Abstract
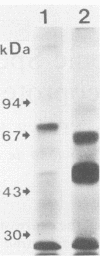
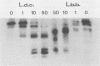
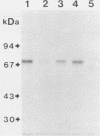
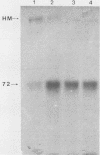
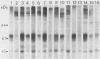
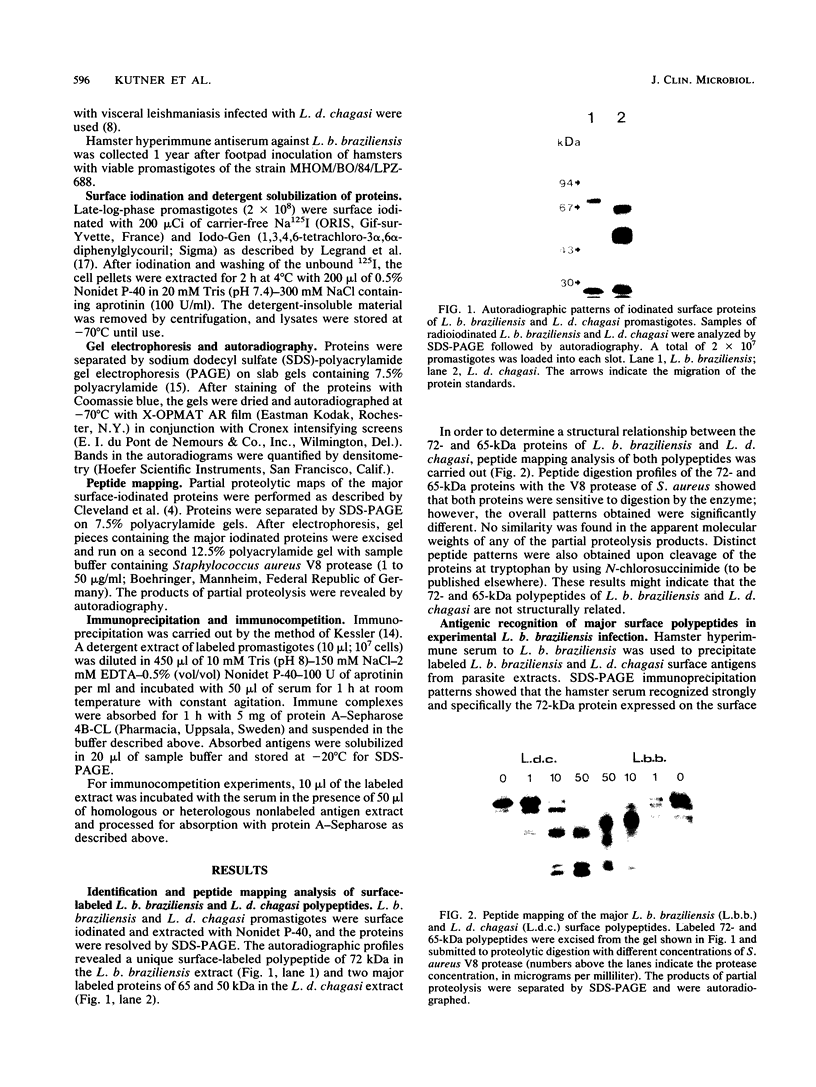
We examined the expression and the antigenicity of the major surface polypeptides of Leishmania braziliensis braziliensis and Leishmania donovani chagasi, parasites which commonly coexist in the same endemic areas of Bolivia. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiles from surface-iodinated promastigotes showed the presence of a unique iodinatable polypeptide of 72 kDa on the L. b. braziliensis surface and of two major components of 65 and 50 kDa exposed at the surface of L. d. chagasi. Comparison of the peptide digestion profiles of the major iodinated polypeptides of both strains showed no similarity between the maps of the 72- and the 65-kDa polypeptides of L. b. braziliensis and L. d. chagasi, respectively. Immunoprecipitation of surface-labeled L. b. braziliensis Nonidet P-40 extracts with 35 serum specimens obtained from Bolivian patients with cutaneous and mucocutaneous leishmaniasis showed that all serum specimens recognized predominantly the 72-kDa antigen and high-molecular-mass proteins in some cases. The recognition patterns were independent of the geographical origin of the patient, the type of lesion, and the serum antibody titer. Serum specimens from children with visceral leishmaniasis did not precipitate the L. b. braziliensis 72-kDa antigen. Hamster hyperimmune serum against L. b. braziliensis also recognized the 72-kDa surface antigen. However, this recognition was inhibited in the presence of the homologous nonlabeled antigen but not in the presence of heterologous (L. d. chagasi and Trypanosoma cruzi) antigens. The specific recognition of 72-kDa surface antigen in both natural and experimental L. b. braziliensis infections suggests that this antigen could be a good candidate for use in the differential immunodiagnosis and prognosis of the disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Chaudhuri G., Chaudhuri M., Pan A., Chang K. P. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem. 1989 May 5;264(13):7483–7489. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjeux P., Mollinedo S., Le Pont F., Paredes A., Ugarte G. Cutaneous leishmaniasis in Bolivia. A study of 185 human cases from Alto Beni (La Paz Department). Isolation and isoenzyme characterization of 26 strains of Leishmania braziliensis braziliensis [corrected]. Trans R Soc Trop Med Hyg. 1987;81(5):742–746. doi: 10.1016/0035-9203(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Gardiner P. R., Jaffe C. L., Dwyer D. M. Identification of cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect Immun. 1984 Feb;43(2):637–643. doi: 10.1128/iai.43.2.637-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S., Chance M. L., Hommel M., Crampton J. M. Cloning of a gene encoding the immunodominant surface antigen of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Apr;23(3):211–222. doi: 10.1016/0166-6851(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Hernández A. G., Payares G., Misle A., Dagger F. The heterogeneity of Leishmania cell-surface antigens. Parasitol Res. 1989;75(8):583–588. doi: 10.1007/BF00930952. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrand D., Desjeux P., le Pont F., Brénière S. F., Lemesre J. L., Santoro F., Capron A. Identification of a major 72 kilodalton surface antigen in twelve isolates of Leishmania braziliensis braziliensis. Mol Biochem Parasitol. 1987 Jun;24(2):117–124. doi: 10.1016/0166-6851(87)90097-1. [DOI] [PubMed] [Google Scholar]
- Lemesre J. L., Rizvi F. S., Afchain D., Sadigursky M., Capron A., Santoro F. Subspecies-specific surface antigens of promastigotes of the Leishmania donovani complex. Infect Immun. 1985 Oct;50(1):136–141. doi: 10.1128/iai.50.1.136-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]