Abstract
It is difficult to differentiate benign and malignancy in polypoid lesions of the gallbladder (PLG) by solely depending on imaging studies. Therefore clinicopathologic features of benign and malignant polyps are compared in an attempt to identify the risk factors of malignant polypoid lesions. The medical records of 291 patients who were confirmed to have PLG through cholecystectomy were reviewed and analyzed for age, sex, symptom, associated gallstone, morphology of PLG, size of PLG, number of PLG, and preoperative tumor markers. Benign PLG was found in 256 patients (88.0%) and malignant PLG in 35 patients (12.0%). Compared with benign group, the malignant group were older (61.1 yr vs. 47.1 yr, P<0.001), more often accompanied with symptoms (62.9% vs. 28.9%, P<0.001). Malignant PLG tended to be sessile (60.0% vs. 10.5%, P<0.001), larger (28.0 mm vs. 8.6 mm, P<0.001) and single lesion (65.7% vs. 44.1%, P<0.016). Age over 60 yr (P=0.021, odds ratio [OR], 8.16), sessile morphology (P<0.001, OR, 7.70), and size over 10 mm (P=0.009, OR, 8.87) were identified as risk factors for malignant PLG. Careful decision making on therapeutic plans should be made with consideration of malignancy for patients over 60 yr, with sessile morphology of PLG, and with PLG size of over 10 mm.
Keywords: Gallbladder, Polyps, Gallbladder Neoplasms, Risk Factors
INTRODUCTION
The term "polypoid lesion of the gallbladder (PLG)" refers to any elevated lesion of the mucosal surface of the gallbladder (1). The presenting symptoms of PLG are non specific and vague, and in many cases asymptomatic. For such reason, PLGs are often detected incidentally. Along with easier accessibility to routine medical check-ups, recent advancements in imaging modalities such as ultrasonography (USG) and endoscopic ultrasonography, the detection of PLG is becoming more frequent. Although there are some differences according to reports, the prevalence of PLG in healthy subjects is 3% to 7%, and PLG are found in 2-12% of cholecystectomy specimens (2).
PLG is classified according to the classification proposed by Christensen and Ishak (1) in 1970. They are classified into benign tumors such as adenoma, benign pseudotumors such as adenomatous hyperplasia, adenomyoma, inflammatory polyp, cholesterol polyp, and malignant polyps such as adenocarcinoma. The reported prevalence of malignant polyps among PLGs varies from 0% to 27% (3). It is well known that gallbladder cancer at an advanced stage reveals poor prognosis even with radical resection, thus early detection and early surgical intervention is particularly important. Despite many available imaging modalities such as USG, computed tomography (CT), magnetic resonance imaging, endoscopic retrograde cholangiography, and endoscopic ultrasonography, it is still difficult to differentiate benign polyp and malignant polyp in PLGs. Therefore treatment plan of PLG should be established with consideration of many clinicopathologic characteristics altogether with information acquired from imaging studies.
The reported risk factors for malignant PLG are age of the patient, total number of polyp, morphology, size, associated gallstone, and symptomatic polyp (4-9). The purpose of this study is to identify risk factors of malignant PLG by comparing the clinicopathologic characteristics of benign and malignant polyps confirmed by cholecystectomies. Thereby, an adequate treatment may be provided to patients with PLG.
MATERIALS AND METHODS
Data of 396 patients who were confirmed to have PLG through cholecystectomy at Seoul National University Hospital between October 1992 and December 2005 were reviewed, Among patients who underwent cholecystectomy for PLG, those with definite evidence of malignancy such as adjacent organ invasion, metastatic lymphadenopathy, systemic metastasis on preoperative USG or CT were excluded, and the study was limited to 291 patients whose information on clinical and PLG characteristics was well documented. Information on age, sex, symptom, associated gallstone, polyp morphology, size, polyp number, and preoperative serum chorioembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) were retrospectively reviewed.
The patients were divided into benign group and malignant group according to their histopathologic results. Chi-square test and Student t-test statistic analysis was carried out where appropriate to investigate whether significant differences in clinicopathologic characteristics exist between two groups. For continuous variables such as age and PLG size, receiver operation characteristic (ROC) curve was used to acquire cut-off value for stratification. In order to identify risk factors for gallbladder cancer, the odds ratio as obtained using multiple logistic regression analysis in 95% confidence interval on clinicopathologic characteristics that showed significant difference. All statistic values with P value <0.05 were considered significant. SPSS 13.0 for Windows (Chicago, IL, U.S.A.) authorized for Seoul National University Hospital was used for statistic analysis.
RESULTS
Demographic findings and clinical characteristics
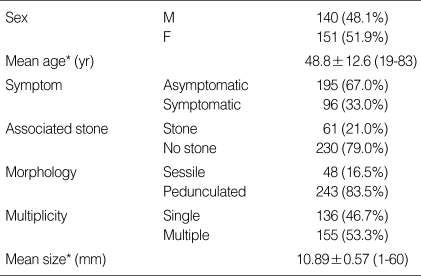
Among 291 patients, 140 (48.1%) were male and 151 (51.9%) were female. The mean age of the patients was 48.8±12.6 yr. Of the total patients, 195 patients (67.0%) were asymptomatic, and 61 patients (21.0%) had associated stone. The general characteristics of PLGs include 48 sessile polyps (16.5%), 136 single polyps (46.7%), and mean size was 10.89±0.57 mm. The demographics and clinical characteristics are summarized in Table 1.
Table 1.
Demographic findings and clinical characteristics of the 291 study subjects
*Continuous variables are described as mean±SEM (standard error of mean).
The polyps were detected by USG alone in 142 patients (48.8%), computed tomography alone in 69 patients (23.7%), by both USG and computed tomography in 80 patients (27.5 %). Most of the USG in the earlier phase of this study was a plain USG, but in more recent times many patients were examined with high resolution USG. In a small portion of patients, endoscopic USG was carried out as an adjunctive examination.
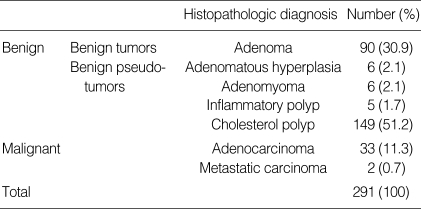
Of 291 patients, benign polyp was discovered in 256 patients (88.0%) and malignant polyp in 35 patients (12.0%). Ninety cases (30.9%) were adenoma, 6 cases (2.1%) adenomatous hyperplasia, 6 cases (2.1%) adenomyomatosis, 5 cases (1.7%) inflammatory polyp, 149 cases (51.2%) cholesterol polyp, 33 cases (11.3%) adenocarcinoma, and 2 cases (0.7%) metastatic cancer (Table 2). The 2 cases of metastatic cancer appearing as PLG are hepatocellular carcinoma and malignant melanoma each.
Table 2.
Histopathologic diagnosis of 291 polypoid lesions of gallbladder
The depths of invasion in 35 malignant polyps were Tis in 5 cases, T1a in 11 cases, T1b in 6 cases, T2 in 12 cases, and T3 in 1 case. Laparoscopic cholecystectomy was done in 14 cases, open cholecystectomy in 1 case, extended cholecystectomy in 18 cases, palliative bypass in 1 case, and 1 pylorus preserving pancreaticoduodenectomy due to concurrent common bile duct cancer. In patients who underwent laparoscopic cholecystectomy, all depths of invasion were Tis and T1, except for 1 patient whose depth was T2.
Metastatic hepatocellular carcinoma was detected in a 64 yr-old male patient with underlying non-B, non-C liver cirrhosis. The patient was diagnosed with hepatocellular carcinoma in S4 and S8 which was treated with a cycle of transarterial chemoembolization 2 yr prior to PLG detection. The other metastatic gallbladder mass was in a 28 yr-old male patient. He underwent a wide excision for a 1.5 cm sized nevus on his left anterior chest which was confirmed as malignant melanoma with a clear resection margin on 9 months prior to the laparoscopic cholecystectomy due to the PLG.
Risk factors of gallbladder cancer
The clinical and pathological features of the patients underwent univariate analysis to investigate statistical differences between benign and malignant PLG. Then using the features that showed significant differences in univariate analysis, multiple logistic regression was done to identify the risk factors for gallbladder cancer.
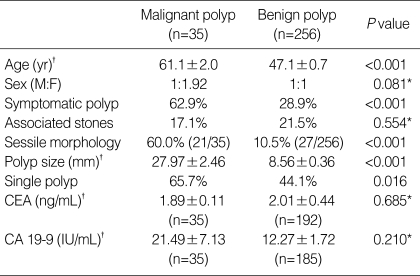
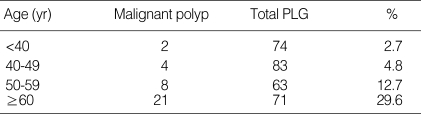
The results of the univariate analysis are summarized in Table 3. In malignant group, there were 12 males (34.3%) and 23 females (65.7%). In benign group, there were 128 males (50.0%) and 128 females (50.0%). The male-to-female ratios in malignant and benign group were 1:1.92 and 1:1, respectively, showing female predominance in malignant group. However, sexual difference in both group was statistically insignificant (P=0.081). The mean age of patients was 61.1±2.0 yr in malignant group, 47.1±0.7 yr in benign group. The patients were significantly older in malignant group (P<0.001). When the frequency of malignant polyp was obtained in accordance with age groups, it tended to increase with age (Table 4).
Table 3.
Comparison of clinicopathologic characteristics between malignant and benign polyp groups
*Statistically not significant; †Continuous variables are described as mean±SEM (standard error of mean).
CEA, chorioembryonic antigen; CA, carbohydrate antigen.
Table 4.
Frequency of malignant polyp according to age groups
*P<0.001
PLG, polypoid lesions of gallbladder.
One hundred and ninety-five patients (67.0%) with PLG were asymptomatic and detected incidentally on routine medical check-up, and the remaining 96 patients (33.0%) discovered PLG during work-up for symptoms such as abdominal pain. Whereas only 28.9% (n=74) of patients sought medical attention due to a symptom in benign group, 62.9% (n=22) of patients in malizgnant group visited medical professionals due to presenting symptoms (P<0.001).
Associated gallstone was discovered in 21.5% (n=55/256) and 17.1% (n=6/35) of patients in benign and malignant group, respectively. Associated gallstone was more frequent in benign group, but without statistical significance (P=0.554).
The information on the morphology, size, and multiplicity of PLG were primarily based on pathology reports. There were 229 (89.5%) pedunculated polyps and 27 (10.5%) sessile polyps in benign group. On the other hand, among 35 patients in malignant group, there were 14 (40.0%) pedunculated polyps and 21 (60.0%) sessile polyps. The frequency of sessile polyp was greater in malignant group compared with benign group (P<0.001).
The mean size was significantly larger in malignant group, the mean size (±standard error of mean [SEM]) being 8.56±0.36 mm and 27.97±2.46 mm for benign and malignant group, respectively (P<0.001). The size of malignant polyp ranged between 8 mm and 60 mm (Table 5), and notably, there were 2 malignant polyps with the size less than 10-8 mm and 9 mm, each.
Table 5.
Frequency of malignant polyp according to size groups
*P<0.001.
PLG, polypoid lesions of the gallbladder.
In benign group, 143 cases (55.9%) demonstrated multiple polyps and 113 cases (44.1%) were single lesion. In malignant group, only 12 cases (34.3%) were multiple lesions and 23 cases (65.7%) were single lesion. Single lesion was more frequent in malignant group (P=0.016).
Preoperative serum CEA and CA 19-9 were measured in most of the patients. Serum CEA was studied in 192 patients of benign group and in 35 of malignant group. In the instance of serum CA 19-9, it was studied in 185 of benign group and 35 of malignant group. The average CEA level (±SEM, reference range: 0-5 ng/mL) in benign group and malignant group was 1.89±0.11 ng/mL and 2.01±0.44 ng/mL, respectively (P=0.685). Only 5.7% (n=11) of benign group and 2.9% (n=1) of malignant group had exceeded the reference level of CEA which also showed no significant difference between the groups (P=0.697). The average CA 19-9 level (±SEM, reference range: 0-37 IU/mL) in the malignant group (21.49±7.13 IU/mL) was higher than that of the benign group (12.14±1.72 IU/mL), although statistically insignificant (P=0.210). Nine patients (4.9%) in benign group and 3 patients (8.6%) in malignant group displayed CA 19-9 level above the reference level, which also lacks statistic significance (P=0.412).
The features that showed statistically significant difference between benign and malignant group were age, present symptom, morphology of polyp, size of polyp, single lesion. However, sex, associated gallstone, preoperative serum CEA and CA 19-9 were statistically insignificant.
Multiple logistic regression was carried out using age, symptom, polyp morphology, polyp size, and multiplicity of PLG, which demonstrated statistical significant difference between the two groups, as covariables.
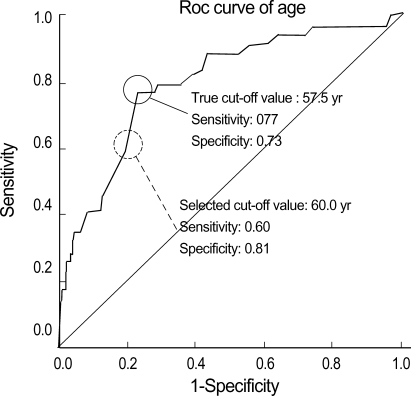
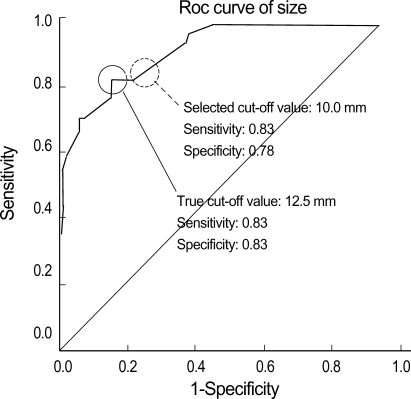
For continuous variables such as age and polyp size, the cut-off values were obtained by ROC curve in order to categorize the values into groups. The cut-off values were 57.5 yr of age and 12.5 mm for the size. The sensitivity and the specificity of the cut-off values were 0.77, 0.73 for age, and 0.83, 0.83 for size, respectively. The cut-off values were rounded off to 60 yr and 10 mm into denary scale to make the clinical application of the values more convenient. The sensitivity and the specificity of the rounded off selected cut-off value were 0.60, 0.81 for age, and 0.83, 0.78 for size, respectively (Fig. 1, 2).
Fig. 1.
ROC curve to determine the cut-off value of age (P<0.001).
Fig. 2.
ROC curve to determine the cut-off value of PLG size (P<0.001).
The result of the multiple logistic regression identified age over 60 yr, sessile morphology, and polyp size over 10 mm as the risk factors of the gallbladder cancer as summarized in Table 6.
Table 6.
Multiple logistic regression to identify risk factors of gallbladder cancer
CI, confidence interval; PLG, polypoid lesions of the gallbladder.
Frequency of malignant PLG in relation to risk factors
The frequency of malignant polyps according to the number of risk factor criteria (age ≥60 yr, sessile morphology, size ≥10 mm) that patient met was reviewed. The frequency of malignant polyp in patients who met all 3 criteria was 77.8% (14/18), 2 criteria was 28.6% (12/42), and only 1 criteria was 7.8% (9/116). For those who did not have any risk factors, there was not a single case of malignant polyp in 115 patients (Table 7).
Table 7.
Frequency of malignant polyp according to corresponding number of risk factors, of age, size of PLG and sesile lesion
PLG, polypoid lesions of the gallbladder.
DISCUSSION
Most of PLGs are asymptomatic and even if there are symptoms, they are non-specific and vague in their nature. Therefore, in the past, only a few patients sought medical attention. However, owing to the development of imaging modality and increased accessibility to medical system, the detection rate of PLG is increasing. The prevalence of PLG in normal population varies from 3% to 7% and PLGs are observed in 2% to 12% of cholecystectomized specimens (3). The reported incidences in Danish, Japanese, and Taiwanese population are 4.3%, 5.2%, and 6.9% (10-12), respectively. Not rare as it is, the importance and the treatment strategy of PLG is still not well understood.
Even though most of the PLGs are benign in nature, malignant polyps are present in some cases. Because advanced gallbladder cancer displays poor prognosis, early detection and appropriate early measure is important for curative treatment and long-term survival. This fact emphasizes the clinical significance of PLGs. Also, though controversial, there are many reports advocating malignant transformation of gallbladder adenoma as in the adenoma-adenocarcinoma sequence of colon cancer (13, 14). Therefore better understanding of clinicopathologic characteristics and further investigations into risk factors for malignant polyps is necessary for establishment of appropriate treatment strategies.
The histopathologic distribution of PLG was cholesterol polyp in 51.2%, adenoma in 30.9%, and malignancy in 12.0% in this study. This result is similar to previous reports on histologic distribution of PLG (9). The reported prevalence of malignant polyp is 0% to 27% (3). Terzi et al. (7) observed 26% malignant polyp in 100 patients, and Kubota et al. (6) reported 22% malignant prevalence. In 2 studies on Chinese population, the reported prevalence was 5.7% and 5.8% (4, 15). The large discrepancy of prevalence of malignancy among investigators is probably due to small sample size and difference in patient selection, and the accepted general prevalence of malignancy is estimated at around 3% to 8% (3).
The first line imaging modality for PLG is USG with its general sensitivity ranging from 36% to 90%, reaching 99% (3). Other conventionally used image modalities include oral cholecystography, computed tomography, endoscopic retrograde cholangiopancreatography which are all known to be inferior to USG (5). But many other new imaging studies such as enhanced helical computed tomography, endoscopic USG, magnetic resonance cholangiography, and positron emission tomography are being introduced and applied into practice for detection of PLG or differential diagnosis of PLG.
In the current study, the mean age of malignant group was significantly older than benign group. This result is in accordance with previous Korean report (16). Also a report that many patients with malignant polyps are over 50 yr of age (5) and another report that malignancy risk of PLG increases with age (13) indicate that old age is an important feature of malignant polyp.
Concerning sexual difference in the incidence of PLG, some authors claim male predominance (11, 12, 17), whereas others observed female predominance (4, 13). In this study, female predominance of PLG was observed, and although statistically insignificant, malignant PLGs occurred more frequently in female.
The clinical symptom of PLG was previously reported to be non-specific and not to differ between benign and malignant polyps. Even if the symptom is present, it is usually similar to that of symptomatic calculous cholecystitis (7, 18). In the present study, 67.0% of all patients were asymptomatic and 33.0% were symptomatic-abdominal pain and epigastric discomfort being the most frequent. Symptoms were significantly associated with malignant polyp compared with benign polyp. The association of symptom and presence of associated gallstone was separately analyzed. Significant association of symptom to associated gallstone was not found (P=0.235). However, symptomatic cases tended to increase with the increase in polyp size (P=0.001). Therefore a speculation can be made that symptom may be associated with the size of the polyp rather than the association of gallstone.
Kubota et al. (6) claimed that sessile morphology is one of the important factors that suggest malignancy, and some scholars also asserted that sessile morphology is a risk factor of gallbladder cancer (9). In this study, sessile morphology was more dominant in the malignant group. Early gallbladder cancer is defined as those confined to mucosa or muscle layer, and the lesions can have several shapes, including pedunculated, sessile, superficial raised, and flat (19). And one of the possible explanations for frequent sessile morphology in malignant PLG may be that most gallbladder cancer arises in-situ from flat, dysplastic epithelium (20).
It is generally agreed upon that polyp size over 10 mm is a predicting factor of malignancy. Koga et al. (21) and Kuota et al. (6) previously reported 37% to 88% prevalence of malignancy in PLG over 10 mm. Csendes et al. (22) observed no adenocarcinoma and no progression to malignancy in PLGs less than 10 mm during mean period of 71 months follow-up. In the current study, the mean polyp size of malignant polyps was by far larger than benign polyps (27.97 mm vs. 8.56 mm). Prevalence of malignant polyp in polyps over 10 mm was 24.4% (33/135). Although this 24.4% prevalence is not in accordance with the 37 to 88% prevalence mentioned above, it is similar to a Korean report of 22.8% prevalence (9). There is a general agreement that polyp size over 10 mm is a risk factor for malignant polyp (4, 5, 7). However, it is worthy of noting that 3 cases and 1 case of malignant polyp less than 10 mm has been reported in two studies (9, 16). Additionally, 2 malignant polyps of less than 10 mm was observed in this study too. Therefore, clinicians should have caution that there may still be malignant polyps even if the sizes of the polyp are less than 10 mm, and the possibility of malignant polyp should not be ruled out on the basis of size alone. In the present study, malignant polyps tended to be a single lesion compared with benign polyps. The similar findings were reported by many others scholars (4-6, 23).
Tumor markers such as CEA and CA 19-9 increases in gallbladder cancer, and CA 19-9 - having high clinical significance with its sensitivity of 60% to 70% in biliary malignancy - is used in diagnosing gallbladder cancer in adjunct to imaging modalities in particular (24). In this study, the mean serum level of CEA and CA 19-9 was within the reference range in both benign and malignant group, and no significant differences existed between two groups. Furthermore, there was no significant difference in the prevalence of patients above the reference range in both groups. Therefore it may be stated that serum tumor markers have little role in differentiating benign and malignancy in PLGs. The impracticality of the tumor markers in differential diagnosis may be explained by the fact that most of the malignant polyps in PLGs are early gallbladder cancers.
Risk factors claimed by other authors are reviewed. Yeh et al. (4) identified age over 50 yr and polyp size over 10 mm as the independent risk factors of malignant polyps. Yang et al. (5) reported that size over 10 mm, single PLG, the presence of gallstone, age over 50 yr, and clinical symptoms were associated with malignancy. Kubota et al. (6) proposed sessile polyp, isoechogenicity with the liver parenchyma, and rapid growth were also important factors predicting malignancy. Terzi et al. (7) noted age over 60 yr, size over 10 mm, and associated gallstone as the risk factors for malignancy. He et al. (8) proclaimed that cholecystectomies should be indicated in polyps with size over 10 mm and age over 50 yr. Jang et al. (9) identified age, polyp size, polyp morphology as the risk factors. In the current study, using significant features of malignant polyp-age, presence of symptom, polyp morphology, polyp size, and multiplicity of PLG-as covariables, multiple logistic regression was carried out. The result of the analysis identified age over 60 yr, sessile morphology, and polyp size over 10 mm as the risk factors for malignant polyp.
No malignant polyp was found in patients without any risk factors identified in the current study. However, 7.8%, 28.6%, and 77.8% of PLGs were malignant in patients with single risk factor, 2 risk factors, and all 3 risk factors, respectively. Therefore, for the risk factors identified in the study, it may be speculated that the risk for malignancy increases as more risk factors are identified on the patient's side. And this speculation may be used as a guidance in deciding the therapeutic strategy of patients with PLG.
In summary, age over 60 yr, sessile morphology, and polyp size over 10 mm was identified as the risk factors for malignant polyps in PLGs. Further, the frequency of malignant polyps in patients with all 3 risk factors was 77.8%, 28.6% in patients with 2 risk factors, and 7.7% in patients with single risk factor. Therefore, clinician should evaluate patients with PLG with the possibility of malignancy in mind if the patient has one or more risk factors. Surgical intervention should be considered in patient with symptomatic PLG and in patients with one or more risk factors.
References
- 1.Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch Pathol. 1970;90:423–432. [PubMed] [Google Scholar]
- 2.Ahrendt SA, Pitt HA. Biliary tract. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. 17th ed. Philadelphia: Elsevier Saunders; 2004. pp. 1597–1641. [Google Scholar]
- 3.Lee KF, Wong J, Li JC, Lai PB. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186–190. doi: 10.1016/j.amjsurg.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Yeh CN, Jan YY, Chao TC, Chen MF. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001;11:176–181. [PubMed] [Google Scholar]
- 5.Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79:227–229. doi: 10.1002/bjs.1800790312. [DOI] [PubMed] [Google Scholar]
- 6.Kubota K, Bandai Y, Noie T, Ishizaki Y, Teruya M, Makuuchi M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117:481–487. doi: 10.1016/s0039-6060(05)80245-4. [DOI] [PubMed] [Google Scholar]
- 7.Terzi C, Sokmen S, Seckin S, Albayrak L, Ugurlu M. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery. 2000;127:622–627. doi: 10.1067/msy.2000.105870. [DOI] [PubMed] [Google Scholar]
- 8.He ZM, Hu XQ, Zhou ZX. Considerations on indications for surgery in patients with polypoid lesion of the gallbladder. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:951–952. [PubMed] [Google Scholar]
- 9.Jang YS, Lee JH, Kim JY, Kim SH, Kim SG, Hwang YJ, Yun YK. Surgical outcomes and risk factors for gallbladder carcinoma of polypoid lesions of gallbladder. Korean J Hepatobiliary Pancreat Surg. 2005;9:164–170. [Google Scholar]
- 10.Jorgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol. 1990;25:281–286. [PubMed] [Google Scholar]
- 11.Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, Hamajima E, Shimodaira M, Ohmiya N. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed] [Google Scholar]
- 12.Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997;92:2066–2068. [PubMed] [Google Scholar]
- 13.Kozuka S, Tsubone M, Yasui A, Hachisuka K. Relation of adenoma to carcinoma in the gallbldder. Cancer. 1982;50:2226–2234. doi: 10.1002/1097-0142(19821115)50:10<2226::aid-cncr2820501043>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77:363–364. doi: 10.1002/bjs.1800770403. [DOI] [PubMed] [Google Scholar]
- 15.Sun XJ, Shi JS, Han Y, Wang JS, Ren H. Diagnosis and treatment of polypoid lesions of the gallbladder: report of 194 cases. Hepatobiliary Pancreat Dis Int. 2004;3:591–594. [PubMed] [Google Scholar]
- 16.Kim SW, Park YH, Park SS. Clinical study of gallbladder polypoid lesions. J Korean Surg Soc. 1994;47:958–966. [Google Scholar]
- 17.Collett JA, Allan RB, Chisholm RJ, Wilson IR, Burt MJ, Chapman BA. Gallbladder polyps: prospective study. J Ultrasound Med. 1998;17:207–211. doi: 10.7863/jum.1998.17.4.207. [DOI] [PubMed] [Google Scholar]
- 18.Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414–417. doi: 10.1046/j.1365-2168.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya Y. Early carcinoma of the gallbladder: macroscopic features and US findings. Radiology. 1991;179:171–175. doi: 10.1148/radiology.179.1.2006272. [DOI] [PubMed] [Google Scholar]
- 20.Albores-Saavedra J, Vardaman CJ, Vuitch F. Non-neoplastic polypoid lesions and adenomas of the gallbladder. Pathol Annu. 1993;28 Pt 1:145–177. [PubMed] [Google Scholar]
- 21.Koga A, Watanabe K, Fukuyama T, Takiguchi S, Nakayama F. Diagnosis and operative indications for polypoid lesions of the gallbladder. Arch Surg. 1988;123:26–29. doi: 10.1001/archsurg.1988.01400250028003. [DOI] [PubMed] [Google Scholar]
- 22.Csendes A, Burgos AM, Csendes P, Smok G, Rojas J. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Ann Surg. 2001;234:657–660. doi: 10.1097/00000658-200111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinkai H, Kimura W, Muto T. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg. 1998;175:114–117. doi: 10.1016/S0002-9610(97)00262-6. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev MS, Tombazzi CR. Tumor markers: an important adjunct to clinical practice. South Med J. 2006;99:107. doi: 10.1097/01.smj.0000198307.88105.c4. [DOI] [PubMed] [Google Scholar]