Abstract
Various pathogenic bacteria, viruses, and protozoan bind to glycosaminoglycan-based receptors on host cells and initiate an infection. Sporozoites of Plasmodium predominantly express circumsporozoite (CS) protein on their surface, which binds to heparan sulfate proteoglycans on liver cell surface that subsequently leads to malaria. Here we show that the interaction of free heparin with this parasite ligand has the potential to be a critical component of invasion. CS protein of P. falciparum contains four cysteines at positions 361, 365, 396, and 401. In this study, all four cysteine residues were mutagenized to alanine both individually and in different combinations. Conversion of cysteine 396 to alanine (protein CS3) led to a 10-fold increase in the binding activity of the protein to HepG2 cells. Replacement of cysteines at positions 361, 365, and 401 either alone or in different combinations led to a near total loss of binding. Surprisingly, activity in these inactive mutants could be effectively restored in the presence of submolar concentrations of heparin. Heparin also up-regulated binding of CS3 at submolar concentrations with respect to the protein but down-regulated binding when present in excess. Given the significantly different concentrations of heparin in different organs of the host and the in vitro results described here one can consider in vivo ramifications of this phenomenon for pathogen targeting of specific organs and for the functional effects of antigenic variation on receptor ligand interaction.
Circumsporozoite (CS) protein is one of the prime candidates being evaluated as an antigen for an antimalarial vaccine (1, 2). CS protein is involved in both invasion of salivary glands in mosquito and the selective invasion of hepatocytes in its human host (3). The mature form of Plasmodium falciparum CS protein possesses four cysteines at positions 361, 365, 396, and 401 (4). These cysteines are conserved in CS across all of the species of Plasmodium, indicating their importance in the biological activity of the protein (5). A conserved 18-aa motif (EWSPCSVTCGNGIQVRIK) constitutes the binding domain of CS known as region II-plus (4, 6). This motif contains a 6-aa consensus sequence (CSVTCG) that is found in a growing family of adhesive molecules such as thrombospondin (7), properdin (8), as well as in malarial proteins like TRAP (thrombospondin-related anonymous protein) (9) and CTRP (CS protein TRAP-related protein) (10). Proteins containing these sequences bind to a variety of sulfated glycoconjugates, in particular to glycosaminoglycans such as heparan sulfate and heparin (6, 11, 12). Whereas cysteines 361 and 365 are present within the binding domain, cysteines 396 and 401 are present further downstream toward the carboxyl terminus.
Whereas some cysteines remain in reduced state, cysteine residues present in close proximity in a three-dimensional structure can be oxidized to form a disulfide bond, which introduces a three-dimensional constraint in the protein's structure. This enhances its stability and provides the requisite structural conformation required for a large number of biological processes. Disulfides can influence the immune response generated against the protein by the host (13, 14), and although CS has been used for making vaccines the pattern of disulfide bonding has not been determined. Information on the pattern of disulfide bridges is all the more important for CS as two known cytotoxic T cell epitopes in protein are nested in between the two-cysteine containing domains (15). To evaluate the role of cysteines in CS, we made 11 different mutants where cysteines have been replaced by alanines either individually or in paired combinations. The proteins were purified to homogeneity and evaluated for their binding potential. Here we show that cysteines are important for the biological activity of the protein. We have shown that heparin can effect change in the CS protein in a way that has ramifications for sporozoite targeting of liver cells. We also describe a mechanism by which inactive mutants can be rejuvenated in the presence of glycosaminoglycans. Further the primary structure of the protein itself can be physically altered, resulting in greater or lesser affinity for cells but its activity essentially can be normalized in the presence of heparin.
Materials and Methods
Materials.
A Quickchange site-directed mutagenesis kit was obtained from Stratagene. Heparin and heparinase I were obtained from Sigma. Heparin Sepharose column was obtained from Amersham Pharmacia. All cell culture supplies were from Life Technologies (Gaithersburg, MD). Hepatoma cell line HepG2 was purchased from American Type Culture Collection. Anti-mouse-alkaline phosphatase conjugate was obtained from Pierce. mAb 2A10 directed against the (NANP)n repeat domain was a kind gift from Robert Wirtz (Centers for Disease Control and Prevention, Atlanta).
Construction of CS Mutants.
Quickchange, a PCR-based site-directed mutagenesis system, was used to introduce mutations in the CS gene without any digestion and ligation of inserts. The reactions were performed as directed by the manufacturer. Plasmid pCS1 was used as template for the construction of plasmids pCS3, pCS4, pCS5, pCS8, pCS9, and pCS10. pCS1 contains DNA sequence encoding for amino acids 27–123[NANPNVDP]3[NANP]21300–411 in a T7 promoter-based Escherichia coli expression vector (16). Plasmids pCS11, pCS12 pCS13, pCS14, and pCS15 used pCS3, pCS4, pCS8, pCS9, and pCS10, respectively as template. Presence of desired mutations was verified by DNA sequencing.
Expression and Purification of Recombinant Proteins.
The proteins were expressed and purified as described (16). Briefly, BL21(λDE3) cells were transformed and the culture was expanded in super broth (pH 7.2), containing 100 μg/ml of ampicillin. At OD600 of 1.0, cells were induced with 2 mM isopropyl β-d-thiogalactoside. After 4 h cells were harvested and the periplasm was isolated by a gentle osmotic shock without disrupting the cell membrane. CS was separated from bacterial contaminants on a heparin sepharose affinity column followed by gel filtration chromatography.
Cell Binding Assay.
Hepatoma cell line HepG2, maintained in RPMI 1640 containing 2 mM glutamine and 10% heat-inactivated FBS was used and the assay was performed as described (17). Briefly, cells at a density of 100,000 cells per well were fixed with 4% paraformaldehyde followed by blocking with TBS containing 1% BSA. Proteins were incubated with cells for 1 h followed by anti-CS mAb for 45 min and alkaline phosphatase-coupled anti-mouse IgG for 30 min. 4-Methylumbelliferyl phosphate (1 mM) was used as substrate, and fluorescence was measured in a fluorometer with excitation at 350 nm and emission at 460 nm. For competition analysis, cells after blocking were incubated with 100 μM of peptide RIIP (EWSPCSVTCGNGIQVRIKPGSANKP) for 2 h before the addition of recombinant protein. All of the experiments were repeated at least three times and had a SD of less than 7.5%.
Heparin and Binding Activity.
For evaluating the effect of heparin on the binding activity of the protein, 50 nM of recombinant proteins was coincubated with different concentrations of heparin for 15 min at 37°C before the addition of this mix onto the cells. For estimating the activation potential, 5 nM of heparin was coincubated with 50 nM of protein. The assay was continued as described above. The sensitivity of fluorometer was adjusted such that the fluorescence activity in a given assay was within the maximum detection limit of the instrument.
Effect of Heparinase I on the Binding Activity of CS-Heparin Complex.
HepG2 cells were treated with 5 units of heparinase I in 50 mM acetate buffer, pH 6.0 or buffer for 2 h at 37°C. The cells were washed three times with Tris-buffered saline (TBS) followed by addition of 50 nM of CS15 or CS15-heparin complex (50 nM:5 nM) and incubation at 37°C for 1 h. Fluorescence was measured as described above.
Effect of Peptide RIIP on the Binding Activity of CS-Heparin Complex.
HepG2 cells after fixing and blocking were incubated with 100 μM of peptide RIIP or BSA for 2 h. This was followed by the addition of CS3 (50 nM)-Heparin (5 nM) complex or CS3 alone. Fluorescence was measured as described above.
Results
Construction, Expression, and Purification of CS Mutants.
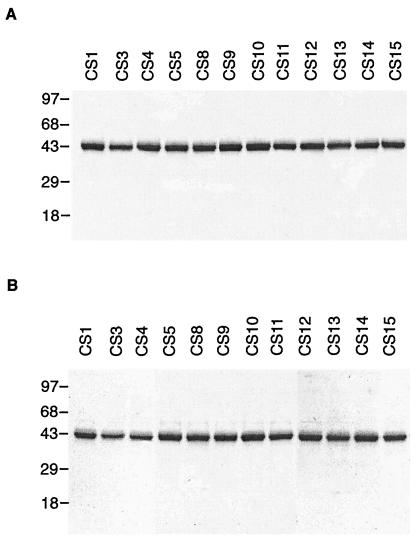
Four cysteines present at positions 361, 365, 396, and 401 in CS protein were mutated to alanine, either alone or in pairs in all possible combinations. This gave rise to 11 different constructs (Table 1). Of these, four constructs (CS3, CS4, CS8, and CS9) had individual cysteines at respective positions 396, 401, 361, and 365 mutated to alanine. Proteins CS5, CS10, CS11, CS12, CS13, and CS14 had two cysteines each converted to alanines, whereas in CS15 all four cysteines were changed into alanines (Table 1). The constructs were expressed in E. coli and even in the absence of a known signal sequence mutant proteins were secreted into the periplasm. Periplasm was used as source to purify the protein. As CS is known to bind to heparin sulfate proteoglycans (HSPG), so a heparin-based affinity column was used for purification (implications of this binding are discussed below). Proteins were purified to apparent homogeneity by using a gel filtration column. Purified protein (3–4 mg) was obtained from a liter of culture. The purified proteins were analyzed on a polyacrylamide gel under reducing and nonreducing conditions (Fig. 1). All 11 proteins migrated as a single band on a nonreducing polyacrylamide gel, indicating that even when cysteines were mutated the protein maintained its preferential monomeric form (Fig. 1B).
Table 1.
Different mutants of CS proteins
| Protein | Cys-361 | Cys-365 | Cys-396 | Cys-401 |
|---|---|---|---|---|
| CS1 | Cys | Cys | Cys | Cys |
| CS3 | Cys | Cys | Ala | Cys |
| CS4 | Cys | Cys | Cys | Ala |
| CS5 | Cys | Cys | Ala | Ala |
| CS8 | Ala | Cys | Cys | Cys |
| CS9 | Cys | Ala | Cys | Cys |
| CS10 | Ala | Ala | Cys | Cys |
| CS11 | Ala | Cys | Ala | Cys |
| CS12 | Cys | Ala | Cys | Ala |
| CS13 | Ala | Cys | Cys | Ala |
| CS14 | Cys | Ala | Ala | Cys |
| CS15 | Ala | Ala | Ala | Ala |
Figure 1.
Analysis of purified CS mutants. Purified proteins were run on a 12% SDS-polyacrylamide gel under (A) reducing and (B) nonreducing conditions. Molecular mass markers are shown in kDa.
Effect of Cysteine Replacement on the Binding Activity of CS Protein.
The binding potential of these recombinant mutant proteins was evaluated on HepG2 cells. Cells were incubated with different concentrations of the protein and the binding activity was checked by fluorometry. CS3, where cysteine at position 396 has been mutated to alanine, showed a 10-fold enhancement in the binding activity with respect to CS1, the native protein where all four cysteine residues were intact (Fig. 2 Left). Whereas CS3 showed a 10-fold enhancement in the binding, conversion of cysteines to alanine at any other position (361, 365, and 401), either individually or in combination led to a loss of 90% of the binding activity (Fig. 2).
Figure 2.
Binding activity of CS mutants. HepG2 cells were incubated with different concentrations of mutants for 1 h followed by addition of anti-CS antibody and alkaline phosphatase-conjugated IgG. Bound enzyme was revealed by a fluorescent substrate.
Competition Binding Analysis of CS3.
CS uses region II-plus as a binding ligand to interact with the hepatocytes. To check whether CS3 also is using region II-plus for binding, binding of CS3 was checked in the presence of peptide RIIP. Peptide RIIP effectively blocked the binding of CS3 in a dose-dependent manner, decreasing binding of CS3 by 65%, indicating that CS3 is using region II-plus as the binding ligand (Fig. 3).
Figure 3.
CS3 binding activity in the presence of peptide RIIP. Different concentrations of CS3 were added to HepG2 cells preincubated with peptide RIIP (○) or buffer (●). Binding activity was measured as described.
Effect of Heparin on the Binding of CS3.
HSPG present on hepatocyte cell surface act as receptors for CS (6). Glycosaminoglycans like heparin and fucoidan can inhibit the binding of CS by binding onto the protein and preventing it from binding to liver cells (6). To evaluate whether CS3 uses HSPG as receptor, its binding activity was evaluated in the presence of heparin. CS3 (50 nM) was preincubated with log dilutions of heparin for 15 min to allow its binding to the protein. The concentrations of heparin ranged from 380,000 nM to 3.8 nM, providing a spectrum of protein to glycosaminoglycan ratio with heparin being present in 7,500-fold molar excess to submolar concentrations with respect to the protein. The protein-heparin complex was added onto the hepatocytes, and the change in the level of binding was evaluated with respect to control where protein was incubated with buffer alone. Heparin inhibited the binding of CS3 and at 380 μM, the highest concentration of heparin tested, fluorescence decreased from 1,610 (control) to 257 resulting in 84% inhibition of binding activity (Fig. 4A). Inhibition was dose-dependent as at 0.38 μM of heparin fluorescence activity was at 1,481, resulting in 8% decrease in binding. This indicated that CS3 was using heparin-based cell surface molecules for binding. Native protein CS1 showed a similar profile for inhibition where fluorescence activity was reduced from 450 (control) to 112 (75% inhibition) at the highest concentration of heparin and reverted back to 432 (4% inhibition) at 0.38 μM of heparin (Fig. 4A). Surprisingly, when present at equimolar and submolar concentrations, heparin started to promote the binding activity of CS protein. At equimolar concentration CS3 binding improved by 175% and at the submolar concentration (0.38 nM) the binding improved by 260%. CS1 also showed a similar profile and at submolar concentration of heparin its fluorescence activity matched with CS3 (Fig. 4A).
Figure 4.
Binding activity of CS3 in the presence of heparin. (A) 50 nM of CS3 (▾, ▿) or CS1 (●, ○) was incubated with different concentrations of heparin before its addition onto HepG2 cells. Fluorescence was measured as described. Curves with filled symbols depict inhibition of binding activity of the CS1 and CS3 with respect to their control preincubated with buffer alone. Curves with open symbols show fluorescence values of the two proteins in the presence of different concentrations of heparin. (B) 50 nM of CS3 was preincubated with 5 nM of heparin or buffer and added onto the HepG2 cells preincubated with buffer (black bars) or peptide RIIP (shaded bars). Fluorescence was measured as described.
Binding Activity of CS3-Heparin Complex in the Presence of Peptide RIIP.
To evaluate whether the enhancement in binding activity of CS3-heparin complex is related to utilization of a different binding ligand in the protein, activity of CS3-heparin complex was evaluated in the presence of peptide RIIP. As described previously, CS3-heparin complex showed a 260% increase in the binding activity in comparison to CS3 alone (Fig. 4B). This improved activity of CS3-heparin complex was reduced by 90% in the presence of peptide RIIP, indicating that the CS3-heparin complex is indeed using region II-plus for binding (Fig. 4B).
Effect of Heparin on the Binding Activity of Inactive Mutants.
As submolar concentrations of heparin promote the binding activity of CS3, an experiment was performed to check whether it could restore the binding activity in any of the inactive mutants of CS. CS4, CS8, CS9, and CS15 (50 nM each) were incubated with 5 nM of heparin before adding the complex onto the cells. Heparin effectively restored the binding of these otherwise inactive proteins (Fig. 5A). The binding activity of these mutants was comparable to the activity of CS1 in the presence of similar concentration of heparin. Proteins alone did not bind onto the cells (Fig. 5A).
Figure 5.
Binding activity of inactive CS mutants in the presence of heparin or heparinase. (A) 50 nM of inactive mutants was coincubated with either 5 nM of heparin (shaded bars) or with buffer alone (black bars) for 15 min followed by their addition onto the cells. Fluorescence was measured as described. (B) HepG2 cells were preincubated with heparinase (shaded bars) or with buffer (black bars) for 2 h followed by addition of 50 nM of CS15 coincubated with buffer or with 5 nM of heparin. Fluorescence was measured as described.
Effect of Heparinase Treatment on the Binding Activity of CS-Heparin Complex.
As heparin was able to restore the binding activity in inactive CS mutants, CS-heparin complex could be using a non-HSPG based receptor for binding. To evaluate this possibility, binding activity of CS15-heparin complex was evaluated on heparinase-treated HepG2 cells. Binding activity of CS15-heparin complex was reduced by 90% on heparinase-treated cells, indicating that the complex is using HSPG-based receptors on hepatocyte surface for binding (Fig. 5B).
Discussion
We have replaced cysteine residues with alanine individually and in various combinations to investigate the role of individual cysteines and disulfides in the binding activity of the CS protein to cells. Because potentially all four cysteines could be making disulfide bridges, 11 different mutants were constructed to disrupt either one or both of the disulfides possible in any given conformation of the protein (Table 1). The constructs were expressed in E. coli, and the proteins were purified to homogeneity (Fig. 1). Replacement of cysteine by alanine either individually or in combination did not lead to dimerization or aggregation of protein (Fig. 1B). This may suggest that cysteines are not easily accessible for the formation of an intermolecular disulfide bond.
An observation of potential importance is that the residues/domains involved in the binding of proteins to a heparin column are not adequate to bind to cells in our assay. Region I and region II-plus of CS are known to interact with heparin (6, 18, 19). All of the mutants bound to the heparin-based affinity column, indicating that the heparin-binding domain in region II-plus doesn't involve cysteine residues or their contribution to the secondary structure. Proteins may have been binding onto the column through region I (20). Alternatively, proteins could be binding through a yet uncharacterized heparin-binding domain.
Cysteines are certainly involved, either directly or structurally, in the binding activity of the protein to cells. Conversion of cysteines 361, 365, and 401 to alanine either alone or in various combinations lead to a near-total loss of binding. Replacement of cysteine 396 with alanine led to a 10-fold increase in the binding activity of the protein. The role of cysteines 361 and 365 in the biological activity of CS protein has been a subject of debate with contradictory reports about the involvement of the domain containing these residues in the binding activity of the protein (6, 18, 21, 22). CS8 (C361A) and CS9 (C365A) were inactive, strongly suggesting that cysteines in region II are contributing toward the binding activity of the protein. These cysteines could either be binding directly and/or providing a proper structural conformation to the residues involved in binding by forming disulfide bridge(s). Because region II motif occurs a number of times in Plasmodium proteins and various organisms and seems to play a universal role in cell–cell interaction, this observation may yield new perspective in understanding its role within varied backgrounds. Cysteines outside of region II-plus can affect binding activity either positively or negatively as shown above.
Considered in isolation, a mutation like that found in CS3 (C396A), which led to an increase in its binding capabilities, would seem to be advantageous. We investigated the reason for this improvement. This enhancement in binding activity can either be attributed to (i) involvement of a different CS binding motif, (ii) association with a previously unrecognized hepatocyte receptor, and (iii) an altered structural conformation of the binding domain.
To evaluate whether CS3 still uses region II-plus for binding, its binding capabilities were evaluated in the presence of peptide representing region II-plus. The peptide successfully competed the binding activity of CS3 on HepG2 cells, indicating that CS3 is indeed using region II-plus for binding (Fig. 3).
CS binds to sulfated glycoconjugates on the hepatocyte cell surface. To evaluate the possibility of a change in specificity toward its receptor, binding capacity of CS3 was evaluated by using heparin as a competitor. Heparin is a highly sulfated, negatively charged mucopolysaccharide and in humans is primarily synthesized by mast cells located in pericapillary connective tissue (23). Heparin is involved in a diverse range of biological processes such as cell attachment, growth factor binding, cell proliferation, migration, morphogenesis, and viral pathogenicity (24). Its molecular mass ranges from 5 to 40 kDa, depending on the number of sugar moieties present in its chain with an average molecular mass of 13 kDa (23). When present in large molar excess, heparin inhibited the binding of CS3, suggesting that CS3 has maintained its specificity for HSPG on liver cells, and an improved structural conformation may be the reason for the enhanced activity (Fig. 4A). At equimolar and submolar levels, heparin unexpectedly started to substantially promote the binding of CS3 and showed a 260% increase in binding when 13 molecules of CS were available for one molecule of heparin. Like CS3, the putative CS3-heparin complex binds through the region II-plus motif, as peptide RIIP was able to block the binding activity of CS3-heparin complex (Fig. 4B).
One plausible interpretation for this phenomenon is that at large molar excess heparin saturates all of the heparin binding sites present on CS and prevents it from binding to target receptors. At submolar concentrations where CS is present in excess, its long chain structures effectively crosslink a large number of CS molecules, resulting in an enhancement of binding. Previously Cerami et al. (17) have shown that CS molecules bind more efficiently if they are present as polymers that may parallel the enhancement of CS-heparin complexes. As CS-heparin complex uses region II-plus, it also suggests the presence of other heparin-binding motifs that enhance but are not directly involved in binding.
This enhancement in binding activity of CS protein cannot be attributed to improved recognition of repeats by the mAbs in the presence of heparin as at submolar concentrations, heparin effectively restored the binding activity in even inactive CS mutants (Fig. 5A). The binding activity of these otherwise inactive mutants in the presence of heparin was comparable to native protein. These inactive turned active mutants were binding to HSPG on liver cells because CS15-heparin complex lost more than 90% of its binding activity on heparinase-treated cells (Fig. 5B). As CS-heparin complex is using region II-plus motif for binding to HSPG receptors present on liver cells, it suggests that interactions of heparin with CS stabilize this binding motif. Heparin could do this by interacting directly with the binding domain or binding through a yet uncharacterized motif in the protein.
In support of the data presented above, it is known that sporozoites adsorb carbohydrate-based components present in host serum and the sporozoites preincubated in media with host serum have an enhanced binding activity (25). The relationship between structural change and altered sporozoite activity has been observed previously. Anti-sporozoite antibodies at extremely low concentration have been shown to promote sporogony in mosquitoes (26, 27). Similarly, heparin-mediated adhesion of Leishmania amistigotes to cellular proteoglycans on Chinese hamster ovary cells also has been observed (28). The potential involvement of heparin in regulating sporozoite targeting and accumulation in specific organs is very plausible as unlike other glycosaminoglycans, its distribution varies more than 1,000-fold, depending on tissue location (24). Our findings could have significant implications in understanding the invasion process of various pathogens, as not only malaria parasites but also a diverse range of organisms use the HSPG-based receptor system for invasion.
Abbreviations
- CS
circumsporozoite
- HSPG
heparan sulfate proteoglycans
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140224597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140224597
References
- 1.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, et al. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 3.Hollingdale M R, Nardin E H, Tharavanij S, Schwartz A L, Nussenzweig R S. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 4.Dame J B, Williams J L, McCutchan T F, Weber J L, Wirtz R A, Hockmeyer W T, Maloy W L, Haynes J D, Schneider I, Roberts D, et al. Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 5.McCutchan T F, Kissinger J C, Touray M G, Rogers M J, Li J, Sullivan M, Braga E M, Krettli A U, Miller L H. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawler J, Hynes R O. J Cell Biol. 1986;103:1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goundis D, Reid K B. Nature (London) 1988;335:82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- 9.Robson K J, Hall J R, Jennings M W, Harris T J, Marsh K, Newbold C I, Tate V E, Weatherall D J. Nature (London) 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 10.Trottein F, Triglia T, Cowman A F. Mol Biochem Parasitol. 1995;74:129–141. doi: 10.1016/0166-6851(95)02489-1. [DOI] [PubMed] [Google Scholar]
- 11.Holt G D, Pangburn M K, Ginsburg V. J Biol Chem. 1990;265:2852–2855. [PubMed] [Google Scholar]
- 12.Muller H M, Reckmann I, Hollingdale M R, Bujard H, Robson K J, Crisanti A. EMBO J. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan A F, Chappel J A, Burghaus P A, Morris J S, McBride J S, Holder A A, Kaslow D C, Riley E M. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Miller L H, Quakyi I A, Keister D B, Houghten R A, Maloy W L, Moss B, Berzofsky J A, Good M F. Nature (London) 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 16.Rathore D, McCutchan T F. Infect Immun. 2000;68:740–743. doi: 10.1128/iai.68.2.740-743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos M J, Nussenzweig V. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 18.Sinnis P, Clavijo P, Fenyo D, Chait B T, Cerami C, Nussenzweig V. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying P, Shakibaei M, Patankar M S, Clavijo P, Beavis R C, Clark G F, Frevert U. Exp Parasitol. 1997;85:168–182. doi: 10.1006/expr.1996.4134. [DOI] [PubMed] [Google Scholar]
- 20.Aley S B, Bates M D, Tam J P, Hollingdale M R. J Exp Med. 1986;164:1915–1922. doi: 10.1084/jem.164.6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich K A, George F W T, Law J L, Martin W J. Science. 1990;249:1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- 22.Gantt S M, Clavijo P, Bai X, Esko J D, Sinnis P. J Biol Chem. 1997;272:19205–19213. doi: 10.1074/jbc.272.31.19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hileman R E, Fromm J R, Weiler J M, Linhardt R J. BioEssays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Nader H B, Dietrich C P. In: Heparin: Chemical and Biological Properties Clinical Applications. Lane D A, Lindahl U, editors. Boca Raton, FL: CRC; 1989. pp. 81–96. [Google Scholar]
- 25.Schulman S, Oppenheim J D, Vanderberg J P. Exp Parasitol. 1980;49:420–429. doi: 10.1016/0014-4894(80)90076-4. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan J A, Do Rosario V, Leland P, Adjepong A, Light J, Woollett G R, Hollingdale M R, Azad A F. Exp Parasitol. 1988;66:171–182. doi: 10.1016/0014-4894(88)90088-4. [DOI] [PubMed] [Google Scholar]
- 27.Hollingdale M R, do Rosario V. Exp Parasitol. 1989;68:365–368. doi: 10.1016/0014-4894(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 28.Love D C, Esko J D, Mosser D M. J Cell Biol. 1993;123:759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]