, in the tracheal carina, midway between the tracheal carina and the right hemidiaphragm, and 1 cm above the right hemidiaphragm. Five patients underwent HRCT twice, with the time interval ranging from 1 to 10 months after the initial CT scan. Two patients underwent HRCT three times, at 1 and 9 months and at 9 and 15 months after the initial CT scan, respectively. One patient underwent HRCT four times due to deteriorating pulmonary function, with the follow-up scans being performed at 6, 12 and 14 months after the initial CT scan. Two radiologists evaluated all of the CT scans independently and then jointly. The final interpretation was made by consensus.
During the analysis of each CT examination, the inspiratory images were reviewed before the expiratory images. The inspiratory HRCT images were assessed for the presence and lobar distribution of decreased lung attenuation. Each lobe of the lung was scored on a scale of 0-5 for the low-attenuating regions, depending on the percentage of each lobe involved: 0 (not affected); 1 (< 10% of lung affected); 2 (10 to 25%); 3 (> 25 to 50%); 4 (> 50% to 75%); and 5 (> 75%). The score of each lobe was summed and averaged for the purpose of comparison. The lobe with the highest score of decreased attenuation was also evaluated. The images were also assessed for segmental or subsegmental bronchial dilatation, diminution of peripheral vascular markings, centrilobular nodules, and branching linear structure (tree-in-bud pattern). Bronchial dilatation was diagnosed on the basis of a bronchial internal diameter greater than that of the adjacent pulmonary artery. Diminution of vascular markings was defined as a reduction in vessel caliber and/or numbers. The prominent lobar distribution of bronchial dilatation was also assessed. The presence of subpleural linear or patchy opacities was also evaluated. The expiratory images were assessed for the presence of air trapping. Air trapping was diagnosable if the lung parenchyma remained lucent on the expiratory CT scans, demonstrating a less than normal increase in attenuation after expiration, or showing little change in cross-sectional area (20). We also evaluated the interval changes of the HRCT findings in those patients who had follow-up images. Contraction or expansion was determined to be present if in the repeated follow-up CT examination there was more than a 10% change in the diameters on at least two scan levels.
RESULTS
Abnormal HRCT findings were present in all cases; the most common HRCT abnormalities consisted of decreased lung attenuation (n=11) (Figs. 1, 2), subsegmental bronchial dilatation (n=6), diminution of peripheral vascularity (n=6), and centrilobular nodules or branching opacities (n=3). Three of the six patients with subsegmental bronchial dilatation showed segmental bronchial dilatation (Fig. 1). Subpleural linear or focal patchy opacity was found in three patients. The averaged score of low attenuation was 1-2 in two patients, 2-3 in five, 3-4 in three, and 4-5 in one. In six patients, the lower lobes obtained the highest score (wide involvement of low attenuation). Four patients showed even lobar distribution of the low attenuating region on the inspiratory images. In the remaining one patient, there was upper lobe predominance of decreased attenuation of the lung parenchyma. In four patients, segmental or subsegmental bronchial dilatations were noted only in the lower lobes (Fig. 1), while in two patients they were noted in all of the lung lobes. Expiratory air trapping was noted in all patients (Fig. 2).
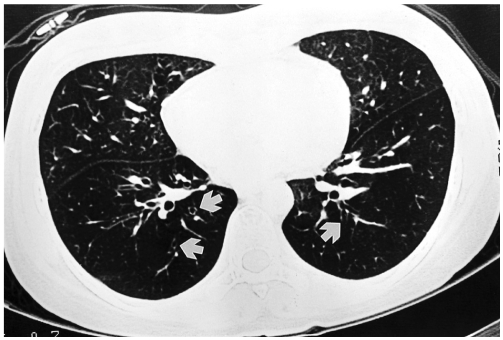
Fig. 1.
A 24-year-old woman with bronchiolitis obliterans. She underwent allogeneic bone marrow transplantation for chronic myelocytic leukemia approximately 11 months previously. Inspiratory high resolution computed tomography at the time of diagnosis of bronchiolitis obliterans shows diffuse hypoattenuation and thin-walled dilated bronchi (white arrows) in both lower
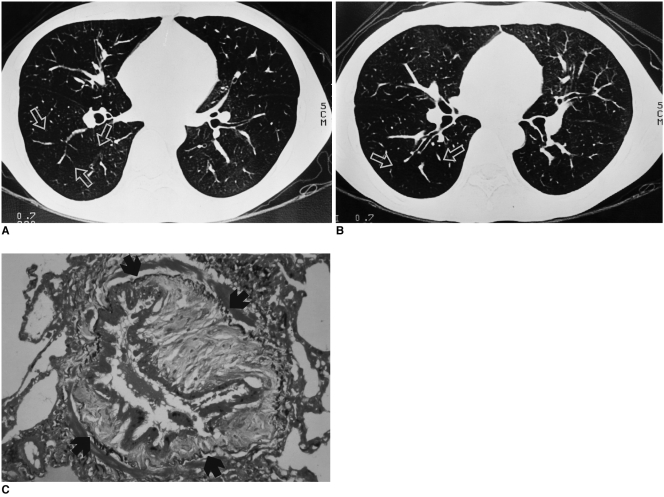
Fig. 2.
A 29-year-old man with bronchiolitis obliterans. He underwent allogeneic bone marrow transplantation for chronic myelocytic leukemia approximately one year previously.
A. Inspiratory high resolution computed tomography shows diffuse decreased lung attenuation. The pulmonary vessels are sparse and attenuated in the region of decreased density (arrows).
B. Expiratory scan shows air trapping within the diseased region (arrows).
C. Photomicrograph (elastic van Gieson stain, ×200) shows narrowing of bronchial lumen due to fibrosis of the lamina propria, surrounded by an elastic bundle (arrows) (from Journal of Thoracic Imaging 2001;16:130-37 "notes from 2000 annual meeting of Korean Society of Thoracic Radiology" with permission).
Centrilobular nodules were noted in three patients. They were seen in two lobes in two patients (both lower lobes) and three lobes in the remaining one patient (right middle lobe and both lower lobes). Tree-in-buds were also noted in all three patients with centrilobular nodules; two lobes in two patients (both lower lobes) and one lobe in one patient (left lower lobe) (Fig. 3).
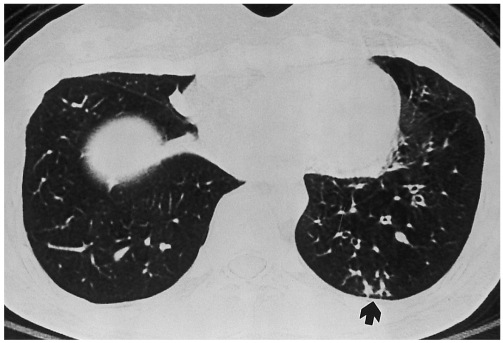
Fig. 3.
Tree-in-buds of bronchiolitis obliterans in a 28-year-old woman. Bronchiolitis obliterans was diagnosed on spirometry and transbronchial lung biopsy 16 months after bone marrow transplantation for chronic myelocytic leukemia. Inspiratory high resolution computed tomography scan shows decreased lung attenuation and subsegmental bronchial dilatations in left lower lobe. Tree-in-bud pattern is noted in subpleural portion of posterior basal segment of left lower lobe (arrow).
Interval changes were found in all eight patients who underwent follow-up HRCT scans. These changes consisted of an increased extent of decreased lung attenuation (n=7), newly developed or progressive bronchial dilatation (n=4), increased lung volume (n=2), bronchial wall thickening and centrilobular nodules (n=1), and diminished vascularity (n=1) (Fig. 4).
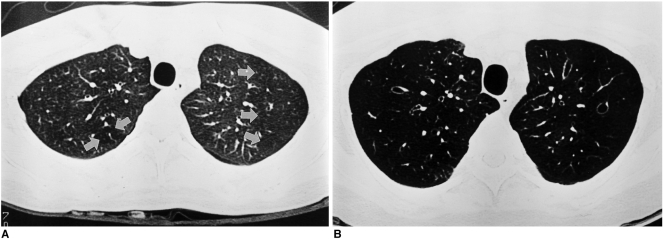
Fig. 4.
Progression of bronchiolitis obliterans on high resolution computed tomography in a 28-year-old woman. Bronchiolitis obliterans was diagnosed on spirometry and transbronchial lung biopsy 16 months after bone marrow transplantation for chronic myelocytic leukemia.
A. Inspiratory high resolution computed tomography scan at the time of diagnosis of bronchiolitis obliterans shows small areas of decreased lung attenuation in both upper lobes (arrows).
B. Inspiratory high resolution computed tomography scan taken nine months later at a similar anatomic level to A, shows markedly increased extent of pulmonary hypoattenuation and new development of subsegmental bronchial dilatations. The lung volume is much increased.
DISCUSSION
Obstructive lung disease complicating bone marrow transplantation was first described in 1982 (3). A similar complication is well known in lung and heart-lung transplant recipients (10, 11). BO appears later than other pulmonary complications, occurring between three and 12 months after BMT. Obstructive lung disease following bone marrow transplantation is more common in patients with chronic graft-versus-host disease (GVHD) (6, 7), occurring in 6-10% of long-term survivors with chronic GVHD and with a mortality rate of more than 50% (7, 21). In our study, ten of the eleven patients with BO had an episode of GVHD, and two of them died of respiratory failure.
The pathogenesis of post BMT BO is unclear. Several theories have been postulated, including immunologic injury caused by GVHD (22), viral infection (21, 23), decreased IgA levels, and chronic aspiration secondary to esophageal abnormalities (12). GVHD is most likely to be responsible for the initial mucosal injury, with further damage being inflicted as a result of repeated aspiration, opportunistic infections and chemotherapy (22).
Although histology provides the most definitive means of diagnosis, the patchy nature of this disease may result in a negative transbronchial biopsy being obtained (21, 24). Many large series use the term post-BMT chronic airflow obstruction, with the diagnosis relying on clinical criteria with pulmonary function tests rather than histology for diagnosis (6, 25, 26).
Obstructive lung disease following bone marrow transplantation has been ascribed to bronchiolitis obliterans (27, 28). Owing to the difficulties in obtaining a histologic specimen and the limitation of the functional diagnosis of BO, HRCT has emerged as a useful tool and as an adjunct to lung function tests in diagnosing BO following organ transplantation. A number of abnormalities have been identified on HRCT in patients with BO, especially in lung transplant recipients (12-14).
Morrish et al. (12) first reported the HRCT findings of four lung transplant patients with BO, who showed bronchial dilatation and decreased peripheral vascular marking. Ikonen et al. (15) reported HRCT findings in patients with long-term follow up after lung transplantation; among the first identifiable chronic changes were volume contraction, decreased peripheral vascular and bronchial markings, and thickening of the septal lines. Later, bronchial dilatation, hyperlucency and mosaic phenomenon were also identified. Recently, expiratory air-trapping on HRCT, a non-specific sign of small-airway disease, has also been demonstrated in patients with BO and is reported to be the most sensitive and accurate radiologic indicator of BO in the lung transplant population (16, 17).
Reports on the HRCT features of BO after BMT are rare. Sargent et al. (9) reported on the HRCT findings of seven child patients with obstructive lung disease following allogeneic bone marrow transplantation. All of these patients showed abnormal findings on the HRCT scan. Areas of parenchymal hypoattenuation affected all 35 lobes of the lung. Expiratory air-trapping was found in all four patients who underwent expiratory cine CT. Subsegmental or segmental bronchial dilatations were noted in five of seven patients.
Ooi et al. (18) reported the HRCT findings of nine adult patients with BO after BMT. They reported normal findings in two patients and abnormal findings in seven patients, with non-specific findings of bronchial dilatation (n=1), hypoattenuated areas (n=4), vascular attenuation (n=4), and consolidation (n=2).
Contrary to Ooi's report, our patients showed abnormalities in all cases, with the findings being parenchymal decreased attenuation, subsegmental bronchial dilatation, diminution of peripheral vascularity, segmental bronchial dilatation, centrilobular nodules, and centrilobular branching structures (tree-in-buds pattern) in order of frequency. Our results are similar to those of Sargent et al. (9).
There are some discrepancies between the results of Ooi et al. (18) and the results of our own and Sargent et al's study (9). There are several possible explanations for these discrepancies. First, the discrepancy could be due to the small numbers of patients in these studies (Sargent et al. = 7 patients, Ooi et al. = 9 patients, and our study = 11 patients). Second, Ooi et al. studied a group of patients with BO after BMT, possibly at the early stage of the disease, since they diagnosed BO through short term follow up using the pulmonary function test (2, 3, 6, 9, 12, 18 and 24 months after BMT), whereas our patients underwent the pulmonary function test at 3-12 months after BMT. Sargent et al. performed the pulmonary function test after the onset of the clinical symptoms. Third, Ooi et al. did not routinely perform the expiratory scan. Sometimes the diagnosis of decreased lung attenuation is difficult on the inspiratory image when the lesion involves the entire lung. The expiratory scan is very helpful in such cases, since a normal increase in lung attenuation is not observed after expiration. Expiratory air-trapping on HRCT is reported to be the most sensitive and accurate radiologic indicator of BO in the lung transplant population (16, 17).
The HRCT findings of BO in BMT patients are very similar to those of BO in lung transplant recipients, except for the volume contraction of the thorax and thickening of the septal lines noted among the chronic changes of BO in lung transplantation patients (12-17).
Centrilobular nodules and tree-in-buds pattern were found somewhat frequently in our study; and they are likely to be caused by inspissated secretions occurring within distal airways or plugging of the terminal airways with granulation tissue, as reported by Padley et al. (29). We also found expiratory air trapping in all of our patients.
Subpleural linear or focal patchy opacities were noted in three patients; these findings are not characteristic of BO, but may in fact represent small areas of linear fibrosis, post-obstructive lobular consolidation or alveolar injury or infarction, due to previous recurrent infection or chemotherapy, as reported by Morrish et al. (12).
In particular, the low attenuation was found to be more prominent in the lower lobes than in the upper or middle lobes, which has not been described in other reports. Similar to the results of our study, Lentz et al. (11) emphasized the diagnostic value of a lower lobe predominance of bronchial dilatation in adult heart-lung transplant recipients who develop BO. Hruban et al. (30) explained the cause of lower lobe predominance of bronchiectasis in lung transplant recipients. They suggested that chronic rejection might preferentially involve the lower lobes of the lungs. Patients with chronic rejection, because of a rejection-associated depletion in the bronchus-associated lymphoid tissue, are more susceptible to the development of pulmonary infection. It is possible, therefore, that the change in the lower lobes may, in part, also be due to the presence of a superimposed infection. These suggestions would also explain the lower lobe predominance of bronchiectasis and low attenuation in patients with BO after BMT. In our study, subsegmental bronchial dilatation was more frequent than segmental bronchial dilatation, which concurs with the report by Padley et al. (29).
Information regarding the interval changes on HRCT of BO after BMT is sparse. Sargent et al. (9) reported interval changes in three cases of BO after BMT. On the 3-9 month follow-up scans, they reported an increased extent of bronchiectasis in one patient and an increased extent of hypoattenuation area in two patients. In our study, interval changes were found in all eight patients in whom follow-up scans were available. Increased extent of pulmonary hypoattenuation was the most frequent interval change in our study. The areas of normal lung attenuation on the earlier study became abnormal on the follow up scans. Progression or new development of bronchial dilatation, increased lung volume, bronchial wall thickening with nodules, and diminished vascularity, were also found in the follow-up scans. After integrating these findings, we were able to speculate on the serial HRCT changes of bronchiolitis obliterans after the onset of functional decrease and clinical symptoms. Initially, HRCT would show a hypoattenuating region, mainly in the lower lobes. Diminished vascularity and subsegmental bronchial dilatations would frequently be associated with this hypoattenuating region. Occasionally, segmental bronchial dilatation would also be noted. Subsequently, the hypoattenuating regions might increase in extent and, later on, hypoattenuated regions would be noted in the entire lobes. In addition, the subsegmental and segmental bronchial dilatations could develop or progress. We speculate that the early change of BO, before the onset of the symptoms, would result in small hypoattenuating areas on HRCT. A prospective study will be required to determine if HRCT can facilitate the diagnosis of obstructive lung disease before the onset of symptoms.
There are some limitations to our study, namely that it was a retrospective study with a small numbers of patients, for which there was an absence of histologic diagnosis of BO except for two patients. Although histologic proof of BO is desirable, transbronchial biopsies are unfortunately known to have a low sensitivity for the diagnosis of BO, and it is clinically not acceptable to submit a large percentage of BMT patients to surgical lung biopsy.
In conclusion, we found that HRCT scans are abnormal in patients with BO after BMT, with the most commonly observed finding being areas of decreased lung attenuation. While the HRCT changes are not specific, it is believed that their characteristic features can assist in the diagnosis of BO in BMT recipients.
References
- 1.Epler GR, Colby TV. The spectrum of bronchiolitis obliterans. Chest. 1983;83:161–162. doi: 10.1378/chest.83.2.161. [DOI] [PubMed] [Google Scholar]
- 2.Ezri T, Kunichezky S, Eliraz A, Soroker D, Halperin D, Schattner A. Bronchiolitis obliterans-current concepts. Q J Med. 1994;87:1–10. doi: 10.1093/oxfordjournals.qjmed.a068855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roca J, Granena A, Rodriguez-Rosin R, Alvarez P, Agusti-Vidal A, Rozman C. Fatal airways disease in an adult with chronic graft-versus-host disease. Thorax. 1982;37:77–78. doi: 10.1136/thx.37.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1998;72:621–627. [PubMed] [Google Scholar]
- 5.Epler GR. Bronchiolitis obliterans and airways obstruction associated with graft-versus-host disease after bone marrow transplantation. Thorax. 1984;39:887–894. doi: 10.1136/thx.39.12.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstruction lung disease after allogeneic bone marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 7.Ralph DD, Springmeyer SC, Sullivan KM, Hackman RC, Storb R, Thomas ED. Rapidly progressive air-flow obstruction in marrow transplant recipients. Possible association between obliterative bronchiolitis and chronic graft-versus-host disease. Am Rev Respir Dis. 1984;129:641–644. [PubMed] [Google Scholar]
- 8.Schultz KR, Green GJ, Wensley D, et al. Obstructive lung disease in children after allogeneic bone marrow transplantation. Blood. 1994;84:3212–3220. [PubMed] [Google Scholar]
- 9.Sargent MA, Cairns RA, Murdoch MJ, Nadel HR, Wensley D, Schultz KR. Obstructive lung disease in children after allogeneic bone marrow transplantation: evaluation with high-resolution CT. AJR Am J Roentgenol. 1995;164:693–696. doi: 10.2214/ajr.164.3.7863896. [DOI] [PubMed] [Google Scholar]
- 10.Paradis I, Yousem S, Griffith B. Airway obstruction and bronchiolitis obliterans after lung transplantation. Clin Chest Med. 1993;14:751–763. [PubMed] [Google Scholar]
- 11.Lentz D, Bergin CJ, Berry GJ, Stoehr C, Theodore J. Diagnosis of bronchiolitis obliterans in heart-lung transplant patients: importance of bronchial dilatation on CT. AJR Am J Roentgenol. 1992;159:463–467. doi: 10.2214/ajr.159.3.1503006. [DOI] [PubMed] [Google Scholar]
- 12.Morrish WF, Herman SJ, Weisbrod GL, Chamberlain DW. Bronchiolitis obliterans after lung transplantation: findings at chest radiography and high-resolution CT. Toronto Lung Transplant Group. Radiology. 1991;179:487–490. doi: 10.1148/radiology.179.2.2014297. [DOI] [PubMed] [Google Scholar]
- 13.Herman SJ. Radiologic assessment after lung transplantation. Clin Chest Med. 1990;11:333–346. [PubMed] [Google Scholar]
- 14.Skeens JL, Fuhrman CR, Yousem SA. Bronchiolitis obliterans in heart-lung transplantation patients: radiologic findings in 11 patients. AJR Am J Roentgenol. 1989;153:253–256. doi: 10.2214/ajr.153.2.253. [DOI] [PubMed] [Google Scholar]
- 15.Ikonen T, Kivisaari L, Taskinen E, Piilonen A, Harjula AL. High-resolution CT in long-term follow-up after lung transplantation. Chest. 1997;111:370–376. doi: 10.1378/chest.111.2.370. [DOI] [PubMed] [Google Scholar]
- 16.Leung AN, Fisher K, Valentine V, et al. Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest. 1998;113:365–370. doi: 10.1378/chest.113.2.365. [DOI] [PubMed] [Google Scholar]
- 17.Bankier AA, Van Muylem AV, Knoop C, Estenne M, Gevenois PA. Bronchiolitis obliterans syndrome in heart-lung transplant recipients: diagnosis with expiratory CT. Radiology. 2001;218:533–539. doi: 10.1148/radiology.218.2.r01fe09533. [DOI] [PubMed] [Google Scholar]
- 18.Ooi GC, Peh WCG, Ip M. High-resolution computed tomography of bronchiolitis obliterans syndrome after bone marrow transplantation. Respiration. 1998;65:187–191. doi: 10.1159/000029257. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allograft. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 20.Webb WR, Müller NL, Naidich DP. High-resolution CT of the lung. 3rd ed. Philadelphia: Williams & Wilkins; 2001. pp. 599–618. [Google Scholar]
- 21.Chan CK, Hyland RH, Hutcheon MA, et al. Small-airways disease in recipients of allogeneic bone marrow transplants. An analysis of 11 cases and a review of the literature. Medicine. 1987;66:327–340. doi: 10.1097/00005792-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Krowka MJ, Rosenow EC, III, Hoagland HC. Pulmonary complications of bone marrow transplantation. Chest. 1985;87:237–246. doi: 10.1378/chest.87.2.237. [DOI] [PubMed] [Google Scholar]
- 23.Paz HL, Crilley P, Topolsky DC, Coll WX, Patchefsky A, Brodsky I. Bronchiolitis obliterans after bone marrow transplantation: the effect of preconditioning. Respiration. 1993;60:109–114. doi: 10.1159/000196183. [DOI] [PubMed] [Google Scholar]
- 24.Theodore J, Starnes VA, Lewiston NJ. Obliterative bronchiolitis. Clin Chest Med. 1990;11:309–321. [PubMed] [Google Scholar]
- 25.Philit F, Wiesendanger T, Archimbaud E, Mornex JF, Brune J, Cordier JF. Post-transplant obstructive lung disease ("bronchiolitis obliterans") a clinical comparative study of bone marrow and lung transplant patients. Eur Respir J. 1995;8:551–558. [PubMed] [Google Scholar]
- 26.Curtis DJ, Smale A, Thien F, Schwarer AP, Szer J. Chronic airflow obstruction in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;16:169–173. [PubMed] [Google Scholar]
- 27.King TE., Jr Bronchiolitis obliterans. Lung. 1989;167:69–93. doi: 10.1007/BF02714935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLoud TC, Epler GR, Colby TV, Gaensler EA, Carrington CB. Bronchiolitis obliterans. Radiology. 1986;159:1–8. doi: 10.1148/radiology.159.1.3952294. [DOI] [PubMed] [Google Scholar]
- 29.Padley SP, Adler BD, Hansell M, Müller NL. Bronchiolitis obliterans: high-resolution CT findings and correlation with pulmonary function tests. Clin Radiol. 1993;47:236–240. doi: 10.1016/s0009-9260(05)81129-8. [DOI] [PubMed] [Google Scholar]
- 30.Hruban RH, Ren H, Kuhlman JE, et al. Inflation-fixed lungs: Pathologic-radiologic (CT) correlation of lung transplantation. J Comput Assist Tomogr. 1990;14:329–335. [PubMed] [Google Scholar]