Abstract
Objective
Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) is a newly developed MR contrast agent. After intravenous injection, Gd-EOB-DTPA is gradually taken up by the hepatocytes and eventually excreted via the biliary pathway without any change to its chemical structure. Because of these characteristics, it can be used as a tracer for quantitative liver function testing. The purpose of this study is to develop a noninvasive method of quantitation of the hepatic function using Gd-EOB-DTPA through the deconvolution analysis.
Materials and Methods
Adult New Zealand white rabbits (n = 10, average body weight = 3.5 kg) were used in the present study. Hepatic injury was induced to by the intragastric administration of carbon tetrachloride (CCl4) three times a week for three weeks. Liver enzyme (aspartate aminotransferase, AST; alanine aminotransferase, ALT) levels and the plasma indocyanine green (ICG) retention rate 15 minutes after an intravenous injection of ICG (ICG R15), was checked before and after the three-week administration of CCl4. At the end of experimental period, an observer "blinded" to the treatment given the rabbits performed the histological examination. MRI studies were performed before and after the three-week administration of CCl4 on a 1.5 T scanner using a human extremity coil. After intravenous bolus injection of Gd-EOB-DTPA (0.3 mL of Gd-EOB-DTPA freshly prepared in 2.7 mL of normal saline) through the ear vein, the 250 axial single level dynamic MR images were obtained using a fast low angle shot (FLASH, TR/TE = 11/4.2 msec, flip angle = 15, acquisition time 1 second, slice thickness = 5 mm, matrix = 128×128, field of view = 120 mm) sequence with 1.5 sec time intervals. The time-intensity curves were obtained at the abdominal aorta and the liver parenchyma that was devoid of blood vessels. Deconvolution analysis of the aortic (input function) and hepatic parenchymal (output function) time-intensity curves was performed with a modified Fourier transform technique to calculate the hepatic extraction fraction (HEF). The presence and type of hepatic injury were determined by the histopathologic examination and statistical analysis of the changes of the hepatic enzyme levels, the ICG R15 and Gd-EOB-DTPA HEF values between the time before and after CCl4 administration with Wicoxon signed rank test. Correlation between the Gd-EOB-DTPA HEF and the change of the ICG R15 were analyzed with Pearson's correlation coefficient.
Results
Histopathologic examination showed findings that were compatible with hepatic fibrosis caused by chronic liver injury. The initial blood biochemical studies before the administration of carbon tetrachloride showed that the mean AST and ALT levels were 39.8±5.2 IU/L and 59.1±11.7 IU/L, respectively. The AST and ALT levels increased to 138.4±50.5 IU and 172.0±71.6 IU/L, respectively, after the three week administration of CCl4. The ALT and AST levels were significantly increased after the three weeks of CCl4 administration (p = 0.018). The ICG R15 values were 4.47±2.08% and 19.43±3.98% before and after three-week administration of CCl4, respectively. The ICG R15 values were significantly increased after hepatic injury (p = 0.018). After normalizing the HEF as 100% in each rabbit before CCl4 administration, the deconvoluted curve after CCl4 administration revealed less hepatocyte extraction efficiency with a mean value of 77.7±3.6. There was a significant correlation between the HEF and changes of the ICG R15 by the Pearson correlation coefficient assessment (correlation coefficient = -0.965, p = 0.000).
Conclusion
The Gd-EOB-DTPA HEF could be calculated from deconvolution analysis of aortic and hepatic parenchymal time-intensity curves obtained by dynamic MRI. The Gd-EOB-DTPA HEF was well correlated with changes of the ICG R15, which is the most common parameter used in the quantitative estimation of the hepatic function. The Gd-EOB-DTPA HEF is a direct, noninvasive technique for the quantitative evaluation of liver function. It could be a promising alternative for the determination of noninvasive hepatic function in those patients with liver disease.
Keywords: Liver, MR; Liver, contrast media; Magnetic resonance (MR), contrast media
Estimation of the hepatic functional reserve is an essential procedure for preventing postoperative hepatic failure and for the medical management of patients with hepatic dysfunction. Resection of a large volume of the liver may result in postoperative liver failure; therefore, the precise estimation of the functional capacity of the whole liver or future remnant liver is important. The liver function is generally assessed by quantitative blood level measurements of liver enzymes such as the aminotransferases. However, the measurement of liver enzymes reflects an escape of these enzymes from the liver cells, and this is only an indirect measurement of hepatocyte injury (1, 2). The measurement of liver enzymes does not directly reflect hepatocyte function. The liver carries out thousands of biochemical functions (3). It has a virtually infinite capacity for regeneration, and this further complicates quantitative assessment of the hepatic function reserve. In fact, approximately half of its mass can be excised with only a transient change being noted on the contemporary liver function tests (3). For these reasons, it is desirable to have a direct measurement of hepatocyte function, and many indices have been proposed for this purpose (4-8). Recently, the indocyanine green (ICG) plasma clearance test is the most commonly used test for the preoperative evaluation of liver function (9). However, it can occasionally be an unreliable index because the hepatic uptake of ICG is affected by several unpredictable factors (10-13). Evaluating the early phase of ICG plasma disappearance or its direct measurement in the liver has been reported to be more accurate for the estimation of liver function (14). Measuring the hepatic extraction fraction (HEF) of a hepatobiliary radiopharmaceutical agent helps to evaluate hepatic function, and this is generally performed using deconvolution analysis (15). A scintigraphy technique with deconvolution analysis has recently been proposed as a direct intrahepatic measurement of the early phase metabolism of an organic anion, Tc-99m diisopropyl imminodiacetic acid (Tc-99m DISIDA) (16). In this study, the authors developed a noninvasive technique to quantitate the hepatocyte function in rabbits using deconvolution analysis of the dynamic MR images obtained after the administration of a liver-specific contrast agent for the MR imaging, gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid (Gd-EOB-DTPA).
MATERIALS AND METHODS
Hepatic Injury Model
Ten adult New Zealand white rabbits (average body weight = 3.5 kg) were used in this study. The animals were allowed to adapt to the animal care facility for one week, and they were given free access to standard rabbit chow and tap water. Hepatic injury was induced by the intragastric administration of carbon tetrachloride (CCl4) three times a week for three weeks. The dose of CCl4 was 0.4 ml/kg and it was administered after mixing it with 1.6 ml/kg of olive oil. During the period of CCl4 administration, three rabbits died, probably of hepatic failure (n = 1) and other undetermined causes (n = 2).
Biochemical Tests and Histopathologic Examinations
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were checked before and three weeks after starting the CCl4 administration. Indocyanine green (ICG) clearance testing was performed in all animals before and at the end of CCl4 administration as an index of the liver function. A single dose of ICG (0.5 mg/kg; Diagnogreen Inj., Diichi Pharmaceutical Co., Tokyo, Japan) was freshly prepared in saline and then injected into an ear vein. Blood samples (3 mL) were collected into tubes containing EDTA before and 15 minutes after the ICG administration. The samples were centrifuged at 2000 g for four minutes, and the plasma was separated and stored at -20℃ until assay. The plasma ICG concentration (the ICG retention rate 15 minutes after an intravenous injection, ICG R15) was determined using an U2001 spectrophotometer (Hitachi, Kyoto, Japan) at 805 nm.
For the histopathologic examination, the rabbits were sacrificed at the end of the experimental period with an overdose (90 mg/kg) of sodium pentobarbital (Dai Han Pharm. Co., Seoul, Korea). Three fragments from each of the liver lobes were collected and processed for light microscopy. The tissues were fixed in 10% formalin and they were then embedded in paraffin. Serial sections were cut and stained with hematoxylin-eosin and Masson's trichrome stain. An observer, who was blinded to the treatment protocol, performed the histological examination.
MR Imaging
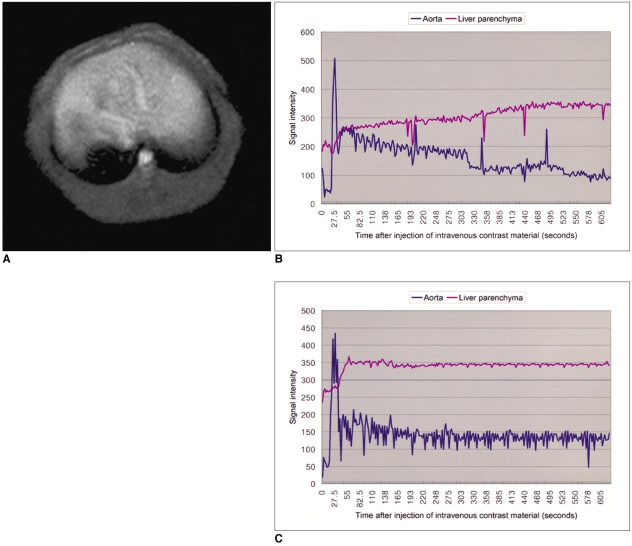
Dynamic MR imaging using Gd-EOB-DTPA (Evovist®, Schering, Berlin, Germany) was performed on the rabbits before and after the three-week administration of CCl4. All the MRI studies were performed on a 1.5T MR scanner (Vision Plus, Siemens Medical System, Erlangen, Germany) with the human extremity coil. The animals were anesthetized with an intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). The initial coronal T1-weighted images were obtained with a turbo fast low angle shot (turbo-FLASH) sequence (TR/TE = 11/4.2 msec, flip angle = 15) for the localization of the liver. On the basis of these coronal images, an optimal level that could give the best visualization of the liver and the abdominal aorta was chosen for repeated axial scans. The other imaging parameters were: slice thickness of 5 mm, matrix size of 128×128, and field of view (FOV) of 120 mm. After an intravenous bolus injection of Gd-EOB-DTPA (0.3 mL of Gd-EOB-DTPA freshly prepared in 2.7 mL of normal saline) through the ear vein, the 250 axial single level dynamic MR images were obtained using the turbo-FLASH sequence (TR/TE = 11/4.2 msec, flip angle = 15, acquisition time: 1 second) with 1.5 sec time intervals (Fig. 1A). The bolus injection was finished within 3 seconds and the MR acquisition was started immediately after this injection. The time-intensity curves were obtained at the abdominal aorta and at the liver parenchyma that was devoid of blood vessels (Figs. 1B, C). For the time-intensity curves, the region of interest (ROI) of 9 mm2 was placed on abdominal aorta and liver parenchyma, and the absolute signal intensities were then measured.
Fig. 1.
MR imaging and time-intensity curves.
A. After intravenous injection of Gd-EOB-DTPA, 250 level dynamic MR imagings were obtained using a turbo-fast low angle shot (turbo-FLASH) sequence (TR/TE = 11/4.2 msec, flip angle = 15, acquisition time 1 second, interscan gap = 1.5 second).
B. Time-intensity curves of aorta and hepatic parenchyma in a rabbit that were obtained before administration of CCl4.
C. Time-intensity curves of aorta and hepatic parenchyma in a rabbit that were obtained three weeks after administration of CCl4.
Deconvolution Analysis
After the intravenous injection, the Gd-EOB-DTPA, which is bound to albumin due to its lipophilic nature, is gradually taken up by the hepatocytes and eventually excreted via the biliary pathway. Since Gd-EOB-DTPA does not change its chemical structure during circulation, it can be used as tracer for quantitative liver function testing. In addition, the Gd-EOB-DTPA is taken up many times because of the recirculation of the hepatic blood flow. Therefore, to quantitate the hepatocyte function, we needed to introduce a convolution model. The concept of convolution is that if we measure the time dependent change of tracer concentration at the last point of the tracer within a finite distance, the final concentration at the last point was affected by the concentration at the initial point and the response function of the interval. Mathematically, it can be expressed as follow.
g(t) = f(t)*h(t) = ∫ f(x)h(t-x)dx
Where f(t) is the input function, which is a tracer concentration at the initial point and g(t) is the output function, which is a tracer concentration at the last point of the interval. H(t) is the response function of the interval and the symbol (*) means convolution of the two functions. In the integral transform of the convolution, both x and t are time variables. Therefore, if we know f(t) and g(t), then the response function, h(t), can be obtained by deconvolution.
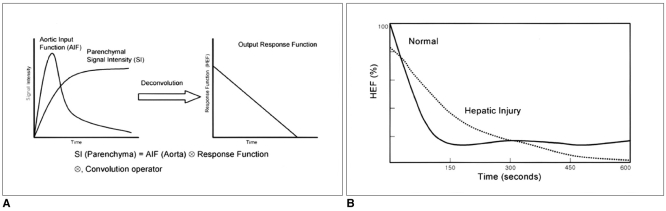
To extract the liver response function, the deconvolution analysis of the aortic (input function) and hepatic parenchymal (output function) time-intensity curves was performed with a modified Fourier transform technique. That is, by measuring the initial input function to the liver and the early phase liver signal intensities, it is possible to extract the hepatic extraction fraction by deconvoluting the early phase liver signal intensities with the aortic signal intensity. Both the aorta and liver curves are extended to many times their original length by appending a low-frequency, smoothly tapering curve to the original data series to compensate for any high-frequency artifact caused by the abrupt termination of data input at the conclusion of the imaging procedure. Finally, the inverse Fourier transform was performed and the resultant deconvoluted curve, which represents the true liver response, was obtained. An exponential curve of best fit was applied to the deconvoluted liver curve and the y-intercept of this curve was used as representing the hepatic extraction fraction (Fig. 2). In each rabbit, the HEF change after CCl4 administration was calculated by normalizing the HEF before CCl4 administration as 100%.
Fig. 2.
Deconvolution analysis and Gd-EOB-DTPA hepatic extraction fraction.
A. Diagram representing the process of deconvolution analysis. Deconvolution analysis of the aortic (input function) and hepatic parenchymal (output function) time-intensity curves was performed with a modified Fourier transform technique.
B. The Gd-EOB-DTPA hepatic extraction fraction after hepatic injury was obtained by normalizing the hepatic extraction fraction of before CCl4 administration as 100% in each rabbit.
Data Analysis
The presence and type of hepatic injury were determined by the histopathologic findings, the levels of the serum enzymes and the ICG R15 value. In each rabbit, the ICG R15 value was correlated with the hepatic extraction fraction measured by MRI using Gd-EOB-DTPA (Gd-EOB-DTPA HEF). The Wilcoxon signed rank test was used to determine the difference of the liver enzyme levels and ICG R15 values before and after the administration of CCl4. The 95% confidence level, p≤0.05, was used to determine a statistically significant difference. Correlation between the Gd-EOB-DTPA HEF and the hepatic enzyme changes and that between the Gd-EOP-DTPA HEF and the ICG R15 change were assessed using the Pearson correlation coefficient.
RESULTS
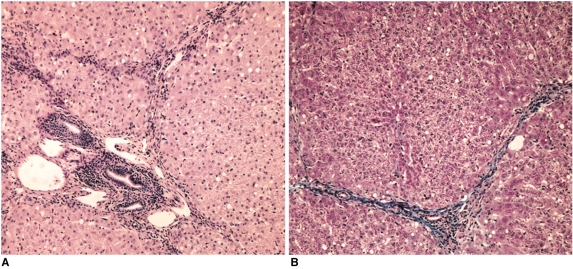
On the histopathologic examination with hematoxylineosin and Masson's trichrome stain, chronic inflammatory cell infiltration was noted around the periportal area, and there was also periportal fibrosis. These findings were compatible with hepatic fibrosis due to chronic liver injury (Fig. 3)
Fig. 3.
Histopathologic findings.
A. Photomicrograph of the liver tissue obtained at the end of experiment shows the chronic inflammatory cell infiltration at the portal and periportal areas (H & E stain; original magnification, ×100).
B. On Masson's trichrome stain, note the periportal fibrosis (original magnification, ×100).
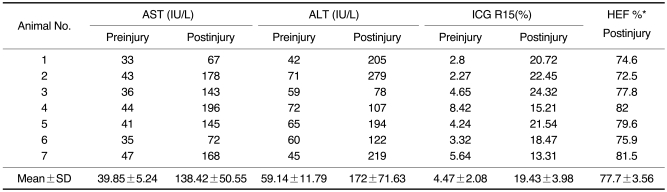
The baseline blood biochemical studies performed before the administration of CCl4 showed that the mean AST and ALT levels were 39.8±5.2 IU/L and 59.1±11.7 IU/L, respectively. After the three-week intragastric administration of CCl4, the AST and ALT levels increased to 138.4±50.5 IU and 172.0±71.6 IU/L, respectively, (Table 1). The increase of the enzyme levels and histopathologic results confirmed the chronic hepatic injury. According to the results of the Wilcoxon signed rank test, there was a significant difference between ALT and AST levels measured before and after the administration of CCl4 at the 95% confidence level (p = 0.018).
Table 1.
Results of Blood Aminotransferase Levels, ICG R15, and Gd-EOB DTPA Hepatic Extraction Fraction
Note.-*Relative Gd-EOB-DTPA hepatic extraction fraction calculated after normalizing the hepatic extraction fraction for before CCl4 administration as 100% in each rabbit. AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, ICG: Indocyanine green, HEF: hepatic extraction fraction
The ICG R15 values before the administration of CCl4 were 4.47±2.08%. After the three-week administration of CCl4 these values increased to 19.43±3.98% (Table 1). According to the results of the Wilcoxon signed rank test, there was a significant difference between the ICG R15 values that were checked before and after the administration of CCl4 at the 95% confidence level (p = 0.018).
Due to the recirculation of Gd-EOB-DTPA, the aorta curve showed small residual signals after the first pass of the contrast agent. However, the calculation of the hepatic response function, by means of deconvolution analysis, corrected the liver time-intensity curve for the constantly changing blood concentration of the contrast agent that was presented to the liver at each moment in time, thus compensating for the systemic recirculation of the agent. Therefore, the resulting hepatic response function mathematically simulates a single bolus injection of contrast agent directly into the hepatic artery. After normalizing the HEF as being 100% in each rabbit before the CCl4 administration, the deconvoluted curves of the dysfunction group after CCl4 administration revealed a reduced hepatocyte extraction efficiency, with a mean value of 77.7±3.6% (Table 1). According to the results of the Wilcoxon signed rank test, there was a significant difference between the HEF calculated before and after the administration of CCl4 at the 95% confidence level (p = 0.018).
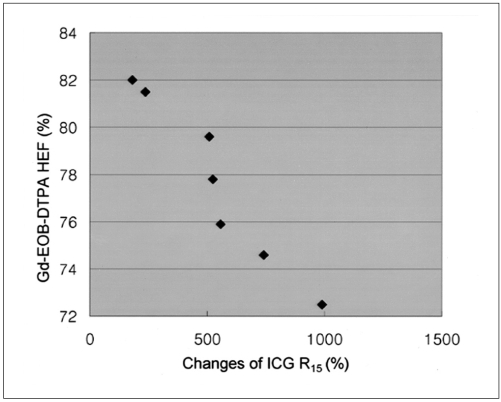
The results of Gd-EOB-DTPA HEF were well correlated with the ICG R15 increase. There was a significant correlation between the HEF and the changes of ICG R15 before and after the administration of CCl4 as accessed by the earson correlation coefficient (correlation coefficient = 0.965, p = 0.000) (Fig. 4). However, no statistically significant correlation was observed between the changes of the ICG R15 and the changes of the liver enzyme levels (AST, correlation coefficient = -0.252, p = 0.568; ALT, correlation coefficient = 0.310, p = 0.499).
Fig. 4.
Scattergram shows the relationship between the Gd-EOB-DTPA hepatic extraction fraction and the changes of indocyanine green R15 before and after hepatic injury. The Gd-EOB-DTPA hepatic extraction fraction decreased as the indocyanine green R15 values increased (correlation coefficient = -0.965, p = 0.000, Pearson correlation coefficient assessment).
DISCUSSION
There have been many indices proposed for the estimation of hepatic functional reserve (4-8). The ICG plasma clearance test has been employed with increasing frequency because ICG, a tricarbocyanine dye, has no reported toxicity and it undergoes neither intrahepatic conjugation nor enterohepatic circulation, and it is removed from the blood stream exclusively by the liver (4). However, it can occasionally be an unreliable index because hepatic uptake of ICG is affected by the hepatic blood flow, the ICG binding lipoprotein in the blood and the exchange of ICG across the hepatocellular membrane (10-13). For this reason, evaluation of the early phase of ICG plasma disappearance or its direct measurement in the liver is desired for an estimation of the "purer" liver function (14). The scintigraphy technique with deconvolution analysis has recently been proposed as a direct intrahepatic measurement of the early phase metabolism of an organic anion, DISIDA (16). In this current study, the authors have developed a noninvasive technique to quantitate hepatocyte function in rabbits by using a deconvolution analysis of the dynamic MR images that were obtained after administration of a liver-specific contrast agent for the MRI.
In this study, CCl4 was used to induce chronic liver disease in the rabbits. Many agents have been used as hepatotoxins. In previous experiment, the most common method of producing experimental cirrhosis has been administering multiple doses of CCl4 (17). As with chloroform and halothane, CCl4 requires activation by oxidases that are involved in the hemolytic breakage of the C-Cl bond. This activation occurs in the hepatic endoplasmic reticulum via an enzyme system of electron transport from reduced nicotinamide adenine dinucleotide phosphate (NADPH) to oxygen, and this leads to formation of a reactive metabolite, the trichloromethyl radical (CCl3). The CCl3 then binds covalently to a series of molecular structures, and particularly to the lipid of endoplasmic reticulum membranes; this initiates lipid peroxidation that reacts with the sulphydryl groups of the cellular proteins. Because the acute toxicity of CCl4 is dependent upon its metabolism by the microsomal hydroxylating hepatic system, inducers of cytochrome p450, such as ethanol or phenobarbitone, can potentiate this toxicity (18-20). The feasibility of using CCl4 for the experimentally induced chronic liver disease has been well described by Brandao et al. (21). The time they required for the development of cirrhosis was relatively long (6 months). In this study, the CCl4 administration period was reduced to three weeks by increasing the dosage. Three of the animals died during the CCl4 administration period, and this was probably because of acute hepatic failure (n = 1) and other uncertain causes (n = 2), yet it was possible to obtain precirrhotic chronic liver injury in seven of the rabbits, which was confirmed as portal fibrosis on the histopathologic examination.
When Gd-EOB-DTPA, gadolinium-diethylenetriaminepentaacetic-acid (DTPA), is covalently linked to the lipophilic ethoxybenzyl (EOB) moiety ([4S]-4-[4-ethoxybenzyl]-3,6,9-tris[carboxylatomethyl]-3,6,9-triazaundecandioic acid) then EOB-DTPA is formed: this is a newly developed MR contrast agent with a preferential uptake by hepatocytes (22). In previously reported animal studies, Gd-EOB-DTPA was cleared from the body by two elimination routes: one route is the receptor-specific uptake via the organic anion transporter (it is also called glutathione-S-transferase), in the hepatocytes with subsequent biliary excretion. The other route is via glomerular filtration in the kidneys with subsequent urinary excretion (22-24). The percentage of hepatobiliary elimination has been shown to differ widely between species (eg, rat, 63-80%; monkeys, 32-34%) (22). The preclinical safety evaluations did not demonstrate any potential for toxic effects on the liver or other tissues after the intravenous injection of Gd-EOB-DTPA (22, 25-26). The results of animal experiments have proven there was complete elimination of the agent, even in the cases where there was severe impairment of hepatobiliary or renal excretory function; the remaining elimination pathway may fully compensate for the virtual absence of the other pathway (23, 27). In a human study, Gd-EOB-DTPA was generally well tolerated with only mild side effects in 22% of the patients who received the contrast agent. No serious adverse effects or significant biochemical changes, including the liver enzymes, were found (28). Another advantage of this new contrast agent is that it can be administered as a bolus, and this makes it very suitable for use in dynamic MR imaging studies. During the first minutes after administration of the contrast agent, liver lesions should show enhancement in accordance with their vascularization and the extent of their extracellular space. Thus, Gd-EOB-DTPA shows promise for improving both the differential diagnosis of liver lesions and the detection of liver metastases in a single examination. Another interesting aspect of Gd-EOB-DTPA is its hepatobiliary excretion and the resultant contrast enhancement of the biliary system. A preclinical study reported that MR images obtained with use of Gd-EOB-DTPA allowed for monitoring the excretory function of hepatocytes (29). After intravenous injection, Gd-EOB-DTPA, which is bound to albumin due to its lipophilic nature, is gradually taken up by hepatocytes and eventually excreted by the biliary pathway. Further, Gd-EOB-DTPA does not change its chemical structure during circulation (22). Owing to these characteristics, Gd-EOB-DTPA could be used not only for hepatic imaging, but also for the measurement of hepatic function.
In this study, we used Gd-EOB-DTPA as a tracer, and the sequential liver MR signal change that corresponded to the tracer concentration at the last point was acquired by the dynamic MR technique. The sequential MR signal change of the aorta, which was assumed to be the tracer concentration at the initial point (f[t]), was also acquired by the dynamic MR technique. Theoretically, the tracer concentration at the initial point, f(t), should be measured at the feeding artery of the liver. However, when we considered the possibilities of the change MR signal regardless of tracer concentration such as angiographic effect, it was practical to measure and define the signal change of the aorta as the tracer concentration at the initial point. Therefore, after measuring f(t) and g(t), the moving function h(t) of liver could be estimated by deconvolution analysis. Since h(t) reflects the hepatocyte uptake of Gd-EOB-DTPA, the liver function can be quantitatively and noninvasively measured through a deconvolution analysis. In order to calculate the deconvolution, either the matrix method or the Fourier transform method can be used. Fourier transform is known to be more accurate, and it is the technique for transforming the data from the time domain into the frequency domain or vice versa. By applying Fourier transform, the convolution integral of equation becomes a simple multiplicity equation. However, the tracer concentration is practically measured during a finite time interval and then abruptly terminated. This abrupt termination of input data introduces a high frequency artifact into the Fourier transform. In this study, we introduced a modified Fourier transform technique. With the modified Fourier transform technique, both the aorta and liver curves are extended to many times their original length by appending a low-frequency, smoothly tapering curve to the original data series to compensate for the high-frequency artifact caused by the abrupt termination of data. Finally, the inverse Fourier transform of f(t) and g(t) was performed and the resultant deconvoluted curve representing the true liver response h(t), was obtained. An exponential curve of the best fit was applied to liver response h(t), and the y-intercept of this curve was used to represent the HEF.
The Gd-EOB-DTPA HEF values calculated by deconvolution analysis of the time-intensity curves obtained from dynamic MRI were lower in the rabbits after they had been treated with CCl4 than in those HEF values before administration of CCl4. The increase of liver enzyme levels had a relatively wide range (AST, 138.4±50.5 IU; ALT, 172.0±71.6 IU/L) and it showed low correlation with the changes of the ICG R15 (AST, correlation coefficient = -0.252, p = 0.568; ALT, correlation coefficient = 0.310, p = 0.499). These results are probably explained by the lack of specificity and directness of the liver enzyme quantification. Yet the HEFs obtained from Gd-EOB-DTPA MRI showed a relatively narrow range of abnormality and a high correlation with the changes of the ICG R15 (correlation coefficient = 0.965, p = 0.000). The histopathologic results and ICG R15 values were compatible with those values resulting from chronic liver disease.
In conclusion, deconvolution analysis with a liver-specific MR agent is a noninvasive technique for the quantitative evaluation of liver function, and this technique may also be valuable for detecting subtle changes in pathophysiology and for comparison of sequential studies. It could be a promising alternative for the noninvasive determination of hepatic function in those patients with liver disease.
References
- 1.Brensilver HL, Kaplan MM. Significance of elevated liver alkaline phosphatase in serum. Gastroenterology. 1975;68:1556–1562. [PubMed] [Google Scholar]
- 2.Cohen JA, Kaplan MM. The SGOT/SGPT ratio: an indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835–838. doi: 10.1007/BF01324898. [DOI] [PubMed] [Google Scholar]
- 3.Almersjo O, Bengmark S, Hafstrom LO, Olsson R. Enzyme and function changes after extensive liver resection in man. Ann Surg. 1969;169:111–119. doi: 10.1097/00000658-196901000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody FG, Rikker LF, Aldrete JS. Estimation of the functional reserve of human liver. Ann Surg. 1974;180:592–598. doi: 10.1097/00000658-197410000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson JM, Warren WD. A method of measuring quantitative hepatic function and hemodynamics in cirrhosis: the changes following distal splenorenal shunt. Jpn J Surg. 1986;16:157–168. doi: 10.1007/BF02471088. [DOI] [PubMed] [Google Scholar]
- 6.Hepner GW, Vesell ES. Quantitative assessment of hepatic function by breath analysis after oral administration of [14C] aminopyrine. Ann Intern Med. 1975;82:632–638. doi: 10.7326/0003-4819-83-5-632. [DOI] [PubMed] [Google Scholar]
- 7.Vesell ES. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979;26:275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]
- 8.Hunton DB, Bollman JL, Hoffman HN. Studies of hepatic functions with indocyanine green. Gastroenterology. 1960;39:713–723. [PubMed] [Google Scholar]
- 9.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M, Watanabe G. Simultaneous measurement of effective hepatic blood flow and systemic circulation. Hepatogastroenterology. 2000;47:1669–1674. [PubMed] [Google Scholar]
- 11.Berk PD, Potter BJ, Stremmel W. Role of plasma membrane ligand binding proteins in the hepatocellular uptake of albumin-bound organic anions. Hepatology. 1987;7:165–176. doi: 10.1002/hep.1840070131. [DOI] [PubMed] [Google Scholar]
- 12.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observation on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolkoff AW. The role of an albumin receptor in hepatic organic anion uptake: the controversy continues. Hepatology. 1987;7:777–779. doi: 10.1002/hep.1840070427. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Watanabe G. Hepatic parenchymal cell volume and the indocyanine green tolerance test. J Surg Res. 2000;92:222–227. doi: 10.1006/jsre.2000.5893. [DOI] [PubMed] [Google Scholar]
- 15.Murase K, Tsuda T, Mochizuki T, Ikezoe J. Hepatic excretion fraction of hepatobiliary radiopharmaceuticals measured using spectral analysis. Nucl Med Commun. 1999;12:1041–1045. doi: 10.1097/00006231-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Tolia V, Kottamasu SR, Tabassum D, Simpson P. The use of hepatocyte extraction fraction to evaluate neonatal cholestasis. Clin Nucl Med. 1999;24:655–659. doi: 10.1097/00003072-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Brensilver HL, Kaplan MM. Significance of elevated liver alkaline phosphatase in serum. Gastroenterology. 1975;68:1556–1562. [PubMed] [Google Scholar]
- 18.Proctor E, Chatamra K. Controlled induction of cirrhosis in the rat. Br J Exp Pathol. 1983;64:320–330. [PMC free article] [PubMed] [Google Scholar]
- 19.Bosma A, Brouwer A, Seifert WF, Knock DL. Synergism between ethanol and carbon tetrachlorid in the generation of liver fibrosis. J Pathol. 1988;156:15–21. doi: 10.1002/path.1711560106. [DOI] [PubMed] [Google Scholar]
- 20.McLean EK, McLean AE, Sutton PM. Instant cirrhosis: an improved method for producing cirrhosis of the liver in rats by simultaneous administration of carbon tetrachloride and phenobarbitone. Br J Exp Pathol. 1969;50:502–506. [PMC free article] [PubMed] [Google Scholar]
- 21.Brandao CG, Ferreira HH, Piovesana H, et al. Development of an experimental model of liver cirrhosis in rabbits. Clin Exp Pharmacol Physiol. 2000;27:987–990. doi: 10.1046/j.1440-1681.2000.03381.x. [DOI] [PubMed] [Google Scholar]
- 22.Schuhmann-Giampieri G, Mahler M, Roll G, Maibauer R, Schmitz S. Pharmacokinetics of the liver-specific contrast agent Gd-EOB-DTPA in relation to contrast-enhanced liver imaging in humans. J Clin Pharmacol. 1997;37:587–596. doi: 10.1002/j.1552-4604.1997.tb04340.x. [DOI] [PubMed] [Google Scholar]
- 23.Muhler A, Oude Elferink RPJ, Weinmann HJ. Complete elimination of the hepatobiliary MR contrast agent in hepatic dysfunction: an experimental study using transport-deficient, mutant rats. MAGMA. 1994;1:134–139. [Google Scholar]
- 24.Clement O, Muhler A, Vexler V, Berthezene Y, Brasch RC. Gadolinium-ethoxybenzly-DTPA: a new liver-specific magnetic resonance contrast agent. Kinetic and enhancement patterns in normal and cholestatic rats. Invest Radiol. 1992;27:612–619. [PubMed] [Google Scholar]
- 25.Muhler A, Clement O, Saeed M, et al. Gadolinium-ethoxybenzyl-DTPA, a new liver-directed magnetic resonance contrast agent. Absence of acute hepatotoxic, cardiovascular, or immunogenic effects. Invest Radiol. 1993;28:26–32. doi: 10.1097/00004424-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn Reson Med. 1991;22:233–237. doi: 10.1002/mrm.1910220214. [DOI] [PubMed] [Google Scholar]
- 27.Muhler A, Heinzelmann I, Weinmann HJ. Elimination of gadolinium-ethoxybenzyl-DTPA in a rat model of severely impaired liver and kidney excretory function. An experimental study in rats. Invest Radiol. 1994;29:213–216. doi: 10.1097/00004424-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Hamm B, Staks T, Muhler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 29.Muhler A, Freise CE, Kuwatsuru R, et al. Acute liver rejection: evaluation with cell-directed MR contrast agents in a rat transplantation model. Radiology. 1993;186:139–146. doi: 10.1148/radiology.186.1.8416555. [DOI] [PubMed] [Google Scholar]