Abstract
Background
It is generally accepted that all transplants are not rejected in the same fashion. However, the extrinsic and intrinsic factors that control the recognition and rejection of a particular allograft by the host are not well characterized.
Methods
We compared the mechanisms underlying the response to donor antigens by T cells activated after transplantation of fully allogeneic skin and corneal grafts in mice.
Results
In corneal-transplanted mice, the CD4+ T cell response was exclusively mediated by T cells recognizing minor antigens in an indirect fashion and producing low levels of IL-2. In contrast, skin grafts elicited both direct and indirect CD4+ T cell responses primarily directed to MHC antigens and characterized by high IL-2 levels. While CD8+ T cells producing γIFN were activated directly in both skin- and corneal-grafted mice, only CD8+ T cells from skin-transplanted mice mounted a cytotoxic response. Next, we investigated whether failure of corneal transplants to induce a CD4+ direct alloresponse is due to their poor immunogenicity or to the site of placement (eye). We observed that corneas transplanted under the skin as well as splenocytes transplanted in the eye were both capable of inducing direct CD4+ T cell alloreactivity.
Conclusions
This shows that, failure of orthotopic corneal allotransplants to elicit a CD4+ T cell direct alloresponse is associated with the combination of two factors, their low immunogenicity and the immune-privileged properties of the eye.
Keywords: skin graft, corneal transplantation, T cells, indirect allorecognition, cytokines
Introduction
Recipient T cells recognizing MHC molecules displayed on donor cells are thought to initiate the immune rejection of allogeneic transplants. In skin transplantation model, allogeneic MHC class II molecules expressed on donor passenger leukocytes activate CD4+ T cells in the recipient’s regional lymph nodes (direct allorecognition) (1). The remarkably high frequency of precursor T cells capable of responding to an allogeneic MHC molecule is usually associated with the strength of in vivo immune response to and acute rejection of allografts (2, 3). In addition, allotransplantation induces an oligoclonal T cell response in which recipient T cells recognize donor peptides presented by recipient APCs; a pathway referred to as indirect allorecognition (4-6). A number of models relying on MHC class II deficient mice and T cell receptor transgenic mice show that either the direct or indirect CD4+ T cell alloresponse is sufficient to mediate acute rejection of skin allografts in rodents, presumably via differentiation of CD8+ cytotoxic T cells (7) (8, 9). However, whether this model pertains to the rejection of all tissue and organ transplants placed in normal recipients requires further investigation.
The susceptibility to T cell-mediated rejection of and tolerance to allografts in defined donor/recipient combinations varies upon the nature of the grafted tissue and its anatomical placement site in the host (10). It is likely that both factors, intrinsic and extrinsic to the graft contribute to its antigenicity and immunogenicity thereby governing the T cell alloresponse and the immune rejection process. In this study, we investigated this question by comparing the mechanisms underlying the recognition by and response of recipient T cells to fully allogeneic skin and corneal allotransplants in mice. We chose these transplants as they represent two extreme situations. Skin allografts in the classical B6 to BALB/c donor/recipient mouse model are highly immunogenic and invariably rejected (8-10 days) via a process resistant to most tolerance protocols. In contrast, in the same mice, fifty percent of corneal transplants are spontaneously accepted while the remainder is slowly rejected (8-10 weeks) via a process easily inhibitable by a variety of immunosuppressive agents. These observations prompted us to investigate the factors governing the T cell alloimmunogenicity of these transplants. We compared the frequency, cytokine pattern, antigen specificity and effector function of CD4+ and CD8+ T cells activated via direct and indirect allorecognition pathways in mice recipient of fully allogeneic skin and corneal transplants placed orthotopically or heterotopically. The mechanisms by which intrinsic and extrensic factors govern the immunogenicity and rejection of these transplants are discussed.
Materials and Methods
Mice
Six- to 8- week-old female BALB/c (H-2d), B10.D2/nSnJ (H-2d), C57BL/10 (H-2b), C57BL/6 (H-2b) and BALB.B (H-2b) as well as B6 MHC class II KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME). They were maintained in our pathogen-free facility at the Massachusetts General Hospital and treated according to institutional guidelines.
Transplantation
Donor central corneas were marked with a 2-mm diameter microcurette, excised by vannas scissors and placed in phosphate-buffered saline (PBS). Recipients were anesthetized by intraperitoneal injection of ketamine and xylazine, and the right eye was excised from a 1.5 mm diameter piece in the central cornea to prepare the graft bed. The donor cornea was placed in the recipient bed and secured with interrupted 11-0 nylon sutures (Sharpoint; Vanguard Houston, TX). After application of antibiotic ointment, the eyelids were closed for 3 days. Seven days later, the sutures were removed. The degree of opacity as well as the degree of neovascularization was assessed using a slit lamp biomicroscopy as previously described (11). The opacification of the graft was quantified using a scoring opacity scale from 0 to 5+. The cornea was considered to be in a rejecting phase with a score equal or beyond 2+. Skin allografts were performed according to the technique previously described by Billingham and Medawar (12).
Preparation of responder cells
Spleen and lymph node cells from naïve, cornea-grafted and skin-grafted BALB/c mice were used as a source of responder cells to measure the total alloresponse as well as the direct and indirect responses. Red blood cells were lyzed for 2 min in Tris-NH4Cl solution. Spleen cells were then washed twice in AIM-V (Gibco BRL, Grand Island, NY) medium containing 0.5 % fetal calf serum (FCS) and resuspended at 107 cells/ml with 0.5 % FCS in AIM-V for use.
T cell and T cell subsets isolation
T cells as well as CD4+ and CD8+ T cell subsets were isolated from the spleen of transplanted and naïve mice by negative selection using commercially available T cell purification columns according to the manufacturer’s instructions (Accurate Chemical & Scientific Corp., Westbury, N.Y) (R & D Systems, Minneapolis, MN). Purified T cells were washed in HBSS and used at 5 × 105 cells/well in ELISPOT assays.
Preparation of antigen-presenting cells
Mitomycin C-treated (MMC) splenocytes from donor and recipient naïve mice were used as allogeneic stimulator cells or syngeneic APCs, respectively. Single cell suspensions of splenocytes devoid of red blood cells were prepared in AIM-V containing 0.5 % FCS and treated with MMC (50 μg/ml) for 30 min at 37° C. The cells were washed once in HBSS, incubated for 10 min at 37° C, and washed once again before resuspension in AIM-V, 0.5 % FCS at 1-3 × 107 cells/ml.
Preparation of sonicates
Stimulator spleen cells were suspended at 3 × 107 cells/ml in AIM-V containing 0.5 % FCS, and sonicated with 10 pulses of 1 sec each. The resulting suspension was frozen in a dry ice/ethanol bath, thawed at room temperature and centrifuged at 300g for 10 min to remove intact cells.
ELISPOT assays
96-well ELISPOT plates (Polyfiltronics, Rockland, MA) were coated with a capture mAb in sterile PBS overnight. Anti-IL-2 (JES6-1A12), -IFNγ (R4-6A2), -IL-4 (11B11) and -IL-5 (TFRK-4) capture mAbs were used at 3, 4, 2 and 5 μg/ml, respectively (Pharmingen, San Diego, CA). On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 h with PBS containing 1 % BSA, then washed 3 times with sterile PBS. Responder cells or purified T cells were added to wells previously filled with either intact donor cells (direct response) or syngeneic APCs together with donor sonicate (indirect response) as previously described (13). The resulting spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (C.T.L., Cleveland, OH).
Cytotoxic T cell assays
Spleen T cells derived from BALB/c recipient mice transplanted with either B6 skin or cornea were collected. CD8+ T cells were isolated and tested for their ability to lyse target cells derived from either allogeneic (B6) or control syngeneic (BALB/c) mice as described elsewhere (14).
In vivo treatment with anti-CD4 and anti-CD8 monoclonal antibodies
Anti-CD4 (GK1.5) and anti-CD8 (53-6.72) monoclonal antibodies (mAbs) were purified from hybridoma culture supernatants using protein G-sepharose gel (Amersham Pharmacia, Piscataway, NJ). Mice were injected with 1 mg of mAb on days -3 and -1 pre-transplantation, and then days 5, 12, 19 and 26 post-transplantation.
Results
Direct and indirect alloresponses by CD4+ T cells in skin- and cornea-transplanted mice
We compared the frequencies and cytokine patterns of CD4+ T cells activated in direct and indirect fashions following skin or corneal allotransplantation. BALB/c mice were grafted with cornea or skin derived from fully allogeneic C57BL/6 (B6) donors. At the time of rejection (10 days for skin and 5 weeks for cornea), recipient purified T cells (from lymph nodes and spleens) were restimulated in vitro with MMC-treated donor APCs (direct pathway) or recipient APCs + donor sonicates (indirect pathway). The frequency of activated inflammatory T cells producing IL-2 and γIFN was then measured by ELISPOT as previously described (13).
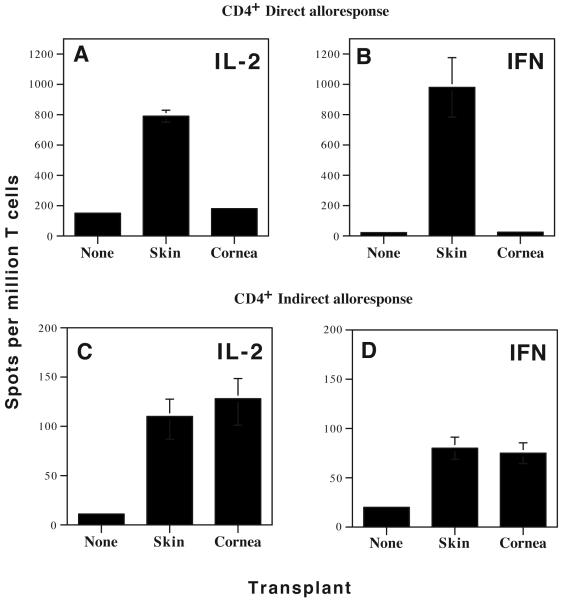
Naïve, CD4+ T cells from non-transplanted mice mounted a direct T cell response typical of a primary in vitro mixed lymphocyte reaction (MLR) characterized by some expansion of IL-2- (> 150 spots/million T cells) but not γIFN-producing T cells (Fig. 1 A-B). No naïve T lymphocytes were primed in vitro via indirect allorecognition, a feature that is thought to reflect the low precursor frequency of these cells in non-transplanted mice (Fig. 1 C-D).
Figure 1. Frequency and cytokine production of inflammatory alloreactive T cells in mice transplanted with fully allogeneic corneal and skin transplants.
Spleen cells were harvested from naïve BALB/c mice (non-transplanted) as well as BALB/c mice grafted with either B6 corneas or B6 skins. CD4+ T cell subsets were isolated and tested for their ability to mount direct and indirect alloresponses. To measure direct alloreactivity (panels A and B), T cells were mixed with MMC-treated B6 APCs (ratio 1:1), and tested in ELISPOT for IL-2 (panels A) and γIFN (panels B) cytokines. To measure indirect alloreactivity (panels C (IL-2) and D (γIFN)), CD4+ T cells were cultured in the presence of recipient APCs and donor-derived sonicates. The frequencies of cytokine-producing T cells are expressed in number of spots per million T cells. Data represent the mean ± SD of three to four independent experiments including at least 5 mice individually tested.
Fully mismatched B6 skin grafts were recognized via both the direct and indirect pathways by CD4+ T cells in BALB/c recipient’s lymphoid organs. As shown in Fig 1 A-B, the direct response was characterized by a massive expansion/activation of inflammatory Th1 cells producing IL-2 and γIFN. CD4+ T cells activated via indirect allorecognition exhibited a similar cytokine pattern while they were present at much lower frequencies. Strikingly, corneal allotransplants failed to induce any direct CD4+ T cell alloreactivity (Fig. 1 A-B). In turn, corneal-transplanted mice which rejected their grafts mounted a significant indirect T cell response mediated by CD4+ T cells (Fig. 1 C-D). Interestingly, no alloresponses were detected in mice that had spontaneously accepted corneal grafts (data not shown).
Differential contribution of minor and MHC antigens to the indirect alloresponse by CD4+ T cells in skin- and cornea-transplanted mice
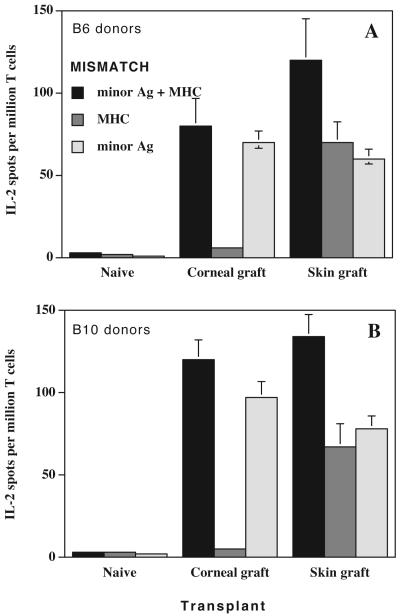
We compared the contributions of minor Ag and MHC antigens to the indirect alloresponse by CD4+ T cells in mice transplanted with either skin or cornea. Corneas and skins from fully mismatched (minor Ag + MHC) C57BL/6 and C57BL/10 (H-2b) mice were grafted onto BALB/c (H-2d) mice. To measure the overall indirect response, splenocytes from grafted mice were challenged in vitro with recipient BALB/c APCs exposed to sonicates prepared from fully allogeneic (C57BL/6 or C57BL/10) donors. We then compared the indirect responses to MHC and minor antigens by restimulating CD4+ T cells with BALB.B (MHC-mismatched) and B10.D2 (minor Ag-mismatched) sonicates, respectively. Following skin transplantation, both MHC and minor antigens induced an indirect alloresponse (Figure 2). In contrast, the entire indirect CD4+ T cell alloresponse in cornea-transplanted mice was directed exclusively to minor antigens (Figure 2).
Figure 2. Indirect alloresponses to MHC and minor antigens in mice transplanted with allogeneic skins and corneas.
BALB/c (BALB background, MHC:H-2d) mice were transplanted with fully allogeneic corneas or skins from either B6 (top panel: B6 background, MHC:H-2b) or B10 (lower panel: B10 background, MHC:H-2b). To measure the overall indirect alloresponse, recipient spleen T cells were isolated and stimulated in vitro with recipient APCs + donor fully allogeneic B6 sonicates. To measure indirect alloresponse to minor antigens, BALB/c recipient’s T cells were stimulated with recipient APCs + minor-mismatched B10.D2 sonicates (B10 background, MHC:H-2d). To measure indirect alloresponse to MHC antigens, BALB/c recipient’s T cells were stimulated with recipient APCs + MHC-mismatched BALB/B sonicates (BALB background, MHC:H-2b)
T cells from transplanted mice exposed to recipient APCs only and T cells from non-transplanted (naïve) mice stimulated with sonicates were used as controls. The number of IL2-producing T cells was measured using an ELISPOT assay. Data represent the mean ± SD of three independent experiments including at least 3 mice individually tested.
Partial activation/differentiation of CD8+ alloreactive T cells in cornea-transplanted mice
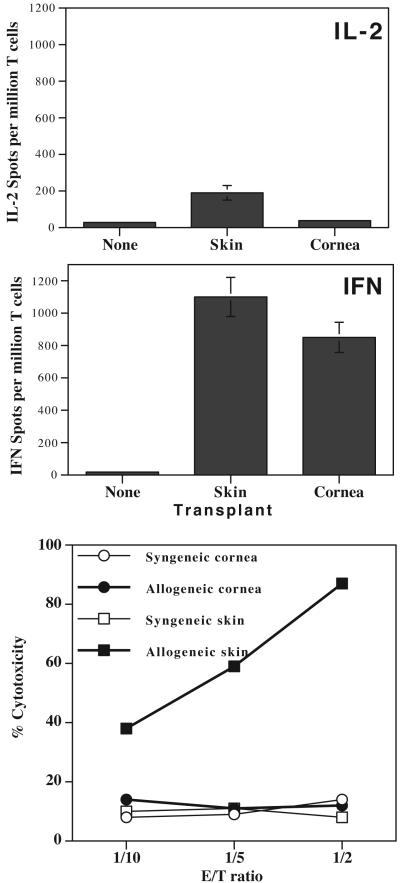
A high frequency (> 800 per million T cells) of IFN-γ-producing CD8+ T cells sensitized through direct allorecognition was observed in BALB/c mice rejecting either corneal or skin allografts (Fig. 3). In contrast, the frequency of CD8+ T cells producing IL-2 was barely higher than that found in naïve mice. In another set of experiments, CD8+ T cells from BALB/c recipients of either B6 skin or cornea grafts were collected at the time of rejection and tested for their ability to kill donor target cells in vitro. As shown in Figure 3, only CD8+ T cells from skin-transplanted mice mounted vigorous allospecific CTL responses. Therefore, while CD8+ T cells from cornea-transplanted mice mount potent direct alloresponses characterized by γIFN production, they do not differentiate into functional cytotoxic T cells.
Figure 3. CD8+ T cell responses in skin- and cornea-grafted mice.
CD8+ T cells from skin- and cornea-transplanted BALB/c mice were isolated. The frequencies of T cells producing IL-2 (top panel) and γIFN (middle panel) in response to donor B6 irradiated splenocytes were measured by ELISPOT. The frequencies of cytokine-producing T cells are expressed in number of spots per million T cells. Data represent the mean ± SD of three to four independent experiments including at least 5 mice individually tested.
The lower panel shows anti-donor cytotoxic response of spleen CD8+ T cells derived from BALB/c mice transplanted with either skin or cornea from allogeneic B6 donors. CD8+ T cells from cornea-transplanted BALB/c mice were tested for their cytolytic activity towards B6 allogeneic target cells (λ) and syngeneic BALB/c control target cells (μ). CD8+ T cells from skin-grafted mice were tested for their ability to kill allogeneic B6 (ν) and syngeneic BALB/c control (θ) target cells. Results are expressed as % cytotoxicity i.e. (experimental - spontaneous 51Cr release) / (total - spontaneous 51Cr release) × 100. The spontaneous release ranged from 10 to 15% of maximal release. The data shown here are representative of 3 separate experiments.
Interrelationships between alloreactive CD4+ and CD8+ T cells in skin- and cornea-grafted mice and their roles in the rejection process
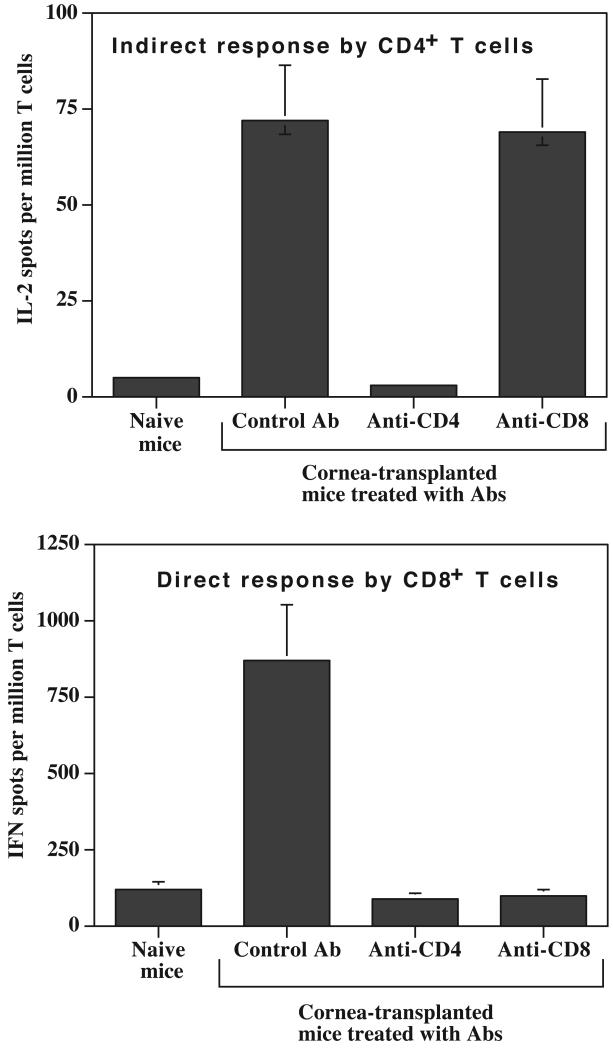
CD4+ alloreactive T cells activated directly or indirectly are capable on their own of eliciting acute rejection of a fully allogeneic skin transplant (15-17). On the other hand, CD8+ CTL can be generated without CD4+ T cell help in certain mouse strains and cause skin allograft rejection on their own (18, 19). Here, we investigated the relationships between CD4+ and CD8+ T cells in cornea-transplanted mice and examined their contribution to the rejection. Recipient BALB/c mice were treated in vivo with either GK1.5 anti-CD4 or 53-6.72 anti-CD8 depleting monoclonal antibodies (mAbs), then transplanted with a B6 cornea. T cell subset depletion, direct and indirect alloresponses and graft rejection were monitored.
In anti-CD4 mAb-treated mice, FACS analysis confirmed the nearly complete depletion (98%) of CD4+ but not CD8+ T cells (data not shown). Removal of CD4+ T cells abrogated both the indirect alloresponse by CD4+ T cells (Figure 4A) and the direct alloresponse by CD8+ T cells (Fig.4B). Aditionally, recipients treated with anti-CD4 mAbs permanently accepted B6 corneal allografts (data not shown). This shows that CD4+ T cell indirect response is required both for the activation of CD8+ T cells through the direct pathway and for the rejection of fully mismatched corneal transplants. Next, recipient BALB/c mice were treated in vivo with the 53-6.72 anti-CD8 antibody. Greater than 85 % of the CD8+ T cells were depleted under these conditions, whereas the CD4+ T cell population was unaffected (data not shown). Despite incomplete depletion of CD8+ T cells in recipients, anti-CD8 mAb treatment resulted in functional elimination of the CD8+ T cell direct alloresponse (Figure 4B). As shown in Figure 4A, the elimination of the CD8+ T cell-mediated direct response cell did not impact the frequency of CD4+ T cells activated indirectly. Finally, removal of CD8+ T cells had no effect on the rejection of corneal allografts (data not shown). Therefore, while skin grafts can be rejected by CD4+ and CD8+ T cells activated directly or indirectly to MHC and minor antigens, corneal allotransplants are strictly rejected by CD4+ T cells activated indirectly to donor minor antigens.
Figure 4. Effects of in vivo depletion of CD4+ and CD8+ T cells on alloresponses in cornea-transplanted mice.
The X axis represents control BALB/c naïve mice (no Ab treatment, no transplant) as well as BALB/c mice transplanted with a B6 cornea and treated in vivo with a control Ab or Abs directed to CD4 and CD8 molecules. The frequencies of activated allospecific T cells were measured using an ELISPOT assay. Panel A represents the indirect IL-2 alloresponse. Panel B represents the direct γIFN alloresponse. Data represent the mean ± SD of two to four independent experiments including at least 3 mice individually tested.
The lack of direct CD4+ T cell alloreactivity in cornea-transplanted mice is associated with the poor immunogenicity of corneas and the immunoprivileged nature of the eye
Unlike skin grafts, orthtopic corneal allotransplants fail to induce a CD4+ T cell-mediated direct alloresponse. We tested whether this is due to the poor immunogenicity of the cornea or to the immune privileged nature of the eye. First, corneal transplants were placed under the skin of a mouse footpad. Ten days later, potent direct CD4+ T cell responses were recorded in draining popliteal lymph nodes and spleen (Table 1). Interestingly, this response was directed to both MHC and minor histocompatibility antigens (data not shown). Therefore, when placed heterotopically in a non immune-privileged site corneal allografts induce CD4+ T cell direct alloreactivity to donor MHC antigens. In another set of experiments, two million B6 splenocytes were injected in the vitreous cavity of BALB/c mice, a site known for its immune privilege nature. Strikingly, ten-twelve days later, robust direct CD4+ T cell responses were found in both cervical lymph nodes and spleen (Table 1), suggesting that the presence of bona fide spleen bone marrow-derived professional APCs can overcome the immune privilege properties of the eye. Altogether, these results show that the failure of orthotopic transplants to elicit CD4+ T cell alloreactivity results from the combination of two factors: 1) the poor immunogenicity of the cornea and, 2) the immune privileged status of the eye.
Table 1.
CD4+ T cell direct alloresponses and sites of graft placement
Allospecific CD4+ T cell alloresponse via the direct pathway was investigated after placement of B6 skin, cornea or splenocytes in a BALB/c recipient’s immune-privileged site (eye) or a nonimmune-privilged site (under the skin). Orthotopic skin and corneal transplants were used as positive and negative controls, respectively. The frequency of activated CD4+ T cells producing IL-2 was measured by an ELISPOT assay. Data represent the mean ± SD of three independent experiments including at least 3 mice individually tested
| CD4+ direct T cell alloresponse (IL-2 spots) | |||
|---|---|---|---|
| Orthotopic | Under the skin | In the eye vitreous cavity | |
| Skin transplant | 540 ± 42 | nd | nd |
| Corneal transplant | 14 ± 3 | 398 ± 39 | nd |
| Splenocytes | nd | 432 ± 67 | 675 ± 49 |
Discussion
MHC vs. minor antigens in indirect allorecognition of corneal and skin transplants
Direct allorecognition by T cells is limited to donor MHC molecules (20, 21). On the other hand, the nature of the antigens governing indirect alloresponses is still controversial. Initial studies on indirect allorecognition focused on MHC peptides based upon the assumption that alloreactive T cells interact preferentially with MHC rather than other proteins (22-25). It is clear, however, that a large variety of non-MHC proteins can serve as a source of minor antigens for processing by recipient APCs and presentation to recipient T cells (26). Our T cell frequency analyses using ELISPOT in the skin transplant model indicate the prevalence of MHC vs. other antigens in indirect alloresponses. It is noteworthy that the processing of MHC molecules seems to be favored in general given that a large proportion of the peptides that have been eluted from the grooves of MHC molecules are actually MHC peptides (27). This phenomenon may bias indirect allorecognition toward determinants derived from MHC rather than minor antigens.
Corneal allografts displaying mismatches at minor histocompatibility loci are more readily rejected than their MHC-disparate counterparts (28). In our study, the absence of indirect alloreactivity to MHC determinants does not result from a competition between MHC- and minor antigen-derived peptides since no indirect response is detectable in MHC-disparate donor/recipient combinations (29). The exclusive restriction of CD4+ T cells to minor antigens might result from a reduced expression of MHC antigens in the normal cornea (absence of MHC class II and reduced MHC class I expression) (30). Additionally, the absence of CD4+ T cell direct alloreactivity in corneal-transplanted mice may account for the bias of the indirect response toward minor antigens. Consistent with this hypothesis is the observation that minor antigens play a dominant role in the rejection of APC-depleted thyroid grafts which do not induce direct CD4+ T cell alloresponses(31). We surmise that the direct CD4+ T cell alloresponse may bias the indirect repose to MHC antigens by increasing both expression and shedding of donor MHC molecules (via IFN-γ and TNFα) thereby boosting the processing of these antigens by recipient APCs.
Roles of CD4+ and CD8+ T cells to the rejection of allogeneic skins and corneas
Following skin transplantation, CD4+ T cells recognizing directly or indirectly donor MHC are sufficient to reject the allografts, presumably by inducing cytotoxic T cell responses. In corneal-grafted individuals, activation of the CD8+ T cell response is strictly dependent upon indirect sensitization of CD4+ T cells to minor antigens (29). These CD8+ T cells do not contribute to rejection of allografts (19, 29, 32, 33). Apparently, in this setting, the help provided by CD4+ T cells is insufficient. Supporting this view, Peeler et al have reported that, in the absence of donor professional APCs, the induction of CTL responses depends upon the diversity of minor antigens presented by recipient APCs (34). In that study, a male cornea expressing a single minor antigen H-Y could not trigger a CTL response in female recipients unless donor-derived Langerhans cells (LCs) were provided along with the graft. In contrast, a CTL response could be initiated independently of graft derived-LCs when recipient and donor were mismatched for multiple minor antigens (34). These observations suggest that in the absence of a cognate CD8+T-APC-CD4+T interaction (direct allorecognition), the quantity of antigens presented through the indirect pathway determines the strength of indirect CD4+ alloreactivity and thereby controls the fate of the CTL response. This situation is reminiscent of that described by Smith et al. during viral infection with HSV in which the ability of non-cognate CD4+-CD8+ T cell interaction to ensure CTL functions depends on the quantities of viral peptides provided exogenously (35). In this model, inefficient “licensing” of APCs was responsible for the lack of productive maturation of CD8+ T cells, a conclusion that presumably pertains to corneal transplantation.
Why cannot orthotopic corneal-transplants elicit a direct CD4+ T cell response?
In skin-grafted mice, the migration of MHC class II+ donor-derived DCs to the recipient’s lymph nodes triggers a potent direct CD4+ T cell alloresponse (36, 37). The most puzzling feature of corneal allografts resides in their inability to trigger direct alloreactivity by CD4+ T cells. Until recently, the cornea was thought to be devoid of bone marrow-derived professional APCs, a conclusion that had emerged from studies showing the absence of MHC class II molecules on any corneal cell (38). However, Liu et al recently reported that cervical lymph nodes draining corneal allografts contain MHC class II+ donor DCs (39). In a subsequent study, Hamrah et al. formally documented the presence of CD11c+ DCs and CD11b+ macrophages in the corneal epithelium (40). In addition, Sonosva et al have reported that CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers (41). The inescapable conclusion is that the cornea does contain mobile bone marrow-derived cells and that the prevailing dogma was incorrect. It is important to note that, DCs, the most potent APC in peripheral tissues, universally do not express MHC class II, CD80 (B7.1), or CD86 (B7.2) in the central cornea, reflecting a highly immature state (40, 42). It has become clear, however, that after transplantation, donor corneal DCs migrate to the recipient’s draining lymph nodes where they express normal levels of MHC class I and II and costimulation receptors on their surface (39). Why don’t these MHC class II+ DCs trigger a CD4+ direct alloresponse? It is possible that the initial lack of expression of MHC class II and/or costimulation receptors on donor corneal DCs leads to recipient T cell anergy. Also, direct alloantigen presentation by these immature DCs may lead to activation of some regulatory T cells (Tregs). In addition, some Tregs may be activated indirectly and suppress direct CD4+ T responses in corneal -transplanted mice. Indeed, studies by W. Streilein and J. Niederkorn show that APCs derived from immunoprivileged sites exert suppressor effects on inflammatory T cells (43, 44). This suggests that any circumstances that would disrupt immune privilege in the eye could render these corneal DCs capable of inducing inflammatory T cell responses. This situation is observed in “high risk” recipients of activated mature corneal transplants displaying an inflamed eye bed environment (45). In these recipients, donor corneal DCs express MHC class II molecules as well as CD40, CD80 and CD86 coreceptors at the time of transplantation (45). These transplants induce potent CD4+ direct alloresponses in draining lymph nodes and undergo acute rejection (1 week), a result suggesting that the lack of immunogenicity of corneal DCs is not an intrinsic property of these cells. Alternatively, it is likely that corneal DCs present in the eye are maintained in a resting immature state that favors their tolerogenic properties. In support of this, previous studies by Niederkorn’s group showed that heterotopic corneal allografts induce a CTL response (46). Here, we show that B6 corneal allografts placed subcutaneously in BALB/c mice trigger CD4+ T cell direct alloreactivity. In addition, we report that spleen cells placed in the vitreous cavity of the eye also induce direct alloresponses by CD4+ T cells. Therefore, the lack of a direct T helper direct response after corneal transplantation is due to combined poor immunogenicity of corneal DCs and immune-privileged properties of the eye. Taken together, these observations indicate that early expression of elevated levels of donor MHC class II on donor DCs promoted by local inflammation is an essential element of the induction of CD4+ T cell direct alloresponses and the differentiation of CD8+ CTL, a phenomenon that is typical of skin but not corneal transplants (47).
Concluding remarks
The mechanisms underlying the recognition of and alloresponse to fully allogeneic skin and corneal allotransplants are fundamentally different. Skin grafts can be rejected either by CD4+ or CD8+ T cells activated directly or indirectly against MHC or minor antigens. On the other hand, only CD4+ T cells activated indirectly by minor antigens reject corneal allografts. In this model, a lack of CD4+ direct alloreactivity is due presumably to delayed expression of MHC class II on donor DCs as well as low alloantigen density. This results in partial activation of CD8+ T cells characterized by γIFN production but no CTL function, a phenomenon associated with the lack of acute rejection of corneal allografts. Therefore, the magnitude and antigen specificity of direct and indirect alloresponses by CD4+ and CD8+ T cells vary considerably with the nature of the transplant and the anatomical site of grafting. Both elements are likely to influence the fate of the alloimmune response by governing the immunogenicty and tolerogenicity of dendritic cells.
Acknowledgments
We would like to thank Ms. Karla Stenger for her assistance in the preparation of the manuscript. We also thank Drs. G. Tocco, A. Alessandrini, H. Winn and J.W. Streilein for helpful discussions.
This work was supported by grants from the NIH/NEI-RO1 EY13310 and the Massachusetts Lions Eye Foundation to GB and the Research to Prevent Blindness foundation and NIH/NEI-R01 EY12963 to MRD. FB was supported by a fellowship from the Fondation pour la Recherche Médicale.
References
- 1.Lechler RI, Lombardi G, Batchelor JR, Reinsmoen N, Bach FH. The molecular basis of alloreactivity. Immunol Today. 1990;11(3):83. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. I. Estimates of the absolute frequency of killer cells generated in vitro. J Exp Med. 1977;145(3):500. doi: 10.1084/jem.145.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 4.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175(1):305. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fangmann J, Dalchau R, Sawyer GJ, Priestley CA, Fabre JW. T cell recognition of donor major histocompatibility complex class I peptides during allograft rejection. Eur J Immunol. 1992;22(6):1525. doi: 10.1002/eji.1830220627. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98(5):1150. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr. Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994;179(3):865. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valujskikh A, Matesic D, Gilliam A, Anthony D, Haqqi TM, Heeger PS. T cells reactive to a single immunodominant self-restricted allopeptide induce skin graft rejection in mice. J Clin Invest. 1998;101(6):1398. doi: 10.1172/JCI893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun MY, Grandjean I, Feunou P, et al. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166(8):4879. doi: 10.4049/jimmunol.166.8.4879. [DOI] [PubMed] [Google Scholar]
- 10.Jones ND, Turvey SE, Van Maurik A, et al. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol. 2001;166(4):2824. doi: 10.4049/jimmunol.166.4.2824. [DOI] [PubMed] [Google Scholar]
- 11.Joo CK, Pepose JS, Stuart PM. T-cell mediated responses in a murine model of orthotopic corneal transplantation. Invest Ophthalmol Vis Sci. 1995;36(8):1530. [PubMed] [Google Scholar]
- 12.Billingham RE, Medawar PD. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385. [Google Scholar]
- 13.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352. [PubMed] [Google Scholar]
- 14.Popov IA, Fedoseyeva EV, Orr PL, Garovoy MR, Benichou G. Direct evidence for in vivo induction of CD8+ cytotoxic T cells directed to donor MHC class I peptides following mouse allotransplantation. Transplantation. 1995;60(12):1621. [PubMed] [Google Scholar]
- 15.Dalloul AH, Chmouzis E, Ngo K, Fung-Leung WP. Adoptively transferred CD4+ lymphocytes from CD8 -/- mice are sufficient to mediate the rejection of MHC class II or class I disparate skin grafts. J Immunol. 1996;156(11):4114. [PubMed] [Google Scholar]
- 16.Krieger Nr YDPFCG CD4+ but not CD8+ cells are essential for allorejection. J Exp Med. 1996;184(5):2013. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valujskikh A, Heeger PS. CD4+ T cells responsive through the indirect pathway can mediate skin graft rejection in the absence of interferon-gamma. Transplantation. 2000;69(5):1016. doi: 10.1097/00007890-200003150-00063. [DOI] [PubMed] [Google Scholar]
- 18.Williams MA, Trambley J, Ha J, et al. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165(12):6849. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 19.Haskova Z, Usiu N, Pepose JS, Ferguson TA, Stuart PM. CD4+ T cells are critical for corneal, but not skin, allograft rejection. Transplantation. 2000;69(4):483. doi: 10.1097/00007890-200002270-00004. [DOI] [PubMed] [Google Scholar]
- 20.Krensky AM, Clayberger C. The nature of allorecognition. Curr Opin Nephrol Hypertens. 1993;2(6):898. doi: 10.1097/00041552-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Lechler R, Lombardi G. Structural aspects of allorecognition. Curr Opin Immunol. 1991;3(5):715. doi: 10.1016/0952-7915(91)90102-7. [DOI] [PubMed] [Google Scholar]
- 22.Benichou G, Fedoseyeva E, Lehmann PV, et al. Limited T cell response to donor MHC peptides during allograft rejection. Implications for selective immune therapy in transplantation. J Immunol. 1994;153(3):938. [PubMed] [Google Scholar]
- 23.Gould DS, Auchincloss H., Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20(2):77. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 24.Sayegh MH, Watschinger B, Carpenter CB. Mechanisms of T cell recognition of alloantigen. The role of peptides. Transplantation. 1994;57(9):1295. doi: 10.1097/00007890-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Suciu-Foca N, Liu Z, Harris PE, et al. Indirect recognition of native HLA alloantigens and B-cell help. Transplant Proc. 1995;27(1):455. [PubMed] [Google Scholar]
- 26.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8(1):75. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 27.Chicz RM, Urban RG, Lane WS, et al. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992;358(6389):764. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 28.Sano Y, Ksander BR, Streilein JW. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl Immunol. 1996;4(1):53. doi: 10.1016/s0966-3274(96)80035-9. [DOI] [PubMed] [Google Scholar]
- 29.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. Journal of Immunology. 2001;167(4):1891. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 30.Whitsett CF, Stulting RD. The distribution of HLA antigens on human corneal tissue. Invest Ophthalmol Vis Sci. 1984;25(5):519. [PubMed] [Google Scholar]
- 31.Bartlett ST, Jennings AS, Yu C, Naji A, Barker CF, Silvers WK. Influence of culturing on the survival of major histocompatibility complex-compatible and -incompatible thyroid grafts in rats. J Exp Med. 1983;157(1):348. doi: 10.1084/jem.157.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegde S, Niederkorn JY. The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest Ophthalmol Vis Sci. 2000;41(11):3341. [PubMed] [Google Scholar]
- 33.Sano Y, Ksander BR, Streilein JW. Analysis of primed donor-specific T cells in recipient mice bearing orthotopic corneal allografts. Transplantation. 2000;70(9):1302. doi: 10.1097/00007890-200011150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Peeler J, Niederkorn J, Matoba A. Corneal allografts induce cytotoxic T cell but not delayed hypersensitivity responses in mice. Invest Ophthalmol Vis Sci. 1985;26(11):1516. [PubMed] [Google Scholar]
- 35.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5(11):1143. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 36.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155(1):31. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen CP, Morris PJ, Austyn JM. Donor dendritic leukocytes migrate from cardiac allografts into recipients’ spleens. Transplant Proc. 1990;22(4):1943. [PubMed] [Google Scholar]
- 38.Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282(5736):326. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195(2):259. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44(2):581. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 41.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23(4):507. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 42.Yamagami S, Usui T, Amano S, Ebihara N. Bone marrow-derived cells in mouse and human cornea. Cornea. 2005;24(8 Suppl):S71. doi: 10.1097/01.ico.0000178732.42921.05. [DOI] [PubMed] [Google Scholar]
- 43.Streilein JW. New thoughts on the immunology of corneal transplantation. Eye. 2003;17(8):943. doi: 10.1038/sj.eye.6700615. [DOI] [PubMed] [Google Scholar]
- 44.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74(2):167. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 45.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173(7):4464. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 46.Matoba AY, Peeler JS, Niederkorn JY. T cell subsets in the immune rejection of murine heterotopic corneal allografts. Invest Ophthalmol Vis Sci. 1986;27(8):1244. [PubMed] [Google Scholar]
- 47.Ross J, Callanan D, Kunz H, Niederkorn J. Evidence that the fate of class II-disparate corneal grafts is determined by the timing of class II expression. Transplantation. 1991;51(2):532. doi: 10.1097/00007890-199102000-00049. [DOI] [PubMed] [Google Scholar]