Abstract
This study evaluated the value of procalcitonin (PCT) levels in pleural effusion to differentiate the etiology of parapneumonic effusion (PPE). Forty-one consecutive PPE patients were enrolled and were divided into bacterial and non-bacterial PPE. Blood and pleural effusion samples were collected for PCT measurement on admission and analyzed for diagnostic evaluation. PCT of pleural fluid was significantly increased in the bacterial PPE group (0.24 ng/mL) compared to the non-bacterial PPE group (0.09 ng/mL), but there was no significant difference for serum PCT. A PCT concentration of pleural fluid >0.174 ng/mL (best cut-off value) was considered positive for a diagnosis of bacterial PPE (sensitivity, 80%; specificity, 76%; AUC, 0.84). Pleural effusion PCT in the bacterial PPE is significantly different from those of the non-bacterial PPE and control groups, so the diagnostic use of PCT still warrants further investigation.
Keywords: Procalcitonin, Pleural Effusion, Pneumonia
INTRODUCTION
Pleural effusion is defined as the accumulation of fluid in the pleural space that exceeds the physiologic amount of 10-20 mL. Heart failure is the main cause of pleural effusion (30-40%). In the non-cardiac effusion, pneumonia is the most common (48%) (1), of which approximately 75% are bacterial. Determining the etiology is greatly facilitated by the ability to analyze the pleural fluid via thoracentesis, a simple bedside procedure. The most practical method for differentiating diagnosis is Light criteria, which separated transudates from exudates by analyzing protein and lactate dehydrogenase (LDH) levels of serum and pleural fluid (1).
However, only a select number of diagnoses can be established definitively by thoracentesis and results of quantitative cultures are not available until 24-72 hr after the procedure. Moreover, bacteriological evidence of infection may not develop simultaneously as clinical signs of sepsis and negative results do not exclude sepsis because of poor sensitivity by culture. Delays in the administration of adequate antimicrobial treatment increase the risk of mortality (2). On the other hand, unnecessary use of antibiotics increases drug resistance, side effects, and expense. Since common clinical and laboratory measurements lack the sensitivity and specificity, other tests are needed to identify the infectious cause of a generalized inflammatory response and to allow early diagnosis and appropriate treatment.
Among the potentially available laboratory parameters, procalcitonin (PCT) has recently become of interest as a possible marker of the systemic inflammatory response to infection (3). It is normally produced in the C-cells of the thyroid gland and is the precursor of calcitonin. A specific protease cleaves PCT to calcitonin, Katacalcin, and an N-terminal residue. Normally, PCT is cleaved and none is released into the blood stream such that levels are undetectable (<0.1 ng/mL) in healthy humans. In severe bacterial infections and sepsis, however, intact PCT is found in blood. PCT levels increase in response to a stimulus within 2-6 hr. A half-life of approximately 20-24 hr is expected for PCT after a single, acute stimulus. Unlike most cytokines, PCT is highly stable in collected blood samples. Thus, it has been described as a good predictor of disease severity and antibiotic efficiency (4).
PCT measurements may be helpful in differentiating between infectious or non-infectious causes in patients presenting with a systemic inflammatory response syndrome, and may differentiate between viral and bacterial infections. Abrupt increases or high PCT values in these patients warrant a search for infection. Numerous clinical studies have also proposed PCT as a specific marker of bacterial infection or general inflammation (5-7). However, localized bacterial infections without systemic manifestations, like bacterial parapneumonic effusion (PPE) without sepsis, may cause only a small to modest increase (0.3-1.5 ng/mL). Therefore, treatment, such as antibiotics, may be necessary despite normal PCT levels (8). If PCT was measured in body fluids other than serum, conflicting results have been reported. PCT values measured in urine fluctuate over a wide range. On average, approximately 25% of the plasma concentrations can be detected in the urine (9). But alveolar PCT does not seem to be a helpful parameter in the early diagnosis of ventilator-associated pneumonia and is not an appropriate marker for predicting mortality (10). This study aimed to determine the usefulness and reliability by PCT level of pleural fluid in the diagnosis of bacterial PPE.
MATERIALS AND METHODS
Subjects
Forty-one consecutive patients with PPE from the Division of Pulmonary/Critical Care Medicine and Infectious Disease of the Department of Internal Medicine at the St. Martin De Porres Hospital in Chiayi from August to December 2006 were included and PPE was diagnosed if they had a score >6 as assessed by clinical pneumonia infection score (CPIS) at baseline or 72 hr (11), and if ultrasound examination showed pleural effusion. All had undergone diagnostic thoracentesis and venipuncture, with measurement of PCT in the pleural fluid and serum, after obtaining ethical research approval from the hospital's Institutional Review Board and informed consent from the patients. Specifically, PPE was categorized as definite bacterial infection if bacterial colonies were identified by blood culture, effusion culture, or quantitative cultures from bronchoscopic broncho-alveolar lavage fluid (BAL). The criteria for the diagnosis of non-bacterial PPE were the negative or non-significant growth in culture of the three kinds of specimens.
Blood and pleural effusion samples were also collected from 9 routine, non-pneumonic effusion patients who acted as the control group. The control group (non-pneumonic) was defined with CPIS ≤6 at baseline and 72 hr, and diagnosed as another disease accounting for the chest radiograph abnormality of pleural effusion. All had either congestive heart failure (n=8) or chronic renal failure (n=1) but not pneumonia.
None of the 50 patients had received systemic or topical antimicrobial therapy 3 days before the study, none had smoked at least one cigarette per day for 1 yr, and none had a diagnosed thyroid disease or small cell lung cancer.
Measurements
Ultrasound-guided thoracentesis was performed to obtain pleural fluid. Blood samples were obtained by venipuncture and specimens were collected in tubes containing ethylenediamine tetraacetic acid (EDTA), centrifuged at 3,000 rpm for 10 min at 4℃, and the supernatants were stored at -20℃ for later determination of PCT if the patient was enrolled after 72 hr. The same technologist running the all PCT assay was blinded of the clinical information. The quantitative determination of PCT was performed with LUMI test PCT, an immunoluminometric assay manufactured by B.R.A.H.M.S Diagnostica GmbH, Hennigsdorf/Berlin (12).
Samplings for blood culture were tested in BD BACTEC lytic/10 anaerobic and standard/10 aerobic. BAL fluid was obtained from fiberoptic bronchoscope Olympus with 200 mL warm sterile saline administrated to the involved lobe. Samples were then plated onto dishes: Trypticase soy agar 5% SB, chocolate II agar, eosin methylene blue agar, and Columbia colistin-nalidoxic acid agar. Bacterial colonies were counted and identified using conventional techniques.
Statistical analysis
Statistical analysis was carried out using SPSS version 10.0 for Windows software. Demographic data was expressed as median with 95% confidence interval. Sensitivity and specificity were expressed in percentages. After confirming normally distributed data, demographic data was compared using the independent t test or the chi-square test when appropriate. The non-parametric Kruskal-Wallis tests or the Bonferroni criteria of Mann-Whitney U tests for continuous variables were used to compare PCT level among groups. The difference was statistical significant if P was <0.05. The receiver-operating characteristic (ROC) curves were analyzed to define the optimal cut-off value and used to compare diagnostic accuracy. Sensitivity, specificity, and area under the ROC curve (AUC) were estimated using a standard formula.
RESULTS
The study population was composed of 50 patients (35 males and 15 females) with a median age of 67 yr. Of these, 25 had bacterial PPE, 16 had non-bacterial PPE, and 9 made up the control group.
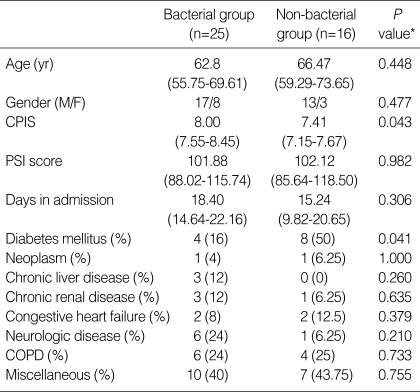
Table 1 showed demographic and clinical data of the PPE patients divided into two subgroups and showed the underlying medical disease. A significant difference occurred for the variables of CPIS and diabetes mellitus.
Table 1.
Demographic and clinical data of the study patients
Results are expressed as mean (95% confidence interval).
*Independent t test and chi-square test for quantitative and categorical variables, respectively.
CPIS, clinical pulmonary infection score; PSI, pneumonia severity index; COPD, chronic obstructive pulmonary disease.
The diagnosis of bacterial PPE was established in 25 cases: 8 patients had gram-negative bacilli, 13 had Gram-positive cocci, and 4 had polymicrobial growth. Overall, pleural fluid culture findings were positive in 9 of 25 patients, bronchoscopic BAL quantitative culture was positive in 16 patients, and 4 had positive blood cultures.
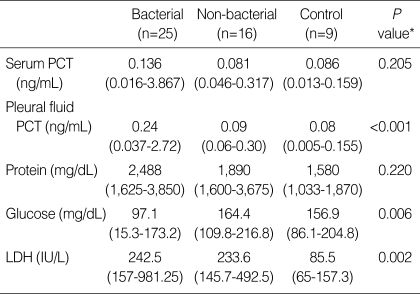
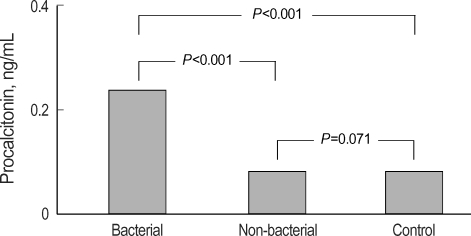
The median PCT levels in pleural fluid were significantly higher in the bacterial PPE (0.24 ng/mL) than in the non-bacterial PPE (0.09 ng/mL) and the control groups (0.08 ng/mL, P<0.001), but no statistical difference was found in serum concentrations among the three groups (P=0.205, Table 2). Regarding non-bacterial PPE diagnosis, no significant differences occurred in the non-bacterial PPE group compared to the control group for pleural PCT (Fig. 1). Others, LDH and glucose levels of pleural fluid also demonstrated significant differences among the three groups, but pleural protein level did not (Table 2).
Table 2.
Serum and pleural procalcitonin levels on hospital admission for the three groups of patients
Results are expressed as median (25th-75th percentiles).
*Kruskal Wallis test of non-parametric test.
PCT, procalcitonin; LDH, lactate dehydrogenase.
Fig. 1.
Comparison of pleural concentrations of procalcitonin among the bacterial parapneumonic effusion (PPE), non-bacterial PPE, and control groups (Bonferroni criteria of Mann-Whitney U test).
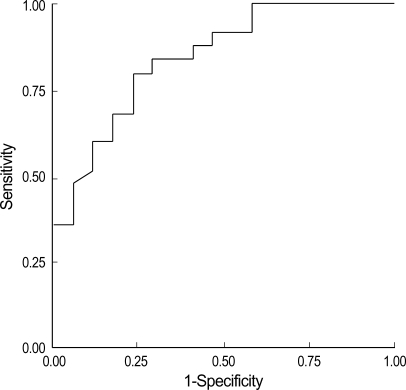
The cut-off value of pleural fluid PCT for diagnosis of bacterial PPE (from the ROC curve; AUC=0.84) was 0.17 ng/mL (Fig. 2). This corresponded to 80% sensitivity and 76% specificity, in contrast to 72% sensitivity and 94% specificity with a well-known serum cut-off value (0.5 ng/mL) for bacterial infection (Table 3) (13).
Fig. 2.
Receiver-operating characteristic (ROC) curve analysis of pleural concentrations of procalcitonin and diagnosis of bacterial parapneumonic effusion.
Table 3.
Measurements of diagnostic accuracy of identifying tests for bacterial parapneumonic effusion
AUC, area under receiver-operation characteristic curves; PCT, procalcitonin.
DISCUSSION
PPE occurs in approximately 10% of patients with community-acquired pneumonia (14) and most bacterial PPE will resolve with antibiotic treatment alone. Thus, identification of the possible etiology represents an initial challenge to clinicians in facilitating PPE. Limitations and inaccuracies in clinical decision-making have motivated the development of techniques to establish the diagnosis of bacterial PPE and guide treatment decisions to significantly reduced antibiotic administration or avoid neglecting bacterial infections. Gram stain and cultures of tracheal secretions have been commonly used but are non-specific and lack the sensitivity for establishing the presence of bacterium partly because of oro-pharyngeal colonization and difficulties in obtaining definite pathogens from an inadequately expectorated specimen. Several methods have been developed in an attempt to improve their diagnostic accuracy, including C-reactive protein (CRP) and bacterial antibody assays. CRP levels are usually lower in viral and superficial bacterial infections than in deep bacterial infections (15) but their role in the etiologic diagnosis of respiratory infections is not well established and their usefulness in distinguishing between bacterial and viral pneumonia have conflicting results (16). Clinical analysis of antibody-coated bacteria is not sensitive enough for the exclusion of bacterial pneumonia. Also its routine use is limited due to the cost and unavailability in clinical laboratory (17). Among these chemical parameters in our study, LDH and glucose in pleural fluid showed significant differences between the three groups, but pleural protein level did not. We believed that the difference of glucose level came from patients with empyema in bacterial PPE group. For the LDH level, the transudative nature of the control group might contribute the statistical difference by Light's criteria for pleural effusion. However, to our knowledge, these parameters could not be a guideline for diagnosis or treatment at present, when they were used in a single.
PCT, a circulating serum calcitonin precursor, is a 116 amino-acid protein stimulated by bacterial inflammation and produced by neuro-endocrine cells of different organs, including the lungs (18). Thus, patients with small cell lung cancer and thyroid disease were excluded for the neurocrine nature in our study. To our knowledge, elevated serum levels of PCT have been found in several diseases such as sepsis syndrome, inhalation burn injury, and infectious pneumonitis (19). It is important that PCT is elevated in bacterial infections but remain low in viral infections (20). It also deserved our attention because that the measurement of PCT with LUMI test is easy and can be completely within two hours. We could absolutely get the information of bacterial infection much more quickly as compared with identifying bacteria by culture. Furthermore, its serum concentration is more sensitive and specific than CRP, interleukin-6 (IL-6) or white blood cell count for differentiating bacterial and viral causes of community-acquired pneumonia (CAP) (12) and is utilized to guide treatment decisions to significantly reduce antibiotic use and costs without adverse effects on outcomes (21). However, localized bacterial infections or infections without systemic manifestations cause only a small to modest increases in PCT levels. Treatment, such as antibiotics, may therefore be necessary in patients with pneumonia despite normal PCT levels. In previous studies, since pneumonia is generally an organ related infection, PCT values are usually low even in the presence of marked clinical symptoms or radiographic findings (22).
In the demographic data, a significant difference occurred in the variables of CPIS and diabetes mellitus between the bacterial and non-bacterial PPE group. CPIS was used to diagnose pneumonia and was not related to PCT levels. Our findings warrant further studies that will take into consideration the association of diabetes mellitus and PCT levels.
PCT has been measured in body fluids other than serum in only few papers and with conflicting results (9). In 2005, Cakir et al. (23) reported that serum and pleural fluid PCT concentrations were statistically different between tuberculous and nontuberculous pleurisy groups, even though they were not elevated up to the cut-off level of 0.5 ng/mL. This study was to verify whether PCT levels measured in pleural effusion could be a marker of bacterial PPE and present with a more sensitive or specific parameter than serum levels to identify non-septic bacterial infection and prevent the unnecessary use of broad-spectrum therapy. Based on the results here, PCT measurement in pleural effusion represents a potential adjunct to the diagnosis of bacterial PPE despite the lack of statistical difference among the three groups in terms of serum levels. To our knowledge, BAL fluid PCT concentrations remained undetectable in previous studies due to the hypothesis that the alveolar membrane could be a strong filter to circulating cytokines (24). The low alveolar PCT release could be the same with some local mediators, like tumor necrosis factor α(25). Otherwise, PCT has been proposed as an indicator of severe generalized infections or sepsis, while localized infections or infections without systemic manifestations cause only a limited increase (26). PCT induction is consequently generally slight in isolated pneumonia due to the organ-related nature of the infection. In our study, the severity of PPE was not taken into consideration and pneumonic severity index score demonstrated a non-significant difference between the bacterial and non-bacterial PPE groups. Thus, we could regard the PPE as a localized infection and our cases might not be so serious to cause sepsis. Then we consequently found that the serum PCT levels in our study were almost lower than the current cutoff value of 0.5 ng/mL and it was not significantly increased in the bacterial PPE group as compared with the non-bacterial or control group. At the time, we believed that pleural fluid might play a role for diagnosis in such the localized infection, like PPE.
This study has limitations. First, the gold standard for evaluating the diagnosis of pneumonia remains the open lung biopsy instead of the CPIS score, although the appropriateness of studying a diagnostic test only among dead patients is debatable. Second, a lower threshold value (0.01 ng/mL) of the quantitative determination by homogeneous immunoassay may be inappropriate for detecting low, meaningful concentrations. Lastly, some bacterial pneumonia, such as Chlamydia, Mycoplasma pathogens, or Legionnaires' disease, could not be cultured easily in traditional media. On the other hand, some culture-positive PPE patients possibly came from contaminations.
In conclusion, this study shows that the pleural fluid PCT levels in bacterial PPE are significantly different from those of the non-bacterial and control groups. However, both the serum and pleural fluid PCT levels in patients with bacterial PPE are below the current cut-off level of 0.5 ng/mL. In a patient with a pleural fluid PCT concentration of <0.17 ng/mL, other confusing reasons of bacterial PPE can be ruled out with a 76% probability. If pleural fluid PCT >0.17 ng/mL, bacterial PPE can be diagnosed with an 80% probability.
References
- 1.Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med. 1980;69:507–512. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 2.Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27:355–362. doi: 10.1007/s001340000640. [DOI] [PubMed] [Google Scholar]
- 3.Ugarte H, Silva E, Mercan D, De Mendonca A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Wanner GA, Keel M, Steckholzer U, Beier W, Stocker R, Ertel W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med. 2000;28:950–957. doi: 10.1097/00003246-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, Ravilly S, Lefèvre H, Royer C, Lacombe C, Palmer P, Bohuon C. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Snider RH, Jr, Nylen ES, Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560. [PubMed] [Google Scholar]
- 7.Oberhoffer M, Bögel D, Meier-Hellmann A, Vogelsang H, Reinhart K. Procalcitonin in higher in non-survivors during the clinical course of sepsis, severe sepsis and septic shock. Intensive Care Med (Abstract) 1996;22:A245. [Google Scholar]
- 8.Giamarellos-Bourboulis EJ, Grecka P, Poulakou G, Anargyrou K, Katsilambros N, Giamarellou H. Assessment of procalcitonin as a diagnostic marker of underlying infection in patients with febrile neutropenia. Clin Infect Dis. 2001;32:1718–1725. doi: 10.1086/320744. [DOI] [PubMed] [Google Scholar]
- 9.Meisner M, Lohs T, Huttemann E, Schmidt J, Hueller M, Reinhart K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. 2001;18:79–87. doi: 10.1046/j.0265-0215.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- 10.Frederi D, Richard D, Guillaume M, Jacques B, Dominique C, Bernard AL. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96:74–79. doi: 10.1097/00000542-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 12.Moulin F, Raymond J, Lorrot M, Marc E, Coste J, Iniguez JL, Kalifa G, Bohuon C, Gendrel D. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child. 2001;84:332–336. doi: 10.1136/adc.84.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dujardin B, Van den Ende J, Van Gompel A, Unger J, Van der Stuyft P. Likelihood ratios: a real improvement for clinical decision making? Eur J Epidemiol. 1994;10:29–36. doi: 10.1007/BF01717448. [DOI] [PubMed] [Google Scholar]
- 14.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 15.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019–1025. doi: 10.1016/s0736-4679(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 16.Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J. 1997;10:1125–1129. doi: 10.1183/09031936.97.10051125. [DOI] [PubMed] [Google Scholar]
- 17.Nohynek H, Eskola J, Kleemola M, Jalonen E, Saikku P, Leinonen M. Bacterial antibody assays in the diagnosis of acute lower respiratory tract infection in children. Pediatr Infect Dis J. 1995;14:478–484. doi: 10.1097/00006454-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Tabassian AR, Nylen E, Giron AE, Snider RH, Cassidy MM, Becker KL. Evidence for cigarette smoke-induced calcitonin secretion from lungs of man and hamster. Life Sci. 1988;42:2323–2329. doi: 10.1016/0024-3205(88)90185-3. [DOI] [PubMed] [Google Scholar]
- 19.Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schuttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24:680–684. doi: 10.1007/s001340050644. [DOI] [PubMed] [Google Scholar]
- 20.Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997;24:1240–1242. doi: 10.1086/513633. [DOI] [PubMed] [Google Scholar]
- 21.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Müller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blind intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 22.Kuse ER, Langefeld I, Jaeger K, Kulpmann WR. Procalcitonin: a new diagnostic tool in complications following liver transplantation. Intensive Care Med. 2000;26(Suppl 2):S187–S192. doi: 10.1007/BF02900736. [DOI] [PubMed] [Google Scholar]
- 23.Cakir E, Deniz O, Ozcan O, Tozkoparan E, Yaman H, Akgul EO, Bilgi C, BilgicH, Ekiz K, Erbil MK. Pleural fluid and serum procalcitonin as diagnostic tools in tuberculous pleurisy. Clin Biochem. 2005;38:234–238. doi: 10.1016/j.clinbiochem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Nelson S, Bagby GJ, Bainton BG, Wilson LA, Thompson JJ, Summer WR. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989;159:189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- 25.Monton C, Torres A, El-Ebiary M, Filella X, Xaubet A, de la Bellacasa JP. Cytokines expression in severe pneumonia: a bronchoalveolar lavage study. Crit Care Med. 1999;27:1745–1753. doi: 10.1097/00003246-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Zeni F, Viallon A, Assicot M, Tardy B, Vindimian M, Page Y, Lafond P, Bertrand JC, Bohuon C. Procalcitonin serum concentrations and severity of sepsis. Clin Intensive Care. 1994;5:89–98. [Google Scholar]