Abstract
Tea made from the leaves of the plant Camellia sinensis is a popular beverage. The possible cancer preventive activity of tea and tea polyphenols has been studied extensively. This article briefly reviews studies in animal models, cell lines, and possible relevance of these studies to the prevention of human cancer. The cancer preventive activity of tea constituents have been demonstrated in many animal models including cancer of the skin, lung, oral cavity, esophagus, stomach, liver, pancreas, small intestine, colon, bladder, prostate, and mammary gland. The major active constituents are polyphenols, of which (-)-epigallocatechin-3-gallate (EGCG) is most abundant, most active, and most studied, and caffeine. The molecular mechanisms of the cancer preventive action, however, are just beginning to be understood. Studies in cell lines led to the proposal of many mechanisms on the action of EGCG. However, mechanisms based on studies with very high concentrations of EGCG may not be relevant to cancer prevention in vivo. The autooxidation of EGCG in cell culture may also produce activities that do not occur in many internal organs. In contrast to the cancer prevention activity demonstrated in different animal models, no such conclusion can be convincingly drawn from epidemiological studies on tea consumption and human cancers. Even though the human data are inconclusive, tea constituents may still be used for the prevention of cancer at selected organ sites if sufficient concentrations of the agent can be delivered to these organs. Some interesting examples in this area are discussed.
Introduction
Tea is a popular beverage made from the leaves of the plant Camellia sinesis, a warm-weather evergreen. Tea plants have been cultivated for thousands of years, and the leaves have been used first for medicinal purposes and then as a popular beverage worldwide. In recent years, both green and black teas have received a great deal of attention from researchers and the general public due to their possible beneficial health effects, such as in the prevention of cancer and cardiovascular diseases. This article discusses our current understanding of the cancer preventive activities of tea, using results mostly from our laboratory to illustrate different points. We will start by introducing the chemical and biochemical properties of tea constituents. Then we will describe the inhibition of tumorigenesis in animal models and possible mechanisms involved as well as studies in cell lines and the interpretation of these results in the light of the bioavailability of tea constituents. Finally, we will discuss the relevance of these results in the prevention of human cancer based on human epidemiological and interventions studies. This topic has been covered by previous reviews (Yang and Wang, 1993; Bushman, 1998; Yang et al., 2002; Hou et al., 2004; Lambert et al., 2005; Clark and You, 2006; Khan et al., 2006; Lu et al., 2006b; Sun et al., 2006b; Sun et al., 2006a; Wu and Yu, 2006; Yang et al., 2006a; Yang et al., 2006b). Because of the citation limitations, we will cite the key reviews instead of some of the original articles. We believe that the elegant studies by Dr. Hasan Mukhtar's group on the inhibition of skin and prostate cancers by green tea polyphenols and the involvement of insulinlike growth factor-1 signaling as well as other excellent work, and that of his former and present colleagues, will be covered in other articles of this special issue in celebration of Dr. Mukhtar's 60th birthday. Therefore, these areas will not be reviewed herein.
Chemical and Biochemical Properties of Tea Constituents
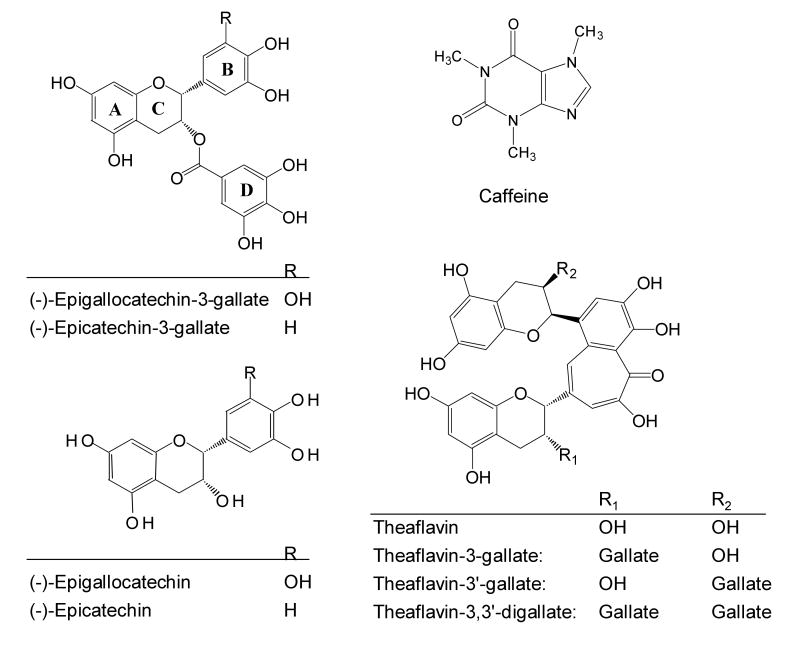
In the manufacture of green tea, tea leaves are heated to inactive the enzymes and dried to preserve their constituents. Tea composition varies with climate, season, horticultural practices, variety, and the age of the leaves. The characteristic polyphenolic compounds are known as catechins, and the structures of the major catechins: (-)-epigallocatechins-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epicatechin (EC), are shown in Fig. 1. Catechin, gallocatechin, epigallocatechin digallates, epicatechin digallate, 3-O-methyl EC and EGC, catechin gallate, and gallocatechin gallate are present in smaller quantities. Flavonols, including quercetin, kaempferol, myricitin, and their glycosides, are also present in tea. Black tea manufacture involves crushing the tea leaves to promote enzymatic oxidation and subsequent condensation of tea polyphenols in a process known as fermentation, which leads to the formation of theaflavins (TFs) and thearubigins, which account for 2% to 6% and 15% to 20%, respectively, of the dry weight of black tea solids. TFs are characterized by the benzotropolone ring structure and the bright red-orange color, and contribute to the unique taste of black tea. The structures of the four major theaflavins are shown in Fig. 1. Thearubigins, which have higher molecular weights, are poorly characterized chemically and biochemically.
Figure 1.
Structures of catechins, theaflavins, and caffeine.
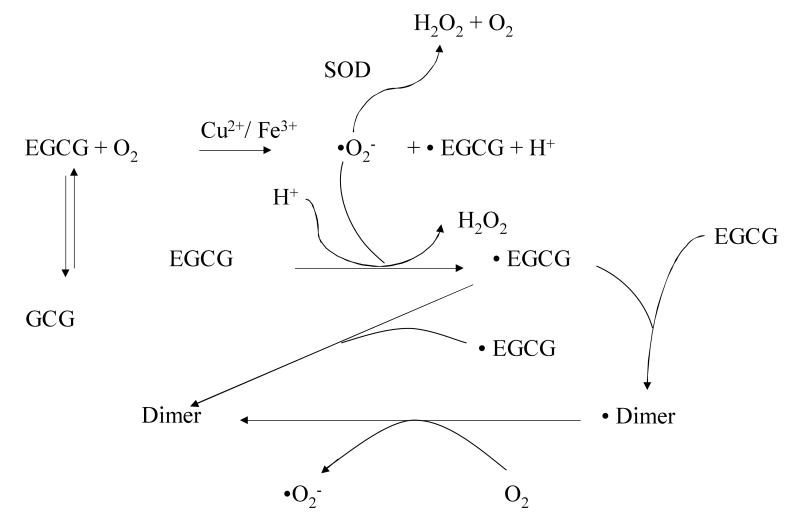
Because of the polyphenolic structure, tea polyphenols are strong antioxidants (reviewed in (Yang et al., 2002)). They are strong metal ion chelators, for example, the chelation of free Fe3+ ions prevents the formation of reactive oxygen species (ROS). Tea polyphenols can trap reactive species such as superoxide radical, singlet oxygen, hydroxyl ROS, nitric oxide, nitrogen dioxide, and peroxynitrite. The vicinal dihydroxy or trihydroxy structures contribute to these antioxidative activities of tea polyphenols, but they also make these compounds susceptible to air oxidation under alkaline or even neutral pH. In the case of EGCG, the autooxidation leads to the generation of superoxide anion and H2O2 and the formation of dimers such as theasinensins. These reactions occur even during cell culture conditions, and we proposed that this is due to superoxide anion-catalyzed chain reactions (Fig. 2), because EGCG can be stabilized by the addition of superoxide dismutase (Hou et al., 2005).
Figure 2.
Proposed mechanism for the autooxidation of EGCG. Under neutral or slightly alkaline pH, EGCG is oxidized by molecular oxygen to form superoxide radical (•O2-) and EGCG radical (•EGCG) in a reaction probably catalyzed by trace metal ions such as Cu2+ and Fe3+. The • O2- can then react with another EGCG molecule to form •EGCG. •EGCG may collide with another •EGCG to form an EGCG dimer. More likely, •EGCG may react with EGCG to form EGCG dimer radical, which has the potential to react with molecular oxygen to generate EGCG dimer and • O2-. An alternative mechanism is that the ·EGCG is oxidized by molecular oxygen to form ·O2− and EGCG quinone, and the quinone would react with another molecule of EGCG to form the dimer. In either case, the • O2- can react with another EGCG molecule to propagate the chain reaction. Addition of superoxide dismutase (SOD) to the culture medium enhances the conversion of •O2- to H2O2 and thus inhibits the autooxidation of EGCG (modified from Hou et al. 2005).
The polyphenolic structures of tea polyphenols also make them good donors for H-bonding. For example, H-bonding of EGCG to water forms a large hydration shell, which reduces the absorbability of EGCG. This H-bonding capacity also enables tea polyphenols to bind strongly to proteins and nucleic acids. For example, EGCG is known to bind to serum proteins such as fibronectin, fibrinogen, and histidine-rich glycoproteins. In our studies on the inhibition of DNA methyltransferase by EGCG, the formation of five H-bonds between EGCG and the amino acids at the enzyme active site was proposed (Fang et al., 2003). More recently, EGCG has been shown to bind strongly to 67-kDa laminin receptor, Bcl-2 proteins, and vimentin, and these proteins have been also proposed to be the target of EGCG of its anti-cancer activities (reviewed in (Yang et al., 2006a; Yang et al., 2006b)).
Inhibition of Tumorigenesis in Animal Models and Possible Mechanisms
In 1987, Yoshizawa et al. first published the inhibitory effects of topically applied EGCG against skin tumor promotion in mice (Yoshizawa et al., 1987). Thereafter, many studies including those by Dr. Hasan Mukhtar's group and by investigators at Rutgers University have been conducted, demonstrating the inhibitory activities of tea and preparations of tea constituents against tumorigenesis in different animal models. As shown in Table 1, the majority of the studies demonstrated cancer protective effects. Nevertheless, there are also studies that did not demonstrate such effects, and the reasons for the negative results are worth considering.
Table 1.
Tea and cancer prevention: results of studies with animal models.a
| Site | Positive Studies | Negative Studies |
|---|---|---|
| Skin | 19 | |
| Lung | 13 | 1 |
| Oral Cavity | 2 | |
| Esophagus | 4 | |
| Stomach | 7 | |
| Small Intestine | 4 | 1 |
| Colon | 11 | 3 |
| Liver | 7 | 1 |
| Pancreas | 2 | |
| Bladder | 3 | |
| Prostate | 6 | |
| Mammary Gland | 4 | 3 |
Updated from Landau et al. (Landau et al., 2005).
At Rutgers University, we have a collaborative group, including Allan H. Conney, Chi-Tang Ho, and Chung S. Yang, studying the prevention of skin, lung, oral, esophageal, forestomach, small intestinal, and colon cancers by tea and tea constituents. The systematic and thorough studies by Allan Conney's group on the inhibition of skin tumorigenesis by tea and caffeine will be covered in a separate article in this journal. Because of page limitations, this article will discuss the inhibition of lung and intestinal/colon carcinogenesis to illustrate progress and existing questions in this area.
Inhibition of lung tumorigenesis
Inhibition of lung tumorigenesis by green tea, black tea and their constituents has been demonstrated in different animal models, including those induced by tobacco smoke related chemical carcinogens such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), benzo[a]pyrene, and N-nitrosodimethylamine as well as spontaneously developed lung tumors in A/J mice (reviewed in (Yang et al., 2005; Clark and You, 2006)). Administration of green tea, black tea, EGCG, or theaflavins during the initiation or promotion stages was shown to significantly decrease NNK-induced lung tumor incidence or volume. However, EGC when given in drinking fluid as 0.1 and 0.3% solution after NNK-treatment did not significantly reduce lung tumorigenesis in this model (unpublished results). Black tea extracts, when given to mice that had already developed NNK-induced lung adenomas, inhibited the progression of adenomas to adenocarcinomas. Oral administration of green tea infusion reduced the number of lung colonies of mouse Lewis lung carcinoma cells in a metastasis system. These results suggest that tea preparations may be preventive agents for all stages of lung carcinogenesis (Yang et al., 2002; Yang et al., 2005). In addition, EGCG was found by Mimoto et al. to be effective in preventing weight loss and lung tumorigenesis in A/J mice induced by cisplatin, and green tea was shown to increase the tumor inhibitory effect of the chemotherapy drug doxorubicin in mice. This indicates the potential of green tea or EGCG to be used in combination with cancer therapeutic drugs to enhance their effects and counteract their toxicity (reviewed in (Yang et al., 2005)).
Our recent studies demonstrated that oral administration of 0.5% Polyphenon E (PPE) or 0.044% caffeine for 32 weeks, to A/J mice that had been treated with one dose of NNK (103 mg/kg b.w., i.p.) 20 weeks earlier, resulted in decreased numbers of lung tumors (Lu et al., 2006a). PPE is a standardized green tea polyphenol preparation containing 65% EGCG, 25% other catechins, and ∼0.5% caffeine. Histopathological analysis indicated that PPE administration significantly reduced the incidence (by 52%) and multiplicity (by 63%) of lung adenocarcinoma. Oral administration of 0.044% caffeine also showed similar inhibitory effect on total tumor formation and marginal inhibitory effect on the incidence and multiplicity of adenocarcinoma (by 48% and 49%, respectively). Immunohistochemical analysis showed that PPE and caffeine treatment inhibited cell proliferation (by 57% and 50%, respectively) in adenocarcinomas, enhanced apoptosis in adenocarcinomas (by 2.6 and 4 fold, respectively) and adenomas (both by 2.5 fold), and decreased levels of c-Jun and Erk1/2 phosphorylation. In the normal lung tissues, neither agent had a significant effect on cell proliferation or apoptosis. In another unpublished study, two-week treatment of adenoma-bearing mice with 0.32% EGCG or 0.044% caffeine resulted in increased apoptotic index (by 130% and 140%, respectively) and reduced c-Jun phosphorylation levels (by 32% and 72%, respectively; P<0.05) in the adenomas. Caffeine administration also reduced Erk1/2 phosphorylation levels by 38% (P<0.05).
Concerning mechanisms of the inhibition of lung carcinogenesis, many studies have been conducted with green tea polyphenols, especially EGCG, in cell culture systems. Inhibition of cell proliferation, induction of apoptosis, and reduction of angiogenesis are generally considered to be mechanisms of cancer prevention for many agents. The existing results (Yang et al., 2005) on the inhibition of lung tumorigenesis are consistent with this concept. The molecular events that trigger these cellular changes, however, are not clear, in spite of the many mechanisms that have been proposed based on studies on cell lines. EGCG may induce the same cellular changes (i.e. apoptosis) by different mechanisms in vivo and in vitro. We have shown that EGCG induces apoptosis of lung cancer cells in culture via a H2O2-dependent mechanisms; whether such a mechanism exists in vivo remains to be demonstrated in animal models or human tissues. Some general problems in the use of data from in vitro experiments to predict cancer prevention mechanisms in vivo will be discussed in a subsequent section.
Lu et al. (Lu et al., 2006b) recently analyzed the gene expression changes caused by the administration of green tea in inhibiting an NNK-induced, or the administration of PPE in inhibiting a benzo[a]pyrene-induced lung tumorigenesis model, in A/J mice. Mouse lung samples were prepared at the time of sacrifice. The authors found that 946 genes were upregulated and 1,096 genes downregulated by the green tea and PPE treatments. Eighty-eight genes that were differentially expressed in tumors (from the normal tissues) were reversed by the treatment. These authors also identified a classifier of 17 genes that were altered by tea and PPE treatments in both normal lungs and lung adenomas, and suggested that these genes may be used as markers for tea exposure. Additional studies are needed to verify the results and solidify the conclusion. Further research is needed to relate these gene expression changes to the changes at the protein and cellular levels that have been observed.
Inhibition of Intestinal/Colon Tumorigenesis
The inhibition of intestinal carcinogenesis by tea and related polyphenols has been demonstrated in different animal models by several research groups (reviewed in (Yang et al., 2002; Landau et al., 2005; Yang et al., 2006a). Green tea extract, alone and in combination with sulindac, decreased intestinal tumor formation in the ApcMin/+ mouse (Suganuma et al., 2001). Orner et al. also demonstrated the inhibition of tumorigenesis in ApcMin/+ mice by orally administered green tea and a greater inhibition by its combination with sulindac (Orner, 2003). Recently, we systematically investigated the effect of the two key constituents of green tea, EGCG and caffeine, on intestinal tumorigenesis in the ApcMin/+ mouse model (Ju et al., 2005). We found that administration of EGCG at doses of 0.08% or 0.16% in drinking fluid significantly decreased small intestinal tumor formation by 37% or 47%, respectively. Caffeine did not inhibit intestinal tumor formation. In another experiment, small intestinal tumorigenesis was inhibited in a dose-dependent manner by oral administration of EGCG in the dose range of 0.02% to 0.32%. Western blot analysis indicated that the oral administration of EGCG resulted in increased levels of E-cadherin and decreased levels of β-catechin in the nuclear, c-Myc, phospho-Akt, and phospho-Erk in the small intestinal tumors (Ju et al., 2005). In another study, we found that PPE administered to ApcMin/+ mice in the diet (0.12% in AIN93G diet) inhibited total intestinal tumor multiplicity (by 70.5%) and the decrease was greater than the decrease observed in the group administered with PPE (0.12%) in the drinking fluid (inhibition by 24.5%) or 0.08% EGCG in the fluid (by 51%) (Hao et al., 2006). ECG (0.08%) had no significant effect on tumor multiplicity. IHC analysis showed that in comparison to the adenomas in the control group, treatment with PPE and EGCG decreased cell proliferation index (based on Ki-67 expression), increased apoptotic index (cleaved caspase-3), decreased nuclear β-catenin levels, and decreased phospho-Akt levels. The results suggest that both EGCG and PPE exert cancer prevention activity by inhibiting cell proliferation, promoting apoptosis, and modulating β-catenin and Akt signaling. The primary molecular events that lead to these cellular and molecular changes are not known.
We previously reported that oral administration of green tea (0.6% green tea solids) inhibited the formation of azoxymethane (AOM)-induced aberrant crypt foci (ACF) in female CF-1 mice on a high-fat diet (Ju et al., 2003). Recently, we have studied the preventive effects of EGCG against colon cancer formation in AOM treated CF-1 female mice (Bose et al., 2006). EGCG (0.1% solution in drinking fluid) administration for 32 weeks decreased tumor incidence by 55% and the number of tumors per tumor-bearing mouse by 26%. Gene expression analysis using Affymetrix U74Av2 Genechip and Microarray Suite 5.0 software revealed distinct alterations by EGCG treatment in the AOM-treated mice (Bose et al., 2006). Many genes, including heat shock protein (Hsp) 86 (a chaperone for some oncogenic proteins), claudin 3 (important for tight junction formation), and RAB8 homologue (possibly important for intracellular trafficking of many membrane proteins), were found to be suppressed by AOM in non-tumorous tissues but up-regulated by EGCG. A group of calcium-regulated genes, including calcium-activated nucleotidase 1, calcium-regulated heat shock protein 1, and endothelial calcium/camodulin-activated myosin light chain kinase and actin interacting protein, were also up-regulated by EGCG. On the other hand, other genes, including Ankyrin repeat domain 10 (important for a variety of protein-protein interactions), were induced by AOM in non-tumorous tissues but down-regulated by EGCG. A small cluster of genes were induced by EGCG, including ATP H+ transporting lysosomal accessing protein 2, Notch-regulated ankyrin repeat protein, and cysteine rich protein 1 in the non-tumorous tissues, but not tumor tissues. Distinct transcription profiles by EGCG in tumor tissue samples were also observed. For example, the gene encoding RhoC, a protein involved in cell motility and whose expression has been associated with the progression of several types of cancer, was down-regulated by EGCG treatment in tumor samples. Some of these gene expression changes may be directly involved in the chemopreventive mechanisms of EGCG.
The above results are consistent with a previous report that tea catechins inhibited 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis in mice (Yin et al., 1994). Thus, the inhibitory effects of green tea and tea polyphenols on intestinal/colon tumorigenesis in mice have been consistently observed in different laboratories.
On the other hand, the effects of tea preparation on colon tumorigenesis in rats have not been consistent. Colon cancer formation induced by AOM in rats was inhibited by green tea polyphenols in the drinking fluid (Kim et al., 1994). In the AOM-treated F344 rats, green tea extract (1 or 2% in drinking fluid) had a marginal inhibitory effect on ACF formation, and its combination with phytic acid appeared to produce a synergistic inhibitory effect (Challa et al., 1997). In a similar model, when different tea preparations were given to F344 rats after AOM-treatment, EGCG and black tea polyphenols were found to inhibit ACF formation; but the effect of green tea polyphenols was marginal and extracts from green or black tea were not effective (Wargovich et al., 2000). Oral administration of green tea extracts (2% in drinking fluid) significantly inhibited ACF formation in 2-amino-3-methylimidazo[4,5-f]quinoline-treated F344 rats, but black tea extracts (1%) did not (Xu et al., 1996). There are also reports on the lack of an inhibitory effect of green tea. For example, Hirose et al. (Hirose et al., 2001; Hirose, 2002) failed to demonstrate an inhibitory effect in the DMH-induced rat model. Weisburger et al. also reported that when black tea extracts, green tea extracts, or EGCG was added in the diet, inhibition of AOM-induced colon tumorigenesis was not observed in the rats (Weisburger, 1998). On the other hand, black tea extracts, when administered to rats in the diet, inhibited AOM-induced colon tumorigenesis, but green tea extracts in the diet failed to show such an inhibition (Caderni et al., 2000). Our recent animal study showed that PPE at 0.12 and 0.24% in the diet significantly inhibited AOM-induced ACF formation in rats by 16 and 37%, respectively (unpublished results).
The reasons for the inconsistency among the different studies are complex and may be related to several factors: 1) the diet used, 2) the protocol of tumor initiation and tumor yield, and 3) the type and dose of tea polyphenols or extracts used, when they were given, and whether they were administered through drinking fluid or through the diet. A key issue that was not discussed in these publications is the stability of the tea polyphenols in solution and in the diet. We know that EGCG is generally less stable in tap water than in deionized water. The unanalyzed constituents in tea extracts may also complicate the matter.
In theory, the intestine is a promising site for chemoprevention with polyphenols that have low systemic bioavailability. EGCG, the major polyphenol in green tea, has only limited systemic bioavailability after oral ingestion. Even the absorbed EGCG is excreted mostly into the intestine through the bile (reviewed in (Yang et al., 2002)). Therefore, the intestine may actually be exposed to high levels of EGCG after ingestion. Our recent results showed that in the mouse, 3 h after intragastric administration of EGCG (200 mg/kg), the intestinal/colon EGCG levels were 9.4-36.3 μg/g (Lambert et al., 2006). These concentrations (approx. 20-70 μM) are similar to those used in our cell culture studies, in which anti-cancer activities have been demonstrated (Ju et al., 2005).
Studies in Cell lines and General Mechanistic Consideration
Since the observation of the inhibitory activity of tea and tea polyphenols in animal models, many studies have been conducted on the inhibition of cell growth and cell transformation by EGCG and other tea polyphenols. Some of these activities have been attributed to the inhibition of AP-1 activity, possibly due to the inhibition of MAP kinases activities. Subsequently, many studies have focused on the effects of EGCG on signal transduction pathways in cell lines and numerous mechanisms for the cancer preventive activity of tea polyphenols have been proposed (reviewed in (Sun et al., 2002; Yang et al., 2002; Hou et al., 2004; Lambert et al., 2005; Khan et al., 2006; Yang et al., 2006b)). These include inhibition of MAP kinases and the PI3K/AKT pathway, inhibition of NFκB and AP-1 mediated transcription, inhibition of growth factor-mediated signaling, inhibition of aberrant arachidonic acid metabolism, and other activities. The end result of these effects may be the inhibition of tumor cell growth, induction of apoptosis, or the inhibition of angiogenesis. Some of these activities have been demonstrated to be associated with the inhibition of carcinogenesis in animal models. In most cases, however, the concentrations of EGCG required to observe these biological effects in vitro exceed the concentrations achievable in plasma and tissues by 10 to 100-fold, and questions remain as to the relevance of these in vitro observations to the mechanisms of the cancer preventive activities in vivo (reviewed in (Hou et al., 2004)).
In general, if an effect can be observed in vitro at concentrations lower or similar to those observed in vivo, then the event may occur in vivo. For example, inhibition of telomerase and matrix metalloproteinases has been demonstrated with rather low concentrations of EGCG (IC50 in the range of 0.5 - 1 μM). EGCG has also been found to bind to Bcl-2 and vimentin with high affinity. However, there are big differences between the effective concentrations determined with pure enzymes and those in cell lines or tissues, possibly due to the non-specific binding of EGCG to many proteins and the limited amount of EGCG that can enter the cells. When a small amount of pure enzyme is used in an enzymatic assay, inhibition may be observed with nanomolar concentrations of EGCG, but it may take much higher concentrations of EGCG to inhibit the activity in cell lines or tissues. This point is illustrated in the inhibition of 20s proteasome chymotryptic activities by EGCG; i.e. the IC50 observed in a cell-free system was 0.1-0.2 μM, but it was 1-40 μM in tumor cell lines (Nam et al., 2001). EGCG was reported to bind to the 67-kDa laminin receptor with a Kd of 0.04 μM, to vimentin with a Kd of 3.3 nM, and interact with Bcl-2 with a Ki of 0.33 μM (Leone et al., 2003; Ermakova et al., 2005). In all these studies, there were experiments demonstrating the biological relevance of the effects in their specific experimental systems, but it required much higher concentrations of EGCG to cause growth inhibition and induce apoptosis. The general applicability of these mechanisms for cancer prevention is still not known.
Another concern in the use of redox sensitive compounds in a cell culture system is the oxidation and stability of the compound. For instance, when added to the cell culture medium, EGCG is oxidized to produce superoxide radical and H2O2 (Hou et al., 2005). We have demonstrated that, depending on the cell lines and culture conditions, the EGCG-induced apoptosis can be completely or partially blocked by the addition of catalase in the culture medium, suggesting that the apoptosis is mediated by H2O2 (reviewed in (Hou et al., 2004)). Autooxidation of EGCG generated reactive species may inactivate epidermal growth factor receptor in cells in culture (Hou et al., 2005). It is not clear whether the EGCG autooxidation-induced effects occur inside animal tissues, because these tissues are endowed with antioxidative enzymes and are usually under lower oxygen partial pressure (<40 mmHg) than the cell culture medium (152 mmHg). This point was discussed in our previous publications (Hou et al., 2004; Hou et al., 2005) and reviewed by Khan et al. (Khan et al., 2006).
Based on the above discussions, we summarize our understanding of the mechanisms of cancer prevention by EGCG as follows: 1) multiple mechanisms are likely to be involved in different experimental systems, 2) some of the proposed mechanisms based on studies in cancer cell lines may not be relevant to cancer prevention; 3) mechanisms of cancer prevention need to be demonstrated in relevant models or human tissues; and 4) many of the observed effects are probably secondary events or downstream events and it is important to identify the direct targets of EGCG action.
Human Studies
The effects of tea consumption on the risk of human cancer have been studied extensively in many different parts of the world. In our review in 1993 (Yang and Wang, 1993; Yang et al., 2002), we summarized that, whereas many studies suggested a reduction of cancer risk by tea consumption, many other studies did not; therefore, no clear-cut conclusion could be drawn. Unfortunately, the lack of conclusion still exists in spite of the more than one hundred papers and ten review articles that have been written on this topic. It is probably not reasonable to expect a simple conclusion concerning tea and cancer prevention from epidemiological studies, because the etiological factors for different types of cancers are not the same, the populations studied are different, and the compositions of green and black tea differ significantly. It is possible that tea consumption can prevent a certain type of cancer in a certain population. In fact, most of the recent publications on the protective effect of tea against cancer were based on studies in Shanghai and the neighboring province of Zhejiang in China, where green tea is usually consumed. The proposed association between green tea consumption and lowered risk of prostate and ovarian cancer (reviewed in (Wu and Yu, 2006)) are intriguing and worth further investigation. Even with the same population consuming the same type of tea, the picture is still not clear. Examples on the epidemiology of tea consumption and cancer risk of the stomach, colon, lung, and breast are discussed below.
As reviewed previously, many case-control studies and some cohort studies observed an association between green tea consumption and lowered risk for stomach cancer (reviewed in (Tsubono et al., 2001; Yang et al., 2002)), and many of the studies were from Japan. However, four recent prospective studies which covered different areas in Japan did not observe a relationship between green tea consumption and stomach cancer (reviewed in (Hoshiyama et al., 2005). The reasons for the discrepancies have been discussed (Tsubono et al., 2001) and remain to be investigated further.
The relationship between tea consumption and colorectal risk has been studied by many investigators, but no clear-cut conclusions can be drawn (reviewed in (Blot et al., 1996; Kohlmeier et al., 1997; Bushman, 1998; Landau et al., 2005). Sun et al. recently conducted a meta-analysis on 21 relevant papers published up to January 2005 (Sun et al., 2006b). Summary odds ratios (ORs) for highest vs. non- or lowest tea consumption levels were calculated based on fixed or random effect models. For green tea, the combined results from eight studies indicated a reduced risk of colorectal cancer with intake (summary OR 0.82, 95% confidence interval = 0.69-0.98). The protective effects were mainly due to the three case-control studies (summary OR = 0.74, 95% CI = 0.60-0.93). For black tea, the summary OR derived from 17 studies was 0.99 (95% interval = 0.87-1.13). Our recent study in collaboration with Drs. Jian-Min Yuan and Mimi C. Yu (University of Minnesota) and Dr. Yu-Tang Gao (Shanghai Cancer Institute) showed some interesting results as follows. A nested case-control study within the Shanghai Cohort Study involving 18,244 men was conducted to investigate the association between prediagnostic urinary tea polyphenols and colorectal cancer risk. After 16 years of follow-up, 117 subjects developed colon cancer. For each case, 5 matched controls were chosen from the cohort. Urinary tea polyphenols, including EGC, 4′-O-methyl-EGC (MeEGC), and EC were measured on all study subjects. Among subjects with at least 5 years of follow-up, higher levels of MeEGC were associated with lower risk of colon cancer. Compared with the lowest quartile of “EGC plus MeEGC”, the multivariate-adjusted ORs (95% CIs) of colon cancer for the 2nd, 3rd, and 4th quartiles were 0.57 (0.29-1.11), 0.39 (0.19-0.80), and 0.43 (0.21-0.88), respectively (Ptrend = 0.007). Urinary EGC and MeEGC are tea consumption biomarkers developed by us, and we are encouraged by these results (Yuan et al., 2006).
The association between tea consumption and lung cancer risk is also unclear; whereas some studies suggested a protective effect, others did not. This is also the conclusion of a recent review, which covers 14 epidemiological studies on green and black tea consumption and lung cancer risk (Clark and You, 2006). In some recent studies, a protective effect of tea consumption against lung cancer was only observed in special subpopulations; for example, green tea in individuals with the Cys(326) allele of 8-oxoguanine-DNA glycosylase (Bonner et al., 2005) and nonsmoking women (Zhong et al., 2001), and black tea in nonsmoking women (Kubik et al., 2004). The results are interesting, but are far from clear because of the many confounding factors.
A recent meta-analysis of 13 papers on breast cancer showed that the combined results of green tea consumption from four studies showed a reduced risk of breast cancer for highest versus lowest intake groups (OR=0.78, 95% CI=0.61-0.98) (Sun et al., 2006b); whereas for black tea, conflicting results were obtained in case-control studies (OR=0.91, 95% CI=0.84-0.98, in 8 studies) versus cohort studies (OR=1.15, 95% CI=1.02-1.31, in 5 studies). A possible mechanism for green tea consumption to reduce breast cancer risk is through the modulation of estrogen levels (Wu and Yu, 2006). Plasma estrone levels were significantly lower in regular green tea drinkers than in non- or irregular green tea drinkers. Tea was found to be more protective against breast cancers in individuals with low catechol O-methyltransferase activity alleles compared those with high activity alleles in one study, but such an association was not found in another study (Wu and Yu, 2006).
In humans as well as in animals, the cancer prevention effects in different organ sites is likely to be determined by the bioavailability of tea constituents. The systemic bioavailability of EGCG is low and that of the non-gallated catechins such as EGC and EC are higher. After ingestion the equivalent of 2 or 3 cups of green tea, peak blood levels in human are in the sub-micromolar range, and levels in internal organs are not higher (Yang et al., 2002). The relationship between tissue levels of tea catechins and cancer prevention at the respective tissues remains to be studied.
The quantity of tea consumption and cancer prevention is a topic of public interest. A study in Shanghai by Gao et al. (Gao et al., 1994) demonstrated that daily consumption of the equivalent of two or three cups of green tea reduced the risk for esophageal cancer among non-smokers/non-drinkers of alcoholic beverages. On the other hand, studies in Japan usually suggest that ten cups of green tea is cancer preventive (Imai et al., 1997). Because of the bitterness of green tea, it may be difficult for some individuals to drink a large amount of green tea. Ingestion of food items than contain tea power or taking tea polyphenol preparations as dietary supplements may be a way to increase the intake of tea constituents. When high doses of tea polyphenols are used, toxicity is a concern (Bonkovsky, 2006).
Concluding Remarks
As discussed in the previous sections, the cancer prevention activity of tea and tea polyphenols has been observed in animal models of carcinogenesis by many investigators. Nevertheless, such an activity has not been convincingly demonstrated in humans based on epidemiological studies. A possible reason for the discrepancy between human epidemiological studies and experiments with animal models is that the human populations are not homogenous in genetic makeup and life style, and the results are influenced by many confounding factors; whereas in animal studies, the experimental conditions are controlled to allow the detection of the treatment effect. A second reason is that the doses of tea or tea polyphenols used in animal studies are usually higher than the amounts consumed by humans. In most epidemiological studies, the quantification of tea consumption is usually inadequate. The use of biomarkers for tea consumption, such as the urinary EGC (or plus 4′-methylEGC), may be a promising approach (Sun et al., 2002; Yuan et al., 2006).
Laboratory research during the past 15 years has characterized the cancer preventive properties and bioavailability of tea constituents. Even though the epidemiological data on the relationship between tea consumption and human cancer are not conclusive, tea may still be used to prevent cancer at selected organ sites, if we can demonstrate such activity in human intervention trials. In terms of organ sites, we believe that the digestive tract, which can have direct contact with tea constituents, holds greater promise. For example, holding tea solution or tea leaves in the mouth can delivery millimolar concentrations of EGCG and other catechins in the oral cavity. Even after vigorously rinsing the mouth, high micromolar concentrations of these catechins can still be measured in the saliva with elimination half-life of greater than 30 minutes (Yang et al., 2002). At these concentrations, EGCG is expected to cause growth inhibition or induce apoptosis in cancer or preneoplastic oral cells (Yang et al., 2002), and this can exert oral cancer preventive activities. In an intervention study in Beijing involving 59 subjects with oral leukoplakia, ingestion and topical application of green tea preparations for 6 months in 29 subjects significantly decreased the size of oral lesions and number of micronuclei, in comparison to the 30 patients in the untreated control (Li et al., 1999). An even more impressive study on the prevention of prostate cancer was recently reported by Bettuzi et al. (Bettuzzi et al., 2006). In this Italian study, 60 subjects with high-grade prostate intraepithelial neoplasia were randomized for a double-blind trial. In the 30 subjects that took 200 mg of green tea polyphenols three times daily for one year, only on subject developed prostate cancer (3%). In the 30 subjects that took placebo, nine subjects developed prostate cancer which is the expected rate (30%) of cancer development. This result is very exciting and should stimulate many similar cancer prevention studies. In this study the dose of 200 mg green tea polyphenols three time a day did not produce significant side or adverse effects. Caution should be applied however, when high doses are used, because there are case reports associating liver toxicity with the use of high dose of green tea extract-based supplements (Bonkovsky, 2006).
We hope the information provided in this review article will help researchers in designing experiments to elucidate the activities and mechanisms of action of tea and tea constituents that are relevant to human cancer prevention. This would help the development of biomarkers on the exposure and biological effects of tea consumption. A fundamental understanding in this area is important for the rational design of future human intervention trials and cohort studies to elucidate the relationship between tea consumption and cancer.
Abbreviation
- EGCG
(-)-epigallocatechin-3-gallate
- EGC
(-)-epigallocatechin
- ECG
(-)-epicatechin-3-gallate
- EC
(-)-epicatechin
- TFs
theaflavins
- ROS
reactive oxygen species
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- PPE
Polyphenon E
- APC
adenomatous polyposis coli
- MIN
multiple intestinal neoplasia
- AOM
azoxymethane
- ACF
aberrant crypt foci
- DMH
dimethylhydrazine
- ORs
odds ratios
- CI
confidence interval
- MeEGC
4′-O-methyl-EGC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Chow WH, McLaughlin JK. Tea and cancer: a review of the epidemiological evidence. Eur J Cancer Prev. 1996;5:425–438. [PubMed] [Google Scholar]
- Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann Intern Med. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- Bonner MR, Rothman N, Mumford JL, He X, Shen M, Welch R, Yeager M, Chanock S, Caporaso N, Lan Q. Green tea consumption, genetic susceptibility, PAH-rich smoky coal, and the risk of lung cancer. Mutat Res. 2005;582:53–60. doi: 10.1016/j.mrgentox.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bose M, Chin KV, Park S, Husain A, Liao J, Ju J, Vittal R, Kopelovich L, Huang MT, Yang CS. Modulation of gene expression by (-)-epigallocatechin-3-gallate and sulindac in an azoxymethane-induced mouse model of colon cancer. Manuscript in preparation 2006 [Google Scholar]
- Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- Caderni G, De Filippo C, Luceri C, Salvadori M, Giannini A, Biggeri A, Remy s, Cheynier V, Dolara P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 2000;21:1965–1969. doi: 10.1093/carcin/21.11.1965. [DOI] [PubMed] [Google Scholar]
- Challa A, Rao DR, Reddy BS. Interactive suppression of aberrant crypt foci induced by azoxymethane in rat colon by phytic acid and green tea (Short Communication) Carcinogenesis. 1997;18:2023–2026. doi: 10.1093/carcin/18.10.2023. [DOI] [PubMed] [Google Scholar]
- Clark J, You M. Chemoprevention of lung cancer by tea. Mol Nutr Food Res. 2006;50:144–151. doi: 10.1002/mnfr.200500135. [DOI] [PubMed] [Google Scholar]
- Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–16890. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- Hao X, Bose M, Lambert JD, Ju J, Lee MJ, Park S, Husain A, Wang S, Sun Y, Yang CS. Inhibition of intestinal tumorigenesis in ApcMin/+ mice by (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin-3-gallate (ECG), and Polyphenon E (PPE). Proceeding Abstract for 2006 AACR Annual Meeting, Abstract # 4894.2006. [Google Scholar]
- Hirose M, et al. Lack of inhibitory effects of green tea catechins in 1,2-dimetylhydrazine-induced rat intestinal carcinogenesis model: comparison of the different formulations, administration routes and doses. Cancer Lett. 2002;188:163–170. doi: 10.1016/s0304-3835(02)00458-5. [DOI] [PubMed] [Google Scholar]
- Hirose M, et al. Hoshiya T, Mizoguchi Y, Nakamura A, Akagi K, Shirai T. Green tea catechins enhance tumor development in the colon without effects in thelung or thyroid after pretreatment with 1,2-dimethylhydrazine or 2,2′-dihydroxy-di-n-propylnitrosamine in male F344 rats. Cancer Lett. 2001;168:23–29. doi: 10.1016/s0304-3835(01)00502-x. [DOI] [PubMed] [Google Scholar]
- Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H, Hayakawa N, Tamakoshi A, Yoshimura T. Green tea and stomach cancer--a short review of prospective studies. J Epidemiol. 2005;15 2:S109–112. doi: 10.2188/jea.15.S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- Ju J, Liu Y, Hong J, Huang MT, Conney AH, Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–178. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kim M, Hagiwara N, Smith SJ, Yamamoto T, Yamane T, Takahashi T. Preventive effect of green tea polyphenols on colon carcinogenesis. In: Huang MT, Osaswa T, Ho CT, Rosen RT, editors. Food Phytochemicals in Cancer Prevention. 1994. pp. 51–55. (ACS Symposium Series 546). [Google Scholar]
- Kohlmeier L, Weterings KG, Steck S, Kok FJ. Tea and cancer prevention: an evaluation of the epidemiologic literature. Nutr Cancer. 1997;27:1–13. doi: 10.1080/01635589709514494. [DOI] [PubMed] [Google Scholar]
- Kubik AK, Zatloukal P, Tomasek L, Pauk N, Havel L, Krepela E, Petruzelka L. Dietary habits and lung cancer risk among non-smoking women. Eur J Cancer Prev. 2004;13:471–480. doi: 10.1097/00008469-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- Landau JM, Lambert JD, Lee MJ, Yang CS. Cancer prevention by tea and tea constituents. CRC Taylor & Francis; New York: 2005. [Google Scholar]
- Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220:218–224. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- Lu G, Liao J, Yang GY, Reuhl K, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis model in a/J mice by tea polyphenols and caffeine. Cancer Res. 2006a doi: 10.1158/0008-5472.CAN-06-1497. Accepted. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yao R, Yan Y, Wang Y, Hara Y, Lubet RA, You M. A gene expression signature that can predict green tea exposure and chemopreventive efficacy of lung cancer in mice. Cancer Res. 2006b;66:1956–1963. doi: 10.1158/0008-5472.CAN-05-3158. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Orner GA, et al. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M, Ohkura Y, Okabe S, Fujiki H. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J Cancer Res Clin Oncol. 2001;127:69–72. doi: 10.1007/s004320000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006a;27:1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006b;27:1301–1309. doi: 10.1093/carcin/bgl024. [DOI] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Lee MJ, Yang CS, Gao YT, Ross RK, Yu MC. Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis. 2002;23:1497–1503. doi: 10.1093/carcin/23.9.1497. [DOI] [PubMed] [Google Scholar]
- Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, Nakatsuka H, Fukao A, Satoh H, Hisamichi S. Green tea and the risk of gastric cancer in Japan. N Engl J Med. 2001;344:632–636. doi: 10.1056/NEJM200103013440903. [DOI] [PubMed] [Google Scholar]
- Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, Price R, Gray K, Kelloff GJ. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- Weisburger JH, et al. Effect of tea extracts, polyphenols, and epigallocatechin gallate on azoxymethane-induced colon cancer. Proc Soc Exp Biol Med. 1998;217:104–108. doi: 10.3181/00379727-217-44211. [DOI] [PubMed] [Google Scholar]
- Wu AH, Yu MC. Tea, hormone-related cancers and endogenous hormone levels. Mol Nutr Food Res. 2006;50:160–169. doi: 10.1002/mnfr.200500142. [DOI] [PubMed] [Google Scholar]
- Xu M, Bailey AC, Hernaez Jf, Taoka CR, Schut HAJ, Dashwood RH. Protection by green tea, black tea, and indole-3-carbinol against 2-amino-3-methylimidazo[4,5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog. 2006a;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- Yang CS, Liao J, Yang GY, Lu G. Inhibition of lung tumorigenesis by tea. Exp Lung Res. 2005;31:135–144. doi: 10.1080/01902140490495525. [DOI] [PubMed] [Google Scholar]
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006b;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- Yin P, Zhao J, Cheng S, Hara Y, Zhu Q, Liu Z. Experimental studies of the inhibitory effects of green tea catechin on mice large intestinal cancers induced by 1,2-dimethylhydrazine. Cancer Lett. 1994;79:33–38. doi: 10.1016/0304-3835(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Yoshizawa S, Horiuchi t, fujiki H, Yoshida T, Okuda t, Sugimura T. Antitumor promoting activity of (-)-epigallocatechin gallate, the main constituent of “tannin” in green tea. Phytother Res. 1987;1:44–47. [Google Scholar]
- Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai cohort study. Inter J Cancer. 2006 doi: 10.1002/ijc.22460. accepted. [DOI] [PubMed] [Google Scholar]
- Zhong L, Goldberg MS, Gao YT, Hanley JA, Parent ME, Jin F. A population-based case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology. 2001;12:695–700. doi: 10.1097/00001648-200111000-00019. [DOI] [PubMed] [Google Scholar]