Abstract
Primary adrenal lymphoma is a very rare extranodal lymphoma; its clinical features consist of a high incidence of bilateral adrenal involvement and diffuse large B-cell lymphoma. We report a patient with primary bilateral adrenal diffuse large B-cell lyphoma who achieved complete remission with R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. A 52-yr-old man presented with fever and progressive fatigue for 3 months. Computed tomography (CT) scans of the abdomen and pelvis demonstrated large bilateral adrenal masses, and a needle biopsy of the left adrenal mass revealed diffuse large B-cell lymphoma. After 6 cycles of R-CHOP chemotherapy, CT scans showed no residual disease. To our knowledge, this is the second report to date of a patient with primary bilateral adrenal diffuse large B-cell lymphoma who achieved complete remission using R-CHOP chemotherapy.
Keywords: Primary Adrenal Lymphoma; Lymphoma, Large B-Cell, Diffuse; R-CHOP; Complete Remission
INTRODUCTION
Although secondary involvement of the adrenal gland has been reported to occur in up to 25% of patients with non-Hodgkin's lymphoma (NHL) (1), primary adrenal lymphoma (PAL) is very rare, with only about 80 cases reported in the English language literature (2). Treatment of PAL is similar to that of other types of lymphoma, but prognosis is poor. Therapeutic modalities include surgery, combination chemotherapy, and/or radiotherapy. Among the chemotherapy regimens utilized are CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), and MACOP-B (adding methotrexate and bleomycin) (2). Here, we describe a patient with primary bilateral adrenal diffuse large B-cell lymphoma who achieved complete remission after treatment with rituximab plus CHOP (R-CHOP).
CASE REPORT
A 52-yr-old man with no prior medical history was admitted for a 3-month history of fever and progressive fatigue. He appeared chronically ill, but alert. Blood pressure was 100/70 mmHg, pulse rate was 102 bpm, respiratory rate was 22 bpm, and body temperature was 38.5℃. Examination of head and neck was unremarkable; no abnormality was found in chest; no hepatosplenomegaly or mass was detected in the abdomen; and there was no evidence of lymphadenopathy or abnormal skin lesions.
A complete blood count showed hemoglobin 10.4 g/dL, hematocrit 30.0%, white blood cell count 5,000/µL, and platelet count 104,000/µL. Sodium level was 126 mM/L (normal range, 133-150 mM/L), but potassium and calcium levels were normal. Serum β2-microglobulin concentration was 4.7 µg/mL (normal range, 1-2.4 µg/mL), serum lactate dehydrogenase (LDH) level was 476 IU/L (normal range, 120-250 IU/L), serum cortisol concentration at 8 AM was 2.9 µg/dL (normal range, 7-25 µg/dL), plasma ACTH level at 8 AM was 210 pg/mL (normal range, 9-52 pg/mL), and serum aldosterone concentration was 56.2 pg/mL (normal range, 20-160 pg/mL). Urinalysis showed that 24 hr excretion of vanillylmandelic acid was 5.9 mg/day (normal range, 0-8 mg/day), of epinephrine was 21.5 µg/day (normal range, 0-20 µg/day), of norepinephrine was 202.1 µg/day (normal range, 1-130 µg/day), and of total metanephrine was 0.6 mg/day (normal range 0-1.3 mg/day). Rapid cosyntropin (analog of ACTH) stimulation test, using 250 µg of intravenous cosyntropin, demonstrated plasma cortisol concentrations at 0, 30 and 60 min of 9.5, 10.0 and 10.5 µg/dL, respectively (reference range, at least 7 µg/dL at 0 min, 16 µg/dL at 30 min, and 18 µg/dL at 60 min), strongly suggesting adrenal insufficiency.
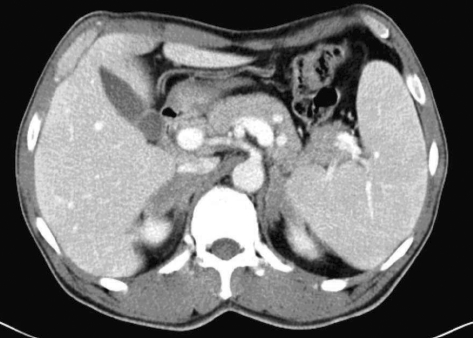
Chest radiography was normal without evidence of hilar lymphadenopathy. Computed tomography (CT) scans of abdomen and pelvis demonstrated large bilateral adrenal masses. The right adrenal gland measured 5.8×2.7 cm, and the left adrenal gland measured 3.7×2.0 cm in its greatest dimension (Fig. 1).
Fig. 1.
Abdominal pelvic CT scan showing bilateral adrenal masses.
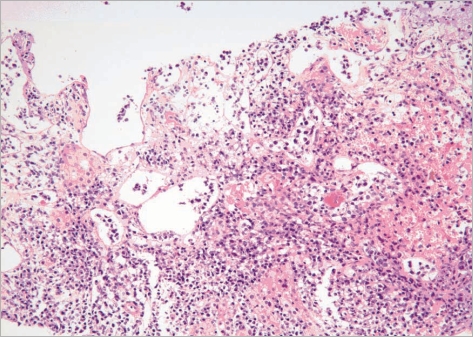
A CT-guided core needle biopsy of the left adrenal mass was performed. The tumor was comprised of large cells, which were immunohistochemically positive for CD20, but negative for CD3 and CD30. The Ki-67 labeling index was about 90%. The tumor was diagnosed as a diffuse large B-cell lymphoma (Fig. 2).
Fig. 2.
CT-guided core needle biopsy of the left adrenal mass showing a tumor comprised of large cells, immunohistochemically positive for CD20 but negative for CD3 and CD30. The Ki-67 labeling index was about 90%. The tumor was diagnosed as a diffuse large B-cell lymphoma.
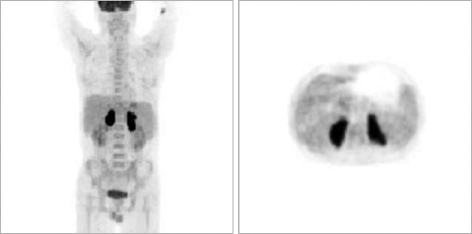
Bone marrow biopsy was negative for lymphomatous involvement. An F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) scan showed intense FDG accumulation in both adrenal glands. There was no abnormal FDG uptake in the rest of the body (Fig. 3).
Fig. 3.
F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) scan showing intense FDG accumulation in both adrenal glands. There was no abnormal FDG uptake in the rest of the body.
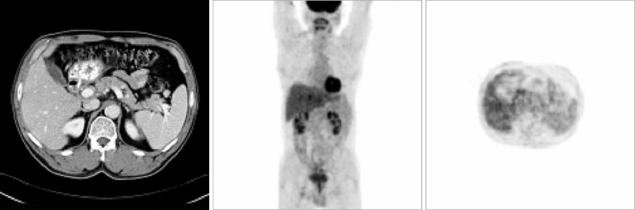
The patient was administered adrenal hormone replacement therapy with 20 mg hydrocortisone at 8 AM and 10 mg at 4 PM. He was also administered R-CHOP chemotherapy, consisting of rituximab (375 mg/m2 i.v.), cyclophosphamide (750 mg/m2 i.v.), doxorubicin (50 mg/m2 i.v.), and vincristine (1.4 mg/m2 i.v.), on day 1, and prednisolone (100 mg p.o.) on days 1-5, every 3 weeks. After completing 6 cycles of R-CHOP chemotherapy, there was no evidence of tumor on follow-up CT and FDG-PET scans (Fig. 4). To date, this patient has been followed regularly at our outpatient clinic for the past 12 months, with no evidence of tumor recurrence.
Fig. 4.
Follow-up CT and FDG-PET scans after 6 cycles of R-CHOP chemotherapy showing no evidence of tumor.
DISCUSSION
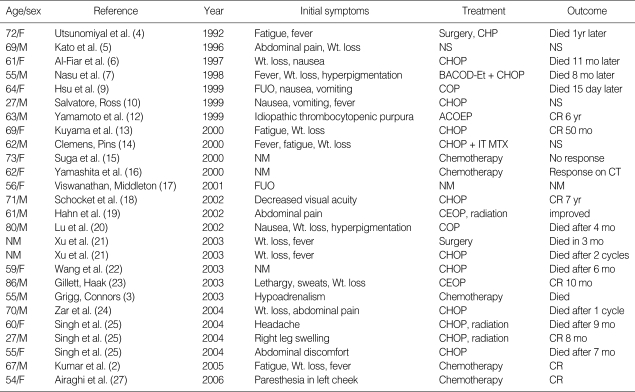
PAL is a very rare lymphoma, with only about 80 cases reported in the English language literature (2, 3). Histologically, the most common type is diffuse large B-cell lymphoma, with most originating in both adrenal glands (Table 1). PAL is most common in elderly men; the mean age at diagnosis is 68 yr and the male-to-female ratio is 2.2:1. Clinical symptoms of PAL include fever, weight loss, local pain, and symptoms of adrenal insufficiency (8). Immune dysfunction may be a predisposing factor in some patients (3). A review of 55 PAL patients showed that 8 (15%) had a concurrent or past history of carcinoma, 2 (4%) had human immunodeficiency virus (HIV) infection, and 7 (13%) had concomitant autoimmune disease (8).
Table 1.
List of documented cases of primary bilateral adrenal diffuse large B-cell lymphoma in the English language literature
A, adriamycin; B, bleomycin; B/L, bilateral; C, cyclophosphamide; CR, complete remission; D, dexamethasone; E, epirubicin; Et, etoposide; FUO, fever of unknown origin; H, doxorubicin; IT, intrathecal; L, left; M, mitozantrone; Met, metastasis; MTX, methotrexate; mo, month; NM, not mentioned; NS, not specified; O, vincristine; P, prednisolone; R, right; Wt, weight.
On CT and magnetic resonance imaging (MRI), PALs tend to appear as complex masses of variable density and often have areas of necrosis and/or hemorrhage, with most tumors being bilateral (8). Because most PAL patients have adrenal involvement only, without any nodal involvement, whole body FDG-PET, which measures increased glucose metabolism and has been used in tumor diagnosis and staging, in monitoring response to treatment, and detecting recurrence, has shown intense FDG uptake only in the adrenal glands (2). In our patient, whole-body FDG-PET demonstrated FDG uptake in the adrenal masses, with no evidence of abnormal uptake in extraadrenal regions.
Tissue samples can be obtained by CT- or ultrasound-guided biopsy or by surgical excisional biopsy (3, 8, 11). Histologically, the most common type of PAL is diffuse large B-cell lymphoma (2, 3, 8). These neoplastic cells express antigens specific to B lymphocytes, such as CD20, CD74, CDw75, and CD79a, while not expressing antigens specific to T lymphocytes, such as CD3, CD5, CD43, and CD45RO (26).
Because of the rarity of PAL, therapeutic regimens have not been defined in detail (2), with most patients treated with regimens similar to those used to treat other types of lymphoma. Therapeutic modalities include surgery, combination chemotherapy, and radiation (8). Most patients with PAL have been treated with CHOP and the remainder with CVP or MACOP-B (2). The roles of radiation therapy and surgical excision of the adrenal glands in the context of bilateral involvement are not known (3). The prognosis of patients with PAL is very poor, with uncertain duration of survival (3, 8, 22). Poor prognosis is thought to be due to advanced age at diagnosis, large tumor size, increased LDH level, bilateral adrenal involvement (regarded as stage IV disease), and adrenal insufficiency at the time of presentation, indicating extensive adrenal destruction (3).
The most common regimen of PAL is CHOP, with a 71-yr-old man with primary bilateral adrenal diffuse large B-cell lymphoma reported to have continued in complete remission for 7 yr after treatment with the CHOP regimen (18). Recent evidence suggests that the outcome of elderly patients with diffuse large B-cell lymphoma is improved by the addition of rituximab to CHOP (28). Our patient, who was administered 6 cycles of R-CHOP every 3 weeks, showed complete remission on follow-up CT and FDG-PET. Although a 62-yr-old man with primary bilateral diffuse large B-cell lymphoma who was treated with a left adrenalectomy, R-CHOP, radiotherapy, and high-dose chemotherapy with autologous peripheral blood stem cell transplantation, has been disease-free for 2 yr, this report was in Japanese (29). Moreover, in contrast to this patient, our patient received only R-CHOP chemotherapy, without surgery, radiation or high-dose therapy.
In conclusion, this may be the second report of a patient with primary bilateral adrenal diffuse large B-cell lymphoma who achieved complete remission using R-CHOP chemotherapy.
References
- 1.Rosenberg SA, Diamond HD, Jaslowitz B, Craver LF. Lymphosarcoma: a review of 1269 cases. Medicine (Baltimore) 1961;40:31–84. doi: 10.1097/00005792-196102000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Xiu Y, Mavi A, El-Haddad G, Zhuang H, Alavi A. FDG-PET imaging in primary bilateral adrenal lymphoma: a case report and review of the literature. Clin Nucl Med. 2005;30:222–230. doi: 10.1097/01.rlu.0000155983.46815.1c. [DOI] [PubMed] [Google Scholar]
- 3.Grigg AP, Connors JM. Primary adrenal lymphoma. Clin Lymphoma. 2003;4:154–160. doi: 10.3816/clm.2003.n.024. [DOI] [PubMed] [Google Scholar]
- 4.Utsunomiya M, Takatera H, Itoh H, Tsujimura T, Itatani H. Bilateral primary non-Hodgkin's lymphoma of the adrenal glands with adrenal insufficiency: a case report. Hinyokika Kiyo. 1992;38:311–314. [PubMed] [Google Scholar]
- 5.Kato H, Itami J, Shiina T, Uno T, Arimizu N, Fujimoto H, Mikata A, Tamaru J, Araki A. MR imaging of primary adrenal lymphoma. Clin Imaging. 1996;20:126–128. doi: 10.1016/0899-7071(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 6.Al-Fiar FZ, Pantalony D, Shepherd F. Primary bilateral adrenal lymphoma. Leuk Lymphoma. 1997;27:543–549. doi: 10.3109/10428199709058323. [DOI] [PubMed] [Google Scholar]
- 7.Nasu M, Aruga M, Itami J, Fujimoto M, Matsubara O. Non-Hodgkin's lymphoma presenting with adrenal insufficiency and hypothyroidism: an autopsy case report. Pathol Int. 1998;48:138–143. doi: 10.1111/j.1440-1827.1998.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Sun NC, Renslo R, Chuang CC, Tabbarah HJ, Barajas L, French SW. Clinically silent primary adrenal lymphoma: a case report and review of the literature. Am J Hematol. 1998;58:130–136. doi: 10.1002/(sici)1096-8652(199806)58:2<130::aid-ajh8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CW, Ho CL, Sheu WH, Harn HJ, Chao TY. Adrenal insufficiency caused by primary aggressive non-Hodgkin's lymphoma of bilateral adrenal glands: report of a case and literature review. Ann Hematol. 1999;78:151–154. doi: 10.1007/s002770050492. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore JR, Ross RS. Primary bilateral adrenal lymphoma. Leuk Lymphoma. 1999;34:111–117. doi: 10.3109/10428199909083386. [DOI] [PubMed] [Google Scholar]
- 11.Fearon P, Rahilly M. Primary adrenal B cell lymphoma. Hosp Med. 1999;60:759–760. doi: 10.12968/hosp.1999.60.10.1224. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto E, Ozaki N, Nakagawa M, Kimoto M. Primary bilateral adrenal lymphoma associated with idiopathic thrombocytopenic purpura. Leuk Lymphoma. 1999;35:403–408. doi: 10.3109/10428199909145746. [DOI] [PubMed] [Google Scholar]
- 13.Kuyama A, Takeuchi M, Munemasa M, Tsutsui K, Aga N, Goda Y, Kanetada K. Successful treatment of primary adrenal non-Hodgkin's lymphoma associated with adrenal insufficiency. Leuk Lymphoma. 2000;38:203–205. doi: 10.3109/10428190009060336. [DOI] [PubMed] [Google Scholar]
- 14.Clemens JQ, Pins MR. NonHodgkin's lymphoma presenting as bilateral testicular and adrenal masses. J Urol. 2000;163:241–242. [PubMed] [Google Scholar]
- 15.Suga K, Ishikawa Y, Matsunaga N, Motoyama K, Hara A. Ga-67 and I-131 adosterol scintigraphic findings of bilateral primary adrenal lymphoma. Clin Nucl Med. 2000;25:718–720. doi: 10.1097/00003072-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita E, Kobayashi T, Suzuki Y. Primary bilateral adrenal involvement of non-Hodgkin's lymphoma shown on a Ga-67 citrate scan. Clin Nucl Med. 2000;25:721–722. doi: 10.1097/00003072-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Viswanathan V, Middleton ML. Primary adrenal lymphoma: a case report. Clin Nucl Med. 2001;26:787–788. doi: 10.1097/00003072-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Schocket LS, Syed NA, Fine SL. Primary adrenal lymphoma with choroidal metastases. Am J Ophthalmol. 2002;134:775–776. doi: 10.1016/s0002-9394(02)01681-1. [DOI] [PubMed] [Google Scholar]
- 19.Hahn JS, Choi HS, Suh CO, Lee WJ. A case of primary bilateral adrenal lymphoma (PAL) with central nervous system (CNS) involvement. Yonsei Med J. 2002;43:385–390. doi: 10.3349/ymj.2002.43.3.385. [DOI] [PubMed] [Google Scholar]
- 20.Lu JY, Chang CC, Chang YL. Adrenal lymphoma and Addison's disease: report of a case. J Formos Med Assoc. 2002;101:854–858. [PubMed] [Google Scholar]
- 21.Xu A, Xiao X, Ye L, Hong B, Wang X. Primary adrenal lymphoma. Leuk Lymphoma. 2003;44:739–740. doi: 10.1080/1042819021000047047. [DOI] [PubMed] [Google Scholar]
- 22.Wang FF, Su CC, Chang YH, Pan CC, Tang KT, Jap TS, Lin HD, Won JG. Primary adrenal lymphoma manifestating as adrenal incidentaloma. J Chin Med Assoc. 2003;66:67–71. [PubMed] [Google Scholar]
- 23.Gillett M, Haak S. Not just another fall in the elderly. Bilateral adrenal lymphoma presenting with adrenal insufficiency causing weakness. Aust Fam Physician. 2003;32:248–250. [PubMed] [Google Scholar]
- 24.Zar T, Khan F, Petit W, Jr, Bernene JR. Primary adrenal lymphoma presenting as adrenal insufficiency. A case report and review of literature. Conn Med. 2004;68:7–10. [PubMed] [Google Scholar]
- 25.Singh D, Kumar L, Sharma A, Vijayaraghavan M, Thulkar S, Tandon N. Adrenal involvement in non-Hodgkin's lymphoma: four cases and review of literature. Leuk Lymphoma. 2004;45:789–794. doi: 10.1080/10428190310001615756. [DOI] [PubMed] [Google Scholar]
- 26.Libe R, Giavoli C, Barbetta L, Dall'Asta C, Passini E, Buffa R, Beck-Peccoz P, Ambrosi B. A primary adrenal non-Hodgkin's lymphoma presenting as an incidental adrenal mass. Exp Clin Endocrinol Diabetes. 2006;114:140–144. doi: 10.1055/s-2005-865913. [DOI] [PubMed] [Google Scholar]
- 27.Airaghi L, Greco I, Carrabba M, Barcella M, Baldini IM, Bonara P, Goldaniga M, Baldini L. Unusual presentation of large B cell lymphoma: a case report and review of literature. Clin Lab Haematol. 2006;28:338–342. doi: 10.1111/j.1365-2257.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 28.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 29.Shirao S, Kuroda H, Kida M, Watanabe H, Matsunaga T, Niitsu Y, Konuma Y, Hirayama Y, Kohda K. Effective combined modality therapy for a patient with primary adrenal lymphoma. Rinsho Ketsueki. 2006;47:204–209. [PubMed] [Google Scholar]