Abstract
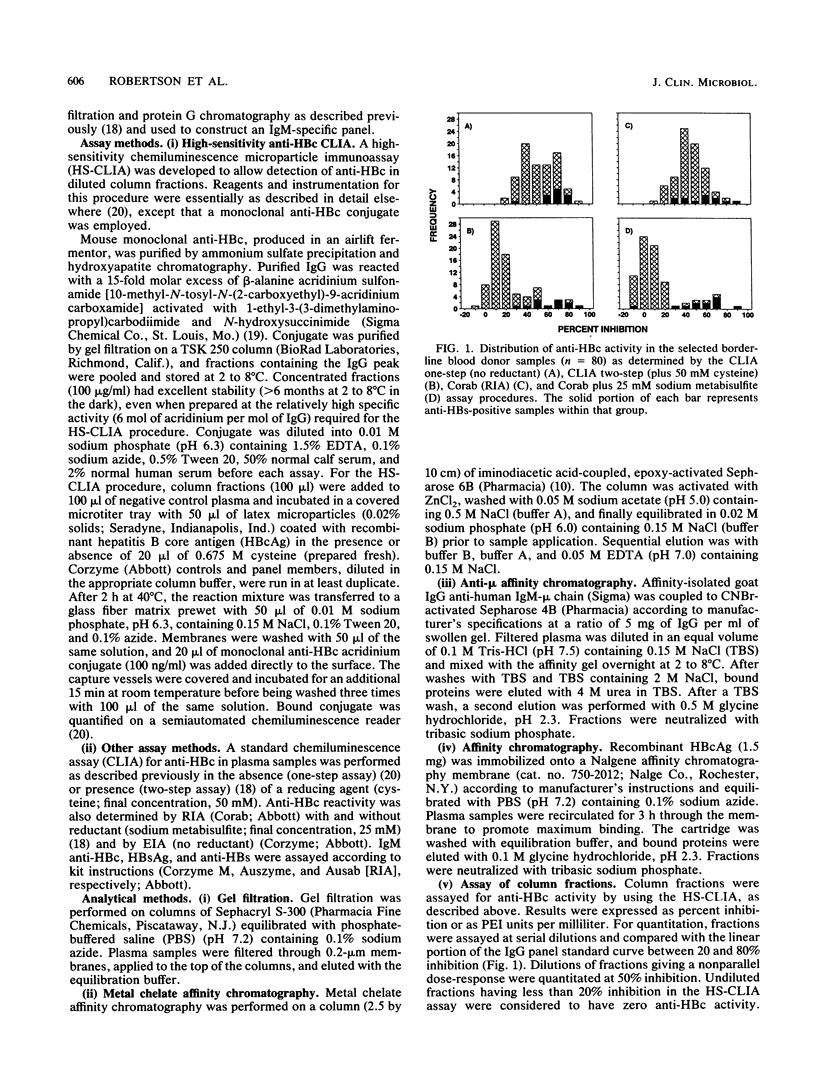
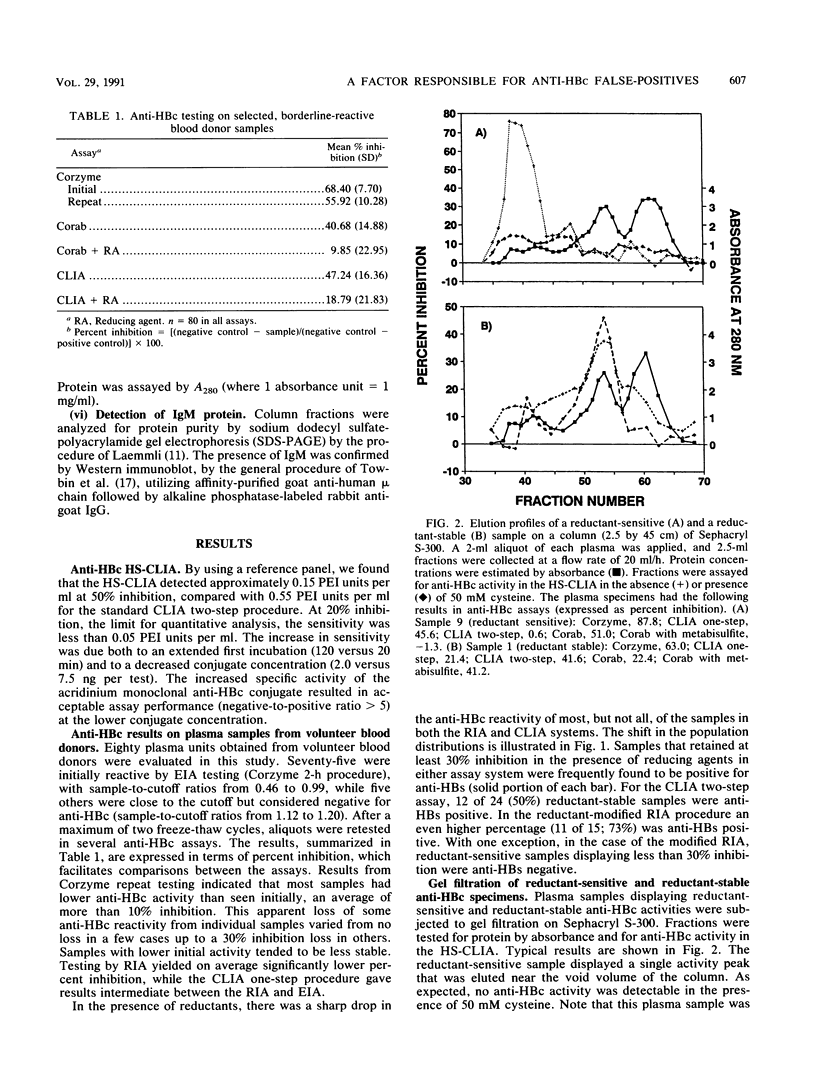
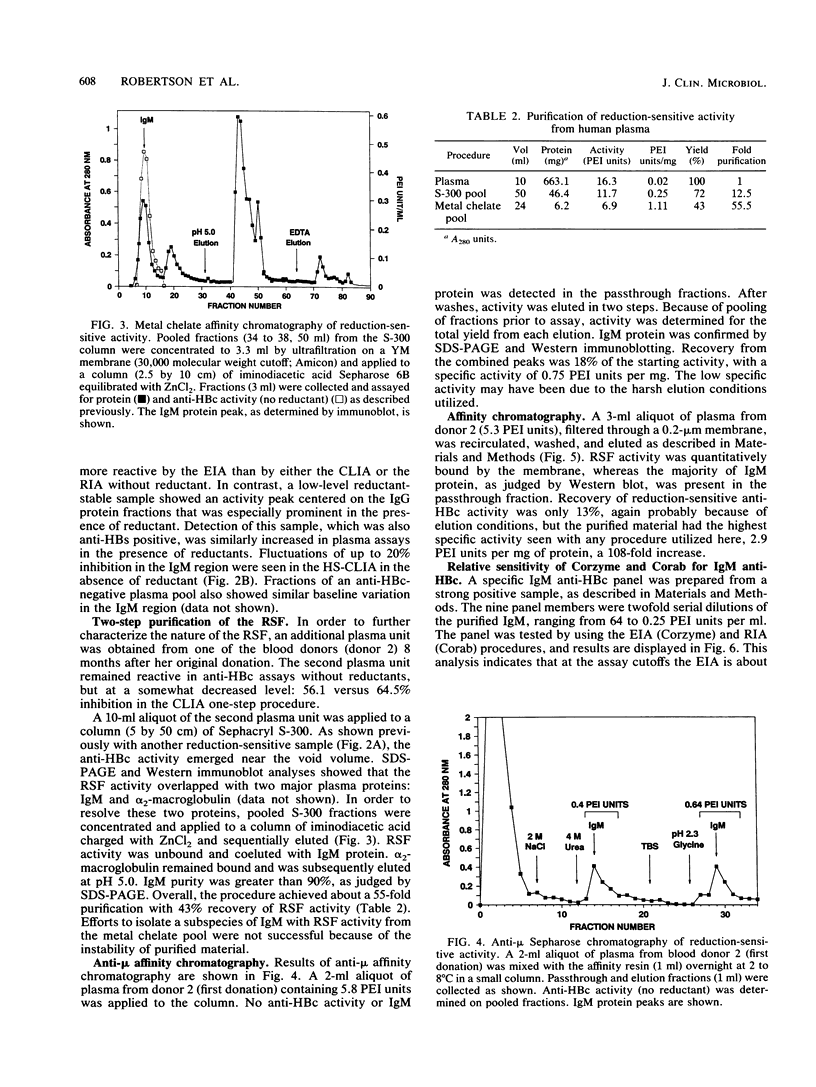
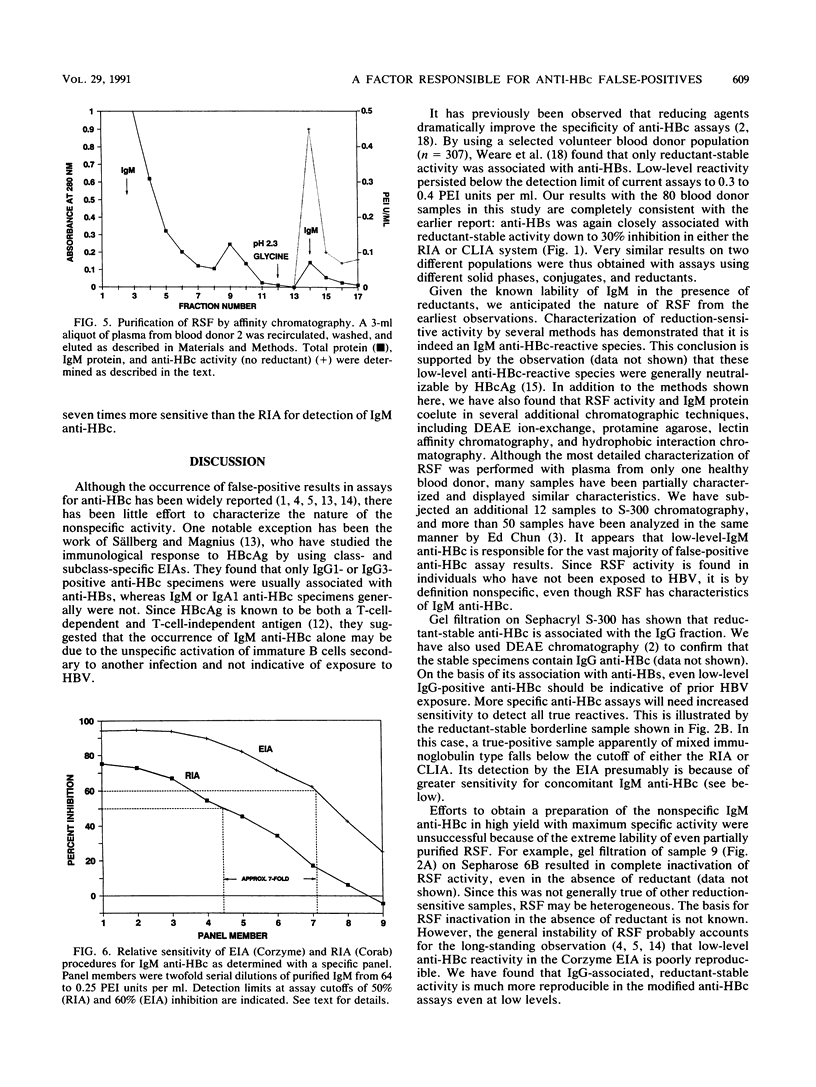
Addition of reducing agents to competitive assays for antibody to hepatitis B core antigen (anti-HBc) eliminates apparent false reactivity of specimens obtained from individuals with no prior history of hepatitis B virus (HBV) infection and without other serological markers of HBV infection. We have purified and characterized a reduction-sensitive factor (RSF) isolated from the plasma of several volunteer blood donors. Column fractions were assayed fro anti-HBc by using a highly sensitive chemiluminescence assay with a detection of 0.15 Paul Ehrlich Institut units per ml at 50% inhibition. Gel filtration on Sephacryl S-300 indicated that reductant-sensitive samples possessed anti-HBc activity that was associated with immunoglobulin M (IgM), whereas reductant-stable activity was associated with IgG. Gel filtration followed by metal chelate affinity chromatography resulted in a 55-fold purification and demonstrated that RSF activity copurifies with IgM. RSF was recovered from a recombinant hepatitis B core antigen matrix and shown to be an IgM species by immunoblot. In addition, RSF activity coeluted with IgM protein from anti-mu-chain Sepharose. Discrepancies between enzyme immunoassay and radioimmunoassay procedures for anti-HBc (Corzyme and Corab, respectively: Abbott Laboratories, North Chicago, Ill.) appear to be due to the relative sensitivity of the enzyme immunoassay for IgM anti-HBc (sevenfold greater than the radioimmunoassay using a specific panel). The biological basis for the occurrence of low levels of nonspecific IgM anti-HBc reactivity in individuals not previously exposed to HBV remains to be elucidated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspari G., Beyer H. J., Elbert G., Koerner K., Muss P., Schunter F. W., Uy A., Gerlich W., Thomssen R., Schmitt H. Unsatisfactory specificities and sensitivities of six enzyme immunoassays for antibodies to hepatitis B core antigen. J Clin Microbiol. 1989 Sep;27(9):2067–2072. doi: 10.1128/jcm.27.9.2067-2072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadler S. C., Murphy B. L., Schable C. A., Heyward W. L., Francis D. P., Kane M. A. Epidemiological analysis of the significance of low-positive test results for antibody to hepatitis B surface and core antigens. J Clin Microbiol. 1984 Apr;19(4):521–525. doi: 10.1128/jcm.19.4.521-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. R., Polesky H. F. Evaluation of routine anti-HBc screening of volunteer blood donors: a questionable surrogate test for non-A, non-B hepatitis. Transfusion. 1987 Jan-Feb;27(1):107–108. doi: 10.1046/j.1537-2995.1987.27187121451.x. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H., Schafer D. F., Ferenci P., Waggoner J. G., Vergalla J., April M., Phillips L. Antibody to hepatitis B surface antigen in nonprimate animal species. Gastroenterology. 1983 Jun;84(6):1478–1482. [PubMed] [Google Scholar]
- Kessler H. A., Harris A. A., Payne J. A., Hudson E., Potkin B., Levin S. Antibodies to hepatitis B surface antigen as the sole hepatitis B marker in hospital personnel. Ann Intern Med. 1985 Jul;103(1):21–26. doi: 10.7326/0003-4819-103-1-21. [DOI] [PubMed] [Google Scholar]
- Koziol D. E., Holland P. V., Alling D. W., Melpolder J. C., Solomon R. E., Purcell R. H., Hudson L. M., Shoup F. J., Krakauer H., Alter H. J. Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Ann Intern Med. 1986 Apr;104(4):488–495. doi: 10.7326/0003-4819-104-4-488. [DOI] [PubMed] [Google Scholar]
- Kurecki T., Kress L. F., Laskowski M., Sr Purification of human plasma alpha 2 macroglobulin and alpha 1 proteinase inhibitor using zinc chelate chromatography. Anal Biochem. 1979 Nov 1;99(2):415–420. doi: 10.1016/s0003-2697(79)80026-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Schmidt P. J., Leparc G. F., Samia C. T. Comparison of assays for anti-HBc in blood donors. Transfusion. 1988 Jul-Aug;28(4):389–391. doi: 10.1046/j.1537-2995.1988.28488265275.x. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Aach R. D., Hollinger F. B., Mosley J. W., Szmuness W., Kahn R., Werch J., Edwards V. Hepatitis B virus antibody in blood donors and the occurrence of non-A, non-B hepatitis in transfusion recipients. An analysis of the Transfusion-Transmitted Viruses Study. Ann Intern Med. 1984 Dec;101(6):733–738. doi: 10.7326/0003-4819-101-6-733. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Magnius L. O. Enzyme immunoassay for anti-hepatitis B core (HBc) immunoglobulin G1 and significance of low-level results in competitive assays for anti-HBc. J Clin Microbiol. 1989 May;27(5):849–853. doi: 10.1128/jcm.27.5.849-853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare J. A., Robertson E. F., Madsen G., Hu R., Decker R. H. Improvement in the specificity of assays for detection of antibody to hepatitis B core antigen. J Clin Microbiol. 1991 Mar;29(3):600–604. doi: 10.1128/jcm.29.3.600-604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks I., Beheshti I., McCapra F., Campbell A. K., Woodhead J. S. Acridinium esters as high-specific-activity labels in immunoassay. Clin Chem. 1983 Aug;29(8):1474–1479. [PubMed] [Google Scholar]
- Wolf-Rogers J., Weare J. A., Rice K., Robertson E. F., Guidinger P., Khalil O. S., Madsen G. A chemiluminescent, microparticle-membrane capture immunoassay for the detection of antibody to hepatitis B core antigen. J Immunol Methods. 1990 Oct 19;133(2):191–198. doi: 10.1016/0022-1759(90)90359-4. [DOI] [PubMed] [Google Scholar]


