Abstract
Objective
We wished to compare the in-vitro efficiency of wet radiofrequency (RF) ablation with the efficiency of dry RF ablation and RF ablation with preinjection of NaCl solutions using excised bovine liver.
Materials and Methods
Radiofrequency was applied to excised bovine livers in a monopolar mode for 10 minutes using a 200 W generator and a perfused-cooled electrode with or without injection or slow infusion of NaCl solutions. After placing the perfused-cooled electrode in the explanted liver, 50 ablation zones were created with five different regimens: group A; standard dry RF ablation, group B; RF ablation with 11 mL of 5% NaCl solution preinjection, group C; RF ablation with infusion of 11 mL of 5% NaCl solution at a rate of 1 mL/min, group D; RFA with 6 mL of 36% NaCl solution preinjection, group E; RF ablation with infusion of 6 mL of 36% NaCl solution at a rate of 0.5 mL/min. In groups C and E, infusion of the NaCl solutions was started 1 min before RF ablation and then maintained during RF ablation (wet RF ablation). During RF ablation, we measured the tissue temperature at 15 mm from the electrode. The dimensions of the ablation zones and changes in impedance, current and liver temperature during RF ablation were then compared between the groups.
Results
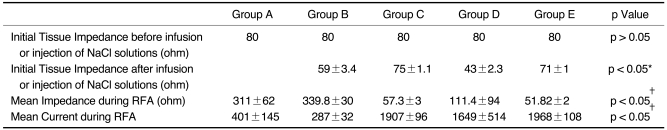
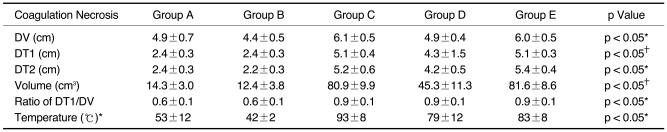
With injection or infusion of NaCl solutions, the mean initial tissue impedance prior to RF ablation was significantly less in groups B, C, D, and E (43-75 Ω) than for group A (80 Ω) (p < 0.05). During RF ablation, the tissue impedance was well controlled in groups C and E, but it was often rapidly increased to more than 200 Ω in groups A and B. In group D, the impedance was well controlled in six of ten trials but it was increased in four trials (40%) 7 min after starting RF ablation. As consequences, the mean current was higher for groups C, D, and E than for the other groups: 401 ± 145 mA in group A, 287 ± 32 mA in group B, 1907 ± 96 mA in group C, 1649 ± 514 mA in group D, and 1968 ± 108 mA in group E (p < 0.05). In addition, the volumes of RF-induced coagulation necrosis were greater in groups C and E than in group D, which was greater than in groups A and B than in group E (p < 0.05); 14.3 ± 3.0 cm3 in group A; 12.4 ± 3.8 cm3 in group B; 80.9 ± 9.9 cm3 in group C; 45.3 ± 11.3 cm3 in group D and 81.6 ± 8.6 cm3 in group E. The tissue temperature measured at 15 mm from the electrode was higher in groups C, D and E than other groups (p < 0.05): 53 ± 12℃ in group A, 42 ± 2℃ in group B, 93 ± 8℃ in group C; 79 ± 12℃ in group D and 83 ± 8℃ in group E.
Conclusion
Wet RF ablation with 5% or 36% NaCl solutions shows better efficiency in creating a large ablation zone than does dry RF ablation or RF ablation with preinjection of NaCl solutions.
Keywords: Experimental study, Interventional procedures, Liver, Radiofrequency ablation
Image-guided percutaneous radiofrequency (RF) ablation has become increasingly popular in recent years, and it has been accepted as an alternative to surgical resection for the treatment of primary and secondary hepatic malignancy in inoperable patients (1-4). However, current RF technology is limited in that only tissue 3.5- to 4.5 cm in diameter could be ablated with a single application of RF energy (5-9). This inherent limitation of monopolar RF ablation, in regards to the small dimension of coagulation necrosis, is attributed to resistive heating at a narrow rim of tissue that surrounds the electrode (1, 2, 10). There is always a rapid increase of temperature at the tissue-electrode interface, and this results in tissue desiccation and charring at the electrode tip. These effects prevent further RF energy conduction beyond the desiccated tissue and this halts further tissue coagulation.
There have been several reported strategies to solve this limitation of monopolar RF ablation including RF ablation with hypertonic saline (HS) preinjection (11-13), wet RF ablation (RF ablation with continuous infusion of HS through the electrode) (14-16), bipolar RF ablation (17, 18), and RF ablation using multiple probes (19-21). Among these approaches, we believe that hypertonic saline-mediated RF ablation, which includes RF ablation with HS preinjection and wet RF ablation, is seemingly a very attractive method because the injected saline could increase both the electrical and thermal conductivities, and the higher boiling points of the hypertonic saline could be helpful in avoiding the rapid boiling of liver tissue adjacent to the electrode (12, 20, 22, 23). Until now, however, there has been no well-designed comparative study between RF ablation with HS preinjection and wet RF ablation using HS infusion. In this context, we conducted a study to verify whether wet RF ablation has better efficiency in creating an ablation zone than does dry RF ablation or RF ablation with HS preinjection, and we did this by measuring the dimensions of the ablation zones in liver tissue, and by measuring the tissue temperature.
MATERIALS AND METHODS
RF Ablation Settings
Based on the previous studies that described consistent results for tissue ablation from ex vivo RF ablation experiments (16, 17, 24, 25), we chose to use excised beef liver purchased from a local butcher as the RF ablation target for our experiment. RF ablation was performed in 25 freshly excised bovine livers weighing, on average, 7.5 Kg each. The livers were cut into several 10 × 10 × 10-cm3 blocks that were dipped into a 50 × 20 × 20-cm3 saline-filled bath at room temperature.
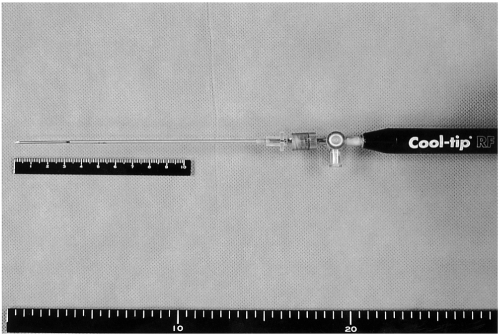
The RF ablation system we used was composed of a 15-gauge perfused-cooled electrode with 3 cm tip exposure, and a 480 kHz generator (CC-3, Radionics, Burlington, Mass) was used at 200 watts. As described in a previous study (24), we developed a perfused-cooled electrode to permit both intra-electrode cooling perfusion and interstitial saline infusion. We modified a 17-gauge cooled-tip electrode with a 3-cm active tip (Radionics) by covering it with a 15-gauge outer sheath, which was made of metal and electrically insulated, except for the 3.5 cm distal portion (Fig. 1).
Fig. 1.
Photograph of the perfused-cooled electrode which was used for wet RF ablation.
The 15-gauge perfused-cooled electrode was advanced at least 4 cm into the target liver tissue. To continuously measure the local tissue temperature during the procedure, a thermocouple was inserted at 15 mm from the electrode. Tissue impedance was monitored using the circuitry incorporated into the generator. A peristaltic pump (Watson-Marlow, Medford, Mass) was used to infuse 0℃ saline solution into the lumen of the electrodes at a rate sufficient to maintain the tip temperature at 20-25℃. The 5% or 36% NaCl solutions were infused through a perfused-cooled electrode using an infusion pump (Pilotec IS; Fresenius Medical Care, Alzenau, Germany). The applied current, power output, and impedance were continuously monitored during the RF ablation, and these parameters were recorded automatically using a computer program (Real Time Graphics Software V 2.0; Radionics).
Ablation Protocol
Radiofrequency was applied to the excised bovine livers in monopolar mode for 10 minutes using a 200 W generator (Radionics) and a perfused-cooled electrode. To compare the performance of the wet RF ablation with dry RF ablation and RF ablation with HS preinjection for creating coagulation necrosis, 50 ablation zones were created at five different regimens: group A; standard dry RF ablation, group B; RF ablation with 11 cc of 5% NaCl solution preinjected, group C; wet RF ablation with infusion of 11cc of 5% NaCl solution at a rate of 1 mL/min, group D; RF ablation with 6 cc of 36% NaCl solution preinjected, and group E; wet RF ablation with a infusion of 6 cc of 36% NaCl solution at a rate of 0.5 mL/min. In groups C and E, infusions of NaCl solutions were started before RF ablation (1 mL) and this was maintained during RF ablation at predetermined infusion rates (wet RF ablation). The initial impedance was set at 80 ohm with the electrode insertion by altering the distance between the electrodes and the dispersive metallic pad. Based on previous studies regarding saline-mediated RF ablation, 5% and 36% NaCl solutions were selected as the test solutions (12, 22, 26). Based on the results of the previous study regarding the optimial concentration and volume of NaCl for RF ablation (12), a volume of 6 mL saturated HS (36% NaCl) was used for comparison between the two RF ablation techniques. To create the same condition between the wet-RF ablation and the RF ablation with preinjection of NaCl solutions, in group E (wet RF ablation), 1 mL of 36% NaCl was infused prior to the RF ablation and 5 mL of the solution were injected during the RF ablation at a rate of 0.5 mL/min. Furthermore, based on other previous studies regarding wet RF ablation (17, 26), 5% NaCl at a rate of 1 mL/min was selected to be infused. To create the same condition between the two techniques, a volume of 11 mL was selected to be preinjected.
The technical aspects of the RF ablation including impedance and wattage changes, tissue temperature measured at 15 mm from the electrode, and the dimensions of the RF-coagulated area were compared for each condition.
Lesion Size Measurement
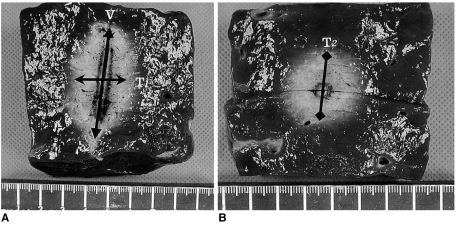
Liver blocks containing the RF ablation lesions were dissected along the longitudinal plane passing through the axes of the probe (the L-plane), and then we cut the blocks transversely and perpendicular to the L-plane (the T-plane). As the white central area of the RF induced ablation zone has been previously shown to correspond with the zone of coagulation necrosis (27, 28), two observers measured the vertical diameter (DV) along the probe, and the transverse diameter (DT1) perpendicular to the DV in the L-plane, and the second transverse diameter of the ablation zone (DT2) in the T-plane (Fig. 2). The volumes of ablation zones were evaluated by approximating the lesion to a sphere using: π(DV × DT1 × DT2)/6.
Fig. 2.
Measurement of the ablated area. Photographs of specimen created by dry RF ablation (group A) in the electrode insertion axis (A) and in the transverse axis perpendicular to the electrode shaft (B). Arrows indicate the three directional diameters of the RF-induced coagulation necrosis: V indicates the vertical diameter; T1, the transverse diameter on the plane along the electrode insertion axis; and T2, the transverse diameter on the plane perpendicular to the electrode shaft.
The shape of the RF-induced ablation zone was assessed by means of the DT1/DV ration.
Statistical Analysis
The dimensions of the thermal ablation area and the technical parameters of the five groups were averaged for each group, and these were compared using a one-way analysis of variance (ANOVA) test. For the comparison between the groups, Bonferroni multiple comparison test was used. Values were expressed as means ± standard deviation. To compare the tissue temperature at 15 mm from the electrode tips, repeated measures of ANOVA test were performed. For all the statistical analyses, a p value of less than 0.05 was considered significant. Statistics were performed using the Instat program (GraphPad Software, Inc., San Diego, Cal).
RESULTS
Electrical Measurements
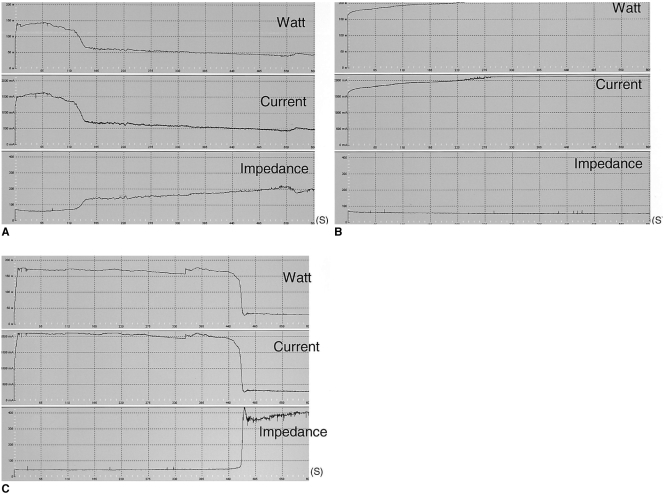
The mean initial tissue impedance prior to the RF ablation was significantly lower in the groups with injection or infusion of NaCl solutions (43-75 Ω) than group A (80 Ω) (Table 1) (p < 0.05). In addition, during the RF ablation, the tissue impedance was well controlled in groups C and E, but it was often rapidly increased to more than 200 Ω in groups A and B (Fig. 3). In group D, the impedance was well controlled in six of ten trials, but it was increased in four (40%) trials 7 min after starting the RF ablation. As a consequence, the mean current was higher for groups C, D, and E than for the other groups: 401 ± 145 mA in group A, 287 ± 32 mA in group B, 1907 ± 96 mA in group C, 1649 ± 514 mA in group D, and 1968 ± 108 mA in group E (p < 0.05).
Table 1.
Measured Values of Electrical Parameters Related to Radiofrequency Ablation for the Five Groups
Note.-RFA = radiofrequency ablation. Group A = dry RFA, group B = RFA with hypertonic saline preinjection (5%), group C = wet-RFA (5%), group D = RFA with hypertonic saline preinjection (36%), group E = wet-RFA (36%). *There were significant differences between dry RFA and other groups, †There were significant differences between groups C, D and E, and groups A and B.
Fig. 3.
Graphic depiction of the changes occurring in tissue impedance, current, and power during radiofrequency ablation. Note that tissue impedance increased markedly and the current decreased during radiofrequency energy instillation in the dry radiofrequency mode. The upper row indicates radiofrequency power (watt), the middle row indicates current (mA) and the lower row indicates impedance (ohm). The horizontal axis indicates ablation time (second).
A. Standard dry radiofrequency ablation (group A).
B. Wet radiofrequency ablation with 5% NaCl infusion (group C).
C. Radiofrequency ablation with 36% NaCl preinjection (group D).
Temperature
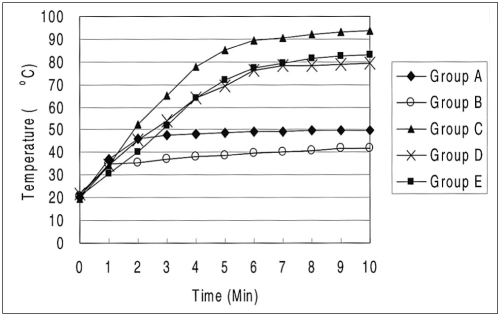
The graphs showing the mean temperature at 15 mm from the electrode tips are shown in Fig. 4. The mean final midpoint temperature values were 53 ± 12℃ in group A, 42 ± 2℃ in group B, 93 ± 8℃ in group C, 79 ± 12℃ in group D and 83 ± 8℃ in group E. The tissue temperatures were higher in groups C, D, and E than in groups A and B (p < 0.05).
Fig. 4.
Graphs of mean tissue temperatures at 15 mm from the electrode in each group. Note that a higher temperature was achieved in groups C, D, and E than in groups A and B.
Dimensions of the Zones of Ablation
After RF ablation, a well-defined area with a central white discoloration was observed in the ablated zone sections. The mean DVs of the RF induced central white zones on the L-plane measured in the gross specimens for each group were larger for groups C, D, and E than for groups A and B: 4.9 ± 0.7 cm in group A, 4.4 ± 0.5 cm in group B, 6.1 ± 0.5 cm in group C, 4.9 ± 0.4 cm in group D and 6.0 ± 0.5 cm in group E (p < 0.05) (Table 2). In addition, the DT1s on the L plane were as follows: 2.4 ± 0.3 cm in group A, 2.4 ± 0.3 in group B, 5.1 ± 0.4 in group C, 4.3 ± 1.5 cm in group D, and 5.1 ± 0.3 cm in group E. The DT1s of the RF-induced coagulation necrosis were greater in groups C and E than in group D, which was greater than in groups A and B (p < 0.05) (Fig. 5). In addition, the mean DT2 of the ablated spheres were also larger in groups C, D, and E than in groups A and B: 2.4 ± 0.3cm in group A; 2.2 ± 0.3 cm in group B, 5.2 ± 0.6 cm in group C, 4.2 ± 0.5 cm in group D and 5.4 ± 0.4 cm in group E, respectively (p < 0.05).
Table 2.
Measured Values of Diameters and Volumes of Radiofrequency-Induced Coagulation and Tissue Temperatures in the Five Groups
Note.-Group A = dry radiofrequency ablation (RFA), group B = RFA with hypertonic saline (HS) preinjection (5%), group C = wet-RFA (5%), group D = RFA with HS preinjection (36%), group E = wet-RFA (36%), DV = vertical diameter, DT1 = transverse diameter on the plane along the electrode, DT2 = transverse diameter on the transverse plane.
*There were significant differences between groups C, D, and E, and groups A and B. †The values in groups C and E were greater than in group D, which was greater than in groups A and B.
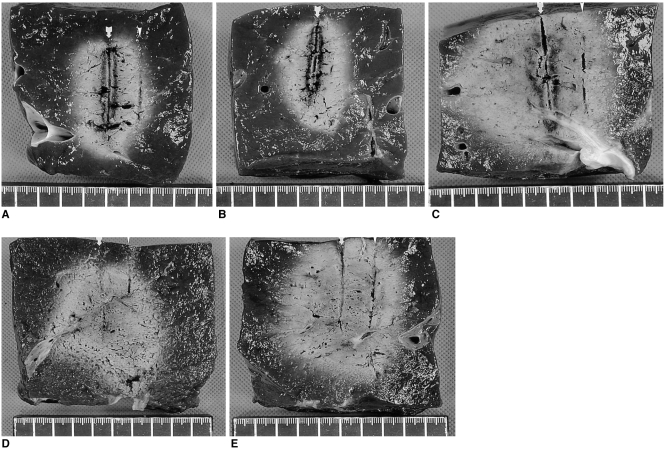
Fig. 5.
Comparison of radiofrequency-induced ablation areas in the five groups. Arrow indicates the electrode insertion site and arrowhead indicates the thermocouple insertion site.
A. Photograph of specimen from group A (standard dry RF ablation).
B. Photograph of specimen from group B (RF ablation with 11 mL of 5% NaCl solution preinjection).
C. Photograph of specimen from group C (wet RF ablation with infusion of 11 mL of 5% NaCl solution).
D. Photograph of specimen from group D (RF ablation with 6 mL of 36% NaCl solution preinjection).
E. Photograph of specimen from group E (wet RF ablation with infusion of 6 mL of 36% NaCl solution).
The volumes of RF-induced coagulation necrosis were greater in groups C and E than in group D, which was greater than in groups A and B (p < 0.05): 14.3 ± 3.0 cm3 in group A, 12.4 ± 3.8 cm3 in group B, 80.9 ± 9.9 cm3 in group C, 45.3 ± 11.3 cm3 in group D, and 81.6 ± 8.6 cm3 in group E. Furthermore, the ratio of DT1/DV were 0.6 ± 0.1 in group A, 0.6 ± 0.1 in group B, 0.9 ± 0.1 in group C, 0.9 ± 0.1 in group D and 0.9 ± 0.1 in group E. The ratio of DT1/DV in groups A and B were significantly less than those of groups C, D and E (p < 0.05).This means that the groups C, D, and E tended to produce a more spherically-shaped coagulation than the other groups (groups A and B).
DISCUSSION
The limitation of the current monopolar RF ablation system is related to the fact that the current density drops off at rate proportional to the inverse square of the distance from the electrode. Therefore, heating preferentially takes places immediately adjacent to the electrode, leading to rapid charring and a decrease of current delivery (1, 2, 20). One of the most effective approaches to overcome this problem is to infuse NaCl solution into target tissue, and this increases both the electrical conductance and thermal conductivity (12, 22, 23). Two ways have been reported for using NaCl solution to increase the efficacy of monopolar RF ablation for creating coagulation necrosis: one is bolus injection of NaCl solution prior to RF ablation and the other way is a continuous infusion of NaCl solution prior to and during the RF ablation. Although several studies have demonstrated that both methods were useful to enlarge the ablation area as compared to the standard dry RF ablation (11-16, 22, 23, 29-31), it would be useful to determine which technique can efficiently induce a larger ablation area.
Our results show that the wet RF ablation with 5% or 36% NaCl solutions or RF ablation with preinjection of 36% NaCl solution created larger ablation zones than did the dry RF ablation (p < 0.05): 14.3 ± 3.0 cm3 in group A, 80.9 ± 9.9 cm3 in group C, 45.3 ± 11.3 cm3 in group D and 81.6 ± 8.6 cm3 in group E. These differences are probably related to the delivery of a higher current throughout the procedure, and this was attributed to the constant low impedance below 100 Ω. Regardless of the delivery methods of the NaCl solutions to the target tissue, the NaCl solutions increase the electrical conductivity and increase the current delivery from the electrode to the tissue (11, 12) (Table 1).
More importantly, the wet RF ablation with 5% or 36% NaCl solutions created larger ablation zones than did RF ablation with a preinjection of 5% or 36% NaCl solutions. The better performance of wet-RFA compared to RF ablation with a preinjection of hypertonic saline could be attributed to several factors. First, in wet RF ablation using the perfused-cooled electrode, some of the infused saline could diffuse out from the electrode, but the diffusing saline is continuously replaced by a slow infusion (25). However, in RF ablation with a preinjection of NaCl solutions, the injected solution could rapidly diffuse out depending upon the tissue pressure. Furthermore, for the in vivo situation, infused saline could be washed-out by perfusion. The less proportion of injected saline that remains in situ, the less enhancement of RF ablation efficacy is expected. Therefore, theoretically at least, wet RF ablation may have the effect to enhance RF ablation efficacy by increasing electrical and thermal conductance in a more stable and predictable way than the pre-RF ablation injection of NaCl solution. Second, for the wet RF ablation, the infused liquid continuously fills the gap between the metal electrode and the adjacent tissue, and this pushes the electrically insulating gases away from the electrode (30). This phenomenon can probably not be expected to happen for RF ablation with a preinjection of NaCl solution. Third, the infused liquid allows for some convective cooling at the tip of the electrode.
When considering the concentration of the injected NaCl solutions, RF ablation with a preinjection of 36% NaCl solution showed a better performance for creating a larger ablation necrosis zone than RF ablation that was done with a preinjection of 5% NaCl solution. This result could be attributed to the following factors. First, the higher ion concentration resulting from 36% NaCl solution could increase the electrical conductance of the tissue, and therefore, this may decrease too much concentration of the electrical energy at tissue adjacent to the electrode and prevent boiling of the tissue. Second, because 36% NaCl has a higher boiling point than 5% NaCl, the charring of the tissue contains 36% NaCl solution could be prevented. Furthermore, we found that RF ablation with a preinjection of 5% NaCl produced a rather smaller ablation zone than the dry RF ablation, although the difference was not statistically significant (p > 0.05). This finding could be attributed to the increased production of electrically insulating gases compared to dry RF ablation; these gases fill the gap between the metal electrode and the tissue and increase the value of the impedance. Although the electrical conductance of the tissue can be increased with an injection of 5% NaCl solution, the 5% saline solution may not be ionic enough to prevent too much concentration of the electrical energy in the tissues adjacent to the electrode.
However, for the wet RF ablation, both groups C and E did not show any difference in the volume of the coagulation necrosis. This suggests that the increased efficacy of wet RF ablation in creating coagulation necrosis is possibly due to both increased electrical conductance and increased hydration. Although the infusion of larger amounts of a lower concentration of NaCl induces less change in electrical conductance than the infusion of a smaller amount of higher concentration NaCl, it may induce a larger hydration effect than does the latter solution. Therefore, infusion of 5% NaCl solution at 1 mL/sec could allow for more gap filling between the metal electrode and the tissue and also it could create some convective cooling at the tip more than the infusion of 36% NaCl solution at 0.5 mL/sec. Furthermore, it could induce better thermal conductivity of the tissue than does an infusion of 36% NaCl solution at 0.5 mL/sec. Further study regarding the optimal concentration and volume of NaCl solution for wet RF ablation will be warranted to provide both the effects of increasing electrical conductance and hydration during RFA.
Although we did not test 36% NaCl at high infusion rates, we believe that decreasing tissue impedance too much would not be helpful to increase RF-induced coagulation necrosis because too large a decease of tissue impedance induces a remarkable increase of current needed to create the heat (23). Furthermore, Ahmed et al. (32) have well demonstrated that the use of RF energy for tumors surrounded by poorly conductive tissues can result in increased heating at the margins of the ablation zone, and these researchers demonstrated this in a previous study regarding the effect of surrounding tissue composition on coagulation necrosis during RF ablation. Therefore, although an increasingly negative linear correlation was observed between the tumor coagulation diameter and the overall baseline system impedance, too great a decrease for the impedance of the surrounding tissue, as well as a decrease of tumoral impedance by the injected HS, could potentially hinder complete tumor destruction.
In our study, wet RF ablation and RF ablation with preinjection of NaCl solutions showed better performance than did the standard dry RF ablation. However, these two methods have a considerable risk of unexpected burn injury to the adjacent vital structures by the boiling saline, as several previous studies have demonstrated (11, 13, 33). Theoretically, the risk of burn injury related to the leaking of hot saline along the electrode and uneven spread of conductive saline along the paths of least resistance may be increased when using too high a volume or too fast an infusion rate. Therefore, further studies concerning the optimal of amount and concentration of the saline are warranted. Furthermore, there is one theoretical concern about wet RF ablation, i.e., saline, contaminated with viable tumor cells, may leak out of the electrode track and causes peritoneal or track seeding (33-35). Another equally unproven concern is that the saline may increase the intratumoral pressure and force tumor cells into the circulation, causing hematogenous seeding (34).
Our experimental study has certain limitations. First, all the ablations involved normal liver parenchyma, not tumor tissue, and they were performed in vitro. Living tumor tissue has a cooling "sink" effect due to the blood flow (36); thus, rapid heat exchange can occur. Therefore, the extent of extrapolation of the results of this experimental study to the real clinical situation is not certain. However, despite these considerations, our work provides a reliable model for comparative study on the efficiency of different RF systems. Second, following a procedure developed in earlier studies, we tested only 5% and 36% NaCl solutions. We believe that further experimental study aimed at optimizing the concentration and the amount of hypertonic saline solution is warranted.
In conclusion, wet RF ablation with infusion of 5% or 36% NaCl solution produced significantly larger ablation zones than the standard dry RF ablation or RF ablation with a preinjection of NaCl solutions. This higher level of coagulation necrosis produced by the wet RF ablation may be due to the combined effect of a greater delivery of energy, which is caused by good control of tissue impedance, and the better heat conductance. The increase of coagulation necrosis by wet RF ablation will likely be of benefit for clinical applications of RF tumor ablation therapy, and especially for a large size tumor.
Footnotes
This study was supported by grant No. 09-2003-012-0 from the Seoul National University Hospital Research Fund.
References
- 1.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 2.McGhana JP, Dodd GD., 3rd Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 3.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 4.Dodd GD, 3rd, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. RadioGraphics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 6.Denys AL, De Baere T, Kuoch V, et al. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003;13:2346–2352. doi: 10.1007/s00330-003-1970-0. [DOI] [PubMed] [Google Scholar]
- 7.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 8.Choi D, Lim HK, Kim MJ, et al. Overlapping ablation using a coaxial radiofrequency electrode and multiple cannulae system: experimental study in ex-vivo bovine liver. Korean J Radiol. 2003;4:117–123. doi: 10.3348/kjr.2003.4.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS. Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in-vivo study in a rabbit liver model. Korean J Radiol. 2003;4:27–34. doi: 10.3348/kjr.2003.4.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg SN, Ahmed M, Gazelle GS, et al. Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology. 2001;219:157–165. doi: 10.1148/radiology.219.1.r01ap27157. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Lee JM, Kim SW, Kim CS. Combined radiofrequency ablation and hot saline injection in rabbit liver. Invest Radiol. 2003;38:725–732. doi: 10.1097/01.rli.0000084360.10254.41. [DOI] [PubMed] [Google Scholar]
- 14.Hansler J, Witte A, Strobel D, et al. Radio-Frequency-Ablation (RFA) with wet electrodes in the treatment of primary and secondary liver tumours. Ultraschall Med. 2003;24:27–33. doi: 10.1055/s-2003-37413. [DOI] [PubMed] [Google Scholar]
- 15.Kettenbach J, Kostler W, Rucklinger E, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003;180:1537–1545. doi: 10.2214/ajr.180.6.1801537. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt D, Trubenbach J, Brieger J, et al. Automated saline-enhanced radiofrequency thermal ablation: initial results in ex vivo bovine livers. AJR Am J Roentgenol. 2003;180:163–165. doi: 10.2214/ajr.180.1.1800163. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Han JK, Kim SH, et al. A comparative experimental study of the in-vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol. 2003;4:163–169. doi: 10.3348/kjr.2003.4.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdio F, Guemes A, Burdio JM, et al. Large hepatic ablation with bipolar saline-enhanced radiofrequency: an experimental study in in vivo porcine liver with a novel approach. J Surg Res. 2003;110:193–201. doi: 10.1016/s0022-4804(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee FT, Jr, Haemmerich D, Wright AS, Mahvi DM, Sampson LA, Webster JG. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–1442. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Rhim H, Han JK, Youn BJ, Kim SH, Choi BI. Dual-probe radiofrequency ablation: an in vitro experimental study in bovine liver. Invest Radiol. 2004;39:89–96. doi: 10.1097/01.rli.0000105041.12347.4b. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed M, Lobo SM, Weinstein J, et al. Improved coagulation with saline solution pretreatment during radiofrequency tumor ablation in a canine model. J Vasc Interv Radiol. 2002;13:717–724. doi: 10.1016/s1051-0443(07)61850-8. [DOI] [PubMed] [Google Scholar]
- 23.Lobo SM, Afzal KS, Ahmed M, Kruskal JB, Lenkinski RE, Goldberg SN. Radiofrequency ablation: modeling the enhanced temperature response to adjuvant NaCl pretreatment. Radiology. 2004;230:175–182. doi: 10.1148/radiol.2301021512. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Han JK, Kim SH, et al. Bipolar radiofrequency ablation using wet-cooled electrodes: an in-vitro experimental study in bovine liver. AJR Am J Roentgenol. doi: 10.2214/ajr.184.2.01840391. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Miao Y, Ni Y, Yu J, Marchal G. A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol. 2000;35:438–444. doi: 10.1097/00004424-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Miao Y, Ni Y, Mulier S, et al. Treatment of VX2 liver tumor in rabbits with "wet" electrode mediated radio-frequency ablation. Eur Radiol. 2000;10:188–194. doi: 10.1007/s003300050031. [DOI] [PubMed] [Google Scholar]
- 27.Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151–158. doi: 10.3348/kjr.2001.2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morimoto M, Sugimori K, Shirato K, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;35:1467–1475. doi: 10.1053/jhep.2002.33635. [DOI] [PubMed] [Google Scholar]
- 29.Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel "cooled-wet" electrode for radiofrequency ablation. Eur Radiol. 2000;10:852–854. doi: 10.1007/s003300051018. [DOI] [PubMed] [Google Scholar]
- 30.Miao Y, Ni Y, Yu J, Zhang H, Baert A, Marchal G. An ex vivo study on radiofrequency tissue ablation: increased lesion size by using an "expandable-wet" electrode. Eur Radiol. 2001;11:1841–1847. doi: 10.1007/s003300100891. [DOI] [PubMed] [Google Scholar]
- 31.Livraghi T, Goldberg SN, Monti F, et al. Saline enhanced radio frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–210. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed M, Liu Z, Afzal KS, et al. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230:761–767. doi: 10.1148/radiol.2303021801. [DOI] [PubMed] [Google Scholar]
- 33.Boehm T, Malich A, Goldberg SN, et al. Radio frequency tumor ablation: Internally cooled electrode versus saline enhanced technique in an aggressive rabbit tumor model. Radiology. 2002;222:805–813. doi: 10.1148/radiol.2223010573. [DOI] [PubMed] [Google Scholar]
- 34.Miao Y, Ni Y, Mulier S, et al. Ex vivo experiment on radiofrequency liver ablation with saline infusion through a screw-tip cannulated electrode. J Surg Res. 1997;71:19–24. doi: 10.1006/jsre.1997.5133. [DOI] [PubMed] [Google Scholar]
- 35.Munver R, Threatt CB, Delvecchio FC, Preminger GM, Polascik TJ. Hypertonic saline-augmented radiofrequency ablation of the VX2 tumor implanted in rabbit kidney: a short-term survival pilot study. Urology. 2002;60:170–175. doi: 10.1016/s0090-4295(02)01667-9. [DOI] [PubMed] [Google Scholar]
- 36.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]