Abstract
We used translation-blocking morpholinos to reduce protein levels in Giardia intestinalis. Twenty-four hours after electroporation with morpholinos targeting either green fluorescent protein or kinesin-2b, levels of these proteins were reduced by 60%. An epitope-tagged transgene can also be used as a reporter for morpholino efficacy with targets lacking specific antibodies.
Giardia intestinalis (synonym Lamblia) is a parasitic protist and a major cause of diarrheal disease in developing countries (1, 16). Certain aspects of giardial biology have proved intractable for researchers seeking to study gene function. The trophozoite contains two diploid nuclei, making the cell effectively tetraploid and gene knockouts infeasible (1a). In addition, although Giardia contains RNA interference genes (homologs of Dicer and Argonaute genes) and this machinery was recently implicated in the control of antigenic variation (13, 15), attempts to manipulate this system for gene knockdown have been unsuccessful (C. C. Wang, personal communication).
While many valuable tools for studying gene function in Giardia have been developed, a fast, reliable method to knock down genes is still lacking. A few researchers have used virus-mediated ribozyme constructs to achieve gene knockdown (3). However, the selection of transformants may eliminate cells in which knockdown is deleterious. Dominant-negative mutants are also used to study gene function (4, 7), but few genes are amenable to this approach. Various levels of knockdown (from 34 to 100%) have been achieved by expressing the antisense sequence of large portions of the open reading frame of the target gene under the control of a strong promoter (6, 9, 10, 13, 20). But because a promoter allowing for the tight control of Giardia gene expression has not been developed, this approach can be applied to study only nonessential (13) or encystation-specific (6, 10) genes. Furthermore, it is not possible to control for off-target effects when using this technique.
Morpholinos are modified antisense oligonucleotides in which a six-membered morpholine ring replaces the deoxyribose ring of DNA and nonionic phosphorodiamidate linkages replace the typical anionic phosphodiester linkages (11). As a result, they cannot be degraded by cellular nucleases and are stable in cell culture (19). When designed to bind between the 5′ cap and a point 25 nucleotides downstream of the translation start site of the target mRNA, morpholinos (typically 25-mers) will sterically block ribosome binding and prevent the translation of the target gene (19). These translation-blocking morpholinos have been used previously to prevent new protein synthesis in trypanosomes (17).
To determine the efficacy of translation-blocking morpholinos in Giardia, we first targeted enhanced green fluorescent protein (enhanced GFP) in a strain expressing the GFP gene under the control of the glutamate dehydrogenase promoter (23). In this strain, diffuse GFP fluorescence is found throughout the cytoplasm (data not shown). The 25-mer morpholino was designed to target the first 24 bases of the GFP open reading frame, plus 1 base upstream of the start codon (Table 1). As a specificity control, we used a morpholino containing five mispaired bases (Table 1). The inclusion of five mispairs has been shown to destabilize the pairing of the morpholino with its target unless the cytoplasmic morpholino concentration is extremely high; therefore, this control can act as a sensor for concentration-dependent off-target effects (11). This control morpholino also shares its chemical properties and base composition with the experimental morpholino.
TABLE 1.
Morpholinos used in this study
| Morpholino | Sequence (5′ to 3′)a |
|---|---|
| Anti-GFP | CAGCTCCTCGCCCTTGCTCACCATG |
| Mispair anti-GFP | CAcCTgCTCGCCgTTGCTgACgATG |
| Anti-GiKIN2b | GCCTTTGCCCTTACTCTTGCTCATC |
| Mispair anti-GiKIN2b | GCgTTTcCCCTTAgTCTTcCTgATC |
Lowercase letters indicate mispaired bases.
Giardia trophozoites were cultured as described previously (14), and the introduction of morpholinos by electroporation was done essentially as described previously for plasmids (18). Lyophilized morpholinos (Gene Tools, LLC, Philomath, OR) were resuspended in sterile water to a concentration of 1 mM. This stock was added directly to a 0.4-cm cuvette with ∼5 × 106 cells in 0.3 ml of medium to produce the desired concentration of morpholinos. For the negative control, a volume of sterile water equal to the volume of the morpholino suspension was added. After electroporation, cells were grown for the amounts of time indicated in the figures and then analyzed by flow cytometry (Fig. 1). For flow cytometry, cells were first incubated in warm HEPES-buffered saline for 30 min to facilitate GFP fluorescence and then fixed with 1% paraformaldehyde and counted on a Beckman-Coulter EPICS XL analyzer. Twenty thousand cells from each sample were counted, enhanced GFP fluorescence was measured, and a gate for GFP-positive cells was created based on comparison to wild-type cells (see Fig. S1 in the supplemental material).
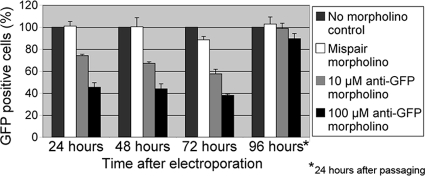
FIG. 1.
Time course of GFP knockdown by morpholinos. Cells were collected at the indicated times after electroporation with water (no-morpholino control), 100 μM mispair anti-GFP morpholino, 10 μM anti-GFP morpholino, or 100 μM anti-GFP morpholino. Fixed samples were subjected to flow cytometry and categorized as GFP positive or GFP negative compared to a wild-type control (see Fig. S1 in the supplemental material). The number of GFP-positive cells in the no-morpholino control culture at each time point was set to 100%. The 96-h culture consisted of cells that were passaged 72 h after electroporation and then grown an additional 24 h before collection. These data are the averages of results for three biological replicates, and error bars represent one standard deviation.
In all cases, the presence of a morpholino had no observable effect on cell growth compared to that of the no-morpholino control (data not shown). GFP levels in the no-morpholino and mispair controls remained approximately equal at all time points (Fig. 1; also see Fig. S2 in the supplemental material for representative flow cytometry histograms). However, 24 h after electroporation, GFP levels in the cultures treated with 100 μM anti-GFP morpholino had decreased by ∼60%, and they remained at approximately this level for the next 2 days, increasing only after the cultures were passaged and allowed to grow for 24 h (Fig. 1, 96 h). In the cultures treated with 10 μM anti-GFP morpholino, protein levels decreased by a maximum of ∼40%. Treatment with a higher concentration of morpholino (200 μM) produced approximately the same level of knockdown as treatment with 100 μM (see Fig. S3 in the supplemental material). For both the GFP and G. intestinalis kinesin-2b (GiKIN2b) experiments described below, maximum knockdown was not achieved until 24 h after electroporation (data not shown). This delay likely reflects the time needed for the turnover of preexisting protein in the cell and/or dilution by cell division.
To determine whether the remaining GFP-positive cells received morpholinos, we treated cells with a fluorescently labeled anti-GFP morpholino by electroporation. Twenty-four hours after electroporation, >99% of cells with 100 or 200 μM morpholino were positive for morpholino fluorescence whereas only ∼37% of cells with 10 μM morpholino were positive (see Fig. S4 in the supplemental material). However, within the morpholino-positive cells, there was no obvious correlation between morpholino fluorescence and GFP levels. The remaining GFP-positive cells in these experiments likely started out with more GFP than the GFP-negative cells (the population is heterogeneous due to variations in plasmid copy number) and did not receive enough morpholinos to reduce the GFP below the level of detection.
Next, we designed a morpholino to target an endogenous protein, G. intestinalis KIN2b (GiKIN2b) (Table 1). This protein is a homolog of the previously characterized G. intestinalis kinesin-2a (GiKIN2a), which is involved in anterograde intraflagellar transport in Giardia (7). In the GiKIN2a study, the expression of a dominant-negative GiKIN2a mutant produced cells with shortened flagella (7). Because the two kinesin-2 homologs likely function in the same complex (21), we chose to target GiKIN2b for morpholino knockdown to compare the resulting phenotype with the known phenotype of the GiKIN2a dominant-negative mutant.
To track the levels of the GiKIN2b protein, we produced a GiKIN2b-specific antibody that recognizes a single ∼72-kDa band in Giardia extracts (Fig. 2A). A 732-nucleotide segment of GiKIN2b (GenBank accession no. XP_001708236) containing the region encoding the C-terminal stalk and tail of the protein was cloned from Giardia genomic DNA by using primers kin-2b forward (5′-ACTGATATCTAATGGGTGCAGGGTTTACGGGCTATAC-3′) and kin-2b reverse (5′-ACTGCGGCCGCTCAACCGAAACCAGCCATGCCACG- 3′) and introduced into the vector pET30c (EMD Biosciences, Gibbstown, NJ), which includes a sequence encoding an N-terminal His tag. Purification on Ni-nitrilotriacetic acid beads under denaturing conditions was performed according to the instructions of the bead manufacturer (Qiagen, Valencia, CA). The purified protein was used to inoculate two New Zealand White rabbits according to a 77-day immunization protocol developed by Covance (Denver, PA).
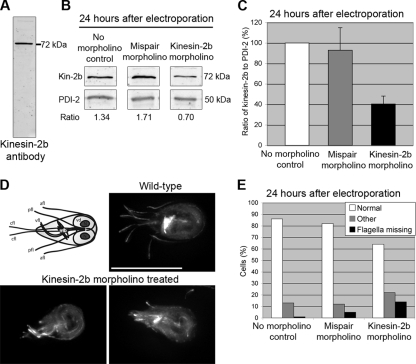
FIG. 2.
Translation-blocking morpholinos reduce GiKIN2b protein levels and cause extreme shortening of the flagella. (A) The GiKIN2b antibody produced for this study recognizes a band of the correct size (72 kDa) on Giardia extract immunoblots. (B) Results from a representative immunoblot analysis of samples 24 h after electroporation with water (no-morpholino control), 100 μM mispair anti-GiKIN2b morpholino, or 100 μM anti-GiKIN2b morpholino. PDI-2 was used as a loading control. For each sample, the ratio of GiKIN2b to PDI-2 is given. (C) Average ratios of GiKIN2b to PDI-2 on immunoblots from three experiments like the one described in the legend to panel B. The GiKIN2b/PDI-2 ratio in the no-morpholino control was set to 100%. Error bars represent one standard deviation from the average for three biological replicates. (D) Diagram of a Giardia cell and immunolabeling of wild-type and anti-GiKIN2b morpholino-treated cells. Fixed cells were labeled with an anti-α-tubulin antibody. In the diagram, the identities of the four pairs of flagella are indicated: afl, anterior flagella; pfl, posteriolateral flagella; vfl, ventral flagella; and cfl, caudal flagella. The median body (mb), a bundle of microtubules with an unknown function; the ventral disc (vd), used to attach to substrates; and two nuclei (N) are also labeled. Scale bar, 11 μm. (E) Distribution of mutant phenotypes in fixed and immunolabeled samples. Cells were collected 24 h after electroporation with water (no-morpholino control), 100 μM mispair anti-GiKIN2b morpholino, or 100 μM anti-GiKIN2b morpholino. Cells were classified as normal, mutant (missing the external portions of at least two pairs of flagella), or other (could not be categorized). One hundred cells from each sample were counted.
The resulting antibody was used on fluorescent immunoblots at a concentration of 1:50,000, along with an anti-protein disulfide isomerase 2 (anti-PDI-2) antibody (a gift from F. D. Gillin [8]) at 1:500,000 as a loading control (9) (Fig. 2B). The ratio of GiKIN2b to PDI-2 was used to compare amounts of GiKIN2b at different time points (Fig. 2B and C). The blots were visualized with a Li-Cor Odyssey infrared imager, and densitometry was performed using the Li-Cor Odyssey software according to the manufacturer's instructions.
When used at 100 μM, the anti-GiKIN2b morpholino achieved a 60% reduction in protein levels in 24 h (Fig. 2C). Mutants with disrupted cytoskeletons were also observed among fixed cells (Fig. 2D). Immunostaining with the TAT1 antibody (22) (a gift from K. Gull), image collection, and deconvolution were performed essentially as described previously (12). The cytoskeleton of a Giardia cell includes four pairs of flagella, a bundle of microtubules called the median body, and several other structures (5) (Fig. 2D). In the mutant cells, the entire external regions of at least two pairs of flagella were missing, with only internal axonemes remaining. The caudal and posteriolateral flagella were most often affected, though three or even all four sets of flagella of some cells were affected. These cells also often had reduced or missing median bodies. After 24 h, approximately 14% of cells in the knockdown cultures displayed mutant phenotypes, compared to 5% in the mispair control culture and 1% in the no-morpholino control culture (Fig. 2E).
When using translation-blocking morpholinos in Giardia, determining the level of knockdown generally requires a specific antibody. However, zebrafish researchers have used an epitope-tagged protein to provide a readout for morpholino efficacy (2). To determine whether this strategy would work for Giardia, we used the GiKIN2b morpholino in a strain carrying a plasmid encoding a C-terminally GFP-tagged GiKIN2b protein under the control of its native promoter (7). Thus, the same morpholino could be used to knock down the endogenous and GFP-tagged GiKIN2b proteins simultaneously.
Although the endogenous protein in this strain was reduced to the same level as that in the wild type after 24 h, GiKIN2b-GFP took a total of 48 h after electroporation to reach that level (Fig. 3A). This delay may be due to a difference in the turnover rate between the endogenous GiKIN2b and the GFP-tagged protein and probably also to high gene dosages in cells with multiple copies of the plasmid. Twenty-four hours after electroporation, 50% of the morpholino-treated cells displayed mutant phenotypes (shortened/missing flagella) (Fig. 3B). In fact, even 15% of the cells of the GiKIN2b::GFP strain without morpholino treatment were mutants. We hypothesize that the GiKIN2b-GFP fusion protein interferes with wild-type GiKIN2b complex function, sensitizing the cells to morpholino knockdown of the remaining GiKIN2b.
FIG. 3.
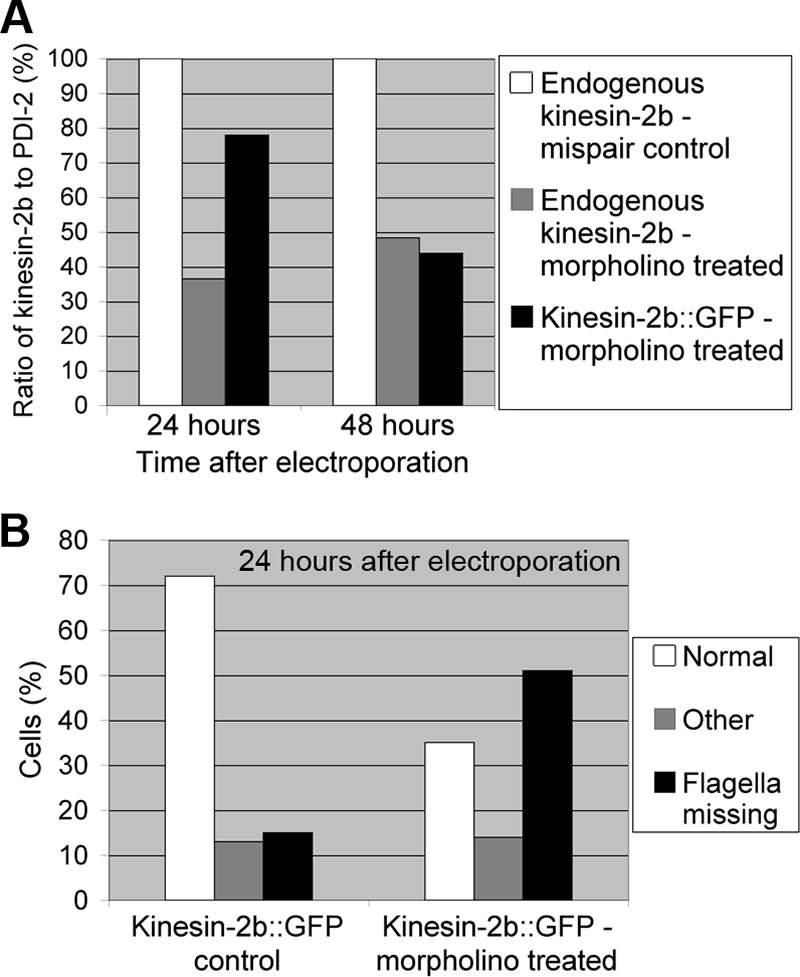
GFP-tagged GiKIN2b is knocked down by the anti-GiKIN2b morpholino. (A) The ratios of GiKIN2b or GiKIN2b-GFP to PDI-2 on immunoblots are plotted, with the ratio in the mispair control sample set to 100%. Cells were collected 24 and 48 h after electroporation with 100 μM mispair anti-GiKIN2b morpholino or 100 μM anti-GiKIN2b morpholino. (B) Distribution of mutant phenotypes in fixed and immunolabeled samples. GiKIN2b::GFP cells were collected 24 h after electroporation with water (control) or 100 μM anti-GiKIN2b morpholino. Cells were classified as normal, mutant (missing the external portions of at least two pairs of flagella), or other (could not be categorized). One hundred cells from each sample were counted.
The phenotype of GiKIN2b knockdown reveals some aspects of kinesin-2 function that the dominant-negative GiKIN2a may have obscured. Induction of the dominant-negative GiKIN2a resulted in a 15 to 30% decrease in flagellar length (7). However, morpholino knockdown of GiKIN2b produced cells missing the entire external regions of their flagella, suggesting that the remaining external flagellar regions of the dominant-negative cells were due probably to residual activity of the wild-type heterotrimeric complex. In any case, some conclusions can be drawn from the results of both studies: that different pairs of flagella are not equally susceptible to kinesin-2 disruption and that the cytoplasmic regions of the flagella are not maintained solely by kinesin-2-mediated intraflagellar transport.
Using translation-blocking morpholinos, we achieved a 60% reduction in protein levels in 24 h for both targets. However, for two different targets, unpublished data suggest that up to 80% knockdown can be achieved (A. R. Paredez and S. C. Dawson, unpublished data). Due to their efficacy, rapid action, specificity, and stability in cell culture, we believe that morpholinos have the potential to become a powerful new tool in the field of Giardia biology.
Supplementary Material
Acknowledgments
We thank members of the Cande lab and especially L. K. Fritz-Laylin and A. R. Paredez for helpful discussion. We also thank E. A. Lee for assistance with experiments, S. C. Dawson for advice and comments on the manuscript, and S. C. Dawson, K. Gull, and F. D. Gillin for reagents.
We gratefully acknowledge funding from the NIH (grant A1054693 to W.Z.C.) and the NSF (predoctoral fellowship to M.L.C.).
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Bernander, R., J. E. Palm, and S. G. Svärd. 2001. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell. Microbiol. 355-62. [DOI] [PubMed] [Google Scholar]
- 2.Collart, C., K. Verschueren, A. Rana, J. C. Smith, and D. Huylebroeck. 2005. The novel Smad-interacting protein Smicl regulates Chordin expression in the Xenopus embryo. Development 1324575-4586. [DOI] [PubMed] [Google Scholar]
- 3.Dan, M., A. L. Wang, and C. C. Wang. 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol. 36447-456. [DOI] [PubMed] [Google Scholar]
- 4.Dawson, S. C., M. S. Sagolla, J. J. Mancuso, L. Fritz-Laylin, and W. Z. Cande. 2007. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot. Cell 3171921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmendorf, H. G., S. C. Dawson, and J. M. McCaffery. 2003. The cytoskeleton of Giardia lamblia. Int. J. Parasitol. 333-28. [DOI] [PubMed] [Google Scholar]
- 6.Gaechter, V., E. Schraner, P. Wild, and A. B. Hehl. 2008. The single dynamin family protein in the primitive protozoan Giardia lamblia is essential for stage conversion and endocytic transport. Traffic 957-71. [DOI] [PubMed] [Google Scholar]
- 7.Hoeng, J. C., S. C. Dawson, S. A. House, M. S. Sagolla, J. K. Pham, J. J. Mancuso, J. Lowe, and W. Z. Cande. 2008. High-resolution crystal structure and in vivo function of a kinesin-2 homologue in Giardia intestinalis. Mol. Biol. Cell 193124-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knodler, L. A., R. Noiva, K. Mehta, J. M. McCaffery, S. B. Aley, S. G. Svärd, T. G. Nystul, D. S. Reiner, J. D. Silberman, and F. D. Gillin. 1999. Novel protein-disulfide isomerases from the early-diverging protist Giardia lamblia. J. Biol. Chem. 27429805-29811. [DOI] [PubMed] [Google Scholar]
- 9.Lauwaet, T., B. J. Davids, A. Torres-Escobar, S. R. Birkeland, M. J. Cipriano, S. P. Preheim, D. Palm, S. G. Svärd, A. G. McArthur, and F. D. Gillin. 2007. Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol. Biochem. Parasitol. 15280-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marti, M., A. Regos, Y. Li, E. M. Schraner, P. Wild, N. Muller, L. G. Knopf, and A. B. Hehl. 2003. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J. Biol. Chem. 27824837-24848. [DOI] [PubMed] [Google Scholar]
- 11.Moulton, J. D., and Y. L. Yan. 2008. Using morpholinos to control gene expression, p. 26.8.1-26.8.29. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, New York, NY. [DOI] [PMC free article] [PubMed]
- 12.Poxleitner, M. K., M. L. Carpenter, J. J. Mancuso, C. J. Wang, S. C. Dawson, and W. Z. Cande. 2008. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 3191530-1533. [DOI] [PubMed] [Google Scholar]
- 13.Prucca, C. G., I. Slavin, R. Quiroga, E. V. Elias, F. D. Rivero, A. Saura, P. G. Carranza, and H. D. Lujan. 2008. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456750-754. [DOI] [PubMed] [Google Scholar]
- 14.Sagolla, M. S., S. C. Dawson, J. J. Mancuso, and W. Z. Cande. 2006. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J. Cell Sci. 1194889-4900. [DOI] [PubMed] [Google Scholar]
- 15.Saraiya, A. A., and C. C. Wang. 2008. snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog. 4e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savioli, L., H. Smith, and A. Thompson. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative.’ Trends Parasitol. 22203-208. [DOI] [PubMed] [Google Scholar]
- 17.Shi, H., C. Tschudi, and E. Ullu. 2007. Depletion of newly synthesized Argonaute1 impairs the RNAi response in Trypanosoma brucei. RNA 131132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer, S. M., J. Yee, and T. E. Nash. 1998. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol. 9259-69. [DOI] [PubMed] [Google Scholar]
- 19.Summerton, J. 1999. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim. Biophys. Acta 1489141-158. [DOI] [PubMed] [Google Scholar]
- 20.Touz, M. C., N. Gottig, T. E. Nash, and H. D. Lujan. 2002. Identification and characterization of a novel secretory granule calcium-binding protein from the early branching eukaryote Giardia lamblia. J. Biol. Chem. 27750557-50563. [DOI] [PubMed] [Google Scholar]
- 21.Wedaman, K. P., D. W. Meyer, D. J. Rashid, D. G. Cole, and J. M. Scholey. 1996. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J. Cell Biol. 132371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines, and K. Gull. 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93491-500. [DOI] [PubMed] [Google Scholar]
- 23.Yee, J., M. R. Mowatt, P. P. Dennis, and T. E. Nash. 2000. Transcriptional analysis of the glutamate dehydrogenase gene in the primitive eukaryote, Giardia lamblia. Identification of a primordial gene promoter. J. Biol. Chem. 27511432-11439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.