Abstract
The effect of sterols from mammals, plants, fungi, and bacteria on model and natural membrane dynamics are reviewed, in the frame of ordering–disordering properties of membranes. It is shown that all sterols share a common property: the ability to regulate dynamics in order to maintain membranes in a microfluid state where it can convey important biological processes. Depending on the sterol class, this property is modulated by molecular modifications that have occurred during evolution. The role of sterols in rafts, antibiotic complexes, and in protecting membranes from the destructive action of amphipathic toxins is also discussed.
Keywords: Cholesterol, Alpha-cholesterol, Cholesterol palmitate, Cholesterol sulphate, Cycloartenol, Sitosterol, Stigmasterol, Ergosterol, Bacteriohopanetetrol, Bacteriohopaneaminotriol, Model and natural membranes, Membrane rafts, Regulating membrane dynamics, Solid-ordered, Liquid-disordered, Liquid ordered, Solid state NMR
Introduction: sterols and the dynamic membrane
It is widely recognized that lipids play multiple roles that either individually or collectively influence cell processes. Glycerolipids and sphingolipids through charge and structure are involved in DNA replication, protein translocation, cell recognition, signalling pathways, energetic, signal transduction, and cell trafficking. Together with diacylglycerols, their collective properties modulate lipid polymorphism, through phase transitions (lamellar, hexagonal, cubic, micelles), which are involved in enzyme conformational changes, cell division, cell fusion, and apoptosis [1].
Sterols, the third lipid class, also regulate biological processes and sustain the domain structure of cell membranes where they are considered as membrane reinforcers [2]. While cholesterol (CHO) is the major sterol of vertebrates, ergosterol (ERG) plays a key role in fungi. Plants usually possess more complex sterol compositions. Stigmasterol (STI) and sitosterol (SIT), two 24-ethyl sterols, are major constituents of the sterol profiles of plant species. They are involved in the embryonic growth of plants [3]. Hopanoids such as bacteriohopanetetrol (BHT) are sterol surrogates of primitive bacteria (archebacteria, cyanobacteria, etc.) that develop in very extreme conditions such as hot springs, very high deep sea pressure, highly saline water, and ice-covered lakes. They are considered as good markers of geological samples containing organic matter [4].
Sterols are critical for the formation of liquid-ordered (lo) membrane states (lipid “rafts” [5]) that are supposed to play an important role in fundamental biological processes such as signal transduction, cellular sorting, cytoskeleton reorganization, asymmetric growth, and infectious diseases. They have been proposed as key molecules to maintain membranes in a state of fluidity adequate for function. For instance, phytosterols have been recently shown to increase membrane cohesion in order to maintain plant membranes in a state of dynamics less sensitive to temperature shocks [6, 7]. In this review, the impact of different classes of sterols on membrane dynamics from mammals to archebacteria will be discussed.
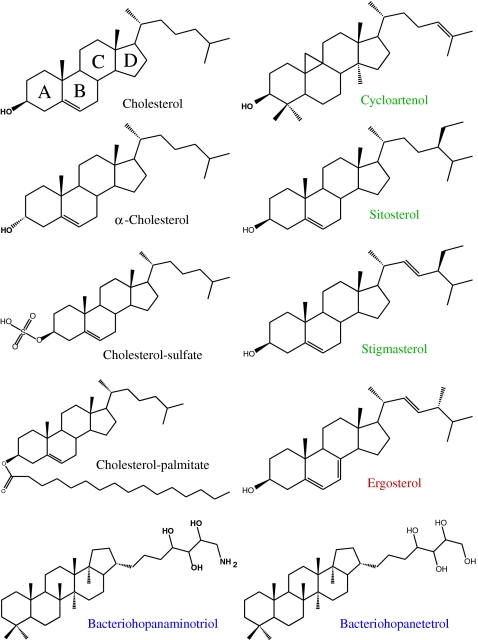
Molecular structure of some sterols in mammals, plants, fungi, and bacteria
In general, sterols are synthesized via the mevalonate pathway of isoprenoid metabolism [3].
Mammalian sterols Cholesterol is found in many biological membranes and is the main sterol of animals. It is often equimolar with phospholipids in many membranes. It has a tetracyclic structure with the OH group in the equatorial position on its first A ring (Fig. 1) and possesses a short aliphatic chain, with two ending methyl groups, branched on the D pentenic ring. The fused ring system is quasi-rigid. The alpha isomer (OH in axial position) is not encountered in nature. Cholesterol sulphate (CHS; Fig. 1), is found in the spermatozoa head and in the stratum corneum (the dry skin outer layer). It has the same structure as cholesterol except for a sulphate group substituting the hydroxyl group, which results to a negative charge. Another class of sterols are ester linked to fatty acids (C16:0, C18:1, and C18:2; Fig. 1). They are found in lipoprotein particles and lipid droplets as extra- or intracellular surplus that subsequently regulate the concentration of free sterols in the membranes.
Fig. 1.
Molecular structures of mammalian, plant, fungus, and bacterial sterols
Plant sterols Plants usually possess more complex sterol compositions. Cholesterol, campesterol, a 24-methyl sterol; stigmasterol and sitosterol, two 24-ethyl sterols, are major constituents of the sterol profiles of plant species. Sterol-C24-methyltransferases are important for plant growth and development [3] and alkylate the side chain on the D-ring of sterols by two successive methylation steps on C24. Therefore, typical phytosterols like sito- and stigmasterol (Fig. 1) possess additional alkyl groups on C24. Stigmasterol has an additional double bond at C22–C23. Both sterols originate from a precursor, cycloartenol, the first cyclic intermediate in the biosynthesis which features a very unusual [9–10] cyclopropyl small ring attached to the B-ring, three additional methyl groups on the fused ring system, and a double bond at the end of the acyl chain. The absence of double bond in position 6–7 in the B-ring confers some flexibility to the molecule [8].
Fungi sterols The main sterol found in fungi is ERG, which structure differs from that of cholesterol by the presence of additional double bonds, on the B-ring and on the acyl chain. A methyl group is also present at C24. As will be shown later, this sterol has peculiar interacting properties with antibiotics that act against fungus, such as polyene antibiotics [9–11].
Bacterial sterols Primitive sterols such as hopanoids are produced by bacteria that develop in very extreme (temperature, acidity, pressure, ionic strength) conditions and are considered as good markers of geological samples containing organic matter [4]. They possess a pentacyclic fused ring structure (Fig. 1) with four OH groups (bacteriohopanetetrol) or three hydroxyls and one NH2 group (bacteriohopaneaminotriol, BHAT) on the branched aliphatic chain. There are no OH group on the A ring, as in cholesterol, conferring to these molecules some kind of inverted polarity, on comparing to cholesterol, i.e., the hydrophilic part of the molecule is no longer on the fused ring system but at the end of the short aliphatic tail.
Measuring membrane dynamics using a noninvasive approach
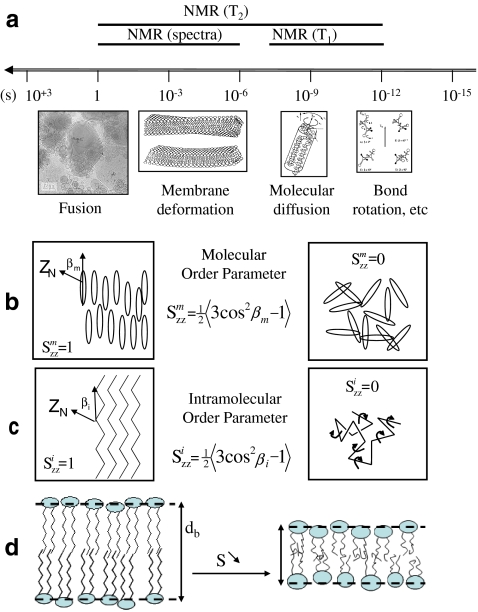
Membrane dynamics is essential for cellular life. A delicate balance between a loose membrane too fluid and akin to great permeability and a rigid membrane forbidding any transfer across the bilayer has been reached through many evolutionary steps in nature. As will be shown in the following, sterols play a central role in this aspect. The rigidity of a membrane is, however, difficult to define. When using macromolecular techniques to probe membrane dynamics, one could state that it is the inverse of fluidity, i.e., a macroscopic quantity that unfortunately hides in some way the molecular aspects. There are a wide variety of molecular techniques, such as spectroscopic methods, that probe dynamics at several scales, atomic, single molecule, and molecular assemblies. Liquid-state nuclear magnetic resonance (NMR) is a very well-known noninvasive technique used to determine the atomic three-dimensional structure of molecules in solution. It is much less known that NMR may also be very powerful for quantitatively measuring molecular or ensemble dynamics. In particular, solid-state NMR is one of the best tools to report in a noninvasive way on several time scales, as pictured in Fig. 2a.
Fig. 2.
a Motional time scales in biomembranes and NMR windows. Drawings and microscopy image depict the spatial scale at which events may occur. Lower panel—order parameter concept. b Molecular order; c intramolecular (bonds) order; d correlation between order and membrane thickness, db—left, ordered membrane with little bond or molecule fluctuations (large db); right, less ordered membrane with bond and molecule fast reorientations within the membrane (small db). Adapted from [12]
Measurement of NMR spin lattice relaxation time (T1) allows sampling the effect of very fast motions, occurring from the picoseconds to the nanoseconds time scale. Spectral lineshapes and transverse relaxation times (T2) are sensitive to slower motional processes occurring at the microsecond to millisecond time scale [12]. For instance, the narrowing of a solid-state deuterium NMR spectrum will reflect the presence of motions faster that the microsecond, due to temperature, hydration, phase changes, formation of complexes between molecules, etc. Measuring the spectral width thus represents a simple and powerful tool to sample dynamics. The membrane microfluidity can then be accessed and the concept of order parameter, S, which originates from the field of liquid-crystal physics, plays a central role. It is best used in biomembranes to define the residual ordering (microfluidity) of molecules. As shown in Fig. 2b,c, membranes made of molecules that have a very low order parameter (near the minimum value of 0) have little internal cohesion: they are almost as a liquid; the solid-state NMR spectrum of a nonoriented sample will be very narrow. In contrary, a membranous molecule that has an elevated molecular order parameter (near the maximum value of 1) is almost perfectly oriented with the main axis of motion (usually the bilayer normal); the solid-state NMR spectrum of a nonoriented sample will be very wide. As illustrated in Fig. 2c,b, the ordering may be described for aliphatic chains, which undergo many internal motions, or for the entire molecule, considered as a rigid rod, as are most of the fused ring systems of sterol molecules. Because the membrane ordering is directly linked to bond or molecule space averages, it can be translated, using an appropriate theory, to average length of a molecule in a membrane and hence to membrane thickness (Fig. 2d) [13, 14]. It is shown that increase/decrease in bilayer order means increase/decrease in membrane thickness.
In this review, the effect of sterols on membrane dynamics will be reported using solid-state deuterium NMR. The dynamic information will originate from lipids or sterols where some hydrogen atoms have been replaced by their isotope, deuterium, allowing making use of the quadrupolar interaction that best reports on internal or molecular order parameters. Of course other spectroscopic techniques could have been used, but many of them present severe drawbacks such as bulky reporter groups and lead only to qualitative information. Because we choose to base our report on deuterium NMR data (the literature is rich of such NMR experiments on sterol/lipid systems), our comparison will not suffer from the inherent time scale differences when using different techniques to report on dynamics.
Effect of mammalian sterols on model membranes
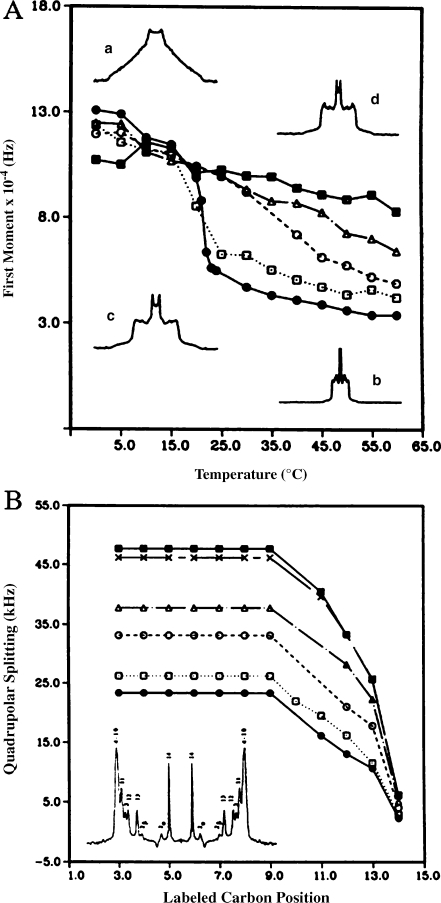
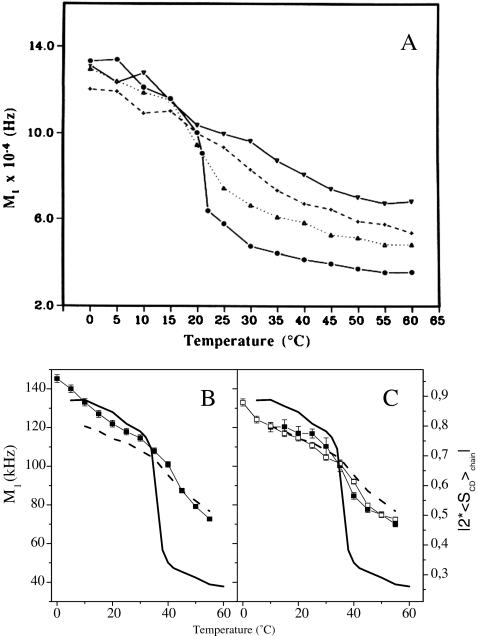
Cholesterol This sterol has been the most studied both in model and natural membranes. It has a well-described ordering–disordering action, which is best seen on model membranes that undergo in its absence a phase transition from a fully rigid state at low temperature, the solid-ordered (so) phase, to a very fluid state at high temperatures, the liquid-disordered (ld) phase. In the presence of cholesterol, the high order at low temperatures is slightly reduced and the low order at high temperatures is markedly increased. This effect is progressive with concentration and for elevated amounts of cholesterol in the membrane, the membrane microfluidity is about the same in the entire temperature range (Fig. 3a). The motional processes responsible for maintaining such a state (molecular rotation, trans-gauche bond isomerization) occur in the microsecond–nanosecond time scale and gently increase in speed with increasing temperature [15]. The lipid bilayer is thus maintained in a liquid-ordered state (lo), where molecules still undergo rotational and lateral diffusion in the membrane but to a lower extent. This state is reached for cholesterol contents greater than 20 mol% [16]. This behavior has been reported so far for saturated or unsaturated lipid chains with variable length and different head groups. In model membranes, it is noteworthy that cholesterol also lowers by approximately 30 °C the temperature of the lamellar-to-hexagonal phase transition of phosphatidylethanolamines (PE), i.e., it stabilizes hexagonal phases of PE lipids and promotes dehydration of both the lamellar and hexagonal phases [17]. A minute look at the increase of internal ordering of the lipid chains at high temperatures shows that all aliphatic carbons, from the glycerol backbone to the methyl terminal, perceive the cholesterol effect in a similar way (Fig. 3b). Because the membrane ordering is directly linked to bond or molecule space averages, it can be translated, as mentioned above, to average length of a molecule in a membrane and hence to membrane thickness. For instance, the presence of 30 mol% cholesterol led to a 7-Å increase in the dimyristoylphosphatidylcholine (DMPC; C14 phosphatidylcholine) bilayer thickness. The same effect is reported using independently chain-labeled phospholipids or ring-labeled cholesterol. Using deuterium-labeled cholesterol offers the advantage of determining the average orientation of the fused ring system with respect to the bilayer normal [18, 19]. Cholesterol sits perpendicular to the membrane surface and keeps this orientation independent of temperature [18]. The depth of embedment in the membrane has been also determined: cholesterol is buried in the bilayer with the OH group facing the carbonyl of the fatty acid lipid chains [20]. The rotational diffusion of cholesterol has been obtained in membranes using NMR relaxation time measurements. The sterol undergoes a fast axial rotation in the bilayer with a frequency of approximately 109 Hz (correlation time of 3 ns at 25 °C) [21]. The activation energy of such a motion is 32 kJ/mol.
Fig. 3.
Temperature variation of the first moment (proportional to chain order parameter), M1, of 2H-labeled DMPC spectra in the presence of various amounts of cholesterol: pure lipid (filled circles), 10% (empty squares), 20% (empty circles), 30% (empty triangles), 50% (filled squares). Inserts show typical 2H-NMR powder spectra: pure lipid at 0 °C (a) and 60 °C (b), lipid plus 50% CHO at 0 °C (d) and 60 °C (c). b Quadrupolar splitting (proportional to bond order parameter) from de-Paked spectra (see insert) of [2H27-DMPC] as a function of labeled carbon position, at T = 50 °C. Same symbols as for a with in addition 40 mol% CHO (crosses). Numbers in the de-Paked spectrum (insert) represent the assignment of labeled carbon positions. Adapted from [42]
Alpha cholesterol This isomer of cholesterol, with the OH group in axial position, does not naturally occur in membranes. It is produced in the course of the chemical synthesis and deuterium labeling of cholesterol. Insertion of comparable amounts of epicholesterol in synthetic membranes produces the order–disorder action as seen for cholesterol but to a lesser extent. Most interesting, the fused ring system is tilted (10–20°) with respect to the bilayer normal, a tilt that tends to reduce when increasing the temperature. Indeed, at high temperatures, the amplitude of allowed motions increases, leading to a decrease of the lateral bilayer pressure; the hydrophobic and hydrophilic interactions are therefore less demanding, and the α-isomer will tend to align its axis of inertia toward the perpendicular to the bilayer surface. Although purely academic, this shows that a small modification in the structure of cholesterol (OH axial or equatorial) leads to important modifications of molecular organization in bilayers: the tilted position of α-cholesterol within the bilayer membrane could be the reason that this isomer of cholesterol is not present in natural membranes, it would disturb too greatly the parallel packing of the lipid chains.
Cholesterol esters The effect of cholesteryl palmitate on the dynamics of dipalmitoylphosphatidylcholine (DPPC) has been investigated. The C16 fatty acid chain folds back into the bilayer hydrophobic interior [22] with the ester adopting a “horseshoe” conformation. The ester linkage is located near the bilayer interface. The orientation of the steroid skeleton of cholesteryl palmitate has been determined using deuterium-labeled molecules (Fig. 4). It is markedly tilted away from the position of that of cholesterol. This reflects the presence of the palmitoyl chain attached to the oxygen at carbon position 3. The palmitoyl chain therefore ‘pulls’ the rigid cholesteryl skeleton away from the average orientation found for cholesterol. From the orientation of the steroid and the values calculated for the carbon–deuterium bond order parameter, the molecular order parameter, Sm, for the steroid moiety of cholesteryl palmitate and of cholesterol were found. At 45 °C, the values Sm = 0.47 for cholesteryl palmitate (5 mol% in the bilayer) and Sm = 0.63 for cholesterol (7 mol%) indicate that fluctuations of the rigid steroid from the axis of motional averaging are greater for cholesteryl palmitate than for cholesterol. This indicates that the ester of cholesterol is less capable of membrane ordering than cholesterol, mainly due to the “horseshoe” conformation that diminishes the favorable van der Waals interactions leading to dense chain packing with cholesterol.
Fig. 4.
Spatial representation of the fused ring system of cholesterol and cholesteryl palmitate. The arrow indicates the direction of the rotation axis, n, collinear with the membrane normal. The symbol (empty circle) is the oxygen attached at C3 and deuterium nuclei of interest are plotted as small open circles. The palmitic chain in cholesteryl palmitate is attached at the oxygen. Molecules are drawn in the overall motional averaging axis system. The figure does not account for the ‘wobbling’ of the steroid skeleton (i.e., the drawing is made for Sm = 1). Adapted from [43]
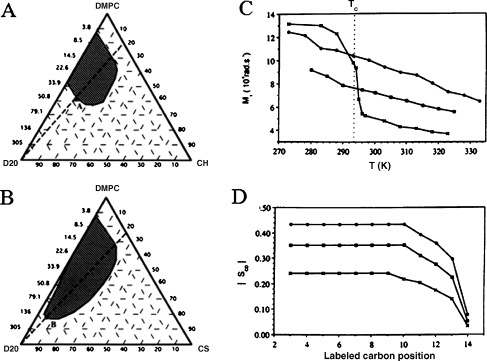
Cholesterol sulphate The comparative effect of cholesterol versus cholesterol sulphate on dimyristoylphosphatidylcholine membranes has been investigated by several techniques including NMR. As shown in Fig. 5a,b, the sulphate analog extends the hydrated lamellar phase domain towards high water contents, and substitution of 30 mol% CHO by CHS in DMPC lamellae induces the trapping of 30 wt.% additional water. 2H-NMR of heavy water allowed to determine that CHS itself binds 12 more water molecules at the interface than CHO, accounting for part of the swelling effect observed in the presence of the sulfated moiety. Like CHO, CHS possesses ordering–disordering properties. The disordering effect is much more pronounced than in the presence of cholesterol whereas the ordering at high temperatures is much less important; the same ordering effect is obtained on the entire bilayer thickness with 30 mol% CHS or 15 mol% CHO (Fig. 5c,d). The order–disorder transition is completely cancelled and there is almost no variation of order on large temperature ranges. These affects are related to the hydrated and negatively charged sulfate group that is much bulkier than the hydroxyl group. Because of this steric hindrance and/or the electrostatic repulsions between like charges, the DMPC acyl chains are further away from the CHS fused ring system than they are from CHO. Therefore, the attractive van der Waals forces are likely to be less intense and, hence, the chains more disordered. These observations shine light in the behavior of some membranes in which both CHO and CHS are present, e.g., the spermatozoon plasma membrane or the stratum corneum, where a good regulation of their relative concentrations is essential. This regulation is ensured by hydrolysis of CHS to CHO via a sulfatase activity. A dysfunction during this step can lead to dramatic changes in membrane properties. Indeed, an accumulation of CHS in the spermatozoa heads results in the incapacity of spermatozoa to penetrate ovum. This contraceptive effect can be accounted for by the CHS-driven lamellar stabilization of the sperm membrane (Fig. 5b), which would thus be less fusogenic, i.e., less capable to undergo nonlamellar structures at the fusion point. At the skin level, an increase in the relative concentration of CHS versus CHO leads to a thickening of the stratum corneum. This clinical pattern of scaling results from disorders in desquamation, and again the swelling and less ordering effect can account for it: the skin retaining more of its elasticity.
Fig. 5.
Partial phase diagrams of DMPC–CHO–D2O (a) and DMPC–CHS–D2O (b) at 25 °C. Hatched areas represent the lamellar phase domain. Compositions are given in weight percentages or corresponding D2O-to-DMPC molar ratio, Ri. Right panel—comparative evolution of the first spectral moment, M1 (chain order parameter), and the bond order parameter, SCD, for DMPC in the presence and absence of 30 mol% steroid. c Thermal evolution of M1, Tc is the DMPC main phase transition temperature. dSCD as a function of the labeled carbon position at 25 °C; DMPC (crosses), DMPC–CHO (30 mol%; filled circles), DMPC–CHS (30 mol%; filled squares). Adapted from [44]
Effect of plant sterols on model membranes
Cycloartenol As in mammals, plant sterols are synthesized via the mevalonate pathway of isoprenoid metabolism. There are, however, particularities of the plant sterol biosynthetic pathway, plants cyclise 2,3-oxidosqualene into cycloartenol (and not lanosterol like in mammals), whose 9b,19-cyclopropane ring is further metabolized. Cycloartenol is therefore a key precursor in the synthesis of other phytosterols. It has a global shape similar to cholesterol, but the absence of a double bond in ring B confers to the molecule some internal flexibility [8] that is not observed with the mammalian sterol. Nonetheless, at equivalent concentrations, the presence of cycloartenol in model membranes generates an ordering–disordering effect that is very similar to that of cholesterol [23] (Fig. 6a). This indicates that the internal flexibility of the fused ring system is of little importance and the global shape appears to be sufficient to make cycloartenol a cholesterol equivalent as far as regulation of membrane dynamics is concerned.
Fig. 6.
a Temperature variation of the first moment (proportional to chain order parameter), M1, of 2H-labeled DMPC spectra in the presence of various amounts of cycloartenol: pure lipid (filled circles), 10% (filled triangles), 20% (crosses), 30% (inverted filled triangles). Lower panel—first moments of 2H-NMR DPPC spectra with plant/fungi sterols versus temperature. First moments of pure DPPC-2H62 spectra (line without symbols) and of DPPC-2H62/CHO (30 mol%) spectra (dashed line without symbols) are shown in all diagrams for comparison. b DPPC-2H62 plus 30 mol% ERG (squares); c DPPC-2H62/STI (30 mol%; filled squares); DPPC-2H/SIT (30 mol%; empty squares). On double y-axis is plotted twice the chain order parameter. Adapted from [23] and [6]
Stigmasterol and sitosterol These two sterols are among the main phytosterol components of plants and are involved in the polarized growth of pollen tube and root hair. The asymmetric growth of plant cells is in general due to the asymmetric distribution of these membrane components. In a way similar to cholesterol, stigmasterol and sitosterol obviously have a strong ordering effect on model membranes above their phase transition temperature. At temperatures below the phase transition, they have a marked disordering effect. Remarkably, the decrease of the total chain ordering in the stigmasterol/sitosterol containing systems has a more sigmoid character compared with cholesterol (Fig. 6). From 25 °C to 45 °C, the chain ordering of DPPC with 30% stigmasterol/sitosterol is in between that of the pure membrane and that of the 30% cholesterol-containing membrane, indicating that in binary lipid systems (phospholipid/sterol), both plant sterols order less the membranes than do cholesterol.
Effect of ergosterol on model membranes
Ergosterol is the major sterol in fungi. Compared to cholesterol, it has an additional double bond in ring B, which brings some aromatic character and another in the short aliphatic chain. A methyl group is also branched at C24. Its ordering–disordering properties are analogous to those found for cholesterol (Fig. 6). The disordering effect is, however, less important than with cholesterol (for similar amounts in the membrane), whereas at high temperatures, the ordering effect is similar to that of cholesterol. As an overall behavior, one can state that ergosterol promotes a quasi-linear decrease of model membrane ordering (DPPC) at the temperature range of 0–60 °C. The order–disorder steep transition is damped down in the presence of this fungus sterol. A detailed two-component phase diagram has been built by Hsueh et al. [24] and shows a very similar shape as that early published with cholesterol, except that the so + lo and ld + lo phase coexistence regions are considerably broader.
Effect of hopanoids on model membranes
Hopanoids (Fig. 1) are made anaerobically from squalene. Unlike mammalian, plant, and fungi main sterols, they are pentacyclic triterpenoids and were discovered in the 1970s, primarily in prokaryotes and in a few plants. Interestingly, hydroxyl and amino groups are located at the end of the short aliphatic chain, providing some kind of “inverted” polarity on comparing to other sterols. They occur almost exclusively in membranes which contain large proton gradients [25]. It has been proposed that they have a role in inhibiting proton leaks through membranes and would reinforce membrane cohesion in bacteria as do eukaryotic sterols [2]. Their dynamical effect on model membranes has been reported using deuterium NMR [23]. Addition of 10–20% hopanoid to DMPC leads also to ordering effects on fluid phases, as seen on Fig. 7a.
Fig. 7.
Hopanes and DMPC membranes. a2H27-DMPC spectra in the absence (lower row) and presence (upper row) of BHT (10 mol%). Lower panel—temperature variation of the first moment (proportional to chain order parameter), M1, of 2H-labeled DMPC spectra in the presence of various amounts of hopanes. Pure lipid (filled circles), b BHAT 10% (filled triangles), 20% (crosses), c BHT 10% (filled triangles)
The disordering action observed at low temperature is, however, very weak, if any, the NMR spectra of pure lipids retaining most of its axial symmetry as usually seen in lo phases. Of interest, some macroscopic orienting properties by magnetic fields are seen as shown by the unusual line shape obtained at 15 °C. Both BHT and BHAT induce similar effects, which are, however, weaker than those observed with cholesterol at similar concentrations, as far as ordering of fluid phase is concerned. Inversion of steroid hydrophobicity, as in the hopanoid, still leads to membrane ordering as seen with cholesterol. This suggests that the hopane fused ring system retains some of the cholesterol properties. However, there is, at present, no information on the orientation of the molecule in the bilayer. One may conjecture from hydrophilic/hydrophobic considerations that the hydroxyl or amino groups that are attached to the short aliphatic chain are located at the interface and that the fused ring system is embedded in the bilayer interior: an inverted situation compared to mammalian, plant, and fungi sterols where the polar groups are attached to ring A.
Sterols and the “raft” concept
A relatively new concept has emerged in biology in relation with the lateral organization of biomembranes: it is proposed that lipid microdomains, also denominated rafts, exist in the plane of the membrane and could explain some complex biological activity [26–28]. These lipid microdomains are rich in cholesterol and saturated chains lipids, such as sphingolipids, and could be stabilized by weak forces such as hydrogen bonding and van der Waals interactions. The rafts also possess distinct protein composition, favoring development of signal transduction or membrane trafficking because of the formation of confined regions. Several techniques have been used to evidence theses membrane domains, the most popular being fluorescence microscopy where fluorescent molecules, thought to be specific of rigid of fluid lipid phases, were added to model or natural membranes. Because one may always question about the possible perturbation of nonnatural probes, we will concentrate in this review on nonperturbing techniques. A general concept, which subtends all the above studies, is the rafts rigidity, which would be due to the presence of increased concentrations of cholesterol and sphingolipids. This very rigid character, as opposed to the rest of the membrane in which rafts diffuse, would play an important role in the functional properties of proteins that are embedded in these regions. As already demonstrated, NMR is a very potent noninvasive technique to probe this aspect.
Cholesterol in rafts Lipid membrane systems that have been reported to be composed of sphingomyelin (SM)/cholesterol microdomains or “rafts” by Dietrich et al. (palmitoyloleoylphosphatidylcholine (POPC)/(SM)/CHO, 1/1/1) [29] and by Schroeder et al. (SCRL: Liver-PC/Liverphosphatidylethanolamine/SM/Cerebrosides/CHO, 1/1/1/1/2) [30] were investigated under the form of fully hydrated liposomes by the 2H NMR method. Liposomes of binary lipid composition POPC/CHO and SM/CHO were also studied as boundary/control systems. All systems at physiological temperatures were found to be in the liquid-ordered phase (lo). Use of deuterium-labeled cholesterol enabled finding both the position of the sterol axis of motion and its molecular order parameter. The axis of anisotropic rotation of cholesterol is such that the molecule is, on average, quasi-perpendicular to the membrane plane, in all of the four systems investigated, its molecular area being minimal with 33 Å2 (Fig. 8a). Cholesterol order parameters greater than 0.8 are observed, indicating that the sterol is in a restricted environment in the temperature range 0–60 °C (Fig. 8b). The binary mixtures present “boundary” situations with the lowest values for POPC/CHO and the highest for SM/CHO. The SCRL raft mixture has the same ordering as the SM/CHO, i.e., the highest order parameter values over the temperature range. It demonstrates that in the SCRL mixture, cholesterol dynamics is as in the binary system SM/CHO, therefore suggesting that it might be depleted from the rest of the membrane to form complexes as if it were alone with SM. On the other hand, the mixture POPC/SM/CHO exhibits intermediate ordering situation between SM/CHO and POPC/CHO. This strongly suggests that cholesterol could be in fast exchange, at the NMR time scale (milli- to microseconds), between two or more membrane regions of different dynamics and questions the statement of “rigid domains” made of SM and cholesterol in the model “raft” system POPC/SM/CHO. To summarize, rafts systems must be considered in a more cautious way and no longer seen as rigid “rocks” floating in fluid lipids. A more dynamic view could be adopted where sterols and other “rigid” molecules exchange between regions of different ordering (microfluidity).
Fig. 8.
aLeft—representation (stick mode) of cholesterol average orientation with respect to its principal axis of motional averaging, n (membrane normal), when n is parallel to the page plane. Right—view with n, perpendicular to the page plane, CPK representation is used here. The dashed line tentatively represents the cholesterol molecular area when viewed from above the membrane surface. b Thermal variation of the cholesterol molecular order parameter, Smol, in the four lipid–cholesterol compositions: POPC/CHO-2H5, SM/CHO-2H5, POPC/SM/CHO-2H5, SCRL/CHO-2H5. Adapted from [45]
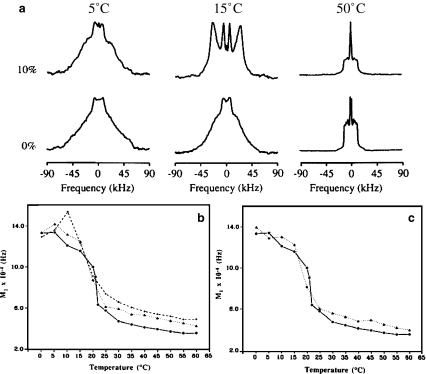
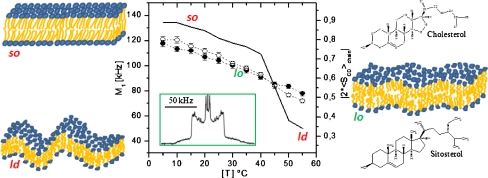
Plant sterols in rafts In plants, specialized lipid domains are involved in the polarized growth of pollen tube and root hair and the asymmetric growth of plant cells is in general due to the asymmetric distribution of membrane components. The effect of sitosterol and stigmasterol, two major plant sterols, on the structure and dynamics of membranes whose composition is representative of domains (rafts) in plants was recently documented [6]. Liposomes of phytosterols associated with glucosylcerebroside (GC) and with deuterium-labeled dipalmitoylphosphatidylcholine (2H-DPPC) were analyzed with deuterium solid-state nuclear magnetic resonance (2H-NMR). For comparison, membrane systems representative of raft composition in fungi and mammals were also investigated. Spectra such as that shown in Fig. 9, insert, allow detection of the lo phase, characteristic of a membrane state half-way between solid-ordered (so) and liquid-disordered (ld) states. The so state, also called “gel”, is found at low temperatures (below 35 °C), when membranes are essentially composed of SMs or GC (Fig. 9). This membrane state allows little biological function because it prevents membrane trafficking due to its very rigid state (order parameter close to 1). In turn, the ld or “fluid” state is found at high temperatures, in the absence of SM, GC, and sterols (low order parameter). On the contrary, such high membrane dynamics may lead to excessive membrane passages. By using 2H-NMR, the temperature behavior of membrane systems containing GC and plant sterols, it was found that the so–ld, order–disorder, transition was totally abolished: sitosterol and stigmasterol fluidized the so state and ordered the ld state to produce the lo state where membrane fluctuations vary smoothly with temperature (Fig. 9, central panel). This effect was already documented with CHO in mammals but on a much narrower temperature range (vide supra). The case or the fungus system was found in between that of plants and mammals. In summary, it appears that plant membranes of “raft” composition are less sensitive to temperature variations than those of animals.This suggests that cell membrane components like sitosterol, stigmasterol, and glucosylcerebrosides, which are typical of plants, are produced in order to extend the temperature range in which membrane-associated biological processes can take place. This observation is well in accordance with the fact that plants have to endure higher temperature variations than animals, which usually can either regulate their body temperature or change their location in order to avoid extreme heat or coldness. Compared to cholesterol, the two phytosterols posses additional ethyl groups branched on C-24 (Fig. 9, right). It has been proposed that the presence of an additional ethyl group may reinforce the attractive van der Waals interactions leading to more membrane cohesion and therefore less temperature sensitivity. These results also suggest that domains of smaller size would be promoted in the presence of phytosterols and especially with sitosterol. Such domains may be viewed as dynamic, with sterols laterally exchanging at the microsecond time scale. In plant cells, enzymes transfer alkyl groups to the C-24 of sterols. If we suppose that the relative activities of the different branches of the plant sterol biosynthesis are regulated, the concentrations of major sterols in plants, like sitosterol, stigmasterol, and cholesterol, could be controlled. This shows the importance of equilibrated sterol concentrations for plant growth and development. It thus appears that a fine tuning of the sterol structure, i.e., the presence of branched ethyl groups in plant sterols increasing membrane cohesion through formation of smaller membrane domains, may be the evolution response for plant adaptation to large temperature variations [7].
Fig. 9.
Regulation of temperature-driven membrane dynamics by plant sterols. Central panel—first spectral moment (left y-axis) or order parameter (right y-axis) as a function of temperature; solid line2H-DPPC with glucosylcerebroside; open circles plus stigmasterol; filled circles plus sitosterol. Insert2H-NMR spectrum typical of a liquid-ordered, lo, state. Left panel—schematics of solid-ordered, so (gel), and liquid-disordered, ld (fluid), membrane states. Right panel—schematics of the lo (raft) membrane state together with the structures of cholesterol and sitosterol. Adapted from [6, 7]
Sterols and toxins in membranes: the protecting effect
Cationic amphipathic α-helical peptides, such as the bee-venom toxin melittin, preferentially disrupt mixed model membranes or natural membranes such as those of red blood cells [31] potentially causing a release of cell contents. The molecular processes by which this happens are multiple and have been summarized in a review by Shai [32]. One of them involves the formation of bilayer discs of 20–40 nm chopped off from the membrane by lateral segregation of α-helical amphipathic peptides [33], see inserts in Fig. 10.
Fig. 10.
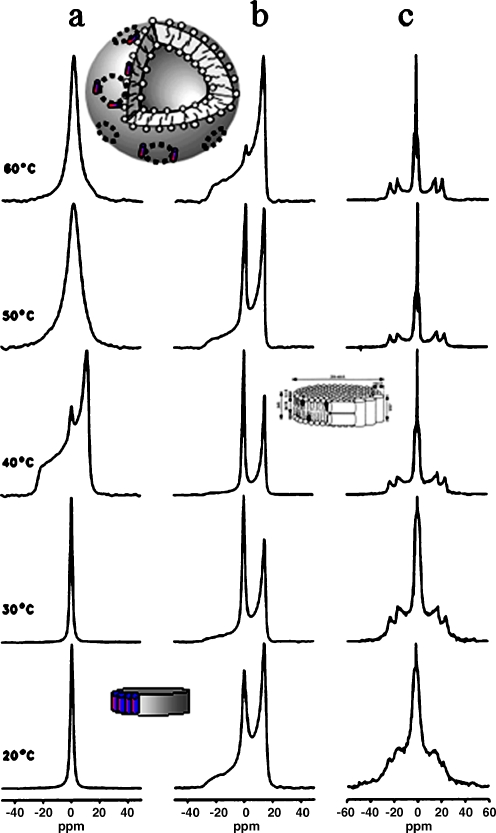
31P- and 2H-NMR spectra of the melittin/DPPC system in the presence and absence of cholesterol. a31P-NMR spectra for selected temperatures of the cholesterol-free system at lipid to melittin molar ratio of 20. b The corresponding 31P-NMR and c2H-NMR spectra of the system containing 30 mol% of 2H-labeled cholesterol. Inserts show the DPPC/melittin discs at low temperatures and the large vesicles at high temperatures. In the presence of CHO, remaining discs contain small amounts of sterol (insert on right hand side). Adapted from [36]
This is translated by the appearance of very sharp and isotropic lines in solid-state 31P or 2H NMR. This phenomenon occurs with saturated chain lipids preferentially in their gel phase (so), i.e., at temperatures below the order disorder transition [34, 35]. Temperatures above the transition lead to the fusion of discs resulting in large vesicles of 200–300 nm (Fig. 10a). In the presence of 30 mol% cholesterol, the lo phase is present and leads to a total inhibition of the toxin-triggered formation of large unilamellar vesicles as seen by the detection or wide NMR powder patterns; the appearance of small discs is also strongly restricted (decrease of amount of isotropic NMR lines) [36]. Use of deuterium-labeled cholesterol shows that remaining discs contain small amounts of sterol, phospholipids being the main component (Fig. 10b,c). Sterols can also protect negatively charged membranes from the disruptive effects of other antimicrobial peptides [37]. This suggests that bacteria (without sterols) are most susceptible to the action of such peptides whereas lower eukaryotes including fungi (containing ergosterol) exhibit an intermediate degree of sensitivity, and higher organisms (containing cholesterol) are largely resistant to antimicrobial peptides.
Sterols and polyene antibiotics in membranes
Polyene antibiotics such as amphotericin B, filipin, and nystatin are known to mediate changes in the membrane permeability of a number of organisms, thus inducing a leakage of important cellular constituents and ultimately lysis and death of the cell. The principal characteristic of these antibiotics is that they apparently require the presence of sterols in the cell membrane to promote such an effect. De Kruijff and coworkers proposed decades ago that polyene antibiotics and sterols formed molecular complexes to create channels or solid patches that disrupt the membrane [9–11]. Amphotericin B has received particular attention. De Kruijff has proposed that amphotericin B and cholesterol form an eight–eight molecular complex, spanning the membrane vertically and creating a pore. The dynamic and structural parameters of the interactions between amphotericin B and sterol-containing model membranes have been monitored by 2H-NMR of deuterium-labeled lipids and cholesterol. The structural parameters of deuterium-labeled cholesterol in CHO/DMPC mixtures did not change upon addition of amphotericin B, i.e., there is no change in microfluidity and rigid domains are not detected, at the microsecond time scale, as could be expected by the presence of a rigid pore where the sterol molecules are segregated away from the lipids. However, measurement of NMR relaxation times for labeled cholesterol showed that the minimum in T1, observed for cholesterol in DMPC at 32–35 °C, was shifted towards 38–40 °C in the presence of amphotericin B (Fig. 11a).
Fig. 11.
a Temperature dependence of the relaxation time, T1z, of [6-2H]-CHO in DMPC, in the presence or absence of amphotericin B (Ampho B). Lower panels—2H-NMR spectra of [2,2,3,4,4,6-2H6]cholesterol in DMPC (30 mol%) in the presence (b) and absence (c) of filipin, at 25 °C. The antibiotic, when present, is equimolar to cholesterol. Adapted from [38]
This minimum allows a direct calculation of the speed of axial rotation of cholesterol in membranes (50 × 109 Hz). The data indicates that the system containing the antibiotic has to be warmed by approximately 5 °C in order to relax with the same efficiency as the system without the drug. In other words, the motions of cholesterol in DMPC are slowed down by the presence of amphotericin B.
The interaction of the polyene antibiotic filipin with membrane sterols is well known and this antibiotic is nowadays well used as a test to detect the presence of sterols in cellular membranes. Although the molecular processes by which this happens are still unclear, filipin has been shown to be particularly efficient in inhibiting fungi growth, through interaction with ergosterol. The effect of filipin on cholesterol containing model membranes has been followed by 2H-NMR of labeled cholesterol [38]. At physiological temperatures, there is evidence of filipin-induced cholesterol immobilization at the membrane. The 2H NMR spectra of cholesterol show two domains in which ordering and dynamics are very different. In one of these, cholesterol is static on the 2H NMR time scale (Fig. 11b), whereas in the other, it undergoes rapid axially symmetric motions similar to those it exhibits in the drug-free membrane. This indicates that the jumping frequency of cholesterol between the labile and immobilized domains is less than 105 Hz. The distribution of cholesterol between these two sites is temperature dependent: at 0 °C, all sterol molecules are immobilized, whereas at 60 °C, they are almost totally in the labile state. To summarize, the effect of polyene antibiotics may be very diverse on membrane sterols: from gentle slowing down of axial rotation to complete immobilization in the membrane, forming “solid” complexes.
Sterols in complex “live” membranes
Natural membranes contain a number of other molecules and in particular membrane proteins that may modulate the sterol behavior as reported above for lipid model membranes. Reporting on the dynamics of entire membranes is a difficult task. Several techniques have been used such as, for instance, fluorescence and electron spin resonance. However, most of these techniques, as well as being invasive, do not directly measure the fluidity of the membrane. Although NMR is not very sensitive, a limited number of experiments have nonetheless been reported in membranes containing all components, i.e., all lipids and proteins. Human red blood cell membranes have been investigated some decades ago. The erythrocyte membrane is known to have approximately 27% of its total lipid weight as cholesterol, which can be exchanged with that from sonicated cholesterol/PC vesicles [39]. Figure 12 shows the spectrum of deuterated cholesterol incorporated in human erythrocytes.
Fig. 12.
2H-NMR spectrum of [2,2,3,4,4,6-2H]cholesterol in membranes of human erythrocytes. Adapted from [39]
Only one average spectrum is detected, very similar to that obtained in model membranes at a concentration of 30 mol%. Analysis in terms of orientational order parameter has shown that the orientation and the anisotropic motion of cholesterol are very similar in natural and model membranes. The other membrane components appear thus not to affect much the regulating role of cholesterol.
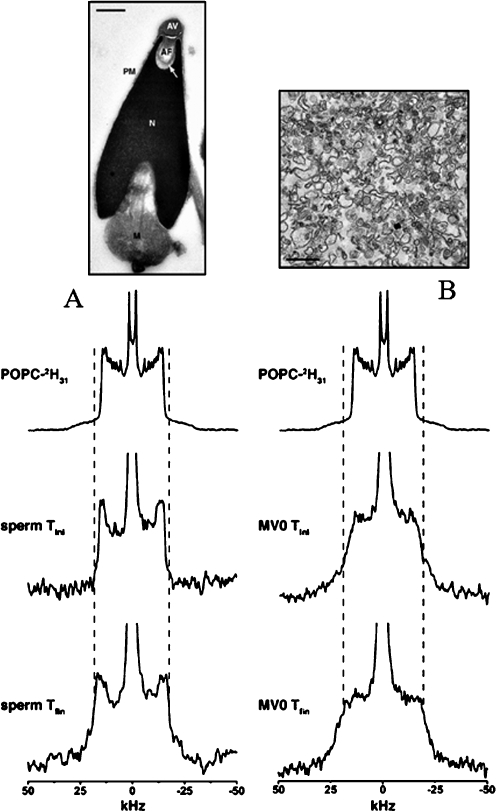
The dynamic properties of whole sea urchin sperm and purified membranes of subcellular compartments have been detected by solid-state 2H-NMR spectroscopy [40, 41]. A deuterium-labeled lipid (POPC) was incorporated into whole cell membranes using an approach similar to that developed for labeled cholesterol (vide supra). Mass spectrometry was used to measure the amount of incorporated lipid and the time it took for the lipid to statistically distribute in all the membranes was determined by recording spectra as a function of time and temperature. Figure 13 shows the resulting data for whole sea urchin sperm cells and nuclear envelope precursor membrane vesicles.
Fig. 13.
Deuterium solid-state NMR spectra of POPC-2H31 labeled sea urchin sperm membranes (a) and precursor egg membrane vesicles (MV0; b). Sperm and MV0 were labeled with MLVs and SUVs of POPC respectively for 30 min at 40 °C and the corresponding deuterium NMR spectra acquired at 10 °C (middle spectra «sperm Tini», «MV0 Tini»). The systems were equilibrated at 40 °C and reacquired at 10 °C (bottom spectra «sperm Tfin», «MV0 Tfin»). The top spectra corresponds to MLVs of POPC and the dashed lines show the plateau quadrupolar splitting enlargement on sperm and MV0 spectra postequilibrium at 40 °C. Upper images are electron microscopy pictures of sperm and egg membrane vesicles. Adapted from [40]
Spectra postequilibrium are typical of the liquid-ordered phase as reported in models. It was demonstrated that whole sperm membranes are more dynamic than nuclear envelope precursor membranes due to the higher cholesterol levels of the latter. This application is rather new and can be exploited as a generic method for monitoring membrane dynamics in whole cells, various subcellular membrane compartments, and membrane domains in subcellular compartments.
Membrane evolution, dynamics, and the role of sterols
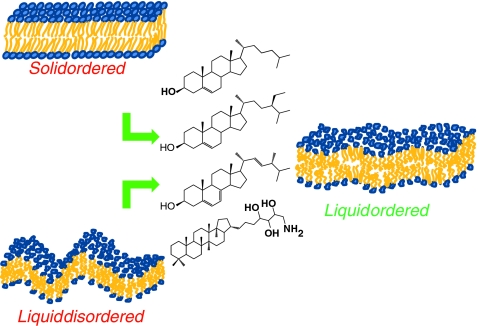
Sterols have been historically considered as membrane reinforcers because they induce a molecular order to membranes. The main motto of this review suggests that they could better be named as “membrane dynamic regulators”, by maintaining the membrane in a state of microfluidity suitable for cell function on large temperature scales (Fig. 14). The so-called “liquid-ordered state”, which was initially considered as a physicist peculiarity, seems to be a state present in “rafts” systems that provides enough “dynamic rigidity” to convey important biological processes.
Fig. 14.
Sterols (mammals, fungi, plants, bacteria) as regulators of membrane dynamics. Left—the solid-ordered and liquid-disordered states in the absence of sterols. Right—the liquid ordered state with sterols
It is interesting to remark that each type of cellular membrane contains a specific sterol as a major component: cholesterol in mammals, ergosterol for fungi, sito-/stigmasterol in plants, and hopanoids in primitive bacteria. The fused ring system (four or five cycles) appears to bear the property of increasing the order of fluid phases. Presence of unsaturations and flexibility of this fused system does not seem to alter the ordering effect. On the contrary, the branched chain seems to have a quite important modulation role. Ethyl groups branched at C24 bring better ordering over large temperature scales, a property used by plant sterols to accommodate thermal shocks. Hydroxyl or amine groups in hopanoids appear to decrease the dynamic regulating capability: the so phase is still observed at low temperatures. However, model membranes in which such sterols are associated with branched chain lipids (phytanoyl chains) have not been investigated. Sterols are undoubtedly key molecules in regulating membrane dynamics but evolution in nature has not only led to sterol modification for adaptation but also synthesis of sterols in association with other lipids (sphingolipids, phosphoinositides) that are only present in specific membranes. Such lipids may serve as fine tuning of the main regulating property, brought by sterols.
References
- 1.Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro N, Streiff S, Heissler D, Elhabiri M, Albrecht-Gary AM, Atsumi M, Gotoh M, Desaubry L, Nakatani Y, Ourisson G. Reinforcing effect of bi- and tri-cyclopolyprenols on ‘primitive’ membranes made of polyprenyl phosphates. Tetrahedron. 2007;63:3395–3407. doi: 10.1016/j.tet.2007.01.076. [DOI] [Google Scholar]
- 3.Schaller H. The role of sterols in plant growth and development. Prog Lipid Res. 2003;42:163–175. doi: 10.1016/S0163-7827(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Suzuki N. Distributions and sources of hopanes, hopanoic acids and hopanols in Miocene to recent sediments from ODP Leg 190, Nankai Trough. Org Geochem. 2007;38:1715–1728. doi: 10.1016/j.orggeochem.2007.05.012. [DOI] [Google Scholar]
- 5.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J. 2007;21:1714–1723. doi: 10.1096/fj.06-7809com. [DOI] [PubMed] [Google Scholar]
- 7.Dufourc EJ. The role of phytosterols in plant adaptation to temperature. Plant Sign Behav. 2008;3:133–134. doi: 10.4161/psb.3.2.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milon A, Nakatani Y, Kintzinger JP, Ourisson G. The conformation of cycloartenol investigated by NMR and molecular mechanics. Helv Chim Acta. 1989;72:1–13. doi: 10.1002/hlca.19890720102. [DOI] [Google Scholar]
- 9.Kruijff B, Demel RA. Polyene antibiotic sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. Part 3: molecular structure of the polyene antibiotic cholesterol complexes. Biochim Biophys Acta. 1974;339:57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- 10.Kruijff B, Gerritsen WJ, Oerlemans A, Demel R, Deenen LLM. Polyene antibiotic sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. Part 2: temperature dependence of the polyene antibiotic sterol complex formation. Biochim Biophys Acta. 1974;339:44–56. doi: 10.1016/0005-2736(74)90331-9. [DOI] [PubMed] [Google Scholar]
- 11.Kruijff B, Gerritsen WJ, Oerlemans A, Demel R, Deenen LLM. Polyene antibiotic sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. Part 1: specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974;339:30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]
- 12.Dufourc EJ. Solid state NMR in biomembranes. In: Larijani B, Woscholski R, Rosser CA, editors. Chemical biology. London: Wiley; 2006. pp. 113–131. [Google Scholar]
- 13.Douliez JP, Leonard A, Dufourc EJ. Restatement of order parameters in biomembranes—calculation of C–C bond order parameters from C–D quadrupolar splittings. Biophys J. 1995;68:1727–1739. doi: 10.1016/S0006-3495(95)80350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douliez JP, Leonard A, Dufourc EJ. Conformational order of DMPC sn-1 versus sn-2 chains and membrane thickness: an approach to molecular protrusion by solid state H-2-NMR and neutron diffraction. J Phys Chem. 1996;100:18450–18457. doi: 10.1021/jp961220v. [DOI] [Google Scholar]
- 15.Weisz K, Grobner G, Mayer C, Stohrer J, Kothe G. Deuteron nuclear-magnetic-resonance study of the dynamic organization of phospholipid cholesterol bilayer-membranes—molecular-properties and viscoelastic behavior. Biochemistry. 1992;31:1100–1112. doi: 10.1021/bi00119a019. [DOI] [PubMed] [Google Scholar]
- 16.Vist MR, Davis JH. Phase equilibria of cholesterol dipalmitoylphosphatidylcholine mixtures: 2H-nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 17.Marinov R, Dufourc EJ. Cholesterol stabilizes the hexagonal type-II phase of 1-palmitoyl-2-oleoyl SN glycero-3-phosphoethanolamine—a solid-state H-2 and P-31 NMR-study. J Chim Phys. 1995;92:1727–1731. [Google Scholar]
- 18.Dufourc EJ, Parish EJ, Chitrakorn S, Smith ICP. Structural and dynamical details of cholesterol lipid interaction as revealed by deuterium NMR. Biochemistry. 1984;23:6062–6071. doi: 10.1021/bi00320a025. [DOI] [Google Scholar]
- 19.Marsan MP, Muller I, Ramos C, Rodriguez F, Dufourc EJ, Czaplicki J, Milon A. Cholesterol orientation and dynamics in dimyristoylphosphatidylcholine bilayers: a solid state deuterium NMR analysis. Biophys J. 1999;76:351–359. doi: 10.1016/S0006-3495(99)77202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard A, Escrive C, Laguerre M, Pebay-Peyroula E, Neri W, Pott T, Katsaras J, Dufourc EJ. Location of cholesterol in DMPC membranes. A comparative study by neutron diffraction and molecular mechanics simulation. Langmuir. 2001;17:2019–2030. doi: 10.1021/la001382p. [DOI] [Google Scholar]
- 21.Dufourc EJ, Smith ICP. A detailed analysis of the motions of cholesterol in biological-membranes by H-2-NMR relaxation. Chem Phys Lipids. 1986;41:123–135. doi: 10.1016/0009-3084(86)90004-6. [DOI] [PubMed] [Google Scholar]
- 22.Valic MI, Gorissen H, Cushley RJ, Bloom M. Deuterium magnetic resonance study of cholesterol esters in membranes. Biochemistry. 1979;18:854–859. doi: 10.1021/bi00572a018. [DOI] [PubMed] [Google Scholar]
- 23.Léonard A, Milon A, Krajewski-Bertrand M-A, Dufourc EJ. Modulation of membrane hydrophobic thickness by cholesterol, cycloartenol and hopanoid. A solid state 2H-NMR study. Bull Magn Reson. 1993;15:124–127. [Google Scholar]
- 24.Hsueh YW, Gilbert K, Trandum C, Zuckermann M, Thewalt J. The effect of ergosterol on dipalmitoylphosphatidylcholine bilayers: a deuterium NMR and calorimetric study. Biophys J. 2005;88:1799–1808. doi: 10.1529/biophysj.104.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haines TH. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res. 2001;40:299–324. doi: 10.1016/S0163-7827(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 26.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 27.London E, Brown DA. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta-Biomembr. 2000;1508:182–195. doi: 10.1016/S0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 28.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder R, London E, Brown D. Interactions between saturated ACYL chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins—GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufourc EJ, Bonmatin JM, Dufourcq J. Membrane-structure and dynamics by H-2-NMR and P-31-NMR—effects of amphipatic peptidic toxins on phospholipid and biological-membranes. Biochimie. 1989;71:117–123. doi: 10.1016/0300-9084(89)90141-7. [DOI] [PubMed] [Google Scholar]
- 32.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta Biomembr. 1999;1462:55–70. doi: 10.1016/S0005-2736(99)00200-X. [DOI] [PubMed] [Google Scholar]
- 33.Dufourc EJ, Faucon JF, Fourche G, Dufourcq J, Gulikkrzywicki T, Lemaire M. Reversible disk-to-vesicle transition of melittin–DPPC complexes triggered by the phospholipid acyl chain melting. FEBS Lett. 1986;201:205–209. doi: 10.1016/0014-5793(86)80609-3. [DOI] [Google Scholar]
- 34.Dufourc EJ, Smith ICP, Dufourcq J. Molecular details of melittin-induced lysis of phospholipid-membranes as revealed by deuterium and phosphorus NMR. Biochemistry. 1986;25:6448–6455. doi: 10.1021/bi00369a016. [DOI] [PubMed] [Google Scholar]
- 35.Pott T, Dufourcq J, Dufourc EJ. Fluid or gel phase lipid bilayers to study peptide membrane interactions? Eur Biophys J Biophys Lett. 1996;25:55–59. [Google Scholar]
- 36.Pott T, Dufourc EJ. Action of melittin on the DPPC–cholesterol liquid-ordered phase—a solid-state H-2-NMR and P-31-NMR study. Biophys J. 1995;68:965–977. doi: 10.1016/S0006-3495(95)80272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason AJ, Marquette A, Bechinger B. Zwitterionic phospholipids and sterols modulate antimicrobial peptide-induced membrane destabilization. Biophys J. 2007;93:4289–4299. doi: 10.1529/biophysj.107.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dufourc EJ, Smith ICP. H-2 NMR evidence for antibiotic-induced cholesterol immobilization in biological model membranes. Biochemistry. 1985;24:2420–2424. doi: 10.1021/bi00331a005. [DOI] [PubMed] [Google Scholar]
- 39.Kelusky EC, Dufourc EJ, Smith ICP. Direct observation of molecular ordering of cholesterol in human-erythrocyte membranes. Biochim Biophys Acta. 1983;735:302–304. doi: 10.1016/0005-2736(83)90306-1. [DOI] [PubMed] [Google Scholar]
- 40.Gamier-Lhomme M, Grelard A, Byrne RD, Loudet C, Dufourc EJ, Larijani B. Probing the dynamics of intact cells and nuclear envelope precursor membrane vesicles by deuterium solid state NMR spectroscopy. Biochim Biophys Acta Biomembr. 2007;1768:2516–2527. doi: 10.1016/j.bbamem.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Garnier M, Dufourc EJ, Larijani B. Characterisation of lipids in cell signalling and membrane dynamics by nuclear magnetic resonance spectroscopy and mass spectrometry. Sign Transduc. 2006;6:133–143. doi: 10.1002/sita.200500077. [DOI] [Google Scholar]
- 42.Leonard A, Dufourc EJ. Interactions of cholesterol with the membrane lipid matrix—a solid-state NMR approach. Biochimie. 1991;73:1295–1302. doi: 10.1016/0300-9084(91)90092-F. [DOI] [PubMed] [Google Scholar]
- 43.Chana RS, Cushley RJ, Wassall SR, Smith ICP, Dufourc EJ. Organization of cholesteryl esters in membranes—a deuterium nuclear magnetic-resonance study. Chem Phys Lipids. 1985;37:345–356. doi: 10.1016/0009-3084(85)90088-X. [DOI] [Google Scholar]
- 44.Faure C, Tranchant JF, Dufourc EJ. Comparative effects of cholesterol and cholesterol sulfate on hydration and ordering of dimyristoylphosphatidylcholine membranes. Biophys J. 1996;70:1380–1390. doi: 10.1016/S0006-3495(96)79696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aussenac F, Tavares M, Dufourc EJ. Cholesterol dynamics in membranes of raft composition: a molecular point of view from H-2 and P-31 solid-state NMR. Biochemistry. 2003;42:1383–1390. doi: 10.1021/bi026717b. [DOI] [PubMed] [Google Scholar]