Abstract
Identification and quantification of multiple proteins from complex mixtures is a central theme in post-genomic biology. Despite recent progress in high-throughput proteomics, proteomic analysis of post-translationally modified (PTM) proteins remains particularly challenging. This mini-review introduces the emerging field of chemical proteomics and reviews recent advances in chemical proteomic technology that are offering striking new insights into the functional biology of post-translational modification.
Keywords: Post-translational modification, Phosphorylation, Glycosylation, Lipidation, Chemical proteomics, Site-specific protein labelling
Introduction
Identification and quantification of multiple proteins from complex mixtures is a central theme in post-genomic biology. Whilst high-throughput proteomics now enables identification and relative quantification of hundreds to thousands of proteins in a single experiment, significant barriers remain for the analysis of specific protein families of central importance in biology and medicine [1–3]. Proteomic analysis of post-translationally modified (PTM) proteins has proven particularly challenging due to the problems of identifying modified peptides by mass spectrometry and interference from the high background of unmodified material. Recent developments in the emerging fields of chemical biology and chemical proteomics [4–8] have resulted in techniques that either take advantage of the cell’s post-translational machinery or exploit subtle differences in the chemical or enzymatic reactivity of specific PTMs to incorporate a small chemical tag specifically at the site of modification. Exquisitely selective chemical reactions can then be used to introduce any combination of secondary labels that enable detection, manipulation and enrichment of proteins bearing a specific PTM [9, 10]. These unique applications of metabolic and protein engineering have opened up a wide range of applications in protein labelling, basic biology, biomarker discovery and drug discovery. This mini-review provides an overview of the techniques available for post-translational chemical proteomics and reviews a selection of key recent advances and applications.
Chemical proteomics: an overview
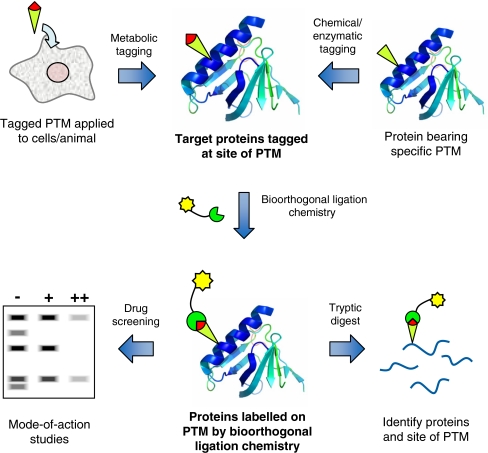
The principle technologies underlying post-translational chemical proteomics are shown in Fig. 1. The key unifying step is the generation of a protein labelled with a small chemical tag at the site of post-translational modification. A highly selective chemical reaction, termed a bioorthogonal ligation, is then performed between the chemical tag and a capture reagent to introduce one or more secondary label(s).
Fig. 1.
An overview of the techniques used for the exploration of PTM using chemical proteomics, and some examples of the downstream applications they enable
The methodology for the initial introduction of the chemical tag is tailored to the PTM of interest, exploiting either in vivo metabolic labelling with a tagged analogue of the PTM or selective (usually post-lysis) modification of a PTM by chemical or enzymatic means. During metabolic incorporation, the small and biologically inert chemical tag remains functionally silent, enabling transfer to the targets and maintaining the wild-type activity of modified proteins. The downstream bioorthogonal ligation technology is generic and remarkably robust, being based largely on the concept of the so-called click chemistry [9–14], a term for reactions between two biologically compatible functional groups that can occur under mild aqueous conditions that do not affect biomolecules. By taking full advantage of the power of organic synthesis, custom capture reagents have been created bearing affinity labels (biotin, His6, FLAG, etc.), reporter dyes, oligonucleotide tags, radiolabels and stable isotope labels. Typically, these labels are organised and separated by the strategic placement of flexible hydrophilic linkers to enhance aqueous solubility and accessibility towards avidin or antibodies. To enable facile and highly selective release after affinity purification, linkers have also been designed bearing an orthogonally cleavable moiety such as a protease cleavage site [15], periodate-cleavable cis-diol [16], reductively cleavable disulphide [17] or diazo-linker [18], or an acid-cleavable acetal [19]. With due regard to the mutual chemical compatibility of each of these functional groups, capture reagents may be designed that couple the advantages of multiple commonly used reporter and affinity purification technologies in a single system.
This chemical approach to post-translational proteomics leads to a unique combination of advantages and applications:
The underlying technology is post-genomic and, thus, is portable between different classes of organism, and it may be applied to profile PTMs both in cells in culture and in live animals [10, 20–23].
The introduction of a fluorescent label can permit direct fixed or live cell imaging of post-translational modification [24–26].
In contrast to traditional metabolic radiolabelling, chemical tagging can result in improvement in signal over background of approximately six orders of magnitude [27] and can provide a versatile handle with which to manipulate the modified proteins.
By modifying a specific member of a large kinase or transferase family so that it will accept a tagged analogue as substrate (also known as orthogonal chemical genetics), the activity and substrate profile of this enzyme may be monitored independently from its isozymes [28]. This approach is evolving into a standard technique in kinase biology [29].
The site of modification may be readily determined in concert with protein identification by virtue of the distinctive mass fingerprint of the tag or secondary label.
Chemical tagging offers superior selectivity and efficiency for the enrichment of PTM proteins when compared to the limited range of existing antibody or affinity column-based enrichment techniques.
Research programmes in a number of chemical biology research groups are aimed at improving and expanding the scope of chemical proteomics to encompass all of the features mentioned above and extending the range of PTMs that are amenable to study. For in vivo applications that involve metabolic engineering, research is ongoing to improve uptake and incorporation of tagged analogues and to enable facile application of bioorthogonal ligation inside living cells. In common with all metabolic labelling techniques, there are potential issues of toxicity and off-target effects that may be elicited by these compounds, although tagged analogues are generally very well tolerated by cells and whole organisms.
In the following sections, key advances in chemical proteomic technology for the analysis of glycosylation, prenylation, acylation and phosphorylation are reviewed. The reader is also referred to related chemical proteomic studies on post-translational sulphenation [30], S-nitrosation [31] and sumoylation [32, 33] that are not covered in detail in this mini-review.
Exploring the post-translational proteome
Glycosylation
Glycosylation was the first PTM to be studied by chemical proteomics [34]. Protein glycosylation plays a role in myriad cellular processes, most notably cellular recognition and signalling, but the enormous complexity and heterogeneity of glycosylation has made the comprehensive analysis of glycoproteins one of the most difficult challenges in post-translational proteomics. Although recent advances in tandem mass spectrometry have enabled the structure of complex polysaccharides to be determined [35], traditional approaches such as lectin or antibody-based affinity purification have met with limited success for the selective enrichment of proteins bearing a specific glycoside and do not assist PTM site identification [36–39]. The development of novel chemical approaches to this problem has, therefore, had a significant impact on our understanding of the functional biology of glycosylation, particularly for O-linked monosaccharide modification. Two distinct approaches have been used to date for the chemical proteomic study of glycosylation (Fig. 2).
Fig. 2.
Tagging/bioorthogonal labelling approach to chemical glycomics. Top metabolic engineering with tagged glycoside analogues, which may also include GalNAc, fucose and ManAc (see text). Bottom post-lysis enzymatic addition of a tagged Gal analogue at O-GlcNac sites using an engineered Gal transferase
In the first approach, pioneered by the group of Carolyn Bertozzi [34, 40, 41], azide- or alkyne-tagged monosaccharides are fed to cells in culture and metabolically incorporated into proteins via a series of in vivo transformations; these chemical tags may then be captured as described above to enable selective enrichment of proteins bearing the target glycoside. Remarkably, this technique may also be applied for the metabolic labelling of cell-surface proteins for engineered cell-surface remodelling and to the labelling of glycosylated proteins in living animals. O-linked glycans studied to date by this approach include those containing sialic acid via metabolism of a tagged N-acetylmannosamine (ManNAc) analogue [42–45], N-acetylgalactosamine (GalNAc) [46], N-acetylglucosamine (GlcNAc) [47–49] and fucose (Fuc) [45]. In the second approach, first reported by the group of Hsieh-Wilson [50, 51], an engineered galactosyltransferase is used to add a tagged glycoside to proteins bearing an O-linked N-acetylglucosamine (O-GlcNAc) PTM at Ser or Thr residues. The tag, a methyl ketone in this instance, can also be captured by bioorthogonal ligation chemistry to install a secondary label for downstream enrichment and analysis. Whilst this latter approach is currently limited to the analysis of O-GlcNAc modifications, it is performed directly on unmodified cell or tissue extracts, and so avoids any interference in the system due to metabolic labelling.
O-GlcNAc modification is modulated by glycosyl transferase and glycosidase activity in much the same way as phosphorylation is controlled by kinases and phosphatases and may play a similarly important role in signal transduction [52–57]. Indeed, phosphorylation of certain proteins can act as a negative regulator of glycosylation, and the protein glycoconjugate plays the biologically active role. Chemical proteomic studies have helped to reveal the role of O-linked GlcNAc, ManNAc and GalNAc mucin-like glycosylation in signalling [58–60] and have shown that chemical proteomic techniques may be used to detect dynamic changes in the glycosylation state of target proteins. Elegant applications of this technology for the direct visualisation of glycosylation in fixed and living cells by fluorescence microscopy also promise to shed light on glycosyl transferase activity and trafficking processes mediated by glycosylation [24–26].
Protein lipidation
Protein lipidation presents a challenge for proteomic analysis because these modifications are difficult to detect using traditional methods and carry limited functionality that could act as a handle for antibody-based recognition or for chemoselective or enzymatic tagging. However, significant progress has been made in the development of novel chemical tagging protocols, in particular for long-chain acylation and prenylation.
Acylation
Protein acylation with long-chain fatty acids plays an important role in mediating membrane association and is involved in the formation of lipid rafts and in signalling and trafficking processes in all eukaryotic cells [61–63]. The two best-studied forms of protein acylation are irreversible co-translational N-myristoylation at an N-terminal glycine residue and reversible post-translational S-palmitoylation at internal cysteine residues. N-myristoyltransferase (NMT) is a prominent drug target in a range of infectious diseases [64–66], and there is increasing evidence that NMT is also implicated in cancer [67, 68]. Palmitoyl transferases have only recently been identified in yeast and mammalian cells, being less amenable to study than NMT as a result of their membrane association and multiple overlapping specificities [69–74]. Techniques for studying the targets of long-chain protein acylation have previously been limited to radiolabelling and hydrophobic affinity chromatography, and it has proven impossible to raise reliable generic antibodies against these non-immunogenic motifs. The pressing need for new techniques in this area has galvanised research in several chemical biology groups, resulting in ‘chemical acylomic’ technologies that can enable whole-proteome isolation and identification of acylated proteins (Fig. 3).
Fig. 3.
Chemical proteomic techniques for the analysis of post-translational and co-translational myristoylation and palmitoylation. Top metabolic labelling with tagged myristic and palmitic acid analogues; in the case of myristoylation, the probe is linked via an amide to the N-terminal glycine (X = N, n = 10 or 11), whilst palmitate probes are linked via a thioester to an internal cysteine (X = S, n = 13). An analogous approach has been developed for prenylomics (see ‘Prenylation’). Bottom a universally applicable method for palmitomics of proteins bearing S-linked palmitoyl cysteine residues
In an analogous manner to chemical glycomics, two approaches have been pioneered for chemical acylomics. In the first, tagged myristic acid or palmitic acid analogues are fed to cells in culture where they are converted to their active acyl-CoA form by endogenous acyl-CoA synthase activity; metabolic incorporation enables subsequent capture for downstream enrichment and identification. One million-fold enhancement in signal is observed over comparable experiments with radiolabelled acids, and visualisation of acylated proteins on a membrane is possible in a matter of minutes as opposed to days or months with radiolabelling [27, 75–77]. Recent work in our laboratories has also shown for the first time that the activity and localisation of key downstream targets of NMT remains unaltered during metabolic labelling (Tate et al., unpublished observations). The second approach, applicable only to S-palmitoylation, the cysteine thioester to which the palmitate is attached acts as a latent tag. Performing a ‘biotin exchange’ protocol allows the introduction of a cysteine-linked biotinylated probe at sites formerly occupied by an S-palmitate group [78, 79]. By taking advantage of an orthogonally cleavable linker, this technique permitted the proteome-wide identification of dozens of novel post-translationally palmitoylated proteins in yeast and should be equally applicable to cells isolated from any organism. In combination with mutant yeast strains, the substrate specificity of several newly identified palmitoyl transferases could also be determined [78]. Although this exchange technique suffers from a relatively high background compared with metabolic labelling approaches [79], it is performed on cell isolates and, thus, there is no danger of affecting the system under study.
We have also demonstrated that chemical tagging of recombinant proteins can be a highly effective technique for site-specific N-terminal labelling with an efficiency that outstrips most currently available techniques [75]. Furthermore, we have shown that protein overexpression and tagging can be performed in a single procedure using readily available chemical tools (Fig. 4). This technology can be expected to have widespread applicability in a range of labelling and protein immobilisation/microarray applications.
Fig. 4.
In vivo tagging and N-terminal labelling of a recombinant protein by NMT in a bacterial coexpression system: a general method for the preparation of labelled proteins
Prenylation
Prenylation, the process of PTM of proteins with lipids of the poly-isoprene class (e.g. geranyl, farnesyl, geranylgeranyl), occurs predominantly at C-terminal cysteine residues and plays a central role in trafficking, membrane association and signalling, particularly in the Ras and Rab superfamilies [61, 80, 81]. Many of the transferases that catalyse protein prenylation in vivo are also under active development as drug targets in cancer and infectious disease [82–84]. Despite the importance of these PTMs, analysis of prenylation, like acylation, has been limited by low-specificity or low-efficiency techniques such as hydrophobic affinity chromatography and radiolabelling; furthermore, the site-specific identification of prenylation has been difficult to achieve due to the poor mass spectrometric characteristics of hydrophobic peptides [3]. Prenyl transferases are remarkably tolerant towards modifications in the prenyl pyrophosphate substrate [85, 86], and the development of chemical proteomic analysis for this PTM using tagged prenyl analogues was, therefore, a logical extension of the metabolic engineering approaches described above. Simply incorporating an azide moiety at the lipid terminus of farnesyl or geranylgeranyl alcohol provides a tagged analogue for metabolic labelling that is readily converted to the activated pyrophosphate form in vivo and subsequently transferred with high efficiency to target proteins without affecting cell viability in culture. To date, several groups have applied this concept for site-specific labelling of recombinant proteins [85, 87–89] and the Zhao group has also reported tagging and subsequent enrichment and analysis of farnesylated and geranylgeranylated proteins from cells in culture [90, 91]. Although chemical ‘prenylomics’ is currently not so developed as chemical glycomics, the pressing need to understand the substrate specificity of the various transferases at the whole-proteome level and how this may be altered by the action of inhibitors will undoubtedly spur further research in this area. For example, such an approach may enable the determination of selectivity and mode of action of putative drugs intended to target specific prenyl transferases and serve to elucidate disease pathways in hereditary retinal diseases involving defective prenyl transferase activity [92].
Phosphorylation
Protein phosphorylation is the most widely studied post-translational modification and yet it remains a challenging system for proteomic study [93]. Current techniques for the enrichment of phosphorylated peptides and proteins are relatively well-developed and are mainly based on various flavours of metal affinity chromatography. However, this system is plagued by non-specific binding, particularly by acidic proteins and peptides, and tends to favour enrichment of poly-phosphorylated species [3]. Enrichment of phosphotyrosine-containing proteins via monoclonal anti-phosphotyrosine antibodies has been a notable recent success in this area [94, 95]. However, non-sequence-specific antibodies towards small PTMs are extremely rare, as demonstrated in a recent study that isolated only a single anti-sulphotyrosine antibody from a large phage antibody library [96].
With a view to overcoming some of these limitations, several chemical proteomic strategies have been developed for phosphoproteomics, the most successful of which involve chemical modification of existing phosphorylated residues (Fig. 5).
Fig. 5.
Chemical proteomic techniques for the analysis of phosphorylation. Top phosphate β-elimination/addition at phosphoserine and threonine; note that prior oxidation of all free thiols (cysteines) to the corresponding sulphonic acid is required to avoid Michael addition by these residues. Bottom direct chemical modification of phosphorylated residues (serine, threonine or tyrosine) via phosphoramidate chemistry
In the first of these, base-mediated β-elimination of phosphate from phospho-Ser or Thr residues permits subsequent Michael addition of biotinylated probes at sites that were formerly phosphorylated [93, 97–99]. Although not applicable to tyrosine phosphorylation, this method has seen some success for the global analysis of phosphorylated proteins from Arabidopsis [97], although it suffers from a low but significant background of β-elimination from glycosylated and free Ser residues. In the second reported approach, a short series of reactions based on reversible phosphoramidate chemistry may be used to introduce a thiol at phosphorylated sites, which can then be used as a handle to introduce secondary labels [100–102]. This method has recently been elaborated for the identification of phosphorylated proteins in Drosophila melanogaster Kc167 cells [100] and has shown great promise for use in general chemical phosphoproteomics. In addition to these two methods, tentative progress has been reported in the analysis of phosphorylated proteins tagged metabolically with a γ-thiophosphate ATP analogue [103].
Perhaps the most exciting potential application for chemical phosphoproteomics is in combination with enzyme–substrate engineering. In this approach, the ATP binding site of a specific kinase is mutated such that it will additionally accept a bulky ATP analogue [28]. If a chemical tag is incorporated into this analogue at the gamma phosphate the protein substrates of the kinase will be tagged at the site of modification, enabling their enrichment and identification [29]. This metabolic/chemical genetic engineering technology would be a potent tool for the analysis of kinase networks because there is no alternative de novo method available to determine the targets of a single kinase against its homologues without resorting to pleiotropic kinase knockouts.
Outlook and future applications
As the numerous examples outlined above illustrate, chemical proteomics is a vibrant and fertile area of research at the interface between chemistry and biology. Recent applications of these technologies to real-world challenges in post-translational proteomics have helped to raise awareness amongst biologists of the potential power of chemical proteomics to enable the study of otherwise intractable systems. Research continues in a growing number of chemical biology groups to improve and widen the scope of chemical proteomics, in particular, recent and current work focuses on:
Application of chemical tagging via PTM as a general and site-specific method for labelling of recombinant proteins.
Overcoming the limitations imposed by the conditions for bioorthogonal ligation to enable general application for labelling in vivo [20, 104].
Improving the uptake and biocompatibility of tagged analogues for metabolic engineering.
Simplification and commercialisation of the requisite chemical reagents to promote increased availability of techniques.
Broadening the range of PTMs that may be studied, in particular to include smaller or less chemically accessible site-specific modifications such as methylation, acetylation, sulphation, oxidation and processing by proteases.
Potentially powerful application of these technologies that are likely to see further development in the near future include the use of chemical tagging for analysis of PTMs as biomarkers [4, 105, 106], determining the effects of transferase inhibitors on downstream target profiles in drug mode-of-action studies and as the basis for enzyme activity assays and screening programmes. Whilst this mini-review has focussed on PTM of proteins, it is worth noting that bioorthogonal ligation technology has also been used for the study of DNA modification [107–110] and activity-based protein profiling (ABPP) [111–113]. In particular, ABPP is a powerful counterpart to the methods described in this mini-review because it can be used to study the activity of enzymes involved in PTM of proteins [114, 115]. In combination, these techniques have the potential to present a full picture of PTM, from transferase to downstream targets, and enable direct observation of the effect of inhibitors or genetic modification on both these processes.
Despite the great progress made to date, probably the most significant barrier to the widespread adoption of chemical proteomic technologies is the unique combination of chemical and biological expertise that is required for its effective application in systems of biomedical importance. However, an increasing number of traditional biology groups are starting to use chemical proteomics in their work through fruitful collaboration with chemists and chemical biologists, and as methods become more reliable, we can expect to see the emergence of commercial kit-based protocols that will result in the acceptance of chemical proteomics as a standard tool for the study of PTMs.
Acknowledgments
The author thanks the Biotechnology and Biological Sciences Research Council (BBSRC), UK for the award of a David Phillips Research Fellowship.
References
- 1.Ahn NG, Shabb JB, Old WM, et al. Achieving in-depth proteomics profiling by mass spectrometry. ACS Chem Biol. 2007;2:39–52. doi: 10.1021/cb600357d. [DOI] [PubMed] [Google Scholar]
- 2.Kislinger T, Emili A. Multidimensional protein identification technology: current status and future prospects. Expert Rev Proteomics. 2005;2:27–39. doi: 10.1586/14789450.2.1.27. [DOI] [PubMed] [Google Scholar]
- 3.Reinders J, Lewandrowski U, Moebius J, et al. Challenges in mass spectrometry-based proteomics. Proteomics. 2004;4:3686–3703. doi: 10.1002/pmic.200400869. [DOI] [PubMed] [Google Scholar]
- 4.Berger AB, Vitorino PM, Bogyo M. Activity-based protein profiling: applications to biomarker discovery, in vivo imaging and drug discovery. Am J Pharmacogenomics. 2004;4:371–381. doi: 10.2165/00129785-200404060-00004. [DOI] [PubMed] [Google Scholar]
- 5.Daub H. Characterisation of kinase-selective inhibitors by chemical proteomics. Biochim Biophys Acta. 2005;1754:183–190. doi: 10.1016/j.bbapap.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery DA, Bogyo M. Chemical proteomics and its application to drug discovery. Curr Opin Biotechnol. 2003;14:87–95. doi: 10.1016/s0958-1669(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 7.Sem DS. Chemical proteomics from a nuclear magnetic resonance spectroscopy perspective. Expert Rev Proteomics. 2004;1:165–178. doi: 10.1586/14789450.1.2.165. [DOI] [PubMed] [Google Scholar]
- 8.Sieber SA, Cravatt BF. Analytical platforms for activity-based protein profiling—exploiting the versatility of chemistry for functional proteomics. Chem Commun (Camb) 2006;22:2311–2319. doi: 10.1039/b600653c. [DOI] [PubMed] [Google Scholar]
- 9.Agard NJ, Baskin JM, Prescher JA, et al. A comparative study of bioorthogonal reactions with azides. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 10.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 11.Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat Chem Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 12.Swieten PF, Leeuwenburgh MA, Kessler BM, et al. Bioorthogonal organic chemistry in living cells: novel strategies for labeling biomolecules. Org Biomol Chem. 2005;3:20–27. doi: 10.1039/b412558d. [DOI] [PubMed] [Google Scholar]
- 13.Sen Gupta S, Kuzelka J, Singh P, et al. Accelerated bioorthogonal conjugation: a practical method for the ligation of diverse functional molecules to a polyvalent virus scaffold. Bioconjug Chem. 2005;16:1572–1579. doi: 10.1021/bc050147l. [DOI] [PubMed] [Google Scholar]
- 14.Kohn M, Breinbauer R. The Staudinger ligation—a gift to chemical biology. Angew Chem Int Ed Engl. 2004;43:3106–3116. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]
- 15.Speers AE, Cravatt BF. A tandem orthogonal proteolysis strategy for high-content chemical proteomics. J Am Chem Soc. 2005;127:10018–10019. doi: 10.1021/ja0532842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melnyk O, Fruchart JS, Grandjean C, et al. Tartric acid-based linker for the solid-phase synthesis of C-terminal peptide alpha-oxo aldehydes. J Org Chem. 2001;66:4153–4160. doi: 10.1021/jo001509f. [DOI] [PubMed] [Google Scholar]
- 17.Gartner CA, Elias JE, Bakalarski CE, et al. Catch-and-release reagents for broadscale quantitative proteomics analyses. J Proteome Res. 2007;6:1482–1491. doi: 10.1021/pr060605f. [DOI] [PubMed] [Google Scholar]
- 18.Verhelst SH, Fonovic M, Bogyo M. A mild chemically cleavable linker system for functional proteomic applications. Angew Chem Int Ed Engl. 2007;46:1284–1286. doi: 10.1002/anie.200603811. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasachar K, Neville DM. New protein cross-linking reagents that are cleaved by mild acid. Biochemistry. 1989;28:2501–2509. doi: 10.1021/bi00432a023. [DOI] [PubMed] [Google Scholar]
- 20.Baskin JM, Prescher JA, Laughlin ST, et al. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang KP, Niessen S, Saghatelian A, et al. An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling. Chem Biol. 2006;13:1041–1050. doi: 10.1016/j.chembiol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Dube DH, Prescher JA, Quang CN, et al. Probing mucin-type O-linked glycosylation in living animals. Proc Natl Acad Sci U S A. 2006;103:4819–4824. doi: 10.1073/pnas.0506855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescher JA, Dube DH, Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 24.Chang PV, Prescher JA, Hangauer MJ, et al. Imaging cell surface glycans with bioorthogonal chemical reporters. J Am Chem Soc. 2007;129:8400–8401. doi: 10.1021/ja070238o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemieux GA, Graffenried CL, Bertozzi CR. A fluorogenic dye activated by the staudinger ligation. J Am Chem Soc. 2003;125:4708–4709. doi: 10.1021/ja029013y. [DOI] [PubMed] [Google Scholar]
- 26.Sawa M, Hsu TL, Itoh T, et al. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin DD, Vilas GL, Prescher JA, et al. Rapid detection, discovery, and identification of post-translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. FASEB J. 2007;22:797–806. doi: 10.1096/fj.07-9198com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alaimo PJ, Shogren-Knaak MA, Shokat KM. Chemical genetic approaches for the elucidation of signaling pathways. Curr Opin Chem Biol. 2001;5:360–367. doi: 10.1016/s1367-5931(00)00215-5. [DOI] [PubMed] [Google Scholar]
- 29.Elphick LM, Lee SE, Gouverneur V, et al. Using chemical genetics and ATP analogues to dissect protein kinase function. ACS Chem Biol. 2007;2:299–314. doi: 10.1021/cb700027u. [DOI] [PubMed] [Google Scholar]
- 30.Charles RL, Schroder E, May G, et al. Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Ruiz A, Lamas S. Proteomic identification of S-nitrosylated proteins in endothelial cells. Methods Mol Biol. 2007;357:215–223. doi: 10.1385/1-59745-214-9:215. [DOI] [PubMed] [Google Scholar]
- 32.Ganesan AK, Kho Y, Kim SC, et al. Broad spectrum identification of SUMO substrates in melanoma cells. Proteomics. 2007;7:2216–2221. doi: 10.1002/pmic.200600971. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Kwon SW, Anselmo A, et al. Broad spectrum identification of cellular small ubiquitin-related modifier (SUMO) substrate proteins. J Biol Chem. 2004;279:20999–21002. doi: 10.1074/jbc.M401541200. [DOI] [PubMed] [Google Scholar]
- 34.Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 35.Parry S, Ledger V, Tissot B, et al. Integrated mass spectrometric strategy for characterizing the glycans from glycosphingolipids and glycoproteins: direct identification of sialyl Le(x) in mice. Glycobiology. 2007;17:646–654. doi: 10.1093/glycob/cwm024. [DOI] [PubMed] [Google Scholar]
- 36.Comer FI, Vosseller K, Wells L, et al. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 37.Haltiwanger RS, Kelly WG, Roquemore EP, et al. Glycosylation of nuclear and cytoplasmic proteins is ubiquitous and dynamic. Biochem Soc Trans. 1992;20:264–269. doi: 10.1042/bst0200264. [DOI] [PubMed] [Google Scholar]
- 38.Yarema KJ, Bertozzi CR. Characterizing glycosylation pathways. Genome Biol. 2001;2:REVIEWS0004. doi: 10.1186/gb-2001-2-5-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaltgrad E, Sen Gupta S, Punna S, et al. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBioChem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 40.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 41.Saxon E, Bertozzi CR. Chemical and biological strategies for engineering cell surface glycosylation. Annu Rev Cell Dev Biol. 2001;17:1–23. doi: 10.1146/annurev.cellbio.17.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Luchansky SJ, Argade S, Hayes BK, et al. Metabolic functionalization of recombinant glycoproteins. Biochemistry. 2004;43:12358–12366. doi: 10.1021/bi049274f. [DOI] [PubMed] [Google Scholar]
- 43.Bussink AP, Swieten PF, Ghauharali K, et al. N-azidoacetylmannosamine-mediated chemical tagging of gangliosides. J Lipid Res. 2007;48:1417–1421. doi: 10.1194/jlr.C700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Laughlin ST, Bertozzi CR. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. Nat Protoc. 2007;2:2930–2944. doi: 10.1038/nprot.2007.422. [DOI] [PubMed] [Google Scholar]
- 45.Hsu TL, Hanson SR, Kishikawa K, et al. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci U S A. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hang HC, Yu C, Kato DL, et al. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc Natl Acad Sci U S A. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vocadlo DJ, Hang HC, Kim EJ, et al. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci U S A. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprung R, Nandi A, Chen Y, et al. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J Proteome Res. 2005;4:950–957. doi: 10.1021/pr050033j. [DOI] [PubMed] [Google Scholar]
- 49.Nandi A, Sprung R, Barma DK, et al. Global identification of O-GlcNAc-modified proteins. Anal Chem. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- 50.Khidekel N, Arndt S, Lamarre-Vincent N, et al. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc. 2003;125:16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 51.Khidekel N, Ficarro SB, Peters EC, et al. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci U S A. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 55.Guinez C, Morelle W, Michalski JC, et al. O-GlcNAc glycosylation: a signal for the nuclear transport of cytosolic proteins? Int J Biochem Cell Biol. 2005;37:765–774. doi: 10.1016/j.biocel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Wells L, Whelan SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–441. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- 57.Hart GW, Greis KD, Dong LY, et al. O-linked N-acetylglucosamine: the “yin–yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- 58.Gama CI, Hsieh-Wilson LC. Chemical approaches to deciphering the glycosaminoglycan code. Curr Opin Chem Biol. 2005;9:609–619. doi: 10.1016/j.cbpa.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Khidekel N, Ficarro SB, Clark PM, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3:339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 60.Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol. 2008;4:97–106. doi: 10.1038/nchembio.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pechlivanis M, Kuhlmann J. Hydrophobic modifications of Ras proteins by isoprenoid groups and fatty acids—more than just membrane anchoring. Biochim Biophys Acta. 2006;1764:1914–1931. doi: 10.1016/j.bbapap.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 63.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 64.Price HP, Menon MR, Panethymitaki C, et al. Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J Biol Chem. 2003;278:7206–7214. doi: 10.1074/jbc.M211391200. [DOI] [PubMed] [Google Scholar]
- 65.Takamune N, Hamada H, Misumi S, et al. Novel strategy for anti-HIV-1 action: selective cytotoxic effect of N-myristoyltransferase inhibitor on HIV-1-infected cells. FEBS Lett. 2002;527:138–142. doi: 10.1016/s0014-5793(02)03199-x. [DOI] [PubMed] [Google Scholar]
- 66.Georgopapadakou NH. Antifungals targeted to protein modification: focus on protein N-myristoyltransferase. Expert Opin Investig Drugs. 2002;11:1117–1125. doi: 10.1517/13543784.11.8.1117. [DOI] [PubMed] [Google Scholar]
- 67.Ducker CE, Upson JJ, French KJ, et al. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol Cancer Res. 2005;3:463–476. doi: 10.1158/1541-7786.MCR-05-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrivastav A, Pasha MK, Selvakumar P, et al. Potent inhibitor of N-myristoylation: a novel molecular target for cancer. Cancer Res. 2003;63:7975–7978. [PubMed] [Google Scholar]
- 69.Budde C, Schoenfish MJ, Linder ME, et al. Purification and characterization of recombinant protein acyltransferases. Methods. 2006;40:143–150. doi: 10.1016/j.ymeth.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell DA, Vasudevan A, Linder ME, et al. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Lam KK, Davey M, Sun B, et al. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J Cell Biol. 2006;174:19–25. doi: 10.1083/jcb.200602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods. 2006;40:177–182. doi: 10.1016/j.ymeth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Ducker CE, Draper JM, Xia Z, et al. In vitro and cellular assays for palmitoyl acyltransferases using fluorescent lipidated peptides. Methods. 2006;40:166–170. doi: 10.1016/j.ymeth.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roth AF, Feng Y, Chen L, et al. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heal WP, Wickramasinghe SR, Bowyer PW, et al. Site-specific N-terminal labelling of proteins in vitro and in vivo using N-myristoyl transferase and bioorthogonal ligation chemistry. Chem Commun (Camb) 2008;4:480–482. doi: 10.1039/b716115h. [DOI] [PubMed] [Google Scholar]
- 76.Kostiuk MA, Corvi MM, Keller BO, et al. Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J. 2007;22:721–732. doi: 10.1096/fj.07-9199com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hang HC, Geutjes EJ, Grotenbreg G, et al. Chemical probes for the rapid detection of fatty-acylated proteins in Mammalian cells. J Am Chem Soc. 2007;129:2744–2745. doi: 10.1021/ja0685001. [DOI] [PubMed] [Google Scholar]
- 78.Roth AF, Wan J, Bailey AO, et al. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roth AF, Wan J, Green WN, et al. Proteomic identification of palmitoylated proteins. Methods. 2006;40:135–142. doi: 10.1016/j.ymeth.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 81.Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. Geranylgeranylation of Rab GTPases. J Lipid Res. 2006;47:467–475. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Flotho C, Kratz C, Niemeyer CM. Targeting RAS signaling pathways in juvenile myelomonocytic leukemia. Curr Drug Targets. 2007;8:715–725. doi: 10.2174/138945007780830773. [DOI] [PubMed] [Google Scholar]
- 83.Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7:297–300. doi: 10.1016/j.ccr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen UT, Cramer J, Gomis J, et al. Exploiting the substrate tolerance of farnesyltransferase for site-selective protein derivatization. ChemBioChem. 2007;8:408–423. doi: 10.1002/cbic.200600440. [DOI] [PubMed] [Google Scholar]
- 86.Dursina B, Reents R, Delon C, et al. Identification and specificity profiling of protein prenyltransferase inhibitors using new fluorescent phosphoisoprenoids. J Am Chem Soc. 2006;128:2822–2835. doi: 10.1021/ja052196e. [DOI] [PubMed] [Google Scholar]
- 87.Rose MW, Xu J, Kale TA, et al. Enzymatic incorporation of orthogonally reactive prenylazide groups into peptides using geranylazide diphosphate via protein farnesyltransferase: implications for selective protein labeling. Biopolymers. 2005;80:164–171. doi: 10.1002/bip.20239. [DOI] [PubMed] [Google Scholar]
- 88.Duckworth BP, Chen Y, Wollack JW, et al. A universal method for the preparation of covalent protein–DNA conjugates for use in creating protein nanostructures. Angew Chem Int Ed Engl. 2007;46:8819–8822. doi: 10.1002/anie.200701942. [DOI] [PubMed] [Google Scholar]
- 89.Duckworth BP, Xu J, Taton TA, et al. Site-specific, covalent attachment of proteins to a solid surface. Bioconjug Chem. 2006;17:967–974. doi: 10.1021/bc060125e. [DOI] [PubMed] [Google Scholar]
- 90.Chan Kim S, Kho Y, Barma D, et al. A tagging-via-substrate technology for genome-wide detection and identification of farnesylated proteins. Methods Enzymol. 2005;407:629–637. doi: 10.1016/S0076-6879(05)07049-7. [DOI] [PubMed] [Google Scholar]
- 91.Kho Y, Kim SC, Jiang C, et al. A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc Natl Acad Sci U S A. 2004;101:12479–12484. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larijani B, Hume AN, Tarafder AK, et al. Multiple factors contribute to inefficient prenylation of Rab27a in Rab prenylation diseases. J Biol Chem. 2003;278:46798–46804. doi: 10.1074/jbc.M307799200. [DOI] [PubMed] [Google Scholar]
- 93.Hjerrild M, Gammeltoft S. Phosphoproteomics toolbox: computational biology, protein chemistry and mass spectrometry. FEBS Lett. 2006;580:4764–4770. doi: 10.1016/j.febslet.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 94.Ding SJ, Qian WJ, Smith RD. Quantitative proteomic approaches for studying phosphotyrosine signaling. Expert Rev Proteomics. 2007;4:13–23. doi: 10.1586/14789450.4.1.13. [DOI] [PubMed] [Google Scholar]
- 95.Liang F, Kumar S, Zhang ZY. Proteomic approaches to studying protein tyrosine phosphatases. Mol Biosyst. 2007;3:308–316. doi: 10.1039/b700704n. [DOI] [PubMed] [Google Scholar]
- 96.Kehoe JW, Velappan N, Walbolt M, et al. Using phage display to select antibodies recognizing post-translational modifications independently of sequence context. Mol Cell Proteomics. 2006;5:2350–2363. doi: 10.1074/mcp.M600314-MCP200. [DOI] [PubMed] [Google Scholar]
- 97.Kwon SJ, Choi EY, Seo JB, et al. Isolation of the Arabidopsis phosphoproteome using a biotin-tagging approach. Mol Cells. 2007;24:268–275. [PubMed] [Google Scholar]
- 98.Leitner A, Lindner W. Chemistry meets proteomics: the use of chemical tagging reactions for MS-based proteomics. Proteomics. 2006;6:5418–5434. doi: 10.1002/pmic.200600255. [DOI] [PubMed] [Google Scholar]
- 99.Oda Y, Nagasu T, Chait BT. Enrichment analysis of phosphorylated proteins as a tool for probing the phosphoproteome. Nat Biotechnol. 2001;19:379–382. doi: 10.1038/86783. [DOI] [PubMed] [Google Scholar]
- 100.Bodenmiller B, Mueller LN, Pedrioli PG, et al. An integrated chemical, mass spectrometric and computational strategy for (quantitative) phosphoproteomics: application to Drosophila melanogaster Kc167 cells. Mol Biosyst. 2007;3:275–286. doi: 10.1039/b617545g. [DOI] [PubMed] [Google Scholar]
- 101.Warthaka M, Karwowska-Desaulniers P, Pflum MK. Phosphopeptide modification and enrichment by oxidation-reduction condensation. ACS Chem Biol. 2006;1:697–701. doi: 10.1021/cb6003564. [DOI] [PubMed] [Google Scholar]
- 102.Zhou H, Watts JD, Aebersold R. A systematic approach to the analysis of protein phosphorylation. Nat Biotechnol. 2001;19:375–378. doi: 10.1038/86777. [DOI] [PubMed] [Google Scholar]
- 103.Kwon SW, Kim SC, Jaunbergs J, et al. Selective enrichment of thiophosphorylated polypeptides as a tool for the analysis of protein phosphorylation. Mol Cell Proteomics. 2003;2:242–247. doi: 10.1074/mcp.M300039-MCP200. [DOI] [PubMed] [Google Scholar]
- 104.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide–alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 105.Bond MR, Kohler JJ. Chemical methods for glycoprotein discovery. Curr Opin Chem Biol. 2007;11:52–58. doi: 10.1016/j.cbpa.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 106.Ferri N, Paoletti R, Corsini A. Lipid-modified proteins as biomarkers for cardiovascular disease: a review. Biomarkers. 2005;10:219–237. doi: 10.1080/13547500500216660. [DOI] [PubMed] [Google Scholar]
- 107.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 108.Burley GA, Gierlich J, Mofid MR, et al. Directed DNA metallization. J Am Chem Soc. 2006;128:1398–1399. doi: 10.1021/ja055517v. [DOI] [PubMed] [Google Scholar]
- 109.Kumar R, El-Sagheer A, Tumpane J, et al. Template-directed oligonucleotide strand ligation, covalent intramolecular DNA circularization and catenation using click chemistry. J Am Chem Soc. 2007;129:6859–6864. doi: 10.1021/ja070273v. [DOI] [PubMed] [Google Scholar]
- 110.Moorhouse AD, Santos AM, Gunaratnam M, et al. Stabilization of G-quadruplex DNA by highly selective ligands via click chemistry. J Am Chem Soc. 2006;128:15972–15973. doi: 10.1021/ja0661919. [DOI] [PubMed] [Google Scholar]
- 111.Sadaghiani AM, Verhelst SH, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr Opin Chem Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 112.Weerapana E, Speers AE, Cravatt BF. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)—a general method for mapping sites of probe modification in proteomes. Nat Protoc. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]
- 113.Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 114.Kumar S, Zhou B, Liang F, et al. Activity-based probes for protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2004;101:7943–7948. doi: 10.1073/pnas.0402323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yee MC, Fas SC, Stohlmeyer MM, et al. A cell-permeable, activity-based probe for protein and lipid kinases. J Biol Chem. 2005;280:29053–29059. doi: 10.1074/jbc.M504730200. [DOI] [PubMed] [Google Scholar]