Abstract
Objectives
We hypothesize that X-ray fused with MRI (XFM) roadmaps might permit direct antegrade crossing and delivery of a VSD closure device and thereby reduce procedure time and radiation exposure.
Background
Percutaneous device closure of membranous ventricular septal defect (VSD) is cumbersome and time-consuming. The procedure requires crossing the defect retrograde, snaring and exteriorizing a guidewire to form an arteriovenous loop, then delivering antegrade a sheath and closure device.
Methods
MRI roadmaps of cardiac structures were obtained from miniature swine with spontaneous ventricular septal defect and registered with live X-ray using external fiducial markers. We compared antegrade XFM-guided VSD crossing with conventional retrograde X-ray guided crossing for repair.
Results
Antegrade XFM crossing was successful in all animals. Compared with retrograde X-ray, antegrade XFM was associated with shorter time to crossing (167 ± 103 seconds versus 284 ± 61 seconds; p = 0.025), shorter time to sheath delivery (71 ± 32 seconds versus 366 ± 145 seconds; p = 0.001), shorter fluoroscopy time (158 ± 95 seconds versus 390 ± 137s; p = 0.003), and reduced radiation dose-area product (2394 ± 1522 mG•m2 versus 4865 ± 1759 mG•m2; p = 0.016).
Conclusions
XFM facilitates antegrade access to membranous VSD from the right ventricle in swine. The simplified procedure is faster and reduces radiation exposure compared with the conventional retrograde approach.
Keywords: Image guided intervention; Interventional magnetic resonance imaging; Congenital heart disease; Multimodality image fusion; Heart Septal Defects, Ventricular
Introduction
Percutaneous closure of membranous ventricular septal defect (VSD) is now feasible using commercial devices (1,2). Conventional percutaneous techniques are cumbersome and require multiple steps — retrograde defect crossing; snare-recovery of a guidewire; formation of an arteriovenous guidewire loop; and placement of an antegrade delivery sheath — all before antegrade delivery of the occluder device (3). Moreover, sheath access may be lost during device delivery and the crossing steps must be repeated. The procedure is technically challenging, mainly because imaging limitations preclude direct antegrade access to the VSD from the right ventricle. As a result, conventional transcatheter VSD closure typically requires prolonged exposure to ionizing radiation.
To enhance image guidance, we combine MRI roadmaps with live X-ray (XFM, X-ray Fused with MRI), based on external fiducial markers (4,5). We overlay MRI-derived features, such as VSD location and endoventricular contours, to position and deliver an asymmetric nitinol occluder device in swine.
We hypothesize that XFM enables direct antegrade crossing, simplifies and shortens procedure conduct, and reduces radiation exposure during closure in animals with congenital membranous VSD.
Methods
Animal Procedures
Animal procedures were approved by the National Heart Lung and Blood Institute Animal Care and Use Committee. Fourteen Yucatan miniature swine with spontaneous ventricular septal defect (Sinclair Research Center, Columbia, MO; 29–67 kg) were anesthetized with atropine, butorphanol, ketamine, and xylazine, and then maintained on isoflurane and mechanical ventilation. Four additional VSD mini-swine were used during technical computer development and were not analyzed further.
Interventional procedures were conducted using percutaneous transfemoral artery and vein access. Left-to-right shunts were measured by oximetry and MRI. VSD dimensions were measured both using MRI and using radiocontrast angiography with calibrated catheters.
MRI and XFM Registration
Experiments were conducted in a dual X-ray and MRI intervention suite (6). Sixteen fiducial markers conspicuous under both X-ray and MRI (Beekley Corp, Bristol, CT), were secured on the thorax. End-expiratory roadmaps were obtained using MRI at 1.5T (Espree, Siemens, Erlangen, Germany) and a standard 8-channel phased array surface coil. Typical ECG-gated segmented steady-state free precession (SSFP) acquisition parameters were repetition time (TR)/echo time (TE), 3/1.5 ms; flip angle, 80°; bandwidth, 930 Hz/pixel; 1.8 × 1.8 × 6 mm voxels. Typical “black-blood” turbo spin echo (TSE) images used TR/TE, 700/34 ms; bandwidth, 292 Hz/pixel; 1.4 × 2.2 × 3–6mm voxels. Typical phase-contrast gradient echo acquisition parameters were: TR/TE, 6.3/2.9 ms; flip angle, 30°; bandwidth, 434 Hz/pixel; 1.4 × 2.5 × 6mm voxels. VSD dimensions were measured on representative long axis SSFP and TSE images.
After MRI, the animals were transferred to a single-plane X-ray system (Axiom Artis FC, Siemens) using a mechanized bidirectional transport table (Miyabe, Siemens). Single-phase MR images were registered to the live X-ray acquisitions (4). Briefly, external fiducial markers, conspicuous under both X-ray and MRI, were matched and the two coordinate systems were aligned with rigid-body registration algorithms using custom image processing software written in MATLAB (Mathworks, Cambridge, MA). MRI regions of interest included contours of the VSD, ventricular cavities, outflow tracts, aortic valve, and aortic root (Figure 1). These were segmented manually while the animals were transferred from MRI to X-ray and then presented to the operator as a static image overlay on real-time X-ray fluoroscopic images. Catheter procedures were conducted only in the X-ray lab.
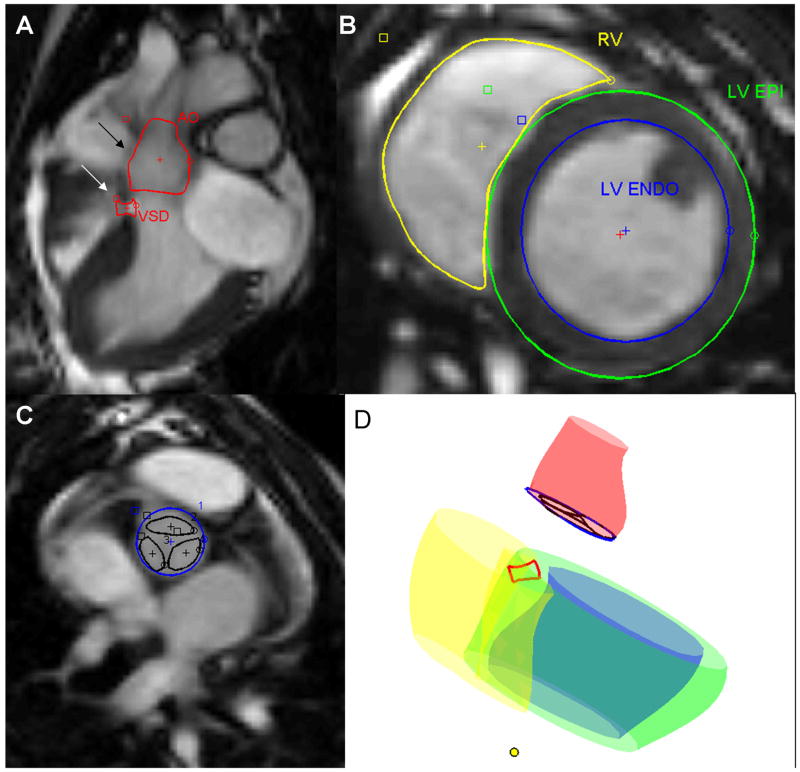
Figure 1. MRI derived contours.
A: Long axis 3 chamber SSFP MRI used to contour VSD (white arrow) and aortic root (black arrow).
B: Short axis SSFP MRI used to contour right (yellow) and left (blue) ventricular endocardial surfaces, and left ventricular epicardial surface (green).
C: Short axis SSFP MRI used to contour aortic valve annulus (blue) and leaflets (black).
D: Surface rendering of MRI-derived contours. The yellow dot indicates the left ventricular apex.
Target registration error (TRE) was defined as the two-dimensional distance between VSD center-of-mass on X-ray cineangiography and SSFP MRI during systole at end-expiration. While the defect could easily be visualized under MRI throughout the cardiac cycle, in cineangiography it was best identified by the jet of contrast ejected across the defect during systole.
VSD Closure procedures
Defect crossing: conventional, retrograde technique (Figure 2A)
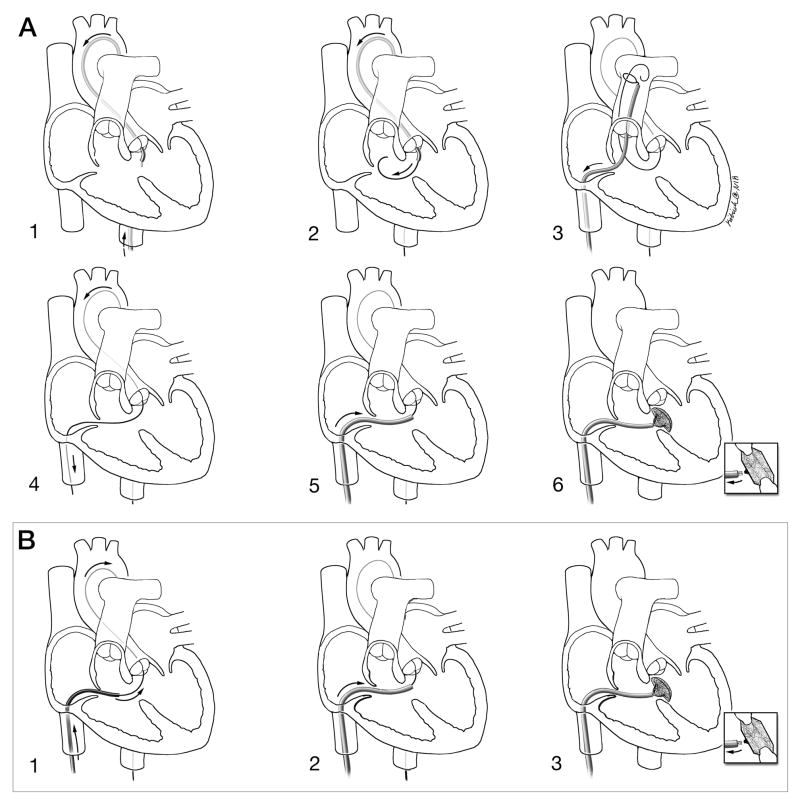
Figure 2. Schematic depictions of conventional and investigational procedure technique.
A: Conventional retrograde X-ray technique. 1: Retrograde, transaortic access to the left ventricle; 2: A guidewire crosses retrograde from the left to right ventricle across the VSD; 3: A transfemoral venous snare retrieves the transaortic guidewire from the pulmonary artery; 4: An arteriovenous loop is exteriorized to provide a rail to deliver the rigid VSD delivery sheath; 5: The delivery sheath is positioned antegrade across the VSD; 6: The VSD occlusion device is positioned and released.
B: Investigational XFM-guided antegrade technique. 1: With enhanced imaging guidance, the guidewire is passed antegrade from the right ventricle through the VSD into the left ventricle and descending aorta; 2: the delivery sheath is positioned in the left ventricle; 3: the VSD occlusion device is positioned and released.
The XFM technique has fewer steps.
Courtesy of Lydia Kibiuk, NIH Medical Arts and Photography Branch.
We used standard crossing technique(3). We first acquired a radiocontrast ventriculography roadmap in a cranial and left anterior oblique projection. We crossed the VSD using retrograde transaortic, braided 6Fr coronary angiographic catheters from the left ventricle and positioned soft guidewires into a distal pulmonary artery branch. We captured and exteriorized the guidewire using a transfemoral venous snare (Amplatz Gooseneck, ev3 Inc, Plymouth, MN) to form an arteriovenous loop.
To complete the procedure, a delivery sheath (Torqueview, AGA Medical, Plymouth, MN) is advanced over this loop from the venous side across the VSD. We did not close the defects until techniques were compared.
Defect crossing: investigational, antegrade technique (Figure 2B)
We crossed antegrade using XFM from the right ventricle into the left ventricle using preformed diagnostic catheters and 0.035″ hydrophilic guidewires (angled Glidewire, Terumo Medical Corporation, Somerset, NJ).
Before closure, with the catheter in the descending aorta, the hydrophilic wire was exchanged for a rigid 0.035″ guidewire (Supra-core, Abbott, Santa Clara, CA).
Percutaneous Membranous VSD device closure
The delivery sheath was advanced across the defect and into the descending aorta. Then the rigid wire was replaced with a flexible guidewire (Noodle, AGA Medical) to redirect the sheath toward the LV apex.
The Amplatzer Membranous VSD Occluder was sized according to manufacturer instructions (1–2mm larger than angiographic VSD diameter). The asymmetric left ventricular disc was oriented based on the XFM representation of the apex. Positioning was confirmed by radiocontrast ventriculography and aortography prior to device release and by MRI and necropsy afterwards.
Experimental Design
In an initial training phase (4 animals), we tested feasibility of XFM guided antegrade VSD crossing.
In the experimental phase (10 animals), we recorded procedure times and radiation exposure. We tested each approach 3–4 times in each animal in random order (conventional-retrograde-followed-by-investigational-antegrade or investigational-followed-by-conventional). First we compared antegrade VSD guidewire crossing under X-ray or XFM guidance using identical catheter technique (3 animals). Next, we compared antegrade XFM guidance with conventional retrograde X-ray guidance for VSD guidewire crossing and sheath delivery (7 animals). To preserve defect anatomy for crossing comparisons, relatively bulky (9Fr) delivery sheaths were not advanced across the defect until all attempts were completed. Animals underwent cardiac MRI before euthanasia and necropsy. All procedures were performed by a single operator. To minimize “operator memory” effects, different image displays (monitors) were used for XFM-guided and conventional X-ray-guided procedures.
The VSD crossing time is defined as the time the femoral sheath is entered until the guidewire is in the distal pulmonary artery (retrograde) or descending aorta (antegrade). The sheath delivery time begins after the VSD is crossed and ends when the arteriovenous loop is formed (retrograde) or the descending aorta is entered with the rigid guidewire (antegrade). Total fluoroscopy time and radiation exposure encompass VSD crossing and sheath delivery.
Data Analysis
For each animal we averaged 3–4 measurements, so as not to exaggerate the statistical degrees of freedom. Results are expressed as mean ± standard deviation. Component procedure times and radiation exposure were compared using two-tailed Student t-tests, adjusted for unequal variances when appropriate (Excel, Microsoft, Redmond, WA). Results were considered to be statistically significant at a value of p<0.05.
Results
Anatomic and physiologic findings
Congenital membranous VSDs were evident on MRI and radiocontrast ventriculography. Defect diameter ranged from 2.5–8mm. Pulmonary-systemic flow ratios ranged from 1.3–3.4 and were similar by oximetry and velocity-encoded MRI. Older animals tended to have smaller VSDs and aneurysmal tricuspid tissue encroaching on the VSD.
Procedural details and complications
In these experiments, 21 additional fluoroscopy frames were performed solely for registration with MRI, adding a total of 0.030 mG•m2 incremental radiation, or <1% of the total average radiation exposure for animal.
During technical development we found that preformed catheters with right-handed 3-dimensional curves (Williams Right, 6Fr Expo, Boston Scientific) simplified antegrade crossing compared with single-plane curve shapes (Judkins right, right coronary bypass). We used Williams Right shaped catheters thereafter for antegrade crossing in the experimental phase.
XFM registration was unavailable in four additional technical development experiments because of computer malfunction. In these, all VSD crossing attempts were unsuccessful despite 15 minutes of fluoroscopy and the procedures were aborted.
There were no procedural complications including conduction or rhythm abnormalities, atrioventricular or semilunar valvular regurgitation, or pericardial effusion. Moreover there was no sheath kinking or device embolization.
Comparison of image-guidance techniques
Antegrade XFM-guided crossing was successful in all 14 animals attempted (Video supplement 1). Figure 3 demonstrates the utility of a MRI roadmap to provide a target for antegrade VSD crossing compared with X-ray alone. The system automatically updated MRI roadmaps “in real time” when table position, X-ray gantry angulation, magnification, and X-ray source-image-distance were changed. This was helpful, for example, to confirm guidewire, sheath, and device position.
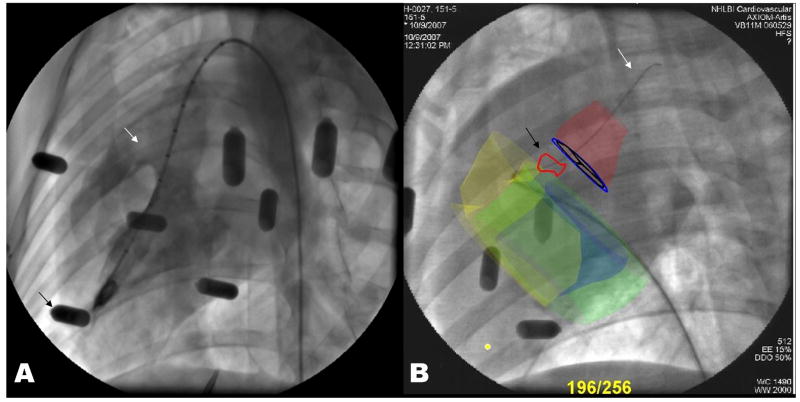
Figure 3. X-ray fluoroscopic landmarks compared with XFM roadmaps.
A: X-ray depicts the ventricular septal defect (white arrow) only during radiocontrast injections. Black arrows indicate the external fiducial beads.
B: A representative image of direct antegrade VSD guidewire crossing using XFM guidance. XFM roadmap elements include the right ventricular endocardial contour (yellow), the left ventricular epicardial contour (green), the left ventricular endocardial contour (blue), the VSD tract (red outline and black arrow), aortic root (red), and aortic valve (blue annulus with black leaflets). The guidewire (white arrow) is delivered across the VSD and into the aorta using a right-handed 3-dimensional curve right coronary artery catheter. The MRI-derived contours adjust automatically when the X-ray system is repositioned.
We found that “blind” antegrade X-ray crossing generally required positioning the catheter near the expected VSD location and probing using a guidewire. By comparison, XFM generally permitted direct entry into the VSD with the catheter.
Compared with retrograde X-ray, antegrade XFM was associated with shorter time to crossing, shorter time to sheath delivery, shorter fluoroscopy time, and reduced radiation dose-area product (Table 1 and Figure 4A).
Table 1.
A comparison of antegrade XFM with conventional retrograde X-ray guided transcatheter VSD repair (see text). XFM: X-ray Fused with MRI; VSD: Ventricular Septal Defect
| Antegrade XFM | Conventional Retrograde X-ray | ||
|---|---|---|---|
| VSD crossing (seconds) | 167 ± 103 | 284 ± 61 | p = 0.025 |
| Sheath positioning (seconds) | 71 ± 32 | 366 ± 145 | p = 0.001 |
| Total fluoroscopy (seconds) | 158 ± 95 | 390 ± 137s | p = 0.003 |
| Radiation dose-area product (DAP, mG•m2) |
2394 ± 1522 | 4865 ± 1759 | p = 0.016 |
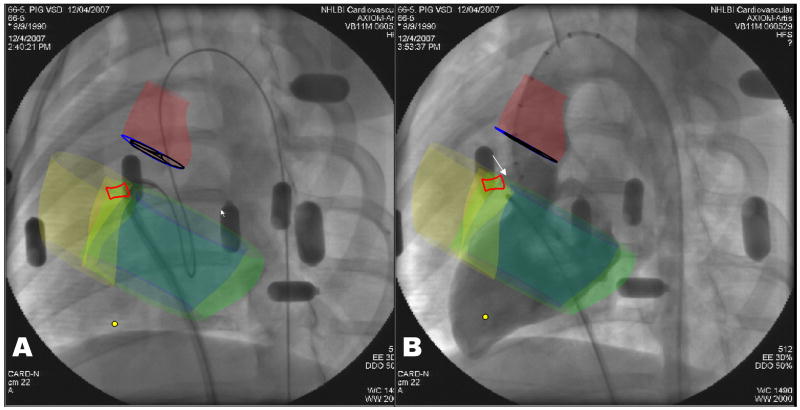
Figure 4. XFM positioning of delivery sheath and VSD occluder deployment.
A: Antegrade sheath delivery guided by XFM. The sheath is directed toward the left ventricular apex using a flexible guidewire. The XFM roadmap includes right (yellow) and left (blue) ventricular endocardial contours, left ventricular epicardial contour (green), aortic annulus (blue), aortic root (red), and VSD tract (bright red).
B: Confirmatory XFM radiocontrast ventriculography before release of VSD occluder device deployed in position. Features include the VSD occluder (white arrow), VSD tract (bright red contour), right (yellow) and left (blue) ventricular endocardial and left ventricular epicardial (green) contours, aortic valve (dark blue) and aortic root (dark red).
Likewise, XFM-guided antegrade VSD crossing was five-fold faster than X-ray guided antegrade technique (itself not a conventional standard; XFM 69 ± 43 seconds versus antegrade X-Ray 366 ± 179 seconds; p = 0.049).
XFM-guided device closure of VSD appropriately attenuated left-to-right flow across the defect without encroaching on the aortic valve leaflets (Figure 4B, Video Supplement 2). Whether XFM or conventional X-ray technique was used first did not affect procedural time or radiation exposure.
Target registration error
Target registration error between center points on cineangiography and SSFP MRI derived VSD contours was 4.9 ± 2.3 mm.
Necropsy
Explanted hearts from five successive animals indicated appropriate device positioning, appropriate device angular orientation relative to the aortic valve, no encroachment on aortic valve leaflets, and freedom from tricuspid valve entrapment.
Discussion
Transcatheter closure of membranous ventricular septal defect is technically challenging (7), primarily because contemporary interventional imaging tools delineate anatomy poorly. Fluoroscopy affords no landmarks to navigate across the VSD. As a result, direct antegrade access to the VSD is generally avoided. In this experiment, XFM enhances image guidance sufficiently to enable direct antegrade crossing and repair. It reduces the number of procedure steps, reduces procedure times, fluoroscopy and radiation exposure. XFM continuously depicts the relationship between structures and their X-ray projection despite changes in gantry angulation and table position (Video Supplement 3). XFM roadmaps also enhance subjective operator confidence by delineating critical intracardiac structures and spatial relationships.
Conventional versus antegrade repair
Right ventricular trabeculation (8), aneurysmal tricuspid valve tissue (9) and related flow perturbations interfere with antegrade percutaneous VSD crossing and closure. XFM mitigates these anatomic and functional obstacles. The VSD target displayed on XFM permits the operator to avoid chordal and trabecular entrapment. XFM allowed the operator directly to enter the VSD with the catheter. Conventional X-ray generally required the operator to find the VSD with a probing guidewire in a process that might entrap chordae.
XFM simplifies VSD closure into a direct antegrade procedure. By contrast, the conventional retrograde approach requires cumbersome crossing from left to right; snaring and then exteriorizing the guidewire to form an arteriovenous loop; followed by positioning and delivering the closure device under fluoroscopic and echocardiographic guidance.
Faster crossing and sheath delivery is particularly helpful during procedures complicated by inadvertent loss of sheath position, in which case these steps must be repeated. Direct antegrade crossing may also be germane to treatment of muscular and post-infarction VSD where defects are often multiple(10). That said, direct retrograde repair of muscular VSD has been reported(11).
Safety
Ionizing radiation is associated with a spectrum of malignancy (12). Children are especially sensitive to radiation exposure and may survive longer to experience radiation-associated toxicity. Children with congenital cardiovascular disease often require multiple exposures to medical radiation. Andreassi et al, found evidence of chromosomal damage in patients with congenital heart disease exposed to radiation (13). The US National Research Council considers cancer risk proportional to exposure even at the lowest levels (14). In this study, XFM halved radiation exposure.
Safe percutaneous VSD closure requires particular attention to critical structures such as the aortic valve (15). Subjectively, detailed soft tissue information from MRI enhances operator confidence navigating the complex spatial relationships among vital intracardiac structures. Indeed, when XFM was technically unavailable, our antegrade approach was not readily accomplished. XFM also facilitated appropriate orientation of the asymmetric occluder device with regard to the aortic valve and cardiac apex, although we offer no quantitative supporting data.
Limitations
We did not use transesophageal echocardiography or biplane fluoroscopy, which are typical adjuncts to conventional retrograde VSD repair. In our clinical experience, transesophageal and intracardiac echocardiography have value in selecting, positioning, and assessing closure devices, but have limited value in actual defect crossing, the procedure tested here. Biplane fluoroscopy might be expected to shorten retrograde crossing times slightly at the expense of additional radiation exposure. We did not compare XFM with newer 3-dimensional surface or transesophageal echocardiography(16). Three-dimensional target registration error is expected to be greater than the two-dimensional measurements we report. These experiments were conducted in swine with congenital VSD and surrounding aneurysmal tricuspid valve tissue; humans have similar anatomy(17).
Future Steps
We measured a target registration error of 4.9 ± 2.3 mm without correction for cardiac and respiratory motion. With further technical development, XFM systems might compensate for respiratory motion; automatically prepare multi-phase MRI roadmaps for ECG-synchronized overlay; register based on intrinsic fiducial landmarks rather than external beads; and incorporate additional imaging modalities such as ultrasound. That said, early generation XFM has been tested in humans (5,18) and is now suitable for clinical application in treatment of membranous and single or multiple muscular VSDs. Clinical endpoints might include technical success and morbidity, radiation exposure, and long-term outcomes.
Conclusion
XFM enhances the familiar X-ray environment with superior MRI soft tissue information to facilitate complex interventions. In this study, XFM simplified and shortened the procedure and reduced radiation exposure. Many congenital structural anomalies, with similarly complex intracardiac 3-D anatomical spatial relationships, might also benefit from such fusion image guidance.
Supplementary Material
Video Supplement 1 – Investigational antegrade XFM wire crossing
Video Supplement 2 – XFM confirmation of VSD occluder position before release
Video Supplement 3 – XFM during a change of X-ray gantry position. The 3D-to-2D projection changes automatically, and the registration is maintained.
Acknowledgments
We thank Pat Russo, John Oslund, and Kurt Amplatz of AGA Medical Corp for providing Amplatzer membranous VSD occluder devices and delivery systems; Victor J. Wright, William H. Schenke, Joni Taylor, and Katherine Lucas for technical assistance.
Sources of Funding
This work was supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL005062-04 CVB).
Abbreviations List
- MRI
Magnetic Resonance Imaging
- SSFP
Steady state free precession MRI
- TE
Echo Time
- TR
Repetition Time
- TSE
Turbo spin echo MRI
- XFM
X-Ray Fused with MRI
- VSD
Ventricular Septal Defect
Footnotes
Disclosures:
Merdim Sonmez was an employee of Siemens Corporate Research.
Michael C. Slack receives compensation as a physician training proctor for Amplatzer Medical Corporation.
Siemens Corporate Research and NHLBI have a Collaborative Research and Development Agreement for specific registration modules to be used in hybrid X-ray-MRI systems.
There are no other potential conflicts of interest.
References
- 1.Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190–7. doi: 10.1161/CIRCULATIONAHA.106.618124. [DOI] [PubMed] [Google Scholar]
- 2.Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007;28:2361–8. doi: 10.1093/eurheartj/ehm314. [DOI] [PubMed] [Google Scholar]
- 3.Mullins CE. Cardiac catheterization in congenital heart disease: pediatric and adult. Malden, Mass.: Blackwell Futura; 2006. [Google Scholar]
- 4.de Silva R, Gutierrez LF, Raval AN, McVeigh ER, Ozturk C, Lederman RJ. X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction. Circulation. 2006;114:2342–50. doi: 10.1161/CIRCULATIONAHA.105.598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez LF, Silva R, Ozturk C, et al. Technology preview: X-ray fused with magnetic resonance during invasive cardiovascular procedures. Catheter Cardiovasc Interv. 2007;70:773–82. doi: 10.1002/ccd.21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick AJ, Raman VK, Raval AN, et al. Invasive human magnetic resonance imaging: feasibility during revascularization in a combined XMR suite. Catheter Cardiovasc Interv. 2005;64:265–74. doi: 10.1002/ccd.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butera G, Chessa M, Carminati M. Percutaneous closure of ventricular septal defects. State of the art. J Cardiovasc Med (Hagerstown) 2007;8:39–45. doi: 10.2459/01.JCM.0000247434.59451.d7. [DOI] [PubMed] [Google Scholar]
- 8.Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation. 1988;78:361–8. doi: 10.1161/01.cir.78.2.361. [DOI] [PubMed] [Google Scholar]
- 9.Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol. 2005;96:52L–58L. doi: 10.1016/j.amjcard.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 10.Holzer R, Balzer D, Cao QL, Lock K, Hijazi ZM. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004;43:1257–63. doi: 10.1016/j.jacc.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Jameel AA, Arfi AM, Arif H, Amjad K, Omar GM. Retrograde approach for device closure of muscular ventricular septal defects in children and adolescents, using the Amplatzer muscular ventricular septal defect occluder. Pediatr Cardiol. 2006;27:720–8. doi: 10.1007/s00246-006-1365-5. [DOI] [PubMed] [Google Scholar]
- 12.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36 Suppl 14:121–5. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–8. doi: 10.1093/eurheartj/ehl014. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council (U.S.) Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, D.C.: National Academies Press; 2006. [PubMed] [Google Scholar]
- 15.Fu YC, Bass J, Amin Z, et al. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006;47:319–25. doi: 10.1016/j.jacc.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Acar P, Abadir S, Aggoun Y. Transcatheter closure of perimembranous ventricular septal defects with Amplatzer occluder assessed by real-time three-dimensional echocardiography. Eur J Echocardiogr. 2007;8:110–5. doi: 10.1016/j.euje.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Ho SY, Thompson RP, Gibbs SR, Swindle MM, Anderson RH. Ventricular septal defects in a family of Yucatan miniature pigs. Int J Cardiol. 1991;33:419–25. doi: 10.1016/0167-5273(91)90072-w. [DOI] [PubMed] [Google Scholar]
- 18.Rhode KS, Sermesant M, Brogan D, et al. A system for real-time XMR guided cardiovascular intervention. IEEE Trans Med Imaging. 2005;24:1428–40. doi: 10.1109/TMI.2005.856731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Supplement 1 – Investigational antegrade XFM wire crossing
Video Supplement 2 – XFM confirmation of VSD occluder position before release
Video Supplement 3 – XFM during a change of X-ray gantry position. The 3D-to-2D projection changes automatically, and the registration is maintained.