Abstract
Maternal environment has been demonstrated to produce considerable impact on offspring growth. However, few studies have been carried out to investigate multi-generational maternal effects of elevated CO2 on plant growth and development. Here we present the first report on the responses of plant reproductive, photosynthetic, and cellular characteristics to elevated CO2 over 15 generations using Arabidopsis thaliana as a model system. We found that within an individual generation, elevated CO2 significantly advanced plant flowering, increased photosynthetic rate, increased the size and number of starch grains per chloroplast, reduced stomatal density, stomatal conductance, and transpiration rate, and resulted in a higher reproductive mass. Elevated CO2 did not significantly influence silique length and number of seeds per silique. Across 15 generations grown at elevated CO2 concentrations, however, there were no significant differences in these traits. In addition, a reciprocal sowing experiment demonstrated that elevated CO2 did not produce detectable maternal effects on the offspring after fifteen generations. Taken together, these results suggested that the maternal effects of elevated CO2 failed to extend to the offspring due to the potential lack of genetic variation for CO2 responsiveness, and future plants may not evolve specific adaptations to elevated CO2 concentrations.
Introduction
Over the next century, the atmospheric CO2 concentration is projected to rise from the current level of about 370 parts per million (ppm) to between 540 and 970 ppm [1]. Given that CO2 is the raw material of photosynthesis, this global change will have profound effects on the structure and function of future plant populations [2]–[8]. A typical experimental approach used in most CO2 experiments to predict how future plants will respond to these changes is to expose individual plants or plant communities to ambient and elevated CO2 within a part, or one generation and compare their responses [3], [9]–[15]. Even several long-term studies of trees, lasting up to 30 years, are still limited to one generation [16]–[18]. A major assumption in such experiments is that the responses of plants to elevated CO2 within one generation can be similar to those observed over many generations. However, an experimental test of the assumption is currently unavailable.
Earlier investigations frequently used seed-propagated annuals as the experimental materials and revealed that maternal environmental conditions became manifest in seed characters which, in turn, may influence the performance of the offspring by altering seed germination, seedling survival and growth [19]–[24]. In other words, plastic response to the environment may extend to an individual's offspring, influencing offspring trait expression [20], [25]. As a consequence, most of the previous studies investigating the responsiveness of plants to elevated CO2 within one generation failed to notice the maternal effects on the offspring growth and the predictions based on the results from such experiments can be challenged. It is necessary, therefore, to illustrate the importance and necessity of examining CO2 response of plants over more than one generation to make accurate predictions about biological consequences of increasingly rising atmospheric CO2 concentration.
Here, we carried out a fifteen-generation selection experiment and a reciprocal sowing experiment (over 5 years in total) using Arabidopsis thaliana (wild-type Columbia) as a model plant to examine multi-generation maternal effects of elevated CO2 on plant growth and development. Arabidopsis is an ideal plant for investigating this issue for three main reasons. First, the short generation time allows us to study the responses of many generations over a reasonably short period of time. Second, its small size makes it possible to grow a large population of plants under controlled CO2 conditions. Finally, the life history and allocation strategy of Arabidopsis is common to numerous annuals that have a short generation time and allocate a high proportion of their resources to reproduction. Thus, the responses of Arabidopsis may provide valuable insights into the responses of various annuals to the rising atmospheric CO2 concentration [2], [26].
On the basis of our studies over the past several years [27]–[29], this study is part of a series examining multi-generational maternal effects of elevated CO2 on Arabidopsis. Our overall aim is to reveal the physiological, cytological, and reproductive responses to elevated CO2, to determine if elevated CO2 can produce maternal effects on plant growth and development across fifteen generations, and to test if the responses of plants to elevated CO2 within one generation will be similar to those observed over many generations.
Results
Reproductive responses to elevated CO2
The date of opening of the first flower was significantly affected by the CO2 treatment (Figure 1). On average, plants grown at elevated CO2 concentrations flowered about three days earlier than those grown at ambient CO2 concentrations in each generation. However, within the same CO2 treatment, the average number of days to first flowering in any two generations was similar, and no significant difference was observed in flowering time among the 15 generations. For example, the average number of days to first flowering in any generation averaged around 40.5 for the populations at elevated CO2 and 44 for those at ambient CO2. Taken together, within an individual generation, CO2 treatment resulted in a significant change in flowering time, whereas no significant changes in flowering time were detected among generations within the same CO2 treatment.
Figure 1. Effects of elevated CO2 on the flowering time of Arabidopsis thaliana over 15 generations.
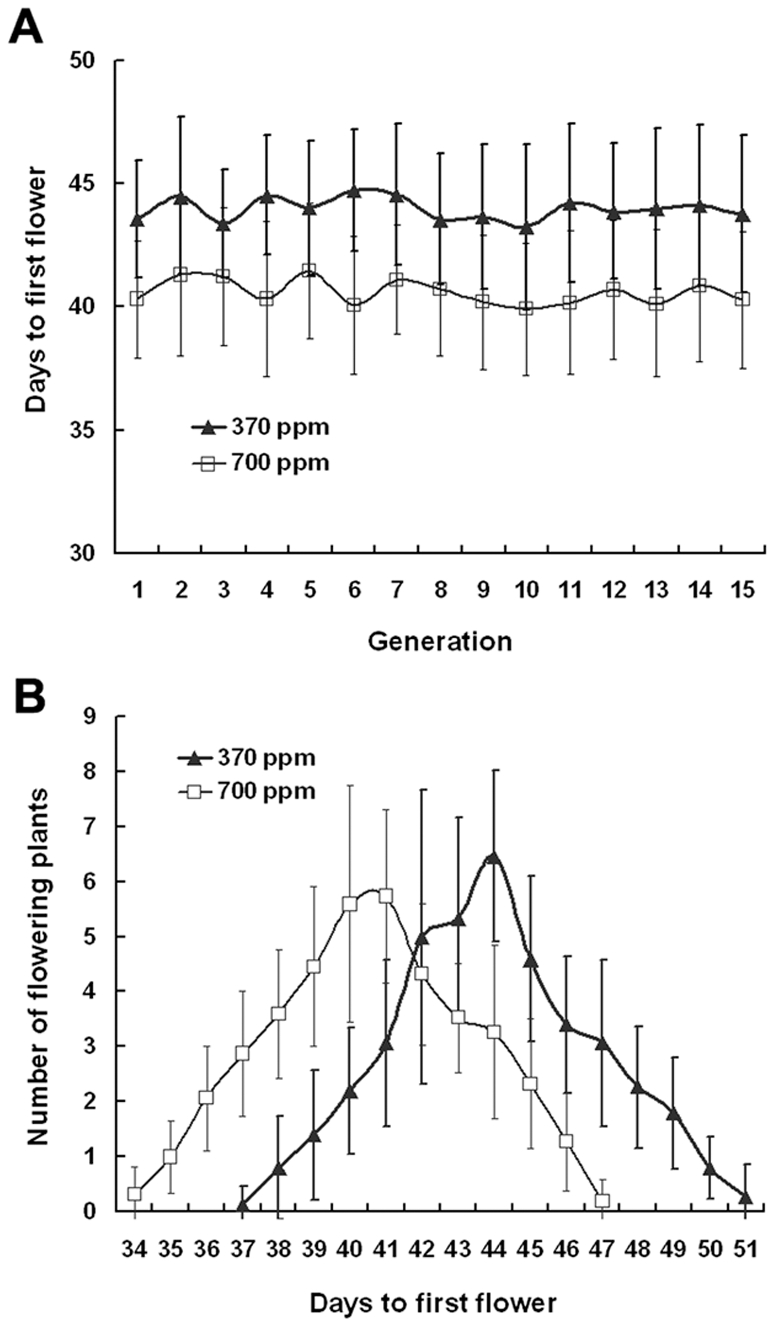
A, On average, plants grown in elevated CO2 flowered significantly earlier than those grown in ambient CO2 concentrations within each generation. B, The number of flowering plants per day was recorded in ambient and elevated CO2 across 15 generations. Error bars represent the standard deviation of the mean.
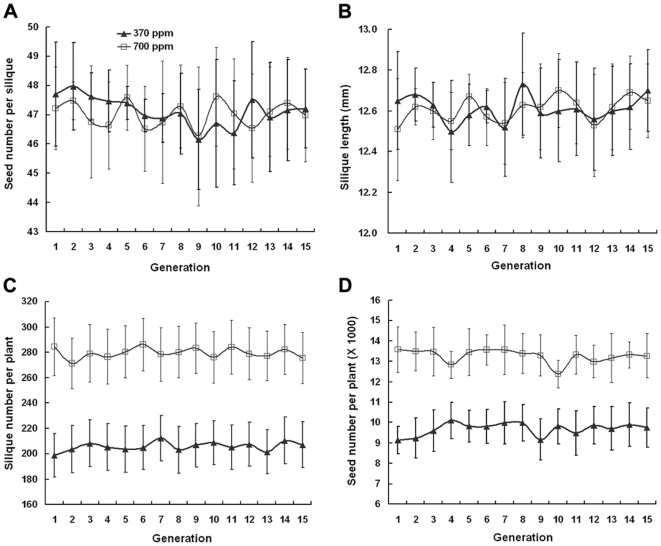
The number of seeds per silique and silique length did not change significantly in response to CO2 treatment (Figure 2A and B). However, we detected significant treatment effects on the number of siliques and the number of seeds per plant (Figure 2C and D). The average number of siliques and seeds per plant across generations in the elevated treatment were significantly higher than those in the ambient treatment. For example, the average number of siliques and seeds per plant exposed to elevated CO2 concentrations were about 36% and 37% higher, respectively, than those exposed to ambient CO2 concentrations. In the same CO2 treatment, however, the number of siliques and the number of seeds per plant did not differ significantly across generations. Across generations, the average number of siliques per plant in the elevated and ambient CO2 treatments averaged around 280 and 206, respectively. Similarly, the average number of seeds per treatment across the 15 generations averaged around 13,000 for elevated CO2 and 9,500 for ambient CO2.
Figure 2. Effects of elevated CO2 on silique length and number of siliques and seeds.
A and B, elevated CO2 had no significant effect on the number of seeds per silique or silique length. C and D, elevated CO2 significantly increased the number of siliques and the number of seeds per plant. Error bars represent the standard deviation of the mean.
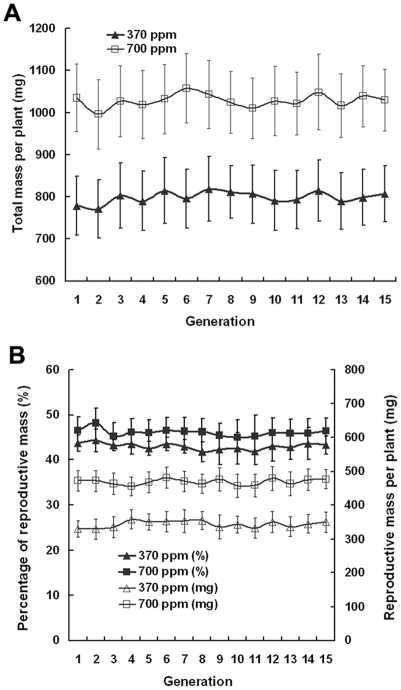
The reproductive mass, total mass per plant, and percentage of reproductive mass per plant were higher with elevated CO2 than with ambient CO2 concentrations. However, these traits did not change significantly across generations in either treatment (Figure 3). On average, the total mass and reproductive mass per plant when grown at elevated CO2 levels were about 1020 and 470 mg, respectively, representing increases of about 27% and 36% over those grown at ambient CO2 concentrations. A similar trend was observed for the relative proportion (%) of reproductive mass per plant, increasing from about 43% in ambient CO2 to about 46% in elevated CO2, indicating that more mass was allocated to reproductive growth at elevated CO2 concentrations. Within the same CO2 treatment, each of these traits was similar across 15 generations, demonstrating that changes in the traits induced by elevated CO2 failed to transfer from one generation to the next via reproduction.
Figure 3. Effects of elevated CO2 on total and reproductive mass.
Elevated CO2 significantly increased total mass (A), reproductive mass, and the relative proportion (%) of reproductive mass per plant (B). Error bars represent the standard deviation of the mean.
Responses of stomatal and photosynthetic traits to elevated CO2
Elevated CO2 significantly reduced stomatal density on both adaxial and abaxial leaf surfaces of plants grown in generations 1, 8, and 15 and in reciprocal sowing experiments (Table 1). For example, on average, stomatal density on the adaxial and abaxial leaf surfaces of plants in these generations was significantly decreased by 15.5% and 12.1% with elevated CO2, respectively. However, stomatal density did not change significantly among these generations in either treatment. For instance, stomatal density on the adaxial leaf surface averaged about 214 per mm2 at ambient CO2 concentrations, ranging from 207.2 to 219.4, and approximately 181 at elevated CO2 concentrations, ranging from 175.4 to 188.4.
Table 1. Stomatal density of leaves of Arabidopsis plants grown at elevated or ambient CO2 in different generations.
| Stomatal density | Adaxial surface | decrease | Abaxial surface | decrease | |||
| Treatment | AC | EC | AC | EC | |||
| Generation | 1 | 216.8±12.6a | 176.3±10.0b | 18.7% | 236.3±12.8a | 204.3±10.5b | 13.5% |
| 8 | 207.2±12.3a | 183.2±9.7b | 11.6% | 231.7±12.6 a | 202.8±11.5b | 12.5% | |
| 15 | 219.4±11.6a | 188.4±10.3b | 14.1% | 237.1±11.8 a | 210.7±10.8b | 11.1% | |
| SA* | 211.6±11.4a | 180.5±9.2b | 14.7% | 240.2±12.1a | 213.2±11.8b | 11.2% | |
| SE* | 215.5±11.1a | 175.4±10.1b | 18.6% | 234.7±10.9a | 205.9±11.2b | 12.3% | |
| P-value | 0.145–0.869 | 0.078–0.886 | / | 0.307–0.921 | 0.197–0.838 | / | |
| Average | 214.1 | 180.8 | 15.5% | 236 | 207.4 | 12.1% | |
The values given indicate means±SD from five plants. Three fully expanded rosette leaves at stage 5.0 were sampled from each of five plants and twenty separate fields were analyzed in each leaf. Mean values were compared by t-test.
The seeds used in the reciprocal sowing experiments were from the fifteenth generation grown in ambient CO2 (SA) and elevated CO2 (SE).
Abbreviations: AC: Ambient CO2; EC: Elevated CO2.
Elevated CO2 also significantly reduced stomatal conductance and transpiration rate, but increased the photosynthetic rate of Arabidopsis leaves in generations 1, 8, and 15 and in the reciprocal sowing experiments (Table 2). Relative to that in ambient CO2, stomatal conductance and transpiration rate in elevated CO2 were on average reduced by about 41.9% and 34.1%, respectively. However, compared to ambient CO2, elevated CO2 significantly increased photosynthetic rate with an average of 17.1% in these generations. Although elevated CO2 significantly affected stomatal conductance, transpiration rate, and photosynthetic rate within each generation, the three traits did not change significantly among these generations in either treatment. For example, stomatal conductance at elevated CO2 ranged from 220.4 to 239.4 mmol m−2 s−1 in these generations, with an average of about 227.9, and no significant difference was detected in stomatal conductance among these generations.
Table 2. Stomatal conductance, transpiration rate and photosynthetic rate of leaves of Arabidopsis plants grown at elevated or ambient CO2 in different generations.
| Photosynthetic features | Stomatal conductance (mmol m−2 s−1) | decrease | Transpiration rate (mmol m−2 s−1) | decrease | Photosynthetic rate (µmol m−2 s−1) | increase | ||||
| Treatment | AC | EC | AC | EC | AC | EC | ||||
| Generation | 1 | 385.6±23.2a | 220.4±14.0b | 42.8% | 8.16±0.41a | 5.18±0.24b | 36.5% | 14.1±1.2a | 16.3±1.4b | 15.6% |
| 8 | 410.4±25.5a | 239.4±15.6b | 41.7% | 8.32±0.40a | 5.46±0.25b | 34.4% | 14.8±1.5a | 17.2±1.3b | 16.2% | |
| 15 | 377.8±21.6a | 221.6±13.9b | 41.3% | 7.79±0.39a | 5.15±0.21b | 33.9% | 13.5±1.4a | 15.8±1.5b | 17.0% | |
| SA* | 382.0±20.4a | 227.8±13.3b | 40.4% | 7.91±0.45a | 5.34±0.28b | 32.5% | 13.9±1.4a | 16.6±1.3 | 19.4% | |
| SE* | 406.2±22.5a | 230.4±16.1b | 43.3% | 8.05±0.36a | 5.38±0.31b | 33.2% | 14.4±1.6a | 16.9±1.7b | 17.4% | |
| P-value | 0.061–0.801 | 0.078–0.895 | / | 0.067–0.650 | 0.067–0.838 | / | 0.204–0.813 | 0.162–0.808 | / | |
| Average | 392.4 | 227.9 | 41.9% | 8.05 | 5.30 | 34.1% | 14.1 | 16.6 | 17.1% | |
The values given indicate means±SD from five plants. Three fully expanded rosette leaves at stage 5.0 were sampled from each of five plants were analyzed for stomatal conductance, transpiration rate and photosynthetic rate. Mean values were compared by t-test.
The seeds used in the reciprocal sowing experiments were from the fifteenth generation grown in ambient CO2 (SA) and elevated CO2 (SE).
Abbreviations: AC: Ambient CO2; EC: Elevated CO2.
Responses of leaf ultrastructure to elevated CO2
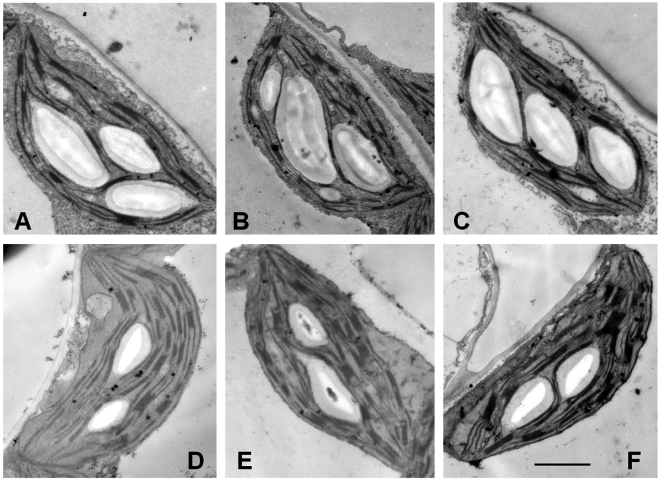
Relative to ambient CO2, elevated CO2 concentrations on average significantly increased the number of starch grains per chloroplast profile and area per starch grain by 42.4% and 51.9%, respectively, in leaves of plants grown in generations 1, 8, and 15 and in reciprocal sowing experiments (Table 3 and Figure 4). However, each of the traits did not change significantly among these generations when exposed to either ambient CO2 or elevated CO2 (Table 3 and Figure 4). For example, the number of starch grains per chloroplast profile averaged around 1.95 at ambient CO2 concentrations, ranging from 1.87 to 2.05, and around 2.77 with elevated CO2, ranging from 2.68 to 2.87. The change in area per starch grain also followed a similar pattern.
Table 3. Chloroplast feature of leaves of Arabidopsis plants grown at elevated or ambient CO2 in different generations.
| Chloroplast feature | Number of starch grains per chloroplast profile | increase | Area per starch grain (µm2) | increase | |||
| Treatment | AC | EC | AC | EC | |||
| Generation | 1 | 1.94±1.28a | 2.76±1.41b | 42.3% | 0.91±0.49a | 1.39±0.73b | 52.7% |
| 8 | 1.90±1.34a | 2.73±1.38b | 43.5% | 0.86±0.52a | 1.24±0.66b | 44.2% | |
| 15 | 1.99±1.41a | 2.87±1.43b | 44.3% | 0.81±0.57a | 1.29±0.72b | 59.3% | |
| SA* | 2.05±1.29a | 2.83±1.38b | 37.9% | 0.93±0.53a | 1.36±0.69 | 46.2% | |
| SE* | 1.87±1.22a | 2.68±1.31b | 43.8% | 0.84±0.51a | 1.32±0.61b | 57.1% | |
| P-value | 0.074–0.750 | 0.103–0.792 | / | 0.079–0.761 | 0.083–0.723 | / | |
| Average | 1.95 | 2.77 | 42.4% | 0.87 | 1.32 | 51.9% | |
The values given indicate means±SD from five plants. Number of starch grains per chloroplast profile was determined according to 300 chloroplasts. Area per starch grain was determined from 150 starch grains. The fully expanded rosette leaves were sampled at stage 5.0. Mean values were compared by t-test.
The seeds used in the reciprocal sowing experiments were from the fifteenth generation grown in ambient CO2 (SA) and elevated CO2 (SE).
Abbreviations: AC: Ambient CO2; EC: Elevated CO2.
Figure 4. Effects of elevated CO2 on leaf chloroplast ultrastructure during different generations.
Plants were grown in elevated CO2 in generations 1 (A), 8 (B), and 15 (C), and under ambient CO2 in generations 1 (D), 8 (E), and 15 (F). Note that more and larger starch grains were observed in chloroplasts of elevated-CO2 grown leaves than in chloroplasts of ambient-CO2 grown leaves in any of the three generations. However, there was no significant difference in the number and size of starch grains in either treatment among the three generations. Scale bar = 1 µm.
Evidence from reciprocal sowing experiments
To evaluate whether Arabidopsis plants exhibited an adaptive response to elevated CO2, we conducted a reciprocal sowing experiment in which seeds from the fifteenth generation in each treatment were grown at both ambient and elevated CO2 concentrations. As a result, we did not detect significant interactions between the maternal CO2 environment and the CO2 transplant environment (Figure 5, and Tables 1, 2, 3). Plants from fifteenth-generation seeds grown under ambient and elevated CO2 were similar, with no significant differences in several traits between the two populations under either CO2 treatment regime. In other words, at a given CO2 concentration, the traits of both populations were similar to those observed at that CO2 level during the selection experiment. For example, the average time to first flowering was about 44 days in both populations when grown at ambient CO2 during the sowing experiment and was similar to that at ambient CO2 during the selection experiment (Figures 1A, 5A). Similarly, the average time to first flowering was about 40.5 days in both populations when plants were grown at elevated CO2, which was not significantly different from that at elevated CO2 during the selection experiment (Figures 1A, 5A). There were similar patterns for the change in silique number per plant, stomatal density in both adaxial and abaxial leaf surfaces, stomatal conductance, transpiration rate, photosynthetic rate, and chloroplast features during the sowing experiment (Figure 5, and Tables 1, 2, 3).
Figure 5. Days to first flower and number of siliques during the reciprocal sowing experiments.
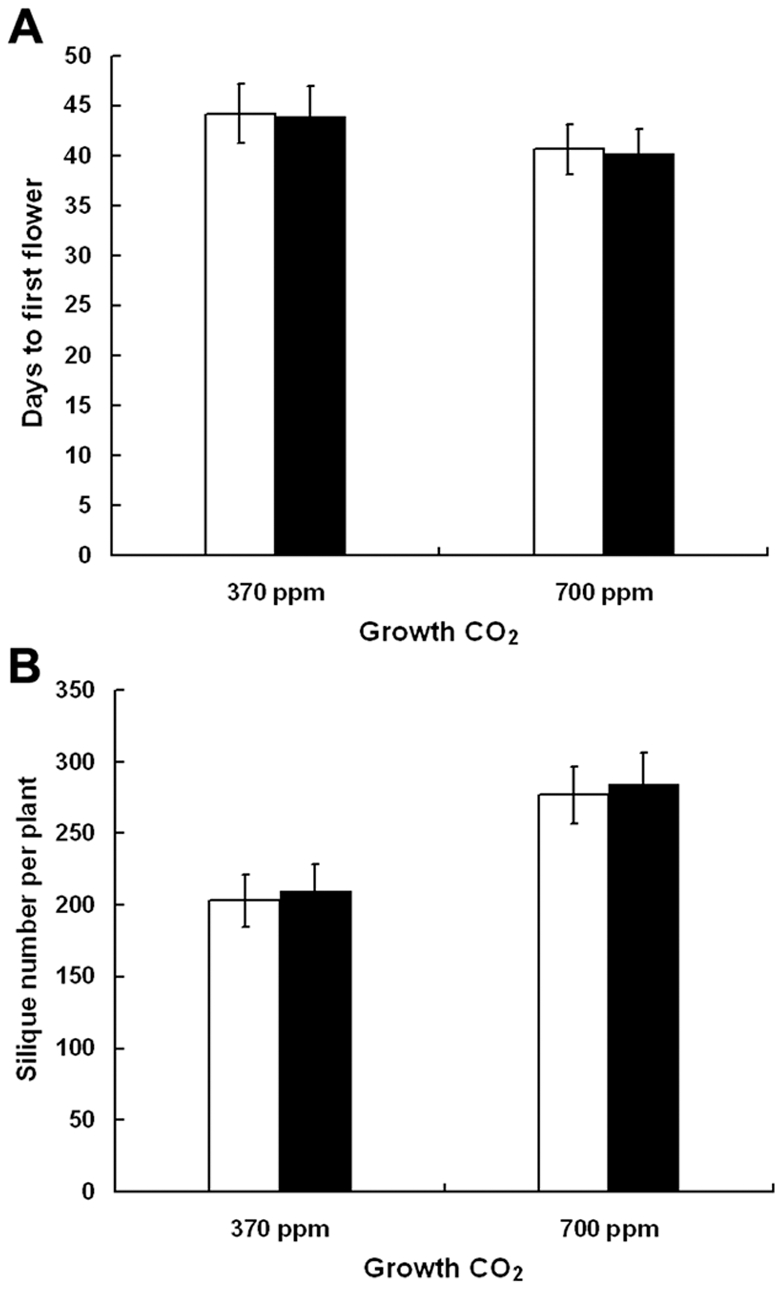
Open and solid bars indicate that seeds were obtained from the plants of the fifteenth generation grown at ambient and elevated CO2 concentrations, respectively. Seed source had no significant effect on days to first flower of plants (A) or number of siliques per plant (B).
Discussion
Many studies have investigated plant responses to elevated CO2 on the ecosystem, community, population, plant, leaf, physiological, biochemical, and molecular levels over the past two decades, most of which were carried out on plants grown only for a single generation of plants [2], [24], [29]–[31]. The main results from those studies indicate that elevated CO2 generally accelerates plant growth and development [27], [32], [33], advances flowering time [31], [34], [35], reduces stomatal density, stomatal conductance, and transpiration rate [6], [27], [36], increases photosynthetic rate and carbohydrate content [4], [27], [31], and enhances reproductive output by altering flower number, fruit set, and seed production [13]. For example, Woodward and Kelly [37] reported an average reduction in stomatal density of 14.3% for 100 species grown under CO2 enrichment. In addition, Jablonski et al. [13] used meta-analysis to integrate data on eight reproductive characteristics from 159 CO2 enrichment papers that provided information on 79 species and found that, on average, elevated CO2 increased fruits, seeds, and total seed mass by 19%, 18%, and 25%, respectively. In the present study, we found that elevated CO2 significantly advanced the flowering time of Arabidopsis, resulted in more siliques and seeds, reduced stomatal density, stomatal conductance, and transpiration rate, and increased photosynthetic rate and the size and number of starch grains in the chloroplast. These results are consistent with previous reports. However, silique length and number of seeds per silique were not influenced by elevated CO2, and it is possible that the two traits are less plastic than other traits in response to elevated CO2. Given that the number of seeds per silique changed only slightly with CO2 enrichment, the significant increase in the number of seeds per plant was mainly attributed to a significant increase in the number of siliques per plant.
In the current study, it was of great interest to find that within each of the 15 generations, elevated CO2 had significant effects on many traits including flowering time, total and reproductive mass, stomatal density and conductance, transpiration rate, and photosynthetic rate, but each of these traits in any two of the 15 generations was similarly responsive to elevated CO2. In other words, no significant difference was observed in each of these traits across the 15 generations within the same CO2 treatment, indicating that maternal CO2 had no significant effect on her offspring performance or transgenerational effects of CO2 were relatively small in this genotype. Our results led us to reject our hypothesis that plants in generation m+n would be more responsive to elevated CO2 than those in generation m (m≥1, n≥1). For instance, according to this hypothesis, if the days to first flowering for elevated CO2-grown plants in generation 1 averaged around 40.5, then the days for elevated CO2-grown plants in generation 1+n (n≥1) would be significantly shorter than 40.5. Our initial hypothesis was based on the assumption that elevated CO2 can exert a selective pressure on plants sufficient to produce genetic variation, and maternal responses to elevated CO2 may extend to the offspring and even accumulate via reproduction, influencing the offspring trait expression [2], [25]. This assumption, however, proved to be false, since all 15 generations were nearly equally responsive to elevated CO2 or maternal CO2 did not produce significant effects on the offspring. The contradictory resulted largely from the fact that elevated CO2 may generate immediate phenotypic change via phenotypic plasticity, but fails to produce genetic change [9], [23], [38]. Therefore, our results from 15 generations demonstrated that elevated CO2 significantly affected many traits and enhanced fitness of Arabidopsis plants within a single generation, but maternal effects of elevated CO2 did not influence the offspring trait expression largely due to the potential lack of genetic variation for CO2 responsiveness. Moreover, the results from the reciprocal sowing experiments confirmed that elevated CO2 did not produce detectable maternal effects on Arabidopsis even after 15 generations
Several studies have used a variety of plant species, including Arabidopsis thaliana [2], Sanguisorba minor [19], Bromus erectus [23], Cerastium glomeratum, Leontodon saxatilis, Poa pratensis and Trifolium repens [39], to investigate maternal effects of elevated CO2 on plant growth, most of which focused on the responses within a single generation. For example, Steinger et al. [23] reported the maternal and direct effects of elevated CO2 on seed provisioning, germination and seedling growth in B. erectus and found that seed germination rate and seedling size were not significantly affected by elevated maternal CO2. Similar results were also observed in algae, C. glomeratum and P. pratensis [39]–[41]. Our results from 15 generations and the reciprocal sowing experiments demonstrated that elevated CO2 failed to produce detectable maternal effects on the Arabidopsis plants. Although elevated CO2 cannot produce significant maternal effects on the offspring or transgenerational effects of elevated CO2 are very small, the mechanism for the non-detectable maternal effects is poorly understood. A possible explanation for this is that the advantages obtained such as increased seed mass at elevated maternal CO2 may be offset by the reduced concentration of nitrogen (and possibly other nutrients) or the increase in the C∶N ratio [23]. Another explanation may be that the selective pressure of elevated CO2 concentration is not high enough to generate genetic changes, unlike certain other factors including heavy metal contamination, drought, biological invasion, and global warming [38], [42]–[44].
In summary, elevated CO2 had a significant positive impact on some reproductive, photosynthetic, and cellular traits of Arabidopsis in the first generation, but the effect was not significantly strengthened after additional generations at elevated CO2. In addition, those traits measured at elevated CO2 were restored when the fifteenth-generation seeds were grown at ambient CO2 in the reciprocal sowing experiment. In other words, Arabidopsis can positively respond to elevated CO2 within each generation, but elevated maternal CO2 had no significant effect on her offspring across 15 generations. Moreover, our study provides convincing evidence to confirm the assumption widely accepted in many previous studies that plant responses to elevated CO2 observed within a single generation are similar to those observed over many generations. Our results also suggest that future plants may not produce specific adaptation to increasing atmospheric CO2 concentrations due to the potential lack of genetic variation for CO2 responsiveness.
Materials and Methods
Experimental design
Arabidopsis thaliana plants of Wild-type Columbia (the Nottingham Arabidopsis Stock Centre, Nottingham University, Nottingham, UK) were continuously grown for fifteen generations, each generation lasting over 14 weeks. Plants were subjected to one of two treatments: (1) ambient CO2 (370 ppm) in each generation or (2) elevated CO2 (700 ppm) in each generation, following a well established protocol [27]. After each generation, we measured various reproductive, photosynthetic and cellular traits and compared those traits between the two CO2 treatments. In addition, the traits in each generation of the same treatment (ambient or elevated) were compared. Furthermore, we performed a reciprocal sowing experiment to test if Arabidopsis had evolved detectable adaptations to elevated CO2 at the end of fifteen generations.
Selection and reciprocal sowing experiments
During the selection experiment, plants were grown for fifteen generations in two environment-controlled growth chambers. The seeds of Arabidopsis thaliana were first grown in the greenhouse, and seeds from the greenhouse-grown plants were used for the first generation. Generation m+1 was sown with seeds of plants from generation m (14≥m≥1) and 10% of the seeds from each individual plant were randomly selected and fully mixed for the next generation. To determine maternal responses at the end of fifteen generations in the selection experiment, we conducted a reciprocal sowing experiment. Seeds from the fifteenth generation at elevated CO2 were grown in both ambient and elevated CO2 growth chambers, as were seeds from the fifteenth generation at ambient CO2. For each generation, 35–45 plants were grown in each CO2 treatment. Plant growth and management followed a well established protocol [27].
Following previously described methods [24], [27], [34], [45], we used two chambers in the experiment: one chamber was controlled at 370±30 ppm and the other at 700±50 ppm. Throughout the experiment, other environmental factors including temperature, light, and relative humidity were identical in both growth chambers. The CO2 concentrations of the two chambers were swopped, and the pots were moved between chambers and randomly re-arranged weekly to negate any possible effects resulting from the chambers and pot position within the chambers and to minimize the potential for interactive effects between the chambers and developmental stages of plants.
Determination of reproductive traits
For each generation, the number of days to reach first flowering was recorded for each plant. Plants were harvested after a 14-week growth period. The number of siliques per plant was determined by counting all intact siliques and central siliques that persisted after seed maturity [2]. The average length (up to 1 mm) of siliques was determined from 30 siliques randomly selected from each of ten plants in each treatment. The total number of seeds per plant was calculated as the total number of siliques per plant multiplied by the mean number of seeds per silique (determined from 30 randomly selected siliques per plant). After plant material was dried to a constant weight at 60°C, vegetative mass, reproductive mass and total mass were determined, respectively.
Determination of stomatal, photosynthetic and cellular traits
When bolting had just commenced, i.e. at stage 5.10, fully expanded rosette leaves of plants in generation 1, 8, 15 and the reciprocal sowing experiment were respectively sampled for the analysis of stomatal density and leaf ultrastructure according to previous reports [27]. In addition, three fully expanded leaves from each of five plants were selected for the measurement of stomatal conductance, leaf transpiration rate as well as photosynthetic rate using an LI-6400 Portable Photosynthesis System (LI-COR Inc., Lincoln, Nebraska, USA). The measurements for ambient CO2-grown plants were carried out at 1500 µmol m−2 s−1 photosynthetically active radiation (PAR), 2.0–2.5 KPa vapour pressure deficit (VPD), 22–24°C and 380 ppm CO2, and for elevated CO2-grown plants at 1500 µmol m−2 s−1 PAR, 2.0–2.5 KPa VPD, 22–24°C and 700 ppm CO2.
Statistics
The data are shown as mean±standard deviation. Data were subjected to one-way analysis of variance and t-test using software SPSS 10.0 (SPSS Inc., Chicago, IL, USA) and Excel 2003 (Microsoft Inc.).
Acknowledgments
We thank Prof. Xinshi Zhang and Prof. Yuxi Hu for their expert guidance for this project, and Dr. Jinsheng He, Dr. Curt M. Peterson and Dr. Seth Pritchard for their valuable discussion at the early stages of these experiments and the preparation of this manuscript. We also thank Ms. Yuan Li and Ms. Guiling Hou for plant care.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Fund of China (30700081, 30730009, 30870436, 90211005), the National Science Fund of China for Distinguished Young Scholars (30225005), and funding from the International Foundation for Science for Dr. Nianjun Teng (Reference No.C/4560-1).
References
- 1.Watson R, Houghton J, Yihui D. Climate Change 2001: The Scientific Basis. Geneva: Intergovernmental Panel on Climate Change; 2001. [Google Scholar]
- 2.Ward JK, Antonovics J, Thomas RB, Strain BR. Is atmospheric CO2 a selective agent on model C3 annuals? Oecologia. 2000;123:330–341. doi: 10.1007/s004420051019. [DOI] [PubMed] [Google Scholar]
- 3.Ceulemans R, Jach ME, Velde R, Lin JX, Stevens M. Elevated atmospheric CO2 alters wood production, wood quality and wood strength of Scots pine (Pinus sylvestris L) after three years of enrichment. Global Change Biol. 2002;8:153–162. [Google Scholar]
- 4.Körner C. Plant CO2 responses: an issue of definition, time and resource supply. New Phytol. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 5.Norby RJ, Ledford J, Reilly CD, Miller NE, O'Neill EG. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA. 2004;101:9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodward FI. Potential impacts of global elevated CO2 concentrations on plants. Curr Opin Plant Biol. 2002;5:207–211. doi: 10.1016/s1369-5266(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 7.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 9.Bazzaz FA, Jasienski M, Thomas SC, Wayne P. Microevolutionary responses in experimental populations of plants to CO2-enriched environments: parallel results from two model systems. Proc Natl Acad Sci USA. 1995;92:8161–8165. doi: 10.1073/pnas.92.18.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang XZ. Reproduction and progeny of Silene latifolia (Caryophyllaceae) as affected by atmospheric CO2 concentration. Am J Bot. 2005;92:826–832. doi: 10.3732/ajb.92.5.826. [DOI] [PubMed] [Google Scholar]
- 11.Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 12.Körner C, Asshoff R, Bignucolo O, Hättenschwiler S, Keel SG, et al. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 2005;309:1360–1362. doi: 10.1126/science.1113977. [DOI] [PubMed] [Google Scholar]
- 13.Jablonski LM, Wang XZ, Curtis PS. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol. 2002;156:9–26. [Google Scholar]
- 14.Thürig B, Körner C, Stöcklin J. Seed production and seed quality in a calcareous grassland in elevated CO2. Global Change Biol. 2003;9:873–884. [Google Scholar]
- 15.Erhardt A, Rusterholz HP, Stöcklin J. Elevated carbon dioxide increases nectar production in Epilobium angustifolium L. Oecologia. 2005;146:311–317. doi: 10.1007/s00442-005-0182-5. [DOI] [PubMed] [Google Scholar]
- 16.Rasse DP, Peresta G, Drake B. Seventeen years of elevated CO2 exposure in a Chesapeake Bay wetland: sustained but contrasting responses of plant growth and CO2 uptake. Global Change Biol. 2005;11:369–377. [Google Scholar]
- 17.Idso SB, Kimball BA. Effects of long-term atmospheric CO2 enrichment on the growth and fruit production of sour orange trees. Global Change Biol. 1997;3:89–96. [Google Scholar]
- 18.Hättenschwiler S, Miglietta F, Raschi A, Körner C. Thirty years of in situ tree growth under elevated CO2: a model for future forest responses? Global Change Biol. 1997;3:436–471. [Google Scholar]
- 19.Wieneke S, Prati D, Brandl R, Stöcklin J, Auge H. Genetic variation in Sanguisorba minor after 6 years in situ selection under elevated CO2. Global Change Biol. 2004;10:1389–1401. [Google Scholar]
- 20.Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- 21.Roach DA, Wulff RD. Maternal effects in plants. Annu Rev Ecol Syst. 1987;18:209–235. [Google Scholar]
- 22.Rossiter MC. Incidence and consequences of inherited environmental effects. Annu Rev Ecol Syst. 1996;27:451–476. [Google Scholar]
- 23.Steinger T, Gall R, Schmid B. Maternal and direct effects of elevated CO2 on seed provisioning, germination and seedling growth in Bromus erectus. Oecologia. 2000;123:475–480. doi: 10.1007/s004420000342. [DOI] [PubMed] [Google Scholar]
- 24.Bezemer TM, Thompson LJ, Jones TH. Poa annua shows inter-generational differences in response to elevated CO2. Global Change Biol. 1998;4:687–691. [Google Scholar]
- 25.Galloway LF. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005;166:93–100. doi: 10.1111/j.1469-8137.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- 26.Ward JK, Kelly JK. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol Lett. 2004;7:427–440. [Google Scholar]
- 27.Teng NJ, Wang J, Chen T, Wu XQ, Wang YH, et al. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006;172:92–103. doi: 10.1111/j.1469-8137.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 28.Teng NJ, Chen T, Lin JX. A review on responses of plant sexual reproduction to elevated CO2. J Plant Ecol. 2006;30:1054–1063. [Google Scholar]
- 29.Hou GL, Teng NJ, Luo MC, Lin JX, Huang Y, et al. Effects of elevated CO2 on the floral stem structure and chemical composition in Arabidopsis thaliana. Acta Bot Boreal-Occident Sin. 2007;27:1499–1506. [Google Scholar]
- 30.Cheng SH, Moore BD, Seemann JR. Effects of short- and long-term elevated CO2 on the expression of ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1998;116:715–723. doi: 10.1104/pp.116.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He JS, Bazzaz FA. Density-dependent responses of reproductive allocation to elevated atmospheric CO2 in Phytolacca americana. New Phytol. 2003;157:229–239. doi: 10.1046/j.1469-8137.2003.00660.x. [DOI] [PubMed] [Google Scholar]
- 32.Jitla DS, Rogers GS, Seneweera SP, Basra AS, Oldfield RJ, et al. Accelerated early growth of rice at elevated CO2: is it related to developmental changes in the shoot apex? Plant Physiol. 1997;115:15–22. doi: 10.1104/pp.115.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris R, Sabatti M, Miglietta F, Mills RF, Taylor G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant Cell Environ. 2001;24:305–315. [Google Scholar]
- 34.Gibeaut DM, Cramer GR, Seemann JR. Growth, cell walls, and UDP-Glc dehydrogenase activity of Arabidopsis grown in elevated carbon dioxide. J Plant Physiol. 2001;158:569–576. [Google Scholar]
- 35.LaDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292:95–98. doi: 10.1126/science.1057547. [DOI] [PubMed] [Google Scholar]
- 36.Kohut R. The long-term effects of carbon dioxide on natural systems: issues and research needs. Environ Int. 2003;29:171–180. doi: 10.1016/S0160-4120(02)00160-5. [DOI] [PubMed] [Google Scholar]
- 37.Woodward FI, Kelly CK. The influence of CO2 concentration on stomatal density. New Phytol. 1995;131:311–327. [Google Scholar]
- 38.Räsänen K, Kruuk LEB. Maternal effects and evolution at ecological time-scales. Funct Ecol. 2007;21:408–421. [Google Scholar]
- 39.Edwards GR, Newton PCD, Tilbrook JC, Clark H. Seedling performance of pasture species under elevated CO2. New Phytol. 2001;150:359–369. [Google Scholar]
- 40.Collins S, Bell G. Evolution of natural algal populations at elevated CO2. Ecol Lett. 2006;9:129–135. doi: 10.1111/j.1461-0248.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 41.Collins S, Bell G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–569. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- 42.Wu L, Bradshaw AD. Aerial pollution and the rapid evolution of copper tolerance. Nature. 1972;238:167–169. [Google Scholar]
- 43.Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett. 2006;9:354–371. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- 44.Donohue K, Schmitt J. Maternal environmental effects in plants: adaptive plasticity? In: Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. Oxford, UK: Oxford University Press; 1998. pp. 137–158. [Google Scholar]
- 45.Leishman MR, Sanbrooke KJ, Woodfin RM. The effects of elevated CO2 and light environment on growth and reproductive performance of four annual species. New Phytol. 1999;144:455–462. doi: 10.1046/j.1469-8137.1999.00544.x. [DOI] [PubMed] [Google Scholar]