Abstract
BACKGROUND
Companion studies using an experimental non-human primate paradigm known as a testicular clamp indicated that the behavior of undifferentiated type A spermatogonia did not conform fully to earlier classical models. This issue was therefore re-examined in normal monkeys.
METHODS
Adult male rhesus monkeys (n = 4) received an i.v. bolus of 5-bromo-2′-deoxyuridine (BrdU): one testis (first) was removed 3 h later and the remaining testis (second) was removed after 11 days and 3 h. Tissue was fixed in Bouin’s solution, and numbers of A dark (Ad), small A pale (Aps) and large A pale spermatogonia, differentiating B spermatogonia, S-phase-labeled and degenerating cells were enumerated. Data are given as mean ± SEM.
RESULTS
During the early stages of the seminiferous epithelial cycle in the first testis, Ap spermatogonia (1.3 cells/cross section) were predominantly Aps (nuclear dia., 7.1 ± 0.1 µm). Aps were never S-phase labeled. Apl (nuclear dia., 8.8 ± 0.5 µm) appeared in Stages IV–VI and were maximal in Stages VII–X when S-phase labeling of this phenotype at 3 h was greatest. The first generation of B spermatogonia appeared in Stages XI–XII (0.84 cells/cross section). Using cells/cross section, the ratio of Ap (Stages I–V):B1:B2:B3:B4:preleptotene spermatocyte was 1:0.7:1.4:2.8:5.6:11.2. In the second testis, labeled Aps (and Apl) were observed. Ad were not BrdU labeled, and degenerating cells were rarely observed.
CONCLUSIONS
The results are not entirely consistent with earlier models of spermatogonial proliferation and differentiation in the monkey. Most notably, our findings suggest that in any one cycle of the seminiferous epithelium only a fraction of Ap spermatogonia is mitotically active.
Keywords: spermatogenesis, primate, undifferentiated spermatogonia, differentiated spermatogonia, testis
Introduction
In man and non-human primates, two distinct types of undifferentiated spermatogonia are generally recognized: dark type A (Ad) and pale type A (Ap) (Clermont and Leblond, 1959; Clermont, 1966, 1969; Clermont and Antar, 1973). Ad spermatogonia have a small, spherical nucleus that, in 5 µm sections of Bouin’s fixed hematoxylin-stained tissue, exhibits dark, dense and homogenous chromatin. The nuclear envelope is generally separated from the chromatin in a clear ‘retraction’ zone (Simorangkir et al., 2005). Ap spermatogonia, on the other hand, typically contain an ovoid and lightly stained nucleus with course granular chromatin; a retraction zone is not observed (Simorangkir et al., 2005). In the adult testis, Ad have been observed to divide only rarely (Clermont, 1972; Clermont and Antar, 1973; Kluin et al., 1983; Fouquet and Dadoune, 1986) and this cell type has thus been referred to as a ‘reserve’ stem cell. In contrast, Ap proliferate extensively and accordingly have been considered as a ‘renewing’ stem cell (Clermont and Antar, 1973). On the basis of earlier studies of the rhesus (Macaca mulatta) and green (Cercopithecus aethiops) monkey, Clermont’s laboratory proposed that, at Stage IX of the seminiferous epithelial cycle, the entire population of Ap divide; half give rise to the first generation of differentiated B spermatogonia and half self-renew (Clermont and Leblond, 1959; Clermont, 1969). In the stump-tail monkey, Macaca arctoides, on the other hand, the same laboratory (Clermont and Antar, 1973) later proposed that during Stages VII–VIII all Ap spermatogonia divide to double their number: two stages later, half of the new generation of Ap divide to give rise to B1 spermatogonia, while the other half remain as Ap (renewal) until Stages VII–VIII of the next cycle when the process is repeated. More recently, Schlatt and co-workers have argued that Ap renewal in the rhesus monkey occurs in the same manner as that described for the stump-tail monkey (Ehmcke et al., 2005a, b).
In studies by this laboratory, in which we examined the action of a selective increment in either pulsatile LH or FSH stimulation to amplify spermatogenesis in the adult rhesus monkey, we found that the behavior of Ap spermatogonia did not conform entirely to either of the Clermont models (Simorangkir et al., 2009). For the latter experiments, the testes were clamped by abolishing endogenous gonadotrophin secretion with a GnRH receptor antagonist and replacing the LH and FSH drive to the testis with an invariant intermittent infusion of recombinant human gonadotrophins. Because of the endocrine perturbations of this experimental design, we felt that it was important to report the behavior of Ap spermatogonia in the testis of normal untreated adult monkeys. Our findings are reported here.
Materials and Methods
Animals
Four adult male rhesus monkeys (age 6–12 years; body weight 9.5–13.5 kg) were used. The monkeys were maintained in accordance with National Institutes of Health Guidelines for the Care and Use of Experimental Animals, and the experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Experimental design
In order to label type A spermatogonia (and other germ cells) in S-phase, an i.v. bolus of 5-bromo-2′-deoxyuridine [BrdU, Sigma Chemical Co., St. Louis, MO, USA; 33 mg/kg body weight as a 2% solution in phosphate-buffered saline (PBS)] was administered while the animals were lightly sedated with ketamine hydrochloride (∼20 mg/kg body weight i.m.; Ketaset, Fort Dodge Laboratories, Fort Dodge, IA, USA). Three hours later, the monkeys were unilaterally orchidectomized using sterile technique after sedation with ketamine hydrochloride (∼20 mg/kg body weight) and the induction of anesthesia by inhalation of 1.5–2.5% isoflurane in oxygen. Two left and two right testes were obtained at this time, and these are referred to as the first testis. The remaining testis (second) was removed 11 days and 3 h later using the same surgical procedure described for the first testis. The rationale for the interval between the first and second castration was based on the available data for the monkey on the duration of the seminiferous epithelial cycle, which has been reported to range between 10.4 and 11.6 days (Clermont and Antar, 1973; de Rooij et al., 1986; Fouquet and Dadoune, 1986; Rosiepen et al., 1997). The 11 days guaranteed that the second testis would have passed through one complete seminiferous epithelial cycle before removal. Post-operatively, each monkey received antibiotic and analgesics as appropriate. Testes were weighed, sliced and cut into small fragments.
For the purpose of this study, fragments representative of the entire testis were fixed overnight in Bouin’s solution, and subsequently stored in 70% ethanol until further processing for paraffin embedding. Only the first testes were analyzed quantitatively, because unilateral castration in the adult rhesus monkey leads to an increase in FSH secretion and compensatory stimulation of the germ cell number in the remaining testis (Ramaswamy et al., 2000). The second testes were used to qualitatively determine the fate of the S-phase cells after one cycle of the seminiferous epithelium. It is to be noted that paraffin embedded testicular tissue from these four monkeys was provided to the laboratory of Dr. Stefan Schlatt for an independent study that has been previously reported (Ehmcke et al., 2005a).
Morphometric analysis
Cell identification and quantification
Morphometric analyses were performed as described previously (Marshall and Plant, 1996; Simorangkir et al., 2005). Five 5-µm sections taken from several paraffin blocks were stained with periodic acid–Schiff (PAS) reagent/hematoxylin. Volume fraction of the seminiferous tubules was determined by the point counting method and used to calculate the total volume of seminiferous tubules per testis. The total length of the seminiferous tubules per testis was calculated using the cross-sectional area, determined by measuring the diameter of 20 seminiferous tubules (using Bioquant software). With the exception of the Sertoli cells, cell number per cross section was determined by counting the number of nuclei of a particular cell type from ∼66 to 106 circular profiles of the seminiferous tubules (from four to six different blocks), systematically across the whole section. Because of the irregularity of the Sertoli cell nuclei, the number of Sertoli cell nucleoli was counted when enumerating this cell type, and for Abercrombie’s correction, nucleoli diameter was used instead of nuclear diameter. For each cross section of seminiferous tubule examined, the stage of the seminiferous epithelial cycle was recorded (Stages I–XII, according to Clermont and Antar, 1973). As previously described by this laboratory in studies of the prepubertal rhesus testis, undifferentiated type A spermatogonia were classified as type Ad, type Ap and type A unclassified (Aunc). Type Aunc could not be unequivocally classified as either type Ad or Ap and they shared some features (a thin nuclear envelope unassociated with heterochromatin and a retraction zone) with type Ad. In contrast to type Ad, however, nuclear size of Aunc was larger and the chromatin was less dense and less homogeneous. Here it is to be noted that in a previous study of the cynomolgus monkey, Fouquet and Dadoune (1986) reported type A spermatogonia that were neither typically Ad nor Ap and referred to them as transition Type A (At). Based primarily on nuclear size, type Ap were further divided into Ap small (Aps) and Ap large (Apl), as described previously (Simorangkir et al., 2005). Differentiated spermatogonia were classified as B1, B2, B3 and B4 as originally reported by Clermont and Antar (1973). The number of cells per cross section was analyzed before and after correction using the Abercrombie formula (Abercrombie, 1946). The number of germ cells per 100 Sertoli cells for a given stage was obtained by dividing the number of a particular germ cell per cross section by the number of Sertoli cells per cross section for the respective stage, and multiplying by 100. The total number of germ cells per testis was derived by multiplying the average of the corrected number of cells per cross section by total length of the seminiferous cord and dividing by thickness of the histological section. The enumeration of cell numbers in testes from normal adult monkeys (present study) was conducted at the same time as cell counts were conducted by one of us (D.R.S.) on the testis from the three groups of testicular clamps described in the companion paper (Simorangkir et al., 2009). D.R.S. was blinded to the identity of the four groups during this evaluation.
S-phase labeling index
BrdU was immunocytochemically identified using a modification of the method described by Simorangkir et al. (2005). For this purpose, two to four sections from a testis of each monkey were deparaffinized in xylene and treated with decreasing concentrations of alcohol, followed by washing in PBS. Sections were then incubated overnight at 4°C with an anti-BrdU antibody (mouse immunoglobulin G monoclonal antibody, Roche Applied Science, Indianapolis, IN, USA) diluted 1:33 in 50 mM PBS containing 0.05% Triton and 1% normal horse serum. After several rinses with PBS, sections were incubated at room temperature with biotinylated horse antimouse antiserum (Vector Laboratories Inc., Burlingame, CA, USA) diluted 1:200 in PBS/Triton and 1% normal horse serum for 1 h. After rinsing, sections were placed in a solution of an avidin–horseradish peroxidase complex (Vectastain ABC Elite Kit, Vector Laboratories Inc., Burlingame, CA, USA) for 1 h at room temperature. Horseradish peroxidase was visualized with 3,3′-diaminobenzidine (SigmaFast DAB/Cobalt, Sigma Chemical Co.). Dark brown precipitate indicated the presence of BrdU. Sections were then counterstained using PAS reagent and hematoxylin. A labeling index (LI) was determined by counting the number of a specific cell type labeled with BrdU, and dividing by the total number of the corresponding cell. For this purpose, each section was examined by moving the microscope stage in a systematic pattern that ensured that seminiferous cords were evaluated only once. As a cross section with a circular profile of a tubule came into the field, each cell nucleus was counted and scored for BrdU labeling. For a given cell type, a total of 1053–1299 cells per testis was evaluated and corresponding stages of the sections were also recorded.
Degenerating germ cell index
The number of degenerating germ cells on or close to the basement membrane was enumerated for each monkey in a 5-µm section stained with PAS reagent/Gill’s hematoxylin. Degenerating germ cells were identified by an accumulation of condensed chromatin at the periphery of the nucleus, which in many cases appeared shrunken (Fig. 1). This distribution of chromatin resulted in a ground glass appearance of material in the middle of the nucleus. Occasionally, degenerating cells were recognized by frankly pyknotic or karyolytic nuclei, and were frequently seen in a close apposition to one another or as a coalescence of three or four cells (Fig. 1). A degenerating index was determined by counting the number of degenerating profiles on the basement membrane using an oil objective lens (100×) coupled with a reticulated ocular lens providing a field of 33 600 µm2. Typically, 200–300 germ cells were observed in a single field. One thousand or more fields were examined for each monkey. When degenerating cells were found, the stage of the seminiferous epithelial cycle was recorded. The degenerating index for each animal was determined by dividing the number of degenerating cells by the number of fields observed and multiplying by 100. The index was expressed as the number of degenerating cells per 100 fields, throughout the 12 stages of the seminiferous cycle.
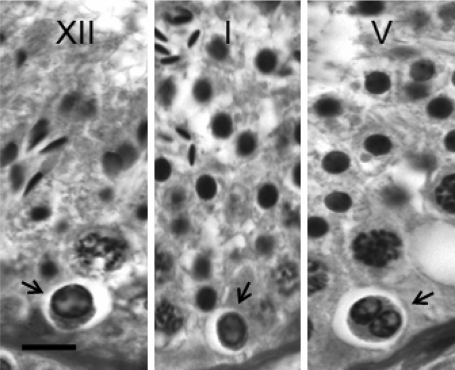
Figure 1.
Photomicrographs of degenerating spermatogonia on the basement membrane of testis from adult rhesus monkeys are shown (arrow) in Stages XII (left), I (middle) and V (right) of the seminiferous epithelial cycle.
Degenerating spermatogonia were typically seen as cells with a small nucleus, characterized by condensed chromatin accumulated at the inner surface of the nuclear membrane surrounding a ground glass-like material. These cells may appear either as solitary (left and middle panel), chains or a coalescence of two or more cells, as shown in the right panel. Bar = 10 µm.
Statistical analysis
Data are expressed as mean ± SE. The significance of differences between means across stages of the seminiferous epithelial cycle was determined using one-way analysis of variance; P ≤ 0.05 was considered significant.
Results
The mean (±SE) volume (testis weight divided by the specific gravity of adult rhesus monkey’s testis tissue, Marshall and Plant, 1996) of the first testes was 22.0 ± 2.2 cm3. The mean diameter, volume and length of seminiferous tubule was 169.3 ± 7.5 µm, 18.1 ± 1.9 cm3 and 804.4 ± 66.1 m, respectively.
In circular cross sections of seminiferous tubule (66–106 per animal), cellular associations indicative of Stages I–XII of the seminiferous epithelial cycle of the monkey were observed on average in 7.4 (I), 7.1 (II), 6.5 (III), 5.9 (IV), 8.3 (V), 9.3 (VI), 15.7 (VII), 9.3 (VIII), 6.8 (IX), 7.7 (X), 6.8 (XI) and 9.3% (XII), respectively, of the cross sections examined.
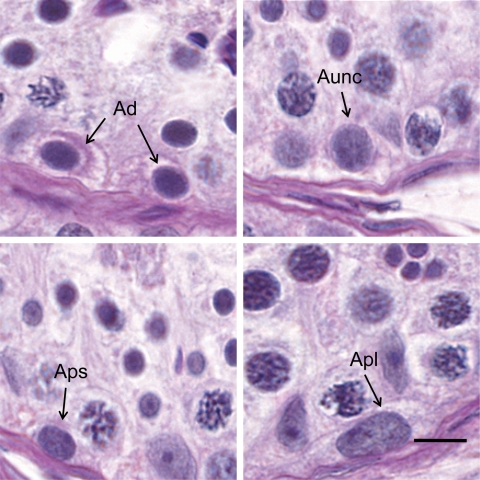
As previously reported (Simorangkir et al., 2005), not all undifferentiated type A spermatogonia could be unambiguously identified as Ad or Ap and these cells were again termed Aunc. Ad were small cells with a spherical and darkly stained nucleus containing dense and homogenous chromatin (Fig. 2). A retraction zone between the nuclear membrane and chromatin was clearly seen. Two or more hemispherical nucleoli were often observed attached to the inner surface of the nuclear envelope, which was separated from the chromatin by a clear halo. The average nuclear diameter of Ad was 6.2 ± 0.1 µm. The cytoplasm of Ad frequently contained PAS-positive material. Ap contained a relatively larger ovoid and lightly stained nucleus with coarse granular chromatin and an elongated nucleolus attached to the nuclear membrane, which was distinct. A retraction zone was not observed. Ap were subdivided into Aps and Apl based primarily on nuclear size. The average nuclear diameter of Aps and Apl was 7.1 ± 0.1 and 8.8 ± 0.2 µm, respectively. In addition, the chromatin in Apl appeared less dense than that of Aps. Aunc shared some morphological similarities to Ad, i.e. a thin nuclear envelope unassociated with heterochromatin and a retraction zone. In contrast to Ad, nuclear size of Aunc was larger (7.4 ± 0.1 µm) and the chromatin was less dense and less homogenously distributed. The mean number of Ad, Ap and Aunc per testis was 95 ± 19, 207 ± 32 and 87 ± 18 × 106, respectively. The mean number of Sertoli cells per testis was 1608 ± 229 × 106. The number of Ad and Aunc per cross section ranged through Stages I–XII of the seminiferous epithelial cycle from 0.4 ± 0.1 to 0.7 ± 0.2 and 0.4 ± 0.1 to 0.7 ± 0.2, respectively, while analogous data per 100 Sertoli cells were 4.1 ± 0.8 to 7.8 ± 1.9 and 4.8 ± 1.4 to 8.5 ± 4.5, respectively. These changes were not significant.
Figure 2.
Photomicrographs obtained from PAS reagent/hematoxylin stained sections from testis of adult rhesus monkeys illustrating the morphological characteristics of type A spermatogonia.
Ad, top left; Aunc, top right; Aps, bottom left; Apl, bottom right. Bar = 10 µm.
Although the number of Ap per cross section (1.3 on average) or per 100 Sertoli cells (14.2) did not change significantly during the seminiferous epithelial cycle, maximal numbers of this cell type (1.5 and 1.6/cross section; 16.5 and 17.6/100 Sertoli cells) were observed in Stages VIII and IX of the cycle (Fig. 3). During Stages I–V (non-mitotic stages; see below), the mean number of Ap per cross section and per 100 Sertoli cells was 1.2 and 13.3, respectively. The number of Ap per cross section and Ap per 100 Sertoli cells before Abercrombie correction was also calculated (Table I).
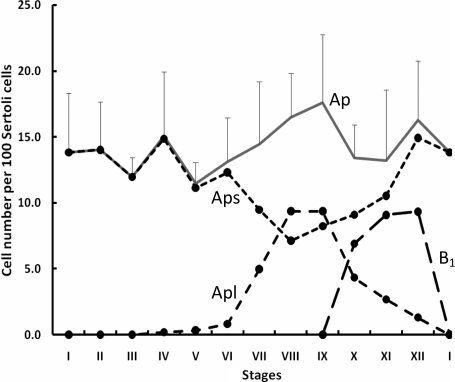
Figure 3.
Changes in the number of Aps, Apl and B1 spermatogonia per 100 Sertoli cells (mean ± SE) during the cycle of the seminiferous epithelium in the adult rhesus monkey (n = 4).
Note peak numbers of Apl in Stages VIII and IX of the cycle were followed by appearance of the first generation of differentiated B spermatogonia in Stages X–XII. The total number of Ap (i.e. Aps + Apl), did not change throughout the cycle (analysis of variance, P > 0.05).
Table I.
Spermatogonia and PL spermatocyte number [corrected (CD) and uncorrected (UC) for Abercrombie (1946)] expressed as cells per cross section and cells per 100 Sertoli cells during the 12 stages of the seminiferous epithelial cycle in adult rhesus monkey
| Stage: | Per cross section (CD) |
Per cross section (UC) |
Per 100 Sertoli cells (CD) |
Per 100 Sertoli cells (UC) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A pale | Bs or PL |
A pale | Bs or PL |
A pale | Bs or PL |
A pale | Bs or PL |
|||||
| I | 1.27 | 1.9 | B2 | 3.1 | 4.7 | B2 | 13.8 | 18.9 | B2 | 21.5 | 31.5 | B2 |
| II | 1.3 | 1.9 | B2 | 3.2 | 4.8 | B2 | 14 | 19.3 | B2 | 21.8 | 32.1 | B2 |
| III | 1.04 | 4 | B3 | 2.5 | 9.7 | B3 | 12 | 44.9 | B3 | 18.7 | 71.2 | B3 |
| IV | 1.39 | 3.8 | B3 | 3.4 | 9.2 | B3 | 15.1 | 38.2 | B3 | 23.5 | 60.6 | B3 |
| V | 1.03 | 6.2 | B4 | 2.5 | 14.4 | B4 | 11.5 | 73.5 | B4 | 18 | 111.7 | B4 |
| VI | 1.19 | 6.7 | B4 | 2.9 | 15.6 | B4 | 13.1 | 71.6 | B4 | 20.6 | 109.2 | B4 |
| VII | 1.28 | 12.9 | PL | 3.3 | 27.5 | PL | 14.4 | 133.4 | PL | 23.5 | 186.3 | PL |
| VIII | 1.56 | 4.1 | 16.5 | 27.7 | ||||||||
| IX | 1.52 | 4.0 | 17.6 | 29.4 | ||||||||
| X | 1.35 | 0.72 | B1 | 3.4 | 2 | B1 | 13.4 | 6.9 | B1 | 21.9 | 12.7 | B1 |
| XI | 1.2 | 0.87 | B1 | 3.0 | 2.4 | B1 | 13.2 | 9.1 | B1 | 21.2 | 16.7 | B1 |
| XII | 1.5 | 0.92 | B1 | 3.7 | 2.6 | B1 | 16.3 | 9.3 | B1 | 25.6 | 17.2 | B1 |
In contrast to total Ap, cyclic fluctuations in Aps and Apl were more striking. Apl began to appear in Stages IV–VI and marked increases in this subclass of Ap spermatogonia were observed in Stages VII–XI (Fig. 3). The appearance of Apl in the second half of the cycle was associated with reduced numbers of Aps. As expected, B1 spermatogonia appeared in Stage X and were maintained in the two subsequent stages as the number of Apl was declining (Fig. 3). The mean number of B1 per 100 Sertoli cells and per cross section in Stages X–XII was 8.5 and 0.84, respectively. This corresponded to values of 19.1, 41.5, 72.5 and 133.4 per 100 Sertoli cells and 1.9, 3.9, 6.4 and 12.9 per cross section for B2 (Stages I and II), B3 (Stages III and IV), B4 (Stages V and VI) and preleptotene (PL) (Stage VII), respectively. The number of differentiated spermatogonia per cross section and per 100 Sertoli cell are also shown before Abercrombie correction in Table I.
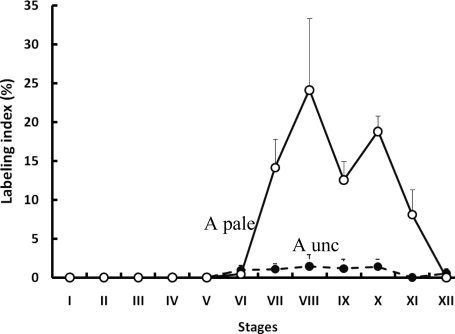
In the first testis, collected 3 h after BrdU injection, labeled Apl and Aunc spermatogonia (Fig. 4) were observed in Stages VI–XII of the seminiferous cycle (Fig. 5). It is to be noted that in the case of Ap spermatogonia only Apl were observed as labeled. Labeling of this cell type was maximal in Stages VIII (24.1%) and X (18.8%), but the fluctuations in LI in Stages VII–XII were not significant (Fig. 5). A low LI (∼1.0%) was observed for Aunc. BrdU-labeled Ad were not observed.
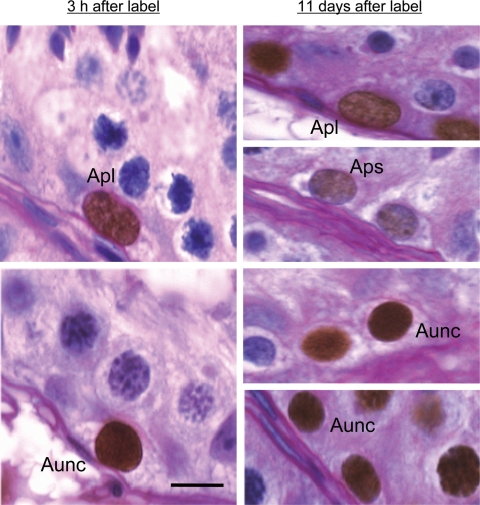
Figure 4.
Photomicrograph of S-phase labeled Apl (left-hand top) and Aunc (left-hand bottom) in the adult rhesus monkeys testis removed 3 h after an i.v. bolus of BrdU.
The undifferentiated progeny of Apl and Aunc may be recognized as label-retaining cells observed in the second testis removed 11 days later (right-hand panels). Presumably, labeled Apl and Aps in the second testis (right-hand top two panels) were derived from Ap in S-phase 11 days earlier. The parents of labeled Aunc in the second testis (right-hand bottom two panels) are less clear. Dark brown precipitate in the nuclei indicates the presence of BrdU. PAS/hematoxylin was used as the counter stain. Bar = 10 µm.
Figure 5.
Mean (±SE) labeling indices of Ap (open circles) and Aunc (filled circles) during the seminiferous epithelial cycle of the adult rhesus monkey (n = 4).
In striking contrast to the BrdU labeling pattern in the first testis, labeled Aps were observed in the second testis removed 11 days, 3 h following the BrdU injection (not shown). Labeled Aps were restricted to Stages VI–XII of the seminiferous epithelial cycle. Labeled Apl and B1 were observed in Stages VII–X and IX–XII, respectively, of the cycle. Labeled Aunc with an apparently smaller nuclear diameter were observed in Stages VI–XI. Labeled Ad were not observed.
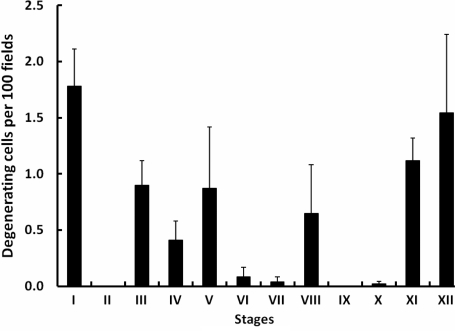
The number of degenerating germ cells on the basement membrane was 7.4 ± 1.5 per 100 fields. This is equivalent to two to three cells per 106 µm2. Degenerating basal germ cells were most frequently observed in Stages XI, XII and I of the seminiferous epithelial cycle (Fig. 6).
Figure 6.
Degenerating germ cells (mean ± SE) on or near the basement membrane per 100 fields across the 12 stages of the seminiferous epithelial cycle of the rhesus monkey (n = 4).
Discussion
The present study utilized BrdU labeling of S-phase cells in combination with standard morphometric procedures to re-examine the proliferation and differentiation of spermatogonia in the adult rhesus monkey. As the morphological features of both undifferentiated and differentiated spermatogonia in the monkey are now generally recognized (Clermont and Leblond, 1959; Clermont, 1969; Clermont and Antar, 1973; Kluin et al., 1983; Fouquet and Dadoune, 1986), and since proliferation of these germ cells throughout the seminiferous epithelial cycle has been investigated on several occasions previously, the present discussion will focus primarily on a critical evaluation of the key similarities and contradictions that exist between results of the present study and those in the literature.
In the rhesus monkey, studies by this laboratory (present study and Ramaswamy et al., 2000) found that the ratio of Ap per cross section during stages of the cycle when Ap spermatogonia were non-mitotic (Stages I–V) to B1 per cross section in Stages X–XII was 1:0.7. Assuming that the differentiation of Ap to B1 occurs with minimal cell death and that there is no amplifying division between Ap to B1 (see later), it must be concluded that approximately one-third of Ap present in the first half of the cycle divided and differentiated into B1. Again, assuming negligible cell death, a similar fraction of Ap would have divided to renew the population of Ap for the next cycle. The remaining third of the Ap population in any given cycle must therefore be quiescent. As in the vervet and stump-tail monkey (Clermont, 1969; Clermont and Antar, 1973), degenerating germ cells were rarely observed in the present study of the rhesus macaque. Assuming that germ cells on the basement membrane were spermatogonia, between two and three spermatogonia per 106 µm2 were found to be degenerating. This compares to a value of 10–20 cells per 106 µm2 that was reported for total germ cells undergoing apoptosis in the cynomolgus monkey (Lue et al., 2002). It should be recognized, however, that the half-life of a degenerating cell may occupy a very small fraction of the entire cycle of the seminiferous epithelium and therefore scarcity of observing a degenerating cell may not be equated to rate of cell turnover.
In contrast to the rhesus monkey where the ratio of Ap per 100 Sertoli cells (Stages I–VII):B1 per 100 Sertoli cells (Stage XI) is 1:0.7, in the stump-tail macaque this ratio is 1:2 indicating in the latter species that: (i) all Ap divide each cycle and (ii) an amplifying division of Ap occurs in Stage VIII (Clermont and Antar, 1973). In contrast to absolute numbers of B1, the ratios of the succeeding generations of differentiating progeny, i.e. B1:B2, B2:B3, B3:B4 and B4:PL spermatocyte in both the rhesus and stump-tail macaque are similar and close to the predicted value of 1:2. The parameter that is fundamentally different between the rhesus and stump-tail monkey is therefore the number of B1 per cross section or per Sertoli cell. That the number of B1 in the rhesus is indeed markedly lower than in the stump-tail monkey is supported by the original study of Clermont and Leblond (1959). Although these early investigators identified only three generations of differentiated B spermatogonia (later recognized as B2, B3 and B4), the uncorrected number of B spermatogonia per cross section reported by Clermont and Leblond (1959) for Stages I, III and V were 3.8, 9.0, and 15.4, respectively: values essentially identical to those reported here for the rhesus monkey (Table I).
A consistent finding of the present study of the rhesus macaque and of the previous investigation of the stump-tail macaque (Clermont and Antar, 1973) was the extensive S-phase labeling of Ap spermatogonia during several stages of the latter half of the seminiferous epithelial cycle. Using 3H-thymidine administered by i.p. injection, Clermont and Antar (1973) reported for three stump-tail monkeys an LI of 26–42% at 3 h post-injection for this cell type in Stages VII–X: compared with 13–23% for the same four stages of the cycle in the rhesus monkey in which BrdU was used to label S-phase cells (present study). Also, using tissue from the four monkeys employed for the present study, Ehmcke et al. (2005a), using a fluorescently tagged secondary antibody, independently reported an LI of 27% for Ap for Stage VII. In addition, Clermont (1969), using 3H-thymidine, reported an LI of 36% for Ap in Stages VII–IX in one Cercopithecus monkey, and Fouquet and Dadoune (1986) found thymidine-labeled Ap from Stages VI to IX of the cynomolgus monkey. More recently, examination of whole mounts of seminiferous tubules obtained from adult rhesus monkeys that had received BrdU 3 h earlier and in which spermatogenesis was driven with recombinant human gonadotrophins revealed labeling of Ap in Stages VII and IX; stages for which data were reported (Ehmcke et al., 2005b).
In macaques, Stages VII–X occupy more than one-third of the seminiferous epithelial cycle (present results; Clermont and Antar, 1973; de Rooij et al., 1986; Fouquet and Dadoune, 1986; Plant and Marshall, 2001), and two hypotheses may be proposed to account for the broad peak of mitotic activity of undifferentiated A spermatogonia during the seminiferous epithelial cycle of the macaque. The first posits that the progeny of S-phase-labeled Ap in Stages VII and VIII are daughter Ap. This being the case, the total number of Ap per cross section or per Sertoli cell would increase in these stages of the cycle. Although this index did not change significantly throughout the cycle in the present study, the uncorrected number of Ap per 100 Sertoli cells in Stage IX (29.4) was 1.4× greater than that in Stages I–V (20.7 on average), when the majority of Ap are presumably in G1 of the cell cycle and are scored as Aps. Clermont and Antar (1973) reported a value of 1.8 for the same metric in the stump-tail monkey. Parenthetically, the present data for the rhesus monkey for the uncorrected number of Ap per 100 Sertoli cells in stages other than Stage IX are essentially identical to those reported previously for the stump-tail monkey. In the case of the cynomolgus monkey (Fouquet and Dadoune, 1986), the comparison is more difficult because the number of Ap per 100 Sertoli cells in this species was, on average, ∼50% of that reported for the rhesus and stump-tail macaque. The French group, however, identified an At, and it may be noted that the combined number (uncorrected) of At and Ap per 100 Sertoli cells during the non-mitotic stages of the cycle, and the changes in this combined group of A spermatogonia throughout the cycle, were reminiscent of those reported for Ap in the other two species of macaque.
According to the first hypothesis, entry of Ap into S-phase would be a poorly synchronized event, occurring across several stages of the epithelial cycle with the initial cohorts of Ap undergoing mitosis to produce daughter Ap; as the cycle progresses and the remaining cohorts of Ap in the cell cycle enter S-phase, these cells do not renew but now differentiate and produce B1. It may be further suggested that as the number of Ap per Sertoli cell increases during Stages VII–IX this expanding population of Ap generates a signal, either directly or indirectly (perhaps via the Sertoli cell), that inhibits further proliferation of Ap and facilitates differentiation to B1. In this manner, the differentiation of Ap leading to the birth of B1 is more tightly synchronized than Ap renewal. This hypothesis eliminates the short-lived population of Ap during Stages VII and IX that is required in the Clermont and Antar model derived from studies of the stump-tail monkey. In this regard, it is difficult to envisage a control mechanism that would allow a single cell type to have such a plastic cell cycle.
The second hypothesis for the broad peak in S-phase labeling of Apl spanning Stages VII–XI of the seminiferous cycle is that Ap divide in Stages VII and perhaps VIII to produce a second distinct, but morphologically similar, generation of undifferentiated A spermatogonia [analogous to A2 spermatogonia in rodents (de Rooij and Russell, 2000)] that are characterized by a short cell cycle such that they all divide in Stage X to produce the first generation of B spermatogonia. If this were the case, it would have to be concluded that the proportion of Ap which remain quiescent during any one cycle of the seminiferous epithelium of the rhesus monkey would be even greater because of the amplifying division. Moreover, it would become appropriate to consider naming the two generations of Ap spermatogonia in the monkey testis, Ap1 and Ap2, in analogy with the terminology used for the rodent (de Rooij and Russell, 2000). It is also interesting to recognize that the lineage Ap1-Ap2-B1-B2-B3-B4 in the monkey would involve the same number of mitoses as the lineage A1-A2-A3-A4-In-B in the rodent (de Rooij and Russell, 2000). The idea that A spermatogonia undergo an amplifying division in the macaque testis is consistent with the model originally proposed by Clermont and Antar (1973) for the stump-tail monkey and more recently applied to the rhesus monkey by Ehmcke et al. (2005a, b). Importantly, however, it differs from these two earlier models in that the amplifying division in Stage VII of the seminiferous epithelial cycle is a differentiating division that produces a distinct spermatogonial type with a relatively short cell cycle. In any event, the unifying model for the proliferation and differentiation of Ap spermatogonia in the primate, as proposed by Ehmcke et al. (2005a, b), may be premature.
Whereas Clermont and Leblond (1959) and Kluin et al. (1969) noted that Ap increase their size during the latter half of the seminiferous epithelial cycle as S-phase is approached and entered, enumeration of Aps and Apl, however, was first reported in a study of the prepubertal rhesus monkey testis (Simorangkir et al., 2005). At this stage of development, Ap proliferate but differentiation is curtailed (Simorangkir et al., 2005). We proposed that Aps are in early to mid G1 of the cell cycle, while Apl are either approaching S-phase, in S-phase or in G2. The present finding that Aps were maximal in the early stages of the cycle (I–IV) and minimal during the second half of the cycle (Stages VII and IX) when the number and LI of Apl were elevated was therefore to be expected. Presumably as the cycle progresses, cohorts of Ap increase their nuclear size as they enter S-phase during Stages VII–IX and, as a result, the proportion of Ap scored as Aps decreases while that scored as Apl increases. The completion of mitosis of the renewing Apl leads to the formation of daughter Aps and, as a result, the relative changes in Aps and Apl are reversed during Stages IX to I to restore the steady state for the next cycle. In striking contrast to the first testis, Aps in the second testis (removed 11 days, 3 h after BrdU injection) were labeled and, moreover, were observed in the same stages in which labeled Apl were recorded in the first testis. As expected and consistent with the previous independent observation of Ehmcke et al. (2005a) using our tissue, labeled Apl were also observed in the second testis at Stages VII–X. These latter findings provide further evidence for the schemata of Ap proliferation proposed here.
The incomplete decline in the number of Ap per cross section from Stages IV to VIII in the present study (∼50%, Fig. 1) is consistent with the notion that during any one cycle of the seminiferous epithelium in the rhesus monkey only two-thirds or less of the Ap population is in the growth fraction. The endocrine determinants and cell biology controlling the proportion of the Ap population that enters the growth fraction remain to be determined (Simorangkir et al., 2009).
The failure to find labeled Ad in the first testis (and also the second testis) is consistent with the generally held dogma that this type of undifferentiated spermatogonia divides rarely in the normal testis (Clermont and Antar, 1973; Kluin et al., 1983). Using a fluorescently tagged second antibody, Ehmcke et al. (2005a), reported strong (LI 0.8%) and very weak, granular (18%) labeling of Ad. We observed neither strong nor weak labeling of this cell type using colorimetric detection of the BrdU antibody.
As previously reported for the juvenile rhesus testis (Simorangkir et al., 2005), not all undifferentiated type A spermatogonia could be unambiguously identified as Ad or Ap and these cells were termed Aunc. Enumeration of the latter category of undifferentiated spermatogonia was important in our earlier study of the prepubertal testis because we were examining whether proliferation of Ad, considered to be essentially non-mitotic in the adult, was responsible for the increase in number of this germ cell from birth to adulthood. Accordingly, unequivocal identification of Ad was required. However, Aunc are morphologically similar to Ad, except that their nuclei are larger with chromatin that is less dense, and it is likely that in previous studies Aunc have been classified as Ad. This is consistent with the present finding that Ad, Aunc and Ap account for ∼25%, 25% and 50%, respectively, of the total population of undifferentiated spermatogonia and with the previously reported 1:1 ratio for Ad:Ap (Clermont and Leblond, 1959; Clermont, 1969; Clermont and Antar, 1973; Kluin et al., 1983; Marshall and Plant, 1996; Ramaswamy et al., 2000). BrdU-labeled Aunc were occasionally observed in both the first and second testis. The LI was low and labeling of this cell type was restricted to Stages VI–X of the seminiferous epithelium when Ap were also observed to divide. In the context of the question of what specific undifferentiated type A spermatogonia comprises the primate spermatogonial stem cell, the progeny of labeled Aunc may prove to be informative. The resolution of this question, however, will require the application of more contemporary methodologies for tracing cell lineages. In this regard, we should like to conclude by reiterating the difficulties in classifying undifferentiated spermatogonia in cross section using classical histochemical methods (Clermont, 1972); a problem that is compounded by the presence of an additional nuclear marker, such as BrdU.
Funding
This research was supported by The Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health, through cooperative agreement U54 HD 08610 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Acknowledgements
We thank the staff of the Primate Core. We are also grateful to Dr Carey D. Balaban, Department of Otolaryngology, University of Pittsburgh School of Medicine, for allowing us to use the Histology Laboratory Core. In this regard, the technical assistance of Gloria J. Limetti and Jean L. Betsch in tissue processing and embedding is also gratefully acknowledged.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Renewal of spermatogonia in man. Am J Anat. 1966;118:509–524. doi: 10.1002/aja.1001180211. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Two classes of spermatogonial stem 510 cells in the monkey (Cercopithecus aethiops) Am J Anat. 1969;126:57–71. doi: 10.1002/aja.1001260106. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals, seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Antar M. Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macaca arctoides. Am J Anat. 1973;136:153–165. doi: 10.1002/aja.1001360204. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Leblond CP. Differentiation and renewal of spermatogonia in the monkey, Macaca rhesus. Am J Anat. 1959;104:237–273. doi: 10.1002/aja.1001040204. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–797. [PubMed] [Google Scholar]
- de Rooij DG, van Alphen MM, van de Kant HJ. Duration of the cycle of the seminiferous epithelium and its stages in the rhesus monkey (Macaca mulatta) Biol Reprod. 1986;35:587–591. doi: 10.1095/biolreprod35.3.587. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Simorangkir DR, Schlatt S. Identification of the starting point for spermatogenesis and characterization of the testicular stem cell in adult male rhesus monkeys. Hum Reprod. 2005;a 20:1185–1193. doi: 10.1093/humrep/deh766. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Leutjens CM, Schlatt S. Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix jacchus. Biol Reprod. 2005;b 72:293–300. doi: 10.1095/biolreprod.104.033092. [DOI] [PubMed] [Google Scholar]
- Fouquet JP, Dadoune JP. Renewal of spermatogonia in the monkey (Macaca fascicularis) Biol Reprod. 1986;35:199–207. doi: 10.1095/biolreprod35.1.199. [DOI] [PubMed] [Google Scholar]
- Kluin MP, Kramer MF, de Rooij DG. Testicular development in Macaca irus after birth. Intl J Androl. 1983;6:25–43. doi: 10.1111/j.1365-2605.1983.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Lue YH, Lasley BL, Laughlin LS, Swerdloff RS, Sina Hikim AP, Leung A, Overstreet JW, Wang C. Mild testicular hyperthermia induces profound transitional Spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis) J Androl. 2002;23:799–805. [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology. 2000;141:18–27. doi: 10.1210/endo.141.1.7276. [DOI] [PubMed] [Google Scholar]
- Rosiepen G, Arslan M, Clemen G, Nieschlag E, Weinbauer GF. Estimation of the duration of the seminiferous epithelium in the non-human primate Macaca mulatta using the 5-bromodeoxyuridine technique. Cell Tissue Res. 1997;288:365–369. doi: 10.1007/s004410050822. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile 554 development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Ramaswamy S, Marshall GR, Pohl CR, Plant TM. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta) Hum Reprod. 2009 doi: 10.1093/humrep/dep052. [DOI] [PMC free article] [PubMed] [Google Scholar]