Abstract
Recent studies have identified development of resistance to tyrosine kinase inhibition (TKI) as a significant roadblock to effective treatment. One mechanism of resistance recently appreciated involves ‘oncogene switching’, or the re-activation of signaling pathways by one or more redundant upstream activators. In breast cancer models, ErbB TKIs such as gefitinib have been shown to lose the ability to modulate ErbB-driven signaling pathways over time, even though ErbB inhibition is maintained. Although incomplete ErB inhibition has been proposed to underlie this phenomenon, our findings suggest that oncogene switching can also re-activate downstream signaling pathways in breast cancer cells, even when ErbB inhibition is complete. We find that ErbB TKI-induced Src activation mediates downstream signaling rebound in SKBR3 cells, and we show that combination of Src and ErbB inhibitors is more effective and longlasting than is either TKI alone. Finally, the Hsp90 inhibitor 17-AAG, by simultaneously and durably inhibiting multiple signaling activators including ErbB and Src kinases, does not permit oncogene switching and results in a more prolonged and robust inhibition of downstream signaling pathways in breast cancer cells than do individual TKIs. These data support the continued clinical evaluation of Hsp90 inhibitors in breast cancer.
Keywords: ErbB tyrosine kinase, Src, Hsp90, oncogene switching, molecularly-targeted drug development
Introduction
A prevailing theory in cancer biology proposes the concept of oncogene addiction namely that cancer cells are “addicted” to one or a few genes that drive the malignancy. 1 Advances in the genetic and proteomic understanding of cancer attribute this to gene mutation or alteration in expression via amplification or epigenetic modification. This improved understanding has allowed the development of therapies targeted to the specific changes in cancer, with the aim of developing treatments that are greatly improved in killing cancer cells while leaving normal cells intact, thus yielding improved survival with decreased side effects for patients. Two such targeted therapies include tyrosine kinase inhibitor (TKI) and heat shock protein 90 (Hsp90) inhibitor therapy.
Protein tyrosine kinases comprise a major oncogene family. Thus, TKIs are predicted to represent a promising treatment modality. Among these is the epidermal growth factor (EGF) receptor inhibitor gefitinib. However, large scale clinical trials in non-small cell lung cancer (NSCLC) yielded limited results, with only about 10% of patients responding to gefitinib treatment (primary resistance). Further, those patients who responded eventually become resistant to gefitinib (secondary resistance). 2 Recently, multiple studies have been elucidating the mechanisms by which resistance occurs, and several mechanisms have been proposed for secondary resistance. These include point mutations of the kinases that confer an inability of the drug to bind to its target, 2 signal pathway reactivation secondary to the over-expression of kinases not normally involved in it, 3 and escape through utilization of redundant activators of a signaling pathway. 4
Recent work by Sergina et al shed more light on how cancers may develop primary resistance to TKI such as gefitinib. By following the effects of in vitro treatment over a longer than typical time course, these researchers found that, in a breast cancer model (SKBR3), gefitinib-induced inactivation of the pro-survival PI3K/Akt signaling pathway is transient, with a rebound in activity noticeable after 48 to 96 hours of treatment. 5 This functional rebound could be a reason for the resistance to gefitinib seen in patients with elevated EGFR, where a response, although expected, is lacking. The relatively short time needed for the rebound to occur suggests it may underlie primary resistance to gefitinib, while its adaptive nature suggests that it may contribute to secondary resistance as well. The rebound of PI3K/Akt activity was shown to be dependent on re-phosphorylation of ErbB3, a member of the ErbB family of kinases which also includes EGFR, ErbB2 and ErbB4. In the Sergina et al. study, ErbB3 re-phosphorylation was proposed to be mediated by ErbB2 kinase activity, concomitant with increased ErbB3 expression and decreased phosphatase activity.
Importantly, however, ErbB receptors also can associate with non-receptor tyrosine kinases. c-Src is one such kinase, with elevated expression or activity shown in a variety of cancers, including breast cancer. 6 In breast carcinoma cells, c-Src phosphorylates the kinase domain of EGFR, 7 and we recently reported that c-Src can similarly directly phosphorylate Tyr877 in the kinase domain of ErbB2. 8 Src has been shown to modulate ErbB2 and ErbB3 complex formation, 9 and a recent study of mammary carcinoma cells expressing ErbB3 suggests that ErbB3 also undergoes compensatory phosphorylation directly mediated by Src family kinases. 7 One goal of the current study was to examine whether Src family kinases may play a role in reactivation of the ErbB3/Akt signaling axis following EGFR/ErbB2 inhibition in SKBR3 cells.
ErbB2 stability and function are both very sensitive to pharmacologic inhibition of Hsp90. 10 Hsp90 is a molecular chaperone that assists the folding, stability and function of a wide variety of cellular proteins, many of which are involved in tumorigenesis. The chaperoning function of Hsp90 requires ATP, whose binding can be blocked by the antibiotic geldanamycin or its semi-synthetic derivative 17-AAG, which is currently undergoing extensive clinical evaluation. Pharmacologic inhibition of Hsp90 results in a rapid and sustained decrease in ErbB2 protein steady-state level and in its autophosphorylation. Hsp90 inhibition also interferes with maturation of nascent EGFR protein, eventually leading to decreased EGFR levels in the cell. 11 Thus, the second goal of this study was to determine whether an Hsp90 inhibitor such as 17-AAG may induce a more durable and robust inhibition of downstream pro-survival signaling mediated by the ErbB receptor family.
Results
17-AAG is superior to gefitinib in chronically inhibiting the ErbB3/PI3K/Akt signaling pathway
EGFR can exert its oncogenic function by dimerizing with and activating ErbB3 which, although lacking kinase activity itself, contains multiple docking sites for PI3 kinase in its C-terminal tail. Phosphorylation in trans of these PI3K docking sites effectively leads to activation of the anti-apoptotic kinase Akt. Thus, inhibition of EGFR (and ErbB2) results in dephosphorylation and inactivation of the PI3K/Akt signaling pathway. However, recent evidence has shown that, while gefitinib treatment initially inactivates the ErbB3/PI3K/Akt pathway, with time ERbB3 phosphorylation rebounds (although EGFR is still effectively inhibited), presumably mediated by ErbB2 re-activation. 5 Our and other groups’ previous research has shown that Hsp90 inhibitors induce rapid ErbB protein degradation and inhibit ErbB kinase activity. 10, 12, 13 We therefore tested whether 17-AAG-induced ErbB inhibition suffers from a similar time-dependent ErbB3 functional rebound.
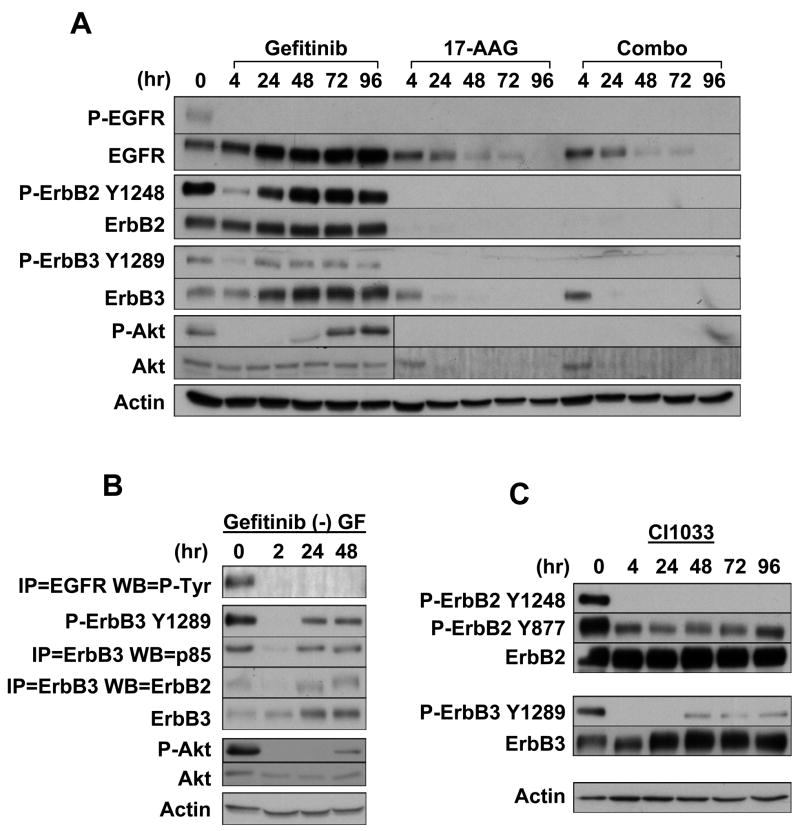
We treated SKBR3 cells with gefitinib alone, 17-AAG alone, or with a combination of the two drugs over a 96-hour period. After 17-AAG, phosphorylation of all ErbB proteins (EGFR, ErbB2, ErbB3) decreased to undetectable levels by 4 hours of treatment, and remained undetectable through 96 hours of incubation (Fig. 1A). This is in direct contrast to the response to gefitinib, where ErbB2 and ErbB3 phosphorylation decreased at 4 hours, but rebounded by 24 hours to levels comparable to those of untreated cells, even though EGFR phosphorylation remained undetectable through 96 hours. Unlike gefitinib, 17-AAG caused rapid loss of the Hsp90-dependent ErbB2 and ErbB3 proteins, while EGFR protein declined more slowly (because of interference in nascent EGFR maturation). These results suggest that 17-AAG is more effective than is gefitinib in inhibiting the signaling of ErbB receptors. Indeed, in the presence of 17-AAG Akt phosphorylation became undetectable by 4 hours and remained so for the remainder of the experiment, while Akt protein was reduced to undetectable levels by 24 hours. In contrast, Akt phosphorylation in gefitinib-treated samples responded as did ErbB2 and ErbB3 (Fig. 1A). Finally, the combination of 17-AAG and gefitinib, at the concentrations used, was not superior to 17-AAG alone.
Figure 1.
17-AAG induces rapid and durable inactivation of ErbB and Akt signaling, as compared to ErbB TKIs. (A) Timecourse of gefitinib, 17-AAG and combination treatments in the human breast cancer cell line SKBR3. Cells were plated in 10 cm dishes and treated for the indicated times with 1 μM gefitinib, 1 μM 17-AAG, or their combination, and examined for the expression and phosphorylation of the indicated proteins. (B) ErbB3 reactivation in the presence of gefitinib occurs independently of serum growth factors. SKBR3 cells were plated in 6-well plates, serum starved for 24 hours and treated for the indicated times with 1 μM gefitinib. (C) Role for a non-ErbB receptor in the activation of ErbB3. SKBR3 cells were plated in a 6-well plate, treated with 1.5 μM CI-1033 for the indicated times and assayed for the expression and phosphorylation of the indicated proteins.
ErbB3 reactivation in the presence of gefitinib is serum-independent
In order to study the mechanism of ErbB3 reactivation in the face of continued EGFR inhibition, we examined gefitinib’s effect on ErbB3-containing ErbB heterodimers, phosphorylation, and downstream activation in the setting of serum starvation. After culturing cells in serum-free medium for 24 hours, we immunoprecipitated ErbB3 proteins from SKBR3 cells treated or untreated with gefitinib for different times, and we immunoblotted for co-precipitation of the regulatory subunit of PI3 kinase, p85. As expected, p85 associated with ErbB3 in untreated samples, but this association was significantly decreased by gefitinib after 2 hours of treatment (Fig. 1B). In agreement with this result, ErbB3 phosphorylation was abolished at this time point. However, with extended gefitinib treatment, ErbB3 phosphorylation rebounded, as did its association with ErbB2 and p85. Accordingly, although Akt phosphorylation was abolished by gefitinib treatment at 2 hours, it became detectable again at 48 hours. Signaling through Stat3, Stat5, Erk1 and Erk2 remained abrogated at all timepoints (not shown). We obtained similar results in the presence of 10% serum, EGF, or heregulin (not shown), suggesting that reactivation of the ErbB3/p85/Akt pathway occurs independently of serum growth factors.
Src kinases mediate ErbB3 reactivation, and this is sensitive to 17-AAG
The rebound of ErbB3 phosphorylation has been speculated to be a function of residual ErbB2 activity. 5 The temporal pattern of ErbB2 re-phosphorylation supported such a notion, as did the transient disruption of the association of ErbB2 with ErbB3 (Fig. 1A, B).
We further investigated the involvement of ErbB2 in ErbB3 re-phosphorylation by employing the more potent and irreversible pan-ErbB inhibitor CI-1033. 14 As expected, CI-1033 blocked ErbB2 autophosphorylation on Tyr1248 for at least 96 hours. However, although CI-1033 visibly decreased phosphorylation on ErbB2 Tyr877, a significant amount of phosphorylation remained, consistent with our recent observation that Tyr877 phosphorylation is at least partly mediated by Src family kinases (SFK). 8 Strikingly, ErbB3 phosphorylation still rebounded detectably in the context of complete and durable inhibition of ErbB2 autophosphorylation (Fig. 1C). These results suggested that though ErbB2 contributes significantly to the rebound of ErbB3 phosphorylation after gefitinib, ErbB3 re-phosphorylation may also occur independently of ErbB kinase activity.
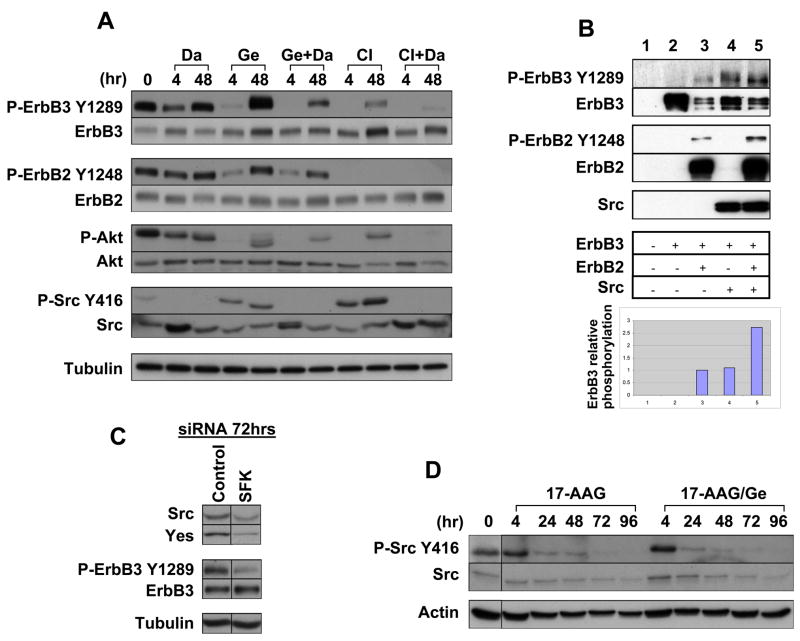
Because ErbB2 Tyr877 phosphorylation is in part mediated by SFK and remained robust in the presence of CI-1033, we investigated a possible role of SFK in this phenomenon. SKBR3 cells were treated with the clinically approved Src-Abl inhibitor dasatinib alone or in combination with gefitinib and CI-1033. ErbB3 phosphorylation decreased to about 30% of control at 4 hours after treatment with dasatinib alone, but returned to baseline by 48 hours (Fig. 2A). PP2, another SFK inhibitor, yielded similar results (data not shown). These results suggest that SFK are involved in ErbB3 phosphorylation. Addition of dasatinib to either gefitinib or CI-1033 more potently decreased ErbB3 phosphorylation at 48 hours than did any single drug treatment, supporting the notion that both SFK and ErbB proteins mediate ErbB3 phosphorylation (Fig. 2A). As expected, decreased ErbB3 phosphorylation was translated into decreased Akt activation, so that the combination of dasatinib with either gefitinib or CI-1033 more profoundly inhibited Akt activation than did any drug alone (Fig. 2A).
Figure 2.
c-Src mediates ErbB3 and PI3K/Akt reactivation in the setting of ErbB TKI, and this can be prevented by 17-AAG. (A) Combination of c-Src and ErbB TKIs is more effective in inactivating ErbB3 and Akt. SKBR3 cells were plated in 6-well plates and treated with 1 μM gefitinib, 1.5 μM CI-1033, and 0.5 μM Dasatinib as shown and for the length of time indicated. (B) c-Src can effect ErbB3 activation independently and in cooperation with ErbB2. c-Src, ErbB2, and ErbB3 were overexpressed in CHO cells alone and in combination. ErbB3 phosphorylation was examined by western blotting and corrected for ErbB3 expression. (C) c-Src family kinase (SFK) knockdown decreases ErbB3 phosphorylation. SKBR3 cells were plated in a 12-well plate, and control, c-Src and Yes siRNAs were transfected (100 nM each). (D) 17-AAG inactivates c-Src alone and in combination with gefitinib. SKBR3 cells were treated for the indicated times with 1 μM 17-AAG or in combination with 1 μM gefitinib, and examined for c-Src activity.
To confirm a role for SFK in phosphorylating ErbB3, we over-expressed these proteins in Chinese hamster ovary (CHO) cells, which express negligible levels of ErbB receptors. When expressed alone ErbB3 remained unphosphorylated, as it lacks intrinsic kinase activity (Fig. 2B). Co-expression of either Src or ErbB2 alone resulted in comparable ErbB3 phosphorylation (when corrected for ErbB3 protein expression, see graph in Fig. 2B). Furthermore, co-expression of both Src and ErbB2 resulted in at least additive phosphorylation of ErbB3, suggesting that the two kinases can cooperatively phosphorylate ErbB3.
Finally, we used siRNA to verify a role for SFK in phosphorylating ErbB3. SKBR3 cells express two of the three ubiquitous Src kinases, Src and Yes, but not Fyn. When Src and Yes were knocked down with specific siRNA, ErbB3 phosphorylation was substantially decreased (Fig. 2C), supporting our hypothesis that SFK are involved in phosphorylating ErbB3.
Interestingly, we noticed that both gefitinib and CI-1033 treatment increased Src activity (p-Src Y416 panel, Fig. 2A), further supporting a compensatory role for SFK in aiding ErbB3 re-phosphorylation in the context of ErbB inhibition. Since 17-AAG was very effective in preventing this response (Fig. 1A), we tested whether 17-AAG would blunt gefitinib-induced Src activation. We treated SKBR3 cells with a combination of the two drugs and immunoblotted for Src phosphorylation on Tyr416, an indicator of Src activation status. 17-AAG prevented gefitinib-induced Src activation. In fact, 17-AAG progressively reduced Src activation in the presence or absence of gefitinib (Fig. 2D). These results support our hypothesis that 17-AAG can be used effectively to target the ErbB3/Akt axis.
17-AAG induces apoptosis in SKBR3 cells
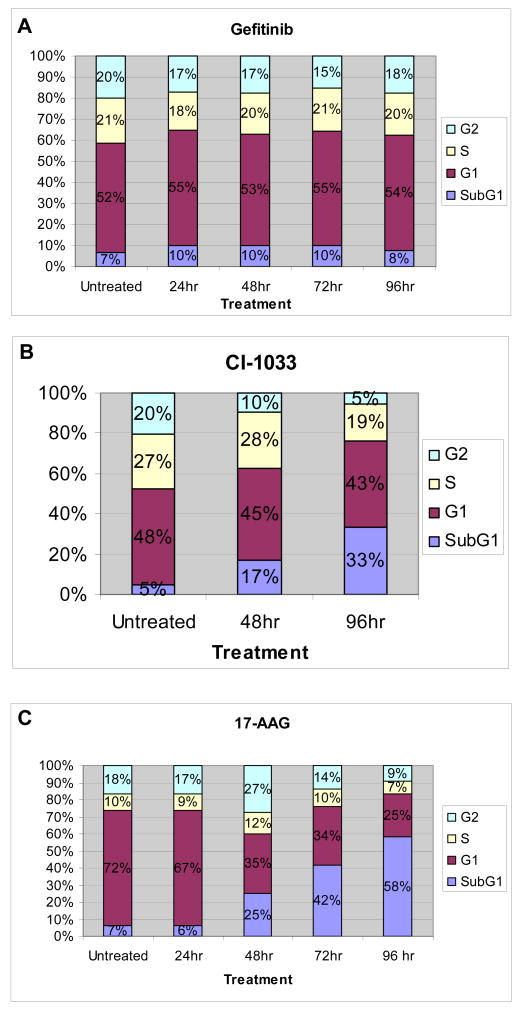
As 17-AAG was superior to ErbB inhibitors in durably blocking the pro-survival ErbB3/Akt signaling pathway, we hypothesized that 17-AAG would be more effective in inducing apoptosis in SKBR3 cells. To test this hypothesis, we observed the SubG1 (apoptotic) fraction in cells treated with each drug. Gefitinib did not affect the cell cycle distribution of SKBR3 cells over a time course of 96 hours (Fig. 3A). By 96 hours, CI-1033 induced a significant increase in the SubG1 fraction and decreases in G2 and S phases (Fig. 3B), consistent with more potent inhibition of ErbB2. However, 17-AAG caused an even more rapid and progressive increase in the SubG1 population, and a decrease in the G1, S, and G2 fractions (Fig. 3C). These data show that 17-AAG is more effective not only in inhibiting the ErbB3/Akt axis but also in inducing apoptosis in SKBR3 cells.
Figure 3.
17-AAG is superior to ErbB TKIs in inducing apoptosis in SKBR3 cells. SKBR3 cells were treated for the indicated times with (A) 1 μM gefitinib, (B) 1.5 μM CI-1033, or (C) 1 μM 17-AAG, and cell cycle analysis was performed using flow cytometry.
17-AAG blocks invasion of SKBR3 cells
Since gefitinib and CI-1033 induced Src activation (Fig. 2A), and because Src kinase has been shown to promote cell invasion, we hypothesized that these ErbB inhibitors may increase the invasiveness of SKBR3 cells. By assessing invasion (cell migration across a fibronectin-coated membrane) in real time, we found that gefitinib induced a transient but significant increase in invasiveness by 15 hours. By 72 hours, invasion of gefitinib-treated and control cells was essentially identical. CI-1033 was only moderately inhibitory by 72 hours and had no effect on invasion after 15 hours (Fig. 4). The potentiation of invasion by gefitinib correlates temporally with its effect on Src activity, suggesting a possible connection. The involvement of Src in SKBR3 invasion in the presence of gefitinib was confirmed by the ability of dasatinib to completely inhibit the process (Fig. 4). Importantly, 17-AAG also fully inhibited the invasion of SKBR3 cells.
Figure 4.
Src-dependent cellular invasion is transiently increased by gefitinib. Real time cell invasion was continuously monitored in SKBR3 cells treated with serum-free medium (negative control, NC), 10% FBS (positive control, PC), 1 μM gefitinib (GE), 1.5 μM CI-1033 (CI-1033), 1 μM 17-AAG (GA), 0.5 μM dasatinib (DA), or the combination 1 μM gefitinib with 0.5 μM dasatinib (GE + DA). Data are expressed as mean cumulative invasion (+/− SD) at 15 hours and at 72 hours after initiation of the experiment.
Discussion
In this study we found that, unlike the ErbB TKIs gefitinib and CI-1033, the Hsp90 inhibitor 17-AAG induced complete and durable inhibition of the ErbB receptor family, as well as the downstream pro-survival PI3K/Akt signaling pathway. The activities of these signaling molecules remained undetectable for 96 hours without evidence of rebound, suggesting that 17-AAG is a potentially robust inhibitor of ErbB-driven cancers. Given the key role played by ErbB3 in modulating PI3K-dependent Akt activation, it is of import that 17-AAG not only rapidly blocked ErbB3 phosphorylation but also directly impacted expression of the kinase. These data are in agreement with those recently published by Citri et al., 15 that identify ErbB3 as a partial Hsp90 client (similar to EGFR). Thus, effects of Hsp90 inhibition on ErbB signaling to downstream effectors is multifactorial, comprising a rapid inhibition of phosphorylation accompanied by a delayed but prolonged impact on protein expression.
Importantly, we also uncovered a novel mechanism of resistance to ErbB TKIs. We found that ErbB inhibition by either gefitinib or CI-1033 induced c-Src activation, which in turn mediated ErbB3 re-phosphorylation and Akt re-activation. This suggests a rapid ability of these cancer cells to compensate for the inactivation of one oncogenic signaling pathway by effectively “switching” to an alternative oncogenic kinase. This model of “oncogene plasticity” does not rely on kinase mutation or drastic changes in gene expression but merely on the inherent redundancy of biological systems, and may explain some of the disappointing results seen in previous TKI clinical trials. It follows that examining oncogenic kinase amplification or activity not only prior to therapy administration, but also soon after treatment initiation may have clinical utility.
Our data support a role for c-Src as a facilitator of receptor tyrosine kinase-driven signaling pathways. 9,16 In the current instance, we have described its role in SKBR3 cells as an auxiliary activator of the ErbB3/PI3K/Akt axis, particularly in the face of TKI-dependent ErbB inhibition. As such, our findings are similar to data recently reported that describe the radioprotective nature of Src-dependent ErbB3 activation. 7 In sum, these data agree in suggesting that in many cases a ‘dirtier’ TKI may ultimately prove more effective clinically than would a highly targeted agent. Importantly, 17-AAG abrogated TKI-induced c-Src activation in our study.
Remarkably, Src activation in SKBR3 cells induced by ErbB TKIs not only provided resistance to the TKI, but also transiently stimulated cell invasion, suggesting that not only can TKIs quickly lose efficacy, but that they might worsen clinical outcomes in certain settings. This observation agrees with previous findings of c-Src playing a central role in breast cancer cell invasion, 17 and offers further support for combination treatment with multiple TKI’s, such as ErbB inhibitors and Src inhibitors in the example reported in this study.
Finally, given their robust ability to inhibit multiple oncogenic kinase-driven signaling pathways, Hsp90 inhibitors may reduce the likelihood that tumor cells will eventually circumvent TKI inhibition. Indeed, the molecular targeting of Hsp90 appears to be one approach to countering the inherent redundancy of biological systems that provides tumor cells with such a pronounced survival advantage.
Materials and Methods
Cell culture and reagents
SKBR3 cells (ATCC, Manassas, VA, USA) were maintained in a 37 °C humidified atmosphere containing 5% CO2 in Dulbecco’s modified Eagle’s minimal essential medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen). 17-AAG (Kosan Biosciences, Hayward, CA, USA) was dissolved in DMSO to a stock concentration of 10 mM and used at a final concentration of 1 μM, representing a plasma concentration readily achieved in vivo. 18 Gefitinib (AstraZeneca, London, England) was dissolved in PBS to a stock concentration of 10 mM and used at a final concentration of 1 μM, the highest clinically achievable plasma level. 19 CI-1033 (Pfizer, Kalamazoo, Michigan, USA) was dissolved in PBS to a stock concentration of 1 mM and used at a final concentration of 1.5 μM. Dasatinib (Bristol-Myers Squibb, Princeton, NJ, USA) was dissolved in DMSO to a stock concentration of 1mM and used at a final concentration of 0.5 μM. PP2 (Calbiochem, La Jolla, CA, USA) was dissolved in DMSO to a stock concentration of 10 mM. For SFK siRNA experiments, SKBR3 cells were plated in a 12-well plate. Src and Yes were knocked down using DharmaFECT 1 and ON-TARGETplus SMARTpool siRNA (Dharmacon, Lafayette, CO, USA).
CHO transfection experiments
Chinese Hamster Ovary (CHO) cells were propagated in a 37 °C humidified atmosphere containing 5% CO2 in DMEM supplemented with 10% FBS. ErbB2, ErbB3, and Src containing plasmids 8 were transfected using FuGene 6 Transfection Reagent (Roche, Nutley, NJ, USA).
Western blotting
Equivalent amounts of protein were separated by polyacrylamide gel electrophoresis (BioRad, Hercules, CA, USA) and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Individual membranes were probed as indicated. Antibodies were purchased as follows: EGFR (BD Transduction; 610016), P-EGFR (Cell Signaling; 4404), ErbB2 (SantaCruzBiotech; sc-284), P-ErbB2 (Neomarkers; Ab-18 (PN2A)), ErbB3 (Santa Cruz Biotech; sc-285), P-ErbB3 (Cell Signaling; 4791), Akt (Cell Signaling; 9272), P-Akt (Cell Signaling; 9271), PI3K p85 (Upstate; 06-497), Actin (Cell Signaling; 4967), Tubulin (Calbiochem; CP06), Src (Cell Signaling; 2108), P-Src, Tyr 416 (Cell Signaling; 2101).
Cell cycle analysis
For cell cycle analysis experiments, SKBR3 cells were plated in 10cm dishes and treated for the indicated times with 1 uM gefitinib, 1 uM 17-AAG, 1.5 uM CI-1033, or their combination. Media containing floating cells were transferred to a tube, adherent cells were trypsinized and then added to the same tube, and cells were fixed overnight in 70% ethanol at −20° C. Cells were washed in phosphate-citrate buffer, treated with 5 ug/ml propidium iodide & 10 ug/ml RNaseA, and analyzed for cell cycle phase by flow cytometry.
Cell invasion assay
The Personal RT-CIM™ System (ACEA Biosystems, San Diego, CA, USA) was used to monitor cell invasion in real time. RT-CIM™ Devices (ACEA Biosystems, DMB16-PET05A, DMT16-PET08B) were used as per the following protocol: well tops and bottoms were coated with 70ul of 10 ul/ml fibronectin (Sigma-Aldrich, #F1141) in PBS and incubated for 1 hour. Wells were washed with serum free media. Cells were serum starved overnight, and plated at a density of 1.2 × 106 SKBR3 cells per well, with 10% FBS added to the bottom chamber as a chemoattractant. Treatments were performed as indicated in the figure, each at least in triplicate, and invasion was monitored continuously in real time. Cummulative area under the curve (amount of invasion) was determined at the two timepoints shown in the figure. Data are displayed as mean +/− SD.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute.
Contributor Information
Itai Pashtan, Howard Hughes Medical Institute, National Institutes of Health Research Scholars, Program, Bethesda, Maryland 20814.
Shinji Tsutsumi, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892.
Suiquan Wang, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892.
Wanping Xu, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892.
Len Neckers, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892.
References
- 1.Weinstein IB, Begemann M, Zhou P, et al. Disorders in cell circuitry associated with multistage carcinogenesis: exploitable targets for cancer prevention and therapy. Clin Cancer Res. 1997;3:2696–2702. [PubMed] [Google Scholar]
- 2.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR Mutation and Resistance of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 4.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of Receptor Tyrosine Kinases Affects the Response of Tumor Cells to Targeted Therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 5.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbeek BS, Vroom TM, Adriaansen-Slot SS, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol. 1996;180:383–8. doi: 10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Contessa J, Abell A, Mikkelsen R, Valerie K, Schmidt-Ullrich R. Compensatory ErbB3/c-Src signaling enhances carcinoma cell survival to ionizing radiation. Breast Cancer Research and Treatment. 2006;95:17–27. doi: 10.1007/s10549-005-9023-9. [DOI] [PubMed] [Google Scholar]
- 8.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–8. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2006;26:3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Neckers L. Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clin Cancer Res. 2007;13:1625–9. doi: 10.1158/1078-0432.CCR-06-2966. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Yuan X, Xiang Z, Mimnaugh E, Marcu M, Neckers L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat Struct Mol Biol. 2005;12:120–6. doi: 10.1038/nsmb885. [DOI] [PubMed] [Google Scholar]
- 12.Tikhomirov O, Carpenter G. Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J Biol Chem. 2000;275:26625–31. doi: 10.1074/jbc.M003114200. [DOI] [PubMed] [Google Scholar]
- 13.Chavany C, Mimnaugh E, Miller P, et al. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 1996;271:4974–7. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- 14.Citri A, Alroy I, Lavi S, et al. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. Embo J. 2002;21:2407–17. doi: 10.1093/emboj/21.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citri A, Harari D, Shohat G, et al. Hsp90 Recognizes a Common Surface on Client Kinases. J Biol Chem. 2006;281:14361–14369. doi: 10.1074/jbc.M512613200. [DOI] [PubMed] [Google Scholar]
- 16.Tan M, Li P, Klos KS, et al. ErbB2 Promotes Src Synthesis and Stability: Novel Mechanisms of Src Activation That Confer Breast Cancer Metastasis. Cancer Res. 2005;65:1858–1867. doi: 10.1158/0008-5472.CAN-04-2353. [DOI] [PubMed] [Google Scholar]
- 17.Hiscox S, Morgan L, Green T, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Research and Treatment. 2006;97:263–274. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–91. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–6. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]