Abstract
The aim of current study was to evaluate the incidence and characteristics of ocular sarcoidosis in a Korean population. We conducted a retrospective study of 104 consecutive patients with biopsy-proven sarcoidosis seen at Asan Medical Center in Seoul, Korea, from 1993 to 2007. Medical records, photographs, and fluorescein angiograms were reviewed. Of 104 patients, 22 (21%) had intraocular involvement with female predominance (86%, M:F=3:19). Of the 39 eyes with ocular involvement, 16 (41%) eyes had isolated anterior uveitis, 12 (31%) eyes had intermediate uveitis, 6 eyes (15%) had panuveitis with retinal vasculitis, and 5 (13%) eyes had panuveitis with punched multifocal choroiditis. Mean duration of ophthalmologic follow-up was 62 months. All ocular inflammation was well managed with topical steroid and/or systemic steroid with relatively good final visual outcomes. Ocular complications such as cataract (12 eyes, 30%), glaucoma (6 eyes, 15%), vitreous opacity (1 eye, 3%), cystoid macular edema (3 eyes, 7%), neovascularization (2 eye, 5%), and epiretinal membrane (4 eye, 10%) were related to ocular sarcoidosis. In Korea, where sarcoidosis is very rare, our study indicates relatively low ocular and predominantly non posterior segment involvement with relatively good visual prognosis.
Keywords: Korean, Ocular Sarcoidosis, Sarcoidosis, Uveitis
INTRODUCTION
Sarcoidosis is a multisystem granulomatous disease of unknown etiology characterized by the presence of noncaseating granulomas in involved organs. Although primarily involving the eyes, lungs, ears, skin, and lymph nodes, many other places in the body are also affected by this disease. Ocular involvement has been observed in 25-60% of patients with systemic sarcoidosis (1). Although sarcoidosis may affect nearly all ocular structures, the most common ocular manifestations are uveitis (30-70%) and conjunctival nodules (40%) (1-5). However, this percentage depends on the population studied and the extent of ocular examination.
Sarcoidosis occurs worldwide but is prominent in certain ethnic and racial groups, including African-Americans, Scandinavians and Irish (6). The prevalence of sarcoidosis in the Korean population is less than one (0.13) in 100,000, which is far less than the 4 per 100,000 observed in the Japanese population (7, 8). Involvement of the eyes also varies according to ethnic background and race, with anterior uveitis more frequent in black patients and posterior uveitis more frequent in whites (1, 2, 4, 5). Ocular sarcoidosis is very rare in southeast Asians, and there has been little analysis in Asian populations (7-9). We have therefore evaluated the incidence and characteristics of ocular sarcoidosis in a Korean population.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 104 consecutive patients (65 women, 39 men) with biopsy-proven sarcoidosis evaluated at Asan Medical Center, Seoul, Korea, from 1993-2007. Sources of tissue for biopsy included lung in 73 patients (71.9%), skin in 15 patients (13.4%), and lymph node in 16 patients (14.7%). The study was approved by the Institutional Review Board at Asan Medical Center. Records of patients diagnosed with sarcoidosis were retrieved from the hospital database. All patients were referred by either a pulmonologist (94 patients) or a general ophthalmologist (10 patients) and a complete history and ophthalmic examination were performed.
Of the 104 patients, 22 patients (39 eyes) had active or inactive ocular involvement. Inactive ocular involvement was based on previous history of ocular involvement and ocular change including keratic precipitates or iris synechiae. For each patient, we recorded demographic data, including gender, age at the time of initial examination, non-ocular and ocular symptom and signs, treatment, and complications.
Ocular involvement was classified anatomically according to International Uveitis Study group criteria as isolated anterior uveitis, intermediate uveitis (presence of anterior vitreous cells, snow-balls, or snowbanks), retinal vasculitis with or without panuveitis (anterior and posterior uveitis), and multifocal choroiditis (punched out chorioretinal scarring with or without evidence of posterior segment inflammation such as vitritis or cystoid macular edema) (10).
Statistical analysis
The prognostic factors considered were age, gender, type of uveitis and initial visual acuity. The relationships between these factors and poor visual outcome were statistically analyzed by means of the Mann-Whitney U test and Spearman rank correlation test. A P value <0.05 was defined as statistically significant.
RESULTS
We identified 39 eyes of 22 patients (19 women, 3 men) with a diagnosis of active (34 eyes) or inactive (5 eyes) ocular sarcoidosis; their clinical and demographic characteristics are shown in Table 1. Of the 22 patients, 9 patients presented to the eye clinic with ocular symptoms as an initial symptom of systemic sarcoidosis. In 12 patients, diagnoses of systemic sarcoidosis preceded ocular sarcoidosis. The mean age at initial examination was 43 yr (range, 23-62 yr). The mean duration of follow up was 49 months (range 4-94) at department of pulmonary medicine and 62 months (range 4-105) at department of ophthalmology. Ten patients (45%) were <40 yr of age, 3 (14%) were 40-54 yr of age, and 9 (40%) were >55 yr showing bimodal peak in age.
Table 1.
Profile of 22 sarcoidosis patients with ocular involvement
*, Total pulmonary follow-up.
O, ocular; NO, non-ocular; LAP, lymphadenopathy at chest radiograph; PI, pulmonary infiltration; ACE (U/L), angiotension converting enzyme level (reference range <40 U/L); NA, not available.
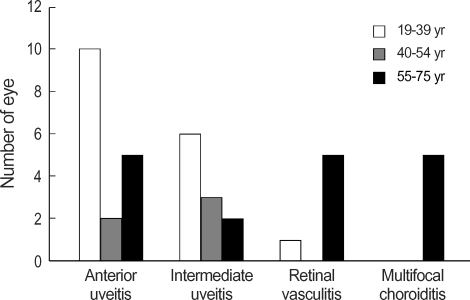
Ophthalmologic manifestations of 22 patients with ocular involvement were summarized in Table 2. Bilateral ocular involvement was seen in 17 patients (77%) and it was not always symmetrical. Of 39 eyes, 16 (41%) had anterior uveitis, 12 (31%) had intermediate uveitis and 11 (28%) had posterior involvement, which was classified as, panuveitis with retina vasculitis in 6 (15%) (Fig. 2) and panuveitis with punched multifocal choroiditis in 5 (13%) (Fig. 3). Age comparisons among the four types of uveitis are presented in Fig. 1. Interestingly, all 3 patients (5 eyes) showing panuveitis with punched multifocal choroiditis were women over 55 yr old.
Table 2.
Ophthalmologic manifestations of 39 eyes of 22 ocular sarcoidosis patients
*, recurrent inflammation; †, total ophthalmologic follow-up; ‡, cataract operation; §, cataract operation, pars plana vitrectomy due to vitreous opacity; ∥, cataract operation, Ahmed valve implantation due to neovascular glaucoma.
AU, anterior uveitis; IU, intermediate uveitis; (U), previous history of uveitis; (AU), previous history of anterior uveitis; (RV), previous history of retinal vasculitis; RV, retinal vasculitis; MC, multifocal choroiditis; Cell, cell in anterior chamber; Flare, flare in anterior chamber; KP, keratic precipitate; Mutton KP, mutton-fal keratic precipitate; D-F nodule, Dalen-Fuchs nodule; T, topical steroid treatment; S, systemic steroid treatment; (S), previous history of systemic steroid treatment; L, local steroid treatment; (PRP), previous history of panretinal photocoagulation; CF, count finger; NLP, no light perception; CAT, cataract; ERM, epiretinal membrane; NV, neovascularization; CME, cystoid macular edema; NVG, neovascular glaucoma.
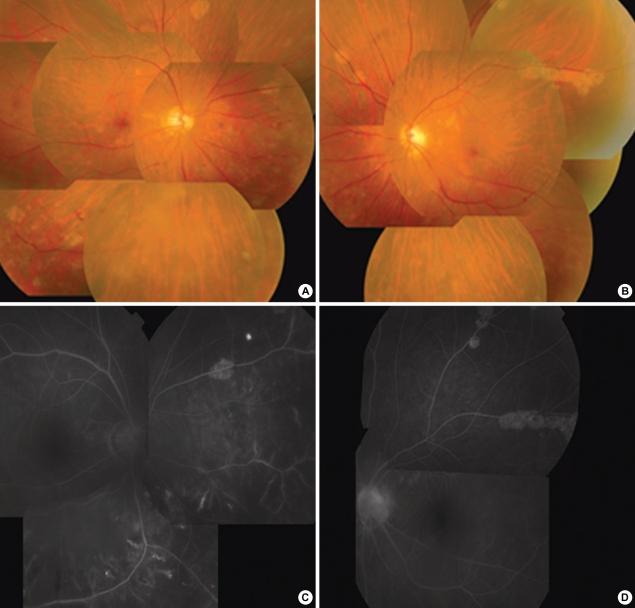
Fig. 2.
Patient No. 17. Fundus photographs (A, B) show retinal vasculitis and multiple depigmented peripheral lesions. Fluorescence angiography (FAG) (C, D) shows late fluorescein leakage from retinal vessels with adjacent capillary nonperfusion.
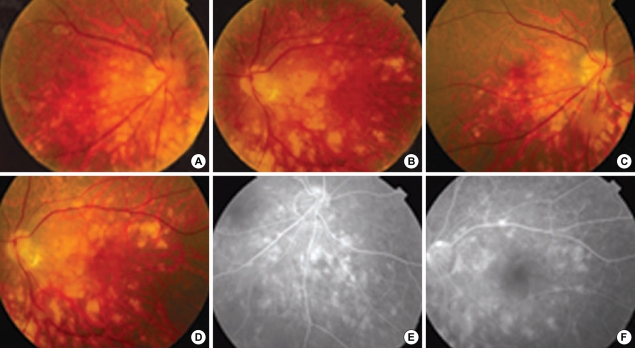
Fig. 3.
Patient No. 21. Fundus photographs show subacute multifocal choroiditis with accompanying vitreous opacity at initial presentation (A, B) and residual choroiditis accompanying atrophic change at one month later (C, D). Fluorescence angiography of right inferonasal aspect and left posterior pole of retina at one month later from initial presentation (E, F) show atrophic choroidal changes.
Fig. 1.
Comparision of age among four ordered types of uveitis. While anterior or intermediate uveitis tend to occur in younger age group, all 3 patients (5 eyes) showing 'panuveitis with punched multifocal choroiditis' were over 55 yr old.
Of 34 eyes with active ocular involvement, four eyes (11%) were exposed to previous systemic steroid treatment. The ophthalmic inflammation was managed with topical steroids alone in 8 eyes (24%) and/or with systemic corticosteroids in 26 eyes (76%). Six (31%) eyes with recurrent panuveitis or advanced to intermediate uveitis were treated with periocular corticosteroids, subtenon injection of triamcinolone, which were sufficient to control the inflammatory response without the use of additional immunosuppressive drugs. Mean duration of illness was 3.8 months (range, 1-35) with recurrence in 11 eyes. The duration of illness only included that of the first episode and with patient No. 19, it was marked as 1 month but the patient was followed up furthermore due to continuing inflammation by local clinic at a place in connection with. All episodes including recurrences were managed with steroid treatment. Systemic cytotoxic agent was not required either for ophthalmological treatment or for pulmonary treatment.
Ten eyes (26%) developed cataract; one of these patients (Patient No. 22) had cataract extraction in both eyes with intraocular lens implantation. Six eyes (15%) had glaucoma, which was treated medically; one of these eye (Patient No. 6) required additional treatment to control the intraocular pressure, but this patient refused surgery and the other patient (Patient No. 22) required Ahmed valve implantation due to neovascular glaucoma. Three eyes developed either cystoid macular edema or epiretinal membrane followed by posterior segment involvements which were observed without further treatment due to the risk of possible further treatment. One eye underwent vitrectomy for vitreous opacity.
Poor visual outcome was significantly more frequent in patients with posterior segment involvement including panuveitis with retinal vasculitis or panuveitis with multifocal choroiditis than in patients with anterior/intermediate uveitis (P<0.05). Also visual outcome was worse in the old aged group (P<0.05). Lower visual acuity at initial examination or gender, however, did not affect the visual outcome at last follow-up.
DISCUSSION
We have reviewed the medical records of patients seen at the Asan Medical Center with biopsy-proven sarcoidosis to investigate the characteristics of ocular sarcoidosis in Koreans. Although sarcoidosis remains very rare in Koreans, only 309 biopsy-proven cases were reported between July 1992 and June 1999 in the second nationwide survey, we identified a sufficient number of patients for analysis having an advantage of utilizing the largest pool of the sarcoidosis patients in Korea (8).
We found that ocular involvement was relatively low in the present study with sarcoidosis (21%), lower than in US and European populations (20-30%) (1, 11-13) and much lower than in Japanese patients with systemic sarcoidosis (50-80%) (1, 11-15). We also observed a significantly higher female predominance in ocular involvement (M:F=3:19), when compared with previous studies (M:F=1:2-3) (11, 16, 17). Furthermore, all 3 patients with panuveitis and multifocal choroiditis were females older than 55 yr. White females also seem to be a distinct clinical subgroup of patients with ocular sarcoidosis (18-20). In elderly white female patients, panuveitis and multifocal choroiditis was predominantly associated with the occurrence of cystoid macular edema (72%) and visual loss (42%) (12, 19). Our findings also suggest that elderly patients are more likely to have relatively poor visual outcomes.
In the present study, posterior segment involvement was 28%, which is comparable to previous results, in which the rate of posterior segment involvement varied from 25% to 94% (12, 21, 22). Although our study showed relatively good visual outcome in patients with ocular involvement, posterior involvement, age and presence of ocular complication related to inflammation were significantly related to relatively visual outcome. Previous studies also found that poor visual outcome was significantly more frequent in patients with panuveitis and multifocal choroiditis and that visual loss was caused by cataract, glaucoma, macular edema, vitreous hemorrhage, and retinal detachment (18).
Corticosteroids are the mainstay of therapy for ocular sarcoidosis. Cycloplegics are frequently needed to prevent syneciae (12). Eye involvement was the most common reason for systemic steroid therapy, followed in order by the lung and heart involvement (23). Eight eyes (24%) were treated with topical steroids alone, with systemic steroids used in patients with ocular disease that included severe or topical treatment resistant posterior uveitis. No further immunosuppressive treatment was needed for management for ocular involvement. However, during the follow-up there was recurrence of ocular inflammation in 11 eyes which were also treated with systemic and topical steroid. Overall, most patients with ocular sarcoidosis in our study responded to topical or systemic steroid therapy.
Although duration between ophthalmologic and non-ophthalmologic diagnosis was remarked as 0 month, this was because of routine referral to pulmonary or ophthalmologic work-up when sarcoidosis is suspicious. Of 22 patients with ocular involvement, 10 patients have been diagnosed with sarcoidosis while management of ocular inflammation. Furthermore, the rest of patients except two patients had active ocular inflammation at the time of referral from pulmonary specialist. Though ocular sarcoidosis is still very rare in Korea, ophthalmic screening examination with high index of suspicion is warranted in patients with sarcoidosis.
Korean sarcoidosis patients seemed to have similar patterns of clinical characteristics such as bimodal peak in age and associated ocular complications compared to western patients. However, in contrast to western population, the rate of ocular involvement was relatively low, with higher female predominance and relatively better visual outcome.
The limitations of this study included its retrospective design, the small numbers of patients, observation in only one institute and a description of only intraocular findings. Prior to this study, however, there was no analysis of clinical characteristics of ocular sarcoidosis in Koreans due to the rarity of sarcoidosis among Koreans. Therefore, the data reported here were accumulated over a 15-yr period and can be recorded as the largest series of ocular sarcoidosis in a Korean population. Moreover, considering that sarcoidosis is very rare in Korea and the incidence of ocular involvement is relatively low, the number of ocular sarcoidosis in our study population may represent the actual incidence of ocular sarcoidosis in Korea.
In conclusion, in Korea where sarcoidosis is very rare, our study shows relatively low ocular involvement and relatively low posterior segment involvement with female predominance. In general, visual prognosis is relatively good. Prompt treatment with high index of suspicion of sarcoidosis-related uveitis may improve visual prognosis.
Footnotes
*This work was presented at the American Academy of Ophthalmology Annual Meeting, November, 2006.
References
- 1.Hunter DG, Foster CS. Ocular manifestations of sarcoidosis. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Opthalmology. Philadelphia: WB Saunders; 1994. pp. 1217–1224. [Google Scholar]
- 2.Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–655. doi: 10.1016/0002-9394(78)90184-8. [DOI] [PubMed] [Google Scholar]
- 3.James DG, Anderson R, Langley D, Ainslie D. Ocular sarcoidosis. Br J Ophthalmol. 1964;48:461–470. doi: 10.1136/bjo.48.9.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karma A. Ophthalmic changes in sarcoidosis. Acta Ophthalmol Suppl. 1979;141:1–94. [PubMed] [Google Scholar]
- 5.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102:297–301. doi: 10.1016/0002-9394(86)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 7.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/ World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 8.Kim DS. Sarcoidosis in Korea: report of the second nationwide survey. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:176–180. [PubMed] [Google Scholar]
- 9.Hosoda Y, Yamaguchi M, Hiraga Y. Global epidemiology of sarcoidosis. What story do prevalence and incidence tell us? Clin Chest Med. 1997;18:681–694. doi: 10.1016/s0272-5231(05)70412-3. [DOI] [PubMed] [Google Scholar]
- 10.BenEzra D, Rorrester JV, Nussenblat RB. Uveitis Scoring System. Berlin: Springer-Verlag; pp. 1–13. [Google Scholar]
- 11.James DG, Neville E, Langley DA. Ocular sarcoidosis. Trans Ophthalmol Soc U K. 1976;96:133–139. [PubMed] [Google Scholar]
- 12.Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Doc Ophthalmol. 1989;72:287–296. doi: 10.1007/BF00153496. [DOI] [PubMed] [Google Scholar]
- 13.Iwata K, Nanba K, Sobue K, Abe H. Ocular sarcoidosis: evaluation of intraocular findings. Ann N Y Acad Sci. 1976;278:445–454. doi: 10.1111/j.1749-6632.1976.tb47057.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Hosoda Y, Sasaki R, Aoki K. Epidemiological study on sarcoidosis in Japan. Recent trends in incidence and prevalence rates and changes in epidemiological features. Sarcoidosis. 1989;6:138–146. [PubMed] [Google Scholar]
- 15.Ohara K, Okubo A, Sasaki H, Kamata K. Intraocular manifestations of systemic sarcoidosis. Jpn J Ophthalmol. 1992;36:452–457. [PubMed] [Google Scholar]
- 16.Lobo A, Barton K, Minassian D, du Bois RM, Lightman S. Visual loss in sarcoid-related uveitis. Clin Experiment Ophthalmol. 2003;31:310–316. doi: 10.1046/j.1442-9071.2003.00666.x. [DOI] [PubMed] [Google Scholar]
- 17.Khalatbari D, Stinnett S, McCallum RM, Jaffe GJ. Demographic-related variations in posterior segment ocular sarcoidosis. Ophthalmology. 2004;111:357–362. doi: 10.1016/S0161-6420(03)00793-0. [DOI] [PubMed] [Google Scholar]
- 18.Thorne JE, Brucker AJ. Choroidal white lesions as an early manifestation of sarcoidosis. Retina. 2000;20:8–15. doi: 10.1097/00006982-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Lardenoye CW, Van der Lelij A, de Loos WS, Treffers WF, Rothova A. Peripheral multifocal chorioretinitis: a distinct clinical entity? Ophthalmology. 1997;104:1820–1826. doi: 10.1016/s0161-6420(97)30021-9. [DOI] [PubMed] [Google Scholar]
- 20.Hershey JM, Pulido JS, Folberg R, Folk JC, Massicotte SJ. Non-caseating conjunctival granulomas in patients with multifocal choroiditis and panuveitis. Ophthalmology. 1994;101:596–601. doi: 10.1016/s0161-6420(94)31296-6. [DOI] [PubMed] [Google Scholar]
- 21.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996;103:1846–1853. doi: 10.1016/s0161-6420(96)30417-x. [DOI] [PubMed] [Google Scholar]
- 22.Bardelli AM, Barberi L, Vanni M, Mazzera L, Traversi C. Eye involvement in sarcoidosis: survey of 197 patients. Sarcoidosis. 1993;10:158–159. [PubMed] [Google Scholar]
- 23.Sugisaki K, Yamaguchi T, Nagai S, Ohmiti M, Takenaka S, Morimoto S, Ishihara M, Tachibana T, Tsuda T. Clinical characteristics of 195 Japanese sarcoidosis patients treated with oral corticosteroids. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:222–226. [PubMed] [Google Scholar]