Abstract
BACKGROUND
Unilateral orchidectomy in monkeys increases spermatogenesis in the remaining testis in association with elevated follicle-stimulating hormone (FSH) secretion and testicular testosterone. The present study examined the relative importance of FSH and testosterone in driving the primate testis toward its spermatogenic ceiling.
METHODS
Adult male rhesus monkeys were treated with a gonadotropin-releasing hormone receptor antagonist to inhibit endogenous FSH and luteinizing hormone (LH) secretion. The gonadotrophin drive to the testis was replaced with a pulsatile recombinant human FSH and LH infusion to maintain testicular volume and circulating testosterone and inhibin B at physiological levels. A selective monotropic elevation of FSH or LH that doubled the concentrations of inhibin B or testosterone, respectively, was then imposed for 4 weeks, each in a group of four monkeys. In a third group (n = 4), the gonadotrophin drive remained clamped at physiological levels. Bromo-deoxyuridine was administered 3 h prior to castration, and the effects of the monotropic hormone increments on germ cell number, S-phase labeling and degeneration were determined.
RESULTS
Increased FSH, but not LH, produced increases in testicular volume (P < 0.05), the proportion of A pale spermatogonia entering the cell cycle and the numbers of differentiated spermatogonia and more advanced germ cells (P < 0.05). Indexes for spermatogonia labeling and germ cell degeneration were not affected.
CONCLUSIONS
Under physiological conditions, circulating concentrations of FSH directly dictate sperm output of the primate testis by regulating the proportion of Ap spermatogonia in the growth fraction. An effect of FSH on survival of the first generation of differentiated B spermatogonia is not excluded.
Keywords: spermatogenesis, primate, testis, testicular clamp, FSH
Introduction
In contrast to several non-primate species, unilateral orchidectomy (UO) in the adult rhesus monkey results in a dramatic increase in the volume of the remaining testis (Medhamurthy et al., 1993; Ramaswamy et al., 2000a), which is associated with an increase in spermatogenesis. In the study by this laboratory, the number of all four generations of differentiated spermatogonia, pachytene spermatocytes and round spermatids in the remaining testis was significantly increased, whereas Sertoli cell number was unchanged as anticipated. The foregoing findings, together with the earlier observation that treatment of adult cynomolgus and rhesus monkeys with human (h) follicle-stimulating hormone (FSH) increased germ cell number in the seminiferous epithelium (van Alphen et al., 1988), indicate that the adult testis of the non-human primate does not operate at its spermatogenic ceiling. This notion is consistent with clinical reports that removal of one testis due to testicular cancer was associated with increased spermatogenesis in the remaining gonad (Jacobsen et al., 2001) and that fertility in men with mono-orchidism appears similar to that in normal controls (Lee and Coughlin, 2002).
The endocrine determinants responsible for driving the remaining testis toward its spermatogenic ceiling following UO are unclear because both circulating FSH levels and testicular testosterone content of the remaining testis, inferred from circulating testosterone concentrations, were increased following this experimental perturbation (Ramaswamy et al., 2000a; Simorangkir et al., 2004). The previous study by van Alphen et al. (1988), however, provides evidence to indicate that increased FSH secretion is the most likely hormonal signal underlying the stimulation of spermatogenesis in the testis that remains following UO. In this early study by van Alphen et al. (1988), adult macaques were treated with daily or twice daily doses of hFSH or hCG, and spermatogenesis was stimulated only during FSH treatment. The doses of gonadotrophin administered in the Alphen study, however, were relatively high (see Discussion), and the confounding possibility that testicular endocrine responses induced by daily administration of one gonadotrophin increased negative feedback on endogenous luteinizing hormone (LH) and FSH secretion and thereby reduced the circulating activity of the other gonadotrophin was not excluded.
Several earlier studies have demonstrated that FSH treatment, either alone or in combination with testosterone, may stimulate male germ cell development. Weinbauer et al. (1991) abolished endogenous gonadotrophin secretion in adult macaques by the administration of a gonadotropin-releasing hormone receptor (GnRH-R) antagonist and observed that FSH administration, alone, retarded depletion of the seminiferous epithelium when treatment with FSH was initiated at the time of the GnRH-R antagonist injections, and partially restored spermatogonia and spermatocyte number when FSH treatment was delayed for 8 weeks. The effect of LH signaling was not investigated by the Münster group. Studies of hypophysectomized or GnRH-R antagonist-treated adult rhesus monkeys, in which spermatogenesis was qualitatively restored by testosterone replacement, have shown that FSH markedly amplifies the stimulatory action of testosterone on the seminiferous tubule (Marshall et al., 1995, 2005). In one of these studies, treatment with FSH, alone, was also shown to stimulate spermatogonial differentiation (Marshall et al., 2005).
The foregoing experimental paradigms, however, have not provided investigators with a model to study the impact on the testis of a selective physiological elevation in either FSH or LH in the absence of a change in the blood levels of the other gonadotrophin. In order to rectify this situation, we have used an experimental model known as a ‘testicular clamp’ that enables gonadotrophin stimulation of the testes to be held constant, and a selective increase in blood levels of one of the gonadotrophins to be imposed, while maintaining that of the other at control levels (Simorangkir et al., 2004). To this end, endogenous gonadotrophin secretion in adult male monkeys was abolished with a GnRH-R antagonist and immediately replaced with recombinant hFSH (rhFSH) and LH (rhLH), administered iv and in a pulsatile manner to mimic the physiological mode of release. Animals were then assigned to one of three groups. The first group was subjected to a selective increase in FSH stimulation by increasing pulse amplitude of this gonadotrophin while clamping that of LH. The second group received a selective increase in LH pulse amplitude, and the third group was clamped at control levels throughout the experiment.
Materials and Methods
Animals
Twelve adult male rhesus monkeys (Macaca mulatta, 8.5–13.9 kg body weight), which were obtained from laboratories within the USA or imported from China, were used. The animals were housed in individual cages and maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals provided by the National Institutes of Health (NIH). All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Surgical procedures
In general, the surgical procedures employed were the same as those described earlier (Simorangkir et al., 2004). In brief, each animal was implanted with an indwelling jugular and a femoral vein catheter. One catheter was dedicated for pulsatile administration of gonadotrophin and the other for remote blood sampling. The monkeys were sedated with ketamine hydrochloride (100 mg iv, Ketaject, Phoenix Scientific Inc., St Joseph, MO, USA), and anesthesia was subsequently achieved with 1.5–2% isoflurane (Abbott Animal House, North Chicago, IL, USA) in oxygen. All surgical procedures were performed under aseptic conditions. Post-surgically, animals received a single im injection of penicillin (300 000 U, Bicillin L-A, Wyeth Laboratories, Philadelphia, PA, USA), and a series of iv injections of cefazolin sodium, a broad-spectrum antibiotic (25 mg/kg body weight, Kefzol, G.C. Hanford Mfg. Co., Syracuse, NY, USA) and an analgesic: either meperidine hydrochloride (1 mg/kg body weight, iv, Demerol, Elkins-Sinn, Cherry Hill, NJ, USA) or ketoprofen (2 mg/kg body weight, im, Ketofen, Fort Dodge Animal House, Fort Dodge, IA, USA), twice a day for 4 days. The catheters were tunneled subcutaneously from the site of venous insertion to the mid-scapular region where they were exteriorized via a small cutaneous fistula. Animals were fitted with a nylon jacket attached to a flexible stainless steel tether and housed in individual specialized remote sampling cages that permitted continuous access to the venous circulation. Castration was also performed under aseptic conditions, with the same pre- and post-surgical treatments.
Hormones and GnRH-R antagonist
rhFSH (7377.6 IU/ml; Batch # BFDA01 522) and rhLH (25 551 IU/ml; Batch # BLCA0102) were kindly provided by Serono, Aubone. The GnRH-R antagonist, acyline (Bioqual, Rockville, MD, USA, 60 µg/kg, daily sc), was kindly provided by the Contraception and Reproductive Health Branch, Center for Population Research, The Eunice Kennedy Shriver National Institute for Child Health and Human Development, NIH. Acyline for injection was prepared in 5% aqueous mannitol (300 kg/ml) and stored at 4°C.
Preparation of the infusate
The gonadotrophin infusates were custom-prepared using procedures described previously (Ramaswamy et al., 2000b; Simorangkir et al., 2004). In brief, rhLH (0.03–0.15 µl/ml) and rhFSH (0.03–0.04 µl/ml) were diluted in Dulbecco’s phosphate-buffered saline (PBS, Invitrogen Corporation, Grand Island, NY, USA). The infusates also contained cefazolin sodium (1 µg/ml, Kefzol, GC Hanford Mfg Co., Syracuse, NY, USA), and the corresponding monkey serum (1%), which had been collected before initiation of the experiments. Infusates were then sterilized by passing them through a 0.22 µm filter (Fisherbrand, Fisher Scientific, Ireland) and stored at 4°C.
Experimental design
The overall experimental design was similar to that employed previously (Simorangkir et al., 2004). Prior to establishing the testicular clamps, the average concentration of circulating testosterone for each animal was determined in plasma samples that had been collected hourly, either for 12 (09:00–21:00) or 24 h (09:00–9:00) on two to three different occasions. Endogenous gonadotrophin secretion was then abolished by daily treatment with acyline (60 µg/kg, sc), and the gonadotrophin drive to the testes was immediately replaced with an intermittent iv infusion of rhFSH and rhLH administered as a brief infusion (pulse) delivered over 1 min every 2.5 h: an inter-pulse interval similar to that observed spontaneously in adult male monkeys (Plant, 1981). The starting pulse dose of rhLH was 150–200 mIU/kg, and, when necessary, was adjusted for individual monkeys to achieve pre-clamp plasma testosterone levels. The final rhLH pulse dose before starting the experiments ranged from 97.5 to 351 mIU/kg. The initial (and final) pulse dose of rhFSH ranged from 20 to 25 mIU/kg and was adjusted to produce blood FSH levels of ∼0.50–0.75 mIU/ml as measured by immunoradiometric assay (IRMA, see below). The bioactivity of these levels of circulating rhFSH in the acyline-treated male monkeys was shown, in selected samples, to be similar to that in adult male monkeys before treatment with the GnRH-R antagonist. Bioactive levels of FSH were kindly measured in the laboratory of Dr William J. Bremner using a previously described procedure employing an in vitro FSH bioassay based on cAMP production by a murine granulosa tumor cell line expressing the inhibin α-promoter/simian virus 40-T Ag fusion gene, and stably transfected with the hFSH receptor (Narula et al., 2002).
Approximately 4–6 weeks were required following the initiation of acyline treatment to adjust the rhFSH and rhLH pulse doses. At this time, a selective elevation in either the FSH (4-fold) or LH (2-fold) dose was then imposed in each of four monkeys for ∼4 weeks. We chose to increase pulse amplitude rather than pulse frequency in order to be able to differentially regulate the gonadotrophin doses. Inhibin B and testosterone responses, respectively, were used to establish that both LH and FSH receptor signaling pathways had been activated to similar degrees (see Results). The remaining four animals served as the control group receiving an invariant FSH and LH infusion throughout the study period. Circulating levels of FSH and testosterone were monitored during two inter-gonadotrophin pulse intervals in samples collected 10 min before and at 5, 20, 40, 60, 100 and 140 min after two sequential and representative pulses once a week. Circulating LH concentrations were monitored using two time points only (10 min before and 5 min after a gonadotrophin pulse). Plasma was separated by centrifugation and stored at −20°C until assayed. Packed cells were re-suspended in sterile saline and returned to the respective animals.
The experiment was terminated by an iv bolus injection of bromo-deoxyuridine (BrdU; Sigma Chemical Co.; 33 mg/kg body weight as a 2% solution in PBS) and 3 h later the animals were orchidectomized. The time from the last pulse infusion of gonadotrophin to castration ranged from 40 min to 2 h 10 min. Testes were sliced and cut into small pieces, placed in Bouin’s fixative overnight and subsequently stored in 70% ethanol until embedding in paraffin.
Hormone assays
Circulating rhFSH and rhLH were measured using IRMA kits purchased from Diagnostic Product Corporation (Los Angeles, CA, USA; #IKFS1) and Diagnostic System Laboratories Inc. (Webster, TX, USA; #DSL-4600), respectively. The sensitivity of the FSH and the LH assays was 0.06 and 0.12 mIU/ml, respectively. The intra- and inter-assay coefficients of variation were <3.8 and <5.7%, respectively, for FSH, and <8.9 and <8.9%, respectively, for LH. Total testosterone levels were measured using a radioimmunoassay (#TKTT5, Diagnostic Product Corporation, Los Angeles, CA, USA) and inhibin B was measured using a specific two-site inhibin B enzyme-linked immunosorbent assay (#DSL-10–84100, Diagnostic System Laboratories, Inc. Webster, TX, USA). The sensitivity of the testosterone and the inhibin B assays was 0.4 ng/ml and 7 pg/ml, respectively. The intra- and inter-assay coefficients of variation were <7.1 and <10.2%, respectively, for testosterone, and <5.6 and <7.6%, for the inhibin B assay.
Testis volume
Testis volume was calculated from weekly measurement of the length and width of the testes determined by one of us (G.R.M.) with calipers (Marshall et al., 1983). During these measurements, monkeys were sedated with ketamine HCl. Because of marked individual differences between animals, testicular volume was expressed relative to the testis volume prior to administration of the GnRH-R antagonist.
Morphometric analysis
Morphometric analyses were performed exactly as described in the companion manuscript (Simorangkir et al., 2009) using six 5 µm sections taken from six randomly chosen paraffin embedded tissue-blocks stained with periodic acid—Schiff’s reagent/Gill’s hematoxylin (Sigma-Aldrich, MO, USA). Cell numbers were determined by one of us (D.R.S.) while blinded to the identity of the animals (see Simorangkir et al., 2009). Undifferentiated type A spermatogonia (Ad, Aps, Apl and Aunc), the four generations of differentiated type B spermatogonia, preleptotene, leptotene, zygotene and pachytene spermatocytes, and round spermatid nuclei were counted from circular profiles of seminiferous tubule cross-sections. Sertoli cell nucleoli were counted. These numbers were then corrected using Abercrombie’s formula (Abercrombie, 1946) and were expressed either as cell number per Sertoli cell or cell number per cross-section.
Degenerating cell and S-phase labeling indexes
A degenerating cell index was determined for the 12 stages of the seminiferous epithelial cycle as described in the companion paper (Simorangkir et al., 2009). Briefly, degenerating germ cells on the basement membrane were identified by an accumulation of condensed chromatin at the periphery of the nucleus or by frankly pyknotic nuclei. A degenerating index was determined by the number of degenerating profiles observed in optical fields of 33 600 µm2 divided by the number of fields examined multiplied by 100. BrdU-labeled cells were recognized immunocytochemically with an anti-BrdU monoclonal antibody visualized with 3,3′-diaminobenzidine as described (Simorangkir et al., 2009), and a labeling index (LI) was determined by counting the number of a specific cell type labeled with BrdU and dividing by the total number of the corresponding cell.
Statistical analysis
The significance of differences between mean hormone concentrations was determined by one-way repeated measure analysis of variance (ANOVA), whereas the significance of differences between mean cell number and other parameters was determined by one-way ANOVA. Both were followed by Student Newman–Keuls method for all pairwise multiple comparison procedures. Statistical significance was accepted at P < 0.05. Data are expressed as mean ± SE.
Results
Endocrine parameters achieved in the clamps
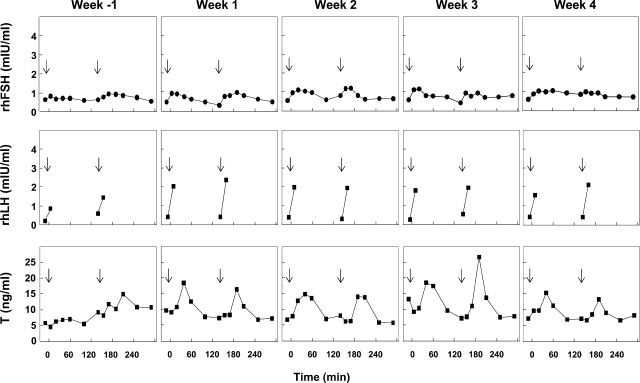
The uninterrupted pulsatile iv infusion of rhLH and rhFSH at a frequency of one pulse every 2.5 h to GnRH-R antagonist-treated adult male monkeys resulted in reproducible profiles of circulating rhLH, rhFSH and testosterone as reflected by hormone levels tracked over two inter-pulse intervals (5 h) every week for the duration of the experiment. Data for a representative control monkey are shown in Fig. 1. Typically, each bolus of rhLH at the control dose produced an increment in the concentration of LH of 2–3 mIU/ml which had declined to basal levels of about 0.5 mIU/ml before the next pulse, and this was followed by an episode of testosterone secretion, which produced peak levels of the steroid in blood ∼40 min later. Circulating FSH levels at the control dose exhibited smaller increments in response to the pulsatile infusion of the gonadotrophin and overall concentrations ranged between 0.4 and 1.2 mIU/ml (Fig. 1).
Figure 1.
An example of a control testicular clamp, as reflected by the time courses of circulating concentrations of recombinant human (rh)FSH (top panels), rhLH (middle panels) and testosterone (T, lower panels), in an adult male rhesus monkey (Macaca mulatta) in which endogenous gonadotrophin secretion was inhibited by treatment with a GnRH receptor antagonist and replaced with an intermittent iv infusion of rhFSH and rhLH administered as a 1 min gonadotrophin pulse (rhFSH, 25 mIU/kg; rhLH, 180 mIU/kg) once every 2.5 h without interruption for 5 weeks. Week −1 corresponds to the week that preceded imposition of a selective elevation in either FSH or LH in the experimental groups. Arrows indicate the time of iv infusion of a gonadotrophin pulse.
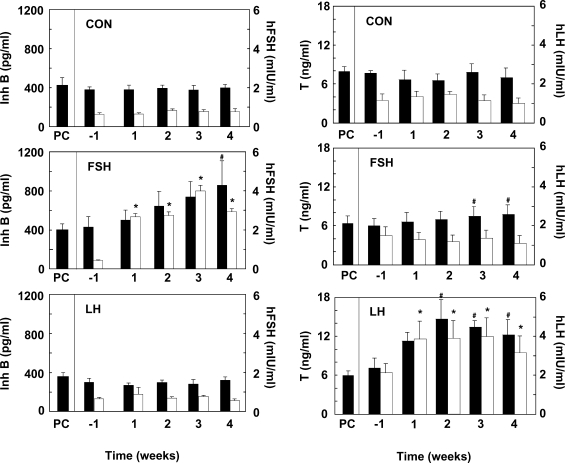
The mean levels of FSH and inhibin B, and of LH and testosterone observed during the 5 h windows of sequential sampling that were conducted throughout the experiment, are shown for the control, increased FSH and increased LH groups in Fig. 2. As expected, selectively increasing the rhFSH pulse dose resulted in a progressive increase in rhFSH concentrations from ∼0.4 to 3–4 mIU/ml by the fourth week of increased FSH administration (Fig. 2). This was associated with a progressive fold increase in circulating inhibin B levels that attained statistical significance by the fourth week of treatment. Interestingly, the selective FSH increase also resulted in a small but significant increase in plasma testosterone concentrations, which occurred in the absence of changes in circulating LH levels (Fig. 2). The selective increase in LH produced elevated circulating levels of this gonadotrophin that had achieved plateau concentrations by the first week of treatment and this was associated with a doubling in plasma testosterone levels. In the control animals, mean circulating levels of the four hormones remained constant throughout the entire experiment.
Figure 2.
Mean circulating concentrations of inhibin B (Inh B, closed histograms) and rhFSH (open histograms) shown in the three left hand panels and testosterone (closed histograms) and rhLH (open histograms) shown in the three right hand panels in testicular-clamped monkeys in which the LH and FSH drive was maintained for 4 weeks at control conditions (CON, top two panels), or in which the FSH (middle two panels) or LH (bottom two panels) drive was selectively elevated, also for a 4-week period. PC, prior to clamping the testis. n = 4 for each group. *Significantly different from week −1 (P < 0.05) and #significantly different from PC and week −1 (P < 0.05).
Testicular parameters
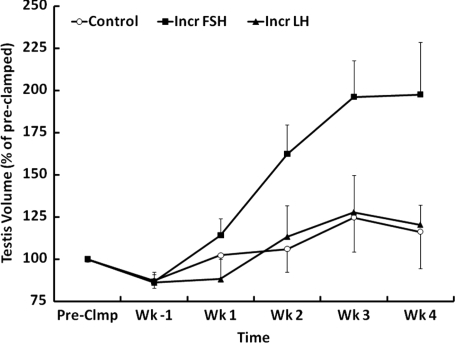
A selective increase in FSH stimulation, but not that of LH, was associated with a progressive increase in testicular volume (Fig. 3). Although this increase did not reach statistical significance, testicular weight at the time of castration was significantly greater in the increased FSH group (Table I). The FSH-induced testicular enlargement was associated with a markedly greater diameter and volume of the seminiferous tubule (Table I). Seminiferous tubule length, however, was not affected (Table I).
Figure 3.
Percentage changes in testicular volume (mean ± SE) from the pre-clamp condition (100%) in three groups of testicular-clamped monkeys. One group received a selective increase in FSH stimulation for 4 weeks (Incr FSH, ▪), one group received a selective increase in LH stimulation for 4 weeks (Incr LH, ▴) and one group was held at control conditions for this duration (Control, ○).
Table I.
Final testicular parameters for the three groups of clamped adult rhesus monkeys (Macaca mulatta)
| Control | Incr FSH | Incr LH | |
|---|---|---|---|
| Weight (g) | 27.1 ± 3.4 | 39.0 ± 3.2* | 26.0 ± 3.1 |
| Vol fraction of seminiferous tubule | 0.84 ± 0.003 | 0.86 ± 0.01 | 0.85 ± 0.01 |
| Seminiferous tubule volume (cm3) | 21.8 ± 2.8 | 32.3 ± 2.6* | 21.2 ± 2.7 |
| Seminiferous tubule diameter (µm) | 177.0 ± 5.2 | 215.9 ± 9.3* | 180.8 ± 7.5 |
| Seminiferous tubule length (m) | 887.5 ± 104.1 | 880.0 ± 30.6 | 813.4 ± 51.8 |
*Significantly different from the other two groups (P < 0.05).
Morphometry of the seminiferous epithelium
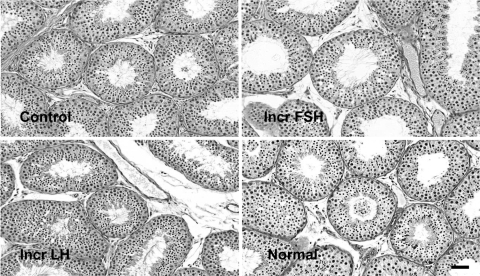
The overall histological appearance of the seminiferous epithelium within and between groups was similar, and the frequency of occurrence of the XII stages of the seminiferous cycle was similar for control, increased FSH and increased LH, with stage VII characteristically exhibiting the highest frequency in all the three groups (Table II). The tubular lumen of the increased FSH group was notably larger than that of the control and increased LH groups (Fig. 4).
Table II.
Percentage of each stage of the seminiferous epithelial cycle
| Stage: | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 8.4 | 7.6 | 7.3 | 7.6 | 7.6 | 8.7 | 15.4 | 7.6 | 7.0 | 7.6 | 7.0 | 8.1 |
| Incr FSH | 8.2 | 7.2 | 6.8 | 7.5 | 8.9 | 8.7 | 15.2 | 8.2 | 7.7 | 7.0 | 6.8 | 7.7 |
| Incr LH | 7.7 | 7.7 | 6.9 | 6.7 | 7.9 | 8.9 | 15.6 | 8.9 | 7.7 | 8.4 | 6.7 | 7.2 |
Figure 4.
Photomicrographs obtained from periodic acid-Schiff’s reagent/hematoxylin stained sections from testis of testicular-clamped adult monkeys under control conditions (top left), selective increase in FSH stimulation (top right) and selective increase in LH stimulation (bottom left) and, for comparison, from a normal adult monkey (bottom right). Bar = 10 µm.
Sertoli and germ cell number per testis
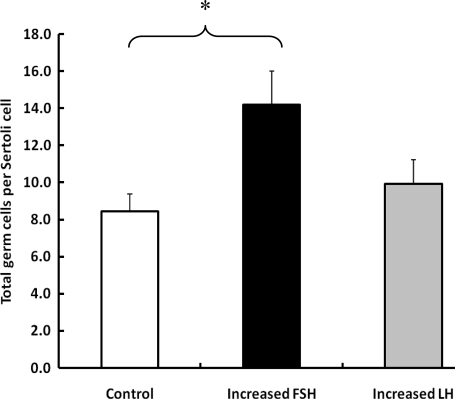
The mean (±SE) number of Sertoli cells per testis in the control, increased FSH and increased LH groups was 1.6 ± 0.1, 1.3 ± 0.1 and 1.3 ± 0.1 × 109, respectively (P > 0.05). The total germ cell number per testis in the increased FSH group (18.8 ± 4.3 × 109) was ∼40% greater than that for either control (13.4 ± 4.0 × 109) or increased LH (13.2 ± 3.5 × 109). Although this difference was not statistically significant, when the total germ cell number was expressed per Sertoli cell, increased FSH, but not increased LH, was found to have significantly increased total germ cell number (Fig. 5).
Figure 5.
Mean (±SE) number of total germ cells per Sertoli cell in testicular-clamped monkeys after 4 weeks of control conditions (open histogram) or after a selective increase in either FSH (closed histogram) or LH (stippled histogram). *P < 0.05.
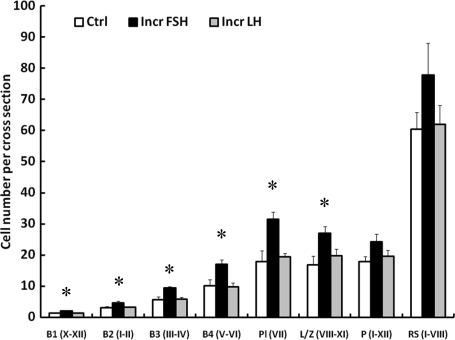
The FSH-induced increase in the total germ cell number was the result of an increase in the number of all four generations of differentiated type B spermatogonia and in the number of preleptotene and leptotene/zygotene spermatocytes (Figs 6 and 7). The number of pachytene and round spermatids, however, was not significantly different between the three groups (Fig. 7). The ratios of the succeeding generations of differentiating progeny, i.e. B1:B2, B2:B3, B3:B4 and B4:Preleptotene spermatocyte in the control group, were close to the predicted value of 1:2 and this was not affected by selective elevations of either FSH or LH (Table III).
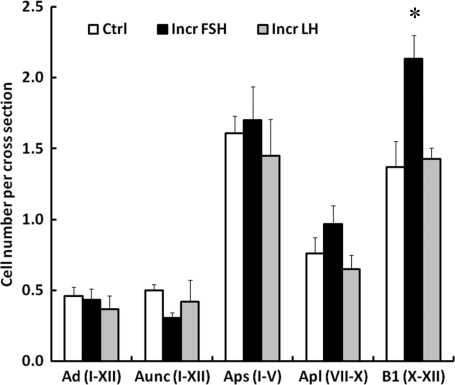
Figure 6.
Mean (±SE) number per cross-section of A dark (Ad) and A unclassified (Aunc) spermatogonia in stages I–XII of the seminiferous epithelial cycle, small A pale spermatogonia (Aps) in stages I–V, large A pale spermatogonia (Apl) in stages VII–X and B1 spermatogonia in stages X–XII in testicular-clamped adult monkeys after 4 weeks of control conditions (open histograms) or after a selective increase in either FSH (closed histograms) or LH (stippled histograms). *Incr FSH greater than control and Incr LH (P < 0.05).
Figure 7.
Mean (±SE) number per cross-section of B1, B2, B3 and B4 spermatogonia, and preleptotene (Pl), leptotene/zygotene (L/Z), and pachytene (P) spermatocytes and round spermatids (RS) in testicular-clamped adult monkeys after 4 weeks of control conditions (open histograms) or after a selective increase in either FSH (closed histograms) or LH (stippled histograms). Roman numerals in parenthesis on the x-axis indicate stages of the seminiferous epithelial cycle analyzed. *Incr FSH greater than control and Incr LH (P < 0.05).
Table III.
Ratio of successive generations of differentiating spermatogonia to their progeny
| B1:B2 | B2:B3 | B3:B4 | B4:Pl | |
|---|---|---|---|---|
| Control | 1:2.2 | 1:1.9 | 1:1.8 | 1:1.8 |
| Incr FSH | 1:2.2 | 1:2.0 | 1:1.8 | 1:1.8 |
| Incr LH | 1:2.4 | 1:1.8 | 1:1.7 | 1:2.0 |
Pl, preleptotene.
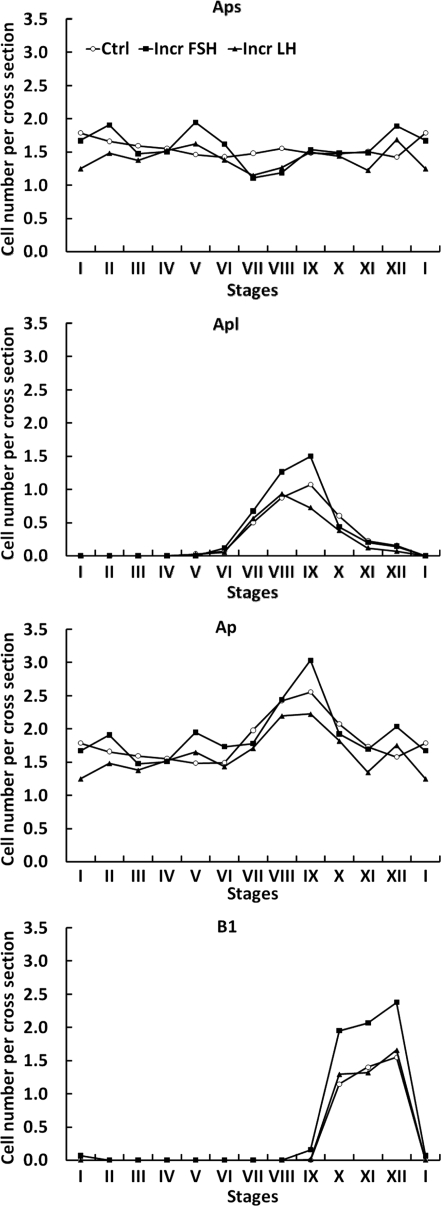
In contrast to differentiated B spermatogonia, there was no significant difference in the mean number of Aps and Apl per cross-section between the three groups of testicular clamps (Fig. 6). For this purpose, the comparison of Aps was restricted to the early stages of the seminiferous epithelial cycle (I–V) before the appearance of Apl. Similarly, Apl were compared during those stages of the cycle (VII–X) when their number is maximal. The mean number of Ad and Aunc per cross-section throughout the seminiferous cycle was similar in all the three groups of clamped animals (Fig. 6). The dynamic changes in Aps, Apl and Ap (Aps + Apl) throughout the seminiferous epithelium cycle and the relationship of these changes to those in B1 spermatogonia are shown in Fig. 8. Although the changes in Aps were small and did not differ between the groups, it is to be noted that the lowest number of this cell type per cross-section was observed in stages VI and VII of the cycle. In all the three groups, Apl appeared in stages VI and VII of the cycle and their number per cross-section was greatest in stage IX, immediately preceding the stage (stage X) in which B1 appeared (Fig. 8). The ratio of Aps (stages I–V):B1 for the control, increased FSH and increased LH was 1:0.8, 1:1.3 and 1:1.0, respectively.
Figure 8.
Changes in the mean number per cross-section of small A pale (Aps, top panel), large A pale (Apl, upper middle panel), total A pale (Ap, lower middle panel) and B1 (lower panel) spermatogonia throughout the XII stages of the seminiferous epithelial cycle in testicular-clamped adult monkeys after 4 weeks of control conditions (○) or after a selective increase in either FSH (▪) or LH (▴).
LI in undifferentiated type A spermatogonia
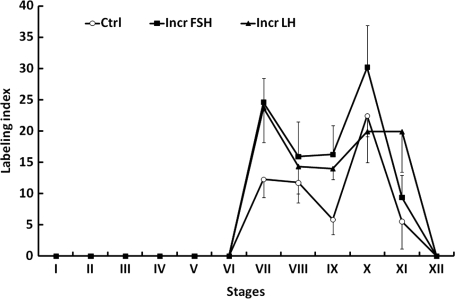
In all the three groups, BrdU-labeled Apl spermatogonia were observed in stages VI–XI of the seminiferous cycle, with maximal labeling in stages VII and X regardless of treatment (Fig. 9). The within group fluctuations in LI for Ap during stages VII–XI and differences in this parameter between groups were not significant (Fig. 9). BrdU-labeled Ad and Aps were not observed. Labeled Aunc were observed occasionally, usually in the latter half of the seminiferous cycle.
Figure 9.
Mean (±SE) bromo-deoxyuridine labeling indexes for A pale spermatogonia throughout the 12 stages of the seminiferous epithelial cycle in testicular-clamped adult monkeys after 4 weeks of control conditions (○) or after a selective increase in either FSH (▪) or LH (▴).
Degenerating germ cells
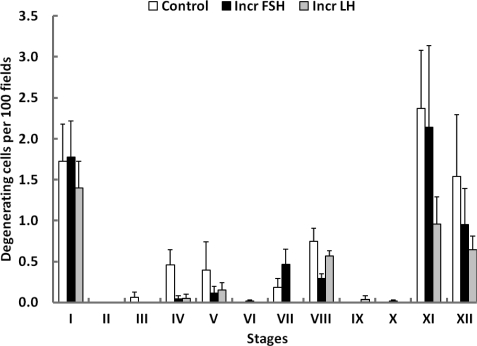
The number of degenerating germ cells on the basement membrane was very low in all the three groups. In control, the mean number of degenerating cells per 100 fields was 7.4 ± 2.2 and this compared with 5.8 ± 1.4 and 4.5 ± 1.1 in increased FSH and increased LH, respectively. These differences were not significant. Interestingly, however, the degenerating index was stage related with the highest levels of degenerating cells being observed at stages I, XI and XII (Fig. 10).
Figure 10.
Changes in the index for degenerating cells on the basement membrane (mean ± SE) throughout the 12 stages of the seminiferous epithelial cycle in testicular-clamped adult monkeys after 4 weeks of control conditions (open histograms) or after a selective increase in either FSH (closed histograms) or LH (stippled histograms).
Discussion
The combined gonadotrophin replacement regimen of brief intermittent infusions of rhFSH and rhLH at a frequency of one pulse every 2.5 h to adult monkeys, in which endogenous gonadotrophin secretion was inhibited with a GnRH-R antagonist, provided in the control conditions a gonadal stimulus that grossly approximated the physiological situation. There are several lines of evidence for this. First, mean plasma testosterone and inhibin B levels before and after the clamp were identical in all the three experimental groups and were similar to those previously reported for normal adult monkeys (Plant, 1981; Ramaswamy et al., 2000a). Second, testicular volume in the control group did not change significantly over the duration of the study, and the mean testicular weight of the control group at the time of castration was similar to those reported previously for adult rhesus monkeys maintained at our primate facilities (Marshall and Plant, 1996). Third, seminiferous tubule diameter and volume in the control group were comparable to those reported in normal adult monkeys (Simorangkir et al., 2009). Lastly, the total germ cell number per testis in the control group (13.4 ± 4.0 × 109) was within the range (8–15 × 109) previously reported for normal adult rhesus monkeys (Ramaswamy et al., 2000a, b). The Sertoli cell of the adult is terminally differentiated (Griswold and McLean, 2006), and therefore, the finding that mean Sertoli cell number per testis was indistinguishable in the increased FSH and increased LH groups, and that both were similar to that in the control group, was to be expected. Similarly, it is generally recognized that the kinetics of the seminiferous epithelial cycle in the adult testis are not subjected to modulation by hormonal factors (Plant and Marshall, 2001), and the finding in the present study that the frequency of the 12 stages of the cycle was similar in the three groups is consistent with this dogma.
The most striking outcome of the present study was the finding that, although a selective increase in FSH drive to the testis resulted in a dramatic stimulation of spermatogenesis as reflected by an increase in testicular volume and the enhanced numbers of differentiating spermatogonia and meiotic germ cells, a selective increase in LH drive was without any discernable effect on the seminiferous tubule. The increased LH drive to the testes achieved by increasing the amplitude of the pulsatile dose of rhLH was unequivocally bioactive because it was associated with increased testosterone secretion, as reflected by a fold increase in circulating levels of the steroid. A similar increase in circulating concentrations of inhibin B, a hormonal indicator of FSH activity (Ramaswamy et al., 2003), was observed in the FSH increased group, and therefore it would seem reasonable to conclude that, to a first approximation, the fold change in bioactivities of the selective increases in either FSH or LH was similar. The foregoing finding that a selective monotropic elevation in circulating FSH concentrations (but not in that of LH) stimulated spermatogenesis in the testicular-clamped adult monkey adds in the following ways to earlier studies, in which alternative and less selective strategies were employed to manipulate the FSH drive to the testis of macaques.
First, the amounts of gonadotrophin employed to provide either an increased FSH (0.65 IU/kg/day on average) or increased LH (2.2 IU/kg/day on average) stimulation in the present study were much lower than those previously used either by this laboratory (Marshall et al., 1995, 2005; FSH, 16–32 IU/kg/day) or by that of the Dutch group (van Alphen et al., 1988; FSH, 3.8–4.6 IU/kg/day; hCG 69 IU/kg/day). Moreover, since an endogenous episodic pattern of blood FSH and LH was mimicked in the present study by the use of an intermittent iv infusion of human gonadotrophin, it seems reasonable to conclude that the endocrine pertubations achieved here were as close to physiological as is experimentally possible. Second, in the present study, the confounding influence of gonadotrophin-induced changes in testicular feedback on endogenous FSH and LH secretion was eliminated, enabling, for the first time, a selective increase in either FSH or LH stimulation to be evaluated in the documented absence of a change in stimulation by the unperturbed gonadotrophin. Third, degenerating cell and S-phase LIs of spermatogonia in the adult macaque testis during selective elevation in FSH or LH stimulation have been quantified for the first time.
From the analysis of germ cell number per cross-section of seminiferous tubule, the earliest step in the process of spermatogenesis that appeared to be stimulated by enhanced FSH signaling was the differentiation of Ap to B1 spermatogonia, as manifest by an increase in the number of the first generation of differentiated B spermatogonia in stages X–XII of the seminiferous epithelial cycle in the increased FSH group. This is reminiscent of UO, which leads to a similar increase in B1 spermatogonia in the remaining testis that is exposed to an increase in circulating FSH concentrations (Ramaswamy et al., 2000a). From findings reported in the latter study, we concluded that amplification of differentiated spermatogonia in the remaining testis was due to increased survival of these germ cells (Plant and Marshall, 2001). The rationale for this view was based on the finding that the total number of Ap spermatogonia in the removed and remaining testis did not change, and on acceptance of the dogma based on Clermont’s earlier work (Clermont, 1972) that, in the monkey, all Ap spermatogonia divide at stage IX of the cycle, with half the population dividing and differentiating to B spermatogonia, and the remainder renewing themselves (Plant and Marshall, 2001). Our recent re-examination of the proliferation and differentiation of A spermatogonia in the rhesus monkey, however, challenges the Clermont dogma by indicating that a third or more of the population of Ap may be quiescent during any one cycle of the seminiferous epithelium (Simorangkir et al., 2009). This being the case, then the increase in the number of B1 spermatogonia in the testes of the increased FSH group (and in the testis remaining following UO) could theoretically result from an increase in the fraction of Ap spermatogonia dividing in each cycle of the seminiferous epithelium. Indeed, the conversion of Ap spermatogonia to B1 spermatogonia, as reflected by the ratio of Aps (stages I–V):B1 per cross-section, was enhanced by selective FSH stimulation. In the control group, the Ap:B1 ratio was 1:0.8, which compared with a ratio of 1:0.7 for normal monkeys (Simorangkir et al., 2009), and this was increased to 1:1.3 by increased FSH. It should be noted that de Rooij and van Beek (1996) have also proposed that FSH may dictate the proportion of Ap spermatogonia that are mitotically active during the seminiferous epithelial cycle of the monkey, although they, like us, accepted the dogma that all Ap spermatogonia divide in each cycle in the normal monkey.
If the action of FSH in controlling spermatogenesis is to regulate the size of the proliferating pool of Ap, then it might be predicted that, in the increased FSH group, the number of Ap approaching or in S-phase of the cell cycle during stages VII–X of the seminiferous epithelial cycle (i.e. Apl) would be increased, while the number of non-dividing Ap (i.e. Aps) in these stages would decrease. Although the number of Apl per cross-section at stages VIII and IX was indeed greatest in the increased FSH group, this difference was not significant. Similarly, although the LI of Ap spermatogonia at stages VII–XI was generally highest in the increased FSH group, between-group differences in this parameter were not statistically significant. In this regard, it is of interest to note that van Alphen et al. (1988) observed an unequivocal increase in the total number of Ap in adult male macaques after 16 days of FSH treatment. Although previous studies by our laboratory found that FSH stimulation was associated with a greater number of Ap at all stages of the seminiferous epithelial cycle, the effect was not significant (Marshall et al., 1995) and, moreover, was not observed in later studies (Ramaswamy et al., 2000a; Marshall et al., 2005). The reasons for these quantitative differences reported for the behavior of Ap in response to increased FSH stimulation remain unclear.
The recognition that a third or more of the population of Ap may be quiescent during any one cycle of the seminiferous epithelium in the normal monkey testis (Simorangkir et al., 2009) also necessitates a comment on our original conclusion regarding FSH action on spermatogonia, which was derived from the studies of testosterone-treated, hypophysectomized adult macaques (Marshall et al., 1995). In this earlier publication, we reported that the number of Ap and B1 per cross-section in the absence of FSH was ∼0.7 and 0.2, respectively. Using the same logic as employed above, these data indicate that ∼30% of Ap were dividing in the absence of FSH. In the presence of FSH, the fraction of Ap dividing increased to ∼45%. This interpretation of the data, which interestingly had been reached earlier by de Rooij and van Beek (1996), would therefore lead to the same conclusion as the present study, i.e. that the role of FSH to stimulate spermatogenesis may be accounted for by its action to recruit non-proliferating Ap into the dividing pool. Data from our more recent study of testosterone-implanted, GnRH-R antagonist-treated adult monkeys (Marshall et al., 2005) are also consistent with the interpretation of de Rooij and van Beek (1996). Until recently, however, our thinking was governed by the classic dogma that posits that all Ap divide at stage IX of each cycle of the seminiferous epithelium.
Although an action of FSH on spermatogonial survival cannot be excluded, the present analysis of degenerating germ cells failed to provide evidence to support this notion. As in normal monkeys (Simorangkir et al., 2009), degenerating germ cells on the basement membrane were rarely observed and showed no relationship to gonadotrophin treatment. Nevertheless, the finding that the degenerating index was greatest at stages XI, XII and I was of interest because these stages are those in which the first generation of differentiated spermatogonia (B1) are born, and it therefore seems reasonable to conclude that the majority of degenerating cells in the monkey testis are probably B1 spermatogonia. Although stages I, XI and XII, therefore, represent a phase of the primate seminiferous epithelial cycle that is a potential target for survival factors, in the present study degeneration of B1 spermatogonia did not appear to be influenced by a selective increase in either FSH or LH stimulation. On the other hand, a reduction in gonadotrophin support, as produced by testosterone administration to normal men, resulted in a reduction in germ cell survival due to increased apoptosis (Ruwanpura et al., 2008). Taken together, these findings suggest that germ cell survival in the primate testis may be near maximal under physiological conditions.
Since it is well established that the FSH receptor is expressed only by the Sertoli cell (Simoni et al., 1997), this somatic cell must be the site of action of FSH that leads to a recruitment of Ap spermatogonia into the mitotic pool. Although a selective increase in FSH resulted in a small, albeit significant, elevation in circulating testosterone concentrations, it is very unlikely that enhanced androgen receptor signaling mediated the FSH-induced activation of Ap spermatognia proliferation and differentiation because the more marked increase in testicular testosterone secretion induced by selectively elevating the LH drive to the testis was without the effect on spermatogonial proliferation and differentiation. It should be noted here that although it is recognized that FSH can amplify LH-stimulated testosterone production by the rodent testis (Odell et al., 1973; Vihko et al., 1991), earlier studies of monkey and man have failed to demonstrate this phenomenon in primates (Majumdar et al., 1997; Young et al., 2000). In the study of the monkey by Majumdar et al. (1997), elevated FSH stimulation was provided for only 48 h and, therefore, the negative result is not inconsistent with the present findings that an increase in testosterone levels was not observed until the third week of increased FSH treatment. However, in the clinical study by Young et al. (2000), LH, alone, or in combination with FSH, was administered to men with post-pubertally acquired hypogonadotropic hypogonadism for a period of 4 weeks. In any event, the effect of FSH to amplify LH-induced testosterone secretion in the present study was subtle, at best, and thus the physiological significance of this phenomenon in primates remains unclear.
Previously, we proposed two models to account for the physiological interaction between FSH and LH in maintaining spermatogenesis in primates (Plant and Marshall, 2001). In the first, intratesticular testosterone is maintained at a level that, in the absence of FSH, results in maximal androgen-dependent germ cell production. In this case, the spermatogenic ceiling may only be achieved when FSH stimulation is combined with that of androgen. In the second model, intratesticular testosterone content is maintained at subthreshold levels, and the spermatogenic ceiling may be reached by an increase in either the FSH or LH (via intratesticular testosterone) drive to the testis. Clearly, the findings of the present study cause the second model to be rejected.
Before concluding, it is worth noting that the failure of an increase in LH activity to stimulate spermatogenesis in the testicular clamp paradigm does not contradict the well established concept that the action of LH on the Leydig cell to generate intratesticular testosterone is essential for spermatogenesis (Plant and Marshall, 2001; McLachlan et al., 2002). For example, in the chemically or surgically hypophysectomized adult monkey, sole replacement with testosterone, the mediator of LH action on the seminiferous tubule, will initiate spermatogenesis (Marshall et al., 1995; 2005), albeit at a level quantitatively below that produced by a combined gonadotrophin drive. To the best of our knowledge, the converse is not true, i.e. the initiation of primate spermatogenesis has not been achieved with FSH in the absence of LH/testosterone.
In summary, using an experimental model known as a testicular clamp, in which the endocrine and spermatogenic functions of the monkey testis are driven at physiological levels by exogenous gonadotrophin, we have shown that a selective increase in FSH stimulation, but not that of LH, is able to take the primate testis to its spermatogenic ceiling by enhancing the growth fraction of Ap spermatogonia that, in turn, leads to an increase in the production of the first generation of differentiated B spermatogonia.
Authors contribution
D.R.S. played the lead role in conducting the in vivo experimental procedures, performed the morphometric and statistical analyses, and contributed to the design of the study and to the writing of the manuscript. S.R. contributed to the conduct of the in vivo experimental procedures and to the measurement of hormone parameters. G.R.M. played a major role in the design of the study and contributed to performing the experimental procedures. C.R.P. was responsible for quality control of the endocrine results. T.M.P. played the lead role in designing the study, analyzing the results and writing the manuscript, and contributed to the conduct of in vivo experimental procedures.
Funding
The Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health, through cooperative agreement U54 HD 08610 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Acknowledgements
The authors would also like to recognize the generous gift of recombinant human gonadotrophins from Serono and the contribution of Dr William J. Bremner’s laboratory in the Department of Medicine, University of Washington, which conducted the bioassays for circulating blood FSH concentrations. We also thank the staff of the Primate and Assay Cores of the Pittsburgh Specialized Cooperative Centers Program in Reproduction and Infertility Research for their help in maintaining the monkeys’ indwelling venous catheters and for conducting the radioimmunoassay (RIA). Drs Nancy J. Alexander and Richard P. Blye and the Eunice Kennedy Shriver National Institute for Child Health and Human Development generously provided acyline. Reagents for the macaque FSH and LH RIAs were obtained from Dr A.F. Parlow, National Hormone and Peptide Program. We are also grateful to Dr Carey D. Balaban, Department of Otolaryngology, University of Pittsburgh School of Medicine, for allowing us to use the Histology Laboratory Core. In this regard, the technical assistance of Gloria J. Limetti and Jean L. Betsch in tissue processing and embedding is also gratefully acknowledged.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals. Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, van Beek MEB. Possibilities to improve primate spermatogenesis by way of hormonal treatment. In: Hamamah S, Mieusset R, editors. Research in Male Gametes: Production and Quality. Paris: INSERM; 1996. pp. 257–268. [Google Scholar]
- Griswold MD, McLean D. The sertoli cell. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd edn. Vol. 1. San Diego, CA: Elsevier; 2006. pp. 949–975. Chapter 19. [Google Scholar]
- Jacobsen KD, Theodorsen L, Fossa SD. Spermatogenesis after unilateral orchiectomy for testicular cancer in patients following surveillance policy. J Urol. 2001;165:93–96. doi: 10.1097/00005392-200101000-00023. [DOI] [PubMed] [Google Scholar]
- Lee PA, Coughlin MT. The single testis: paternity after presentation as unilateral cryptorchidism. J Urol. 2002;168:1680–1683. doi: 10.1097/01.ju.0000028222.74363.ad. [DOI] [PubMed] [Google Scholar]
- Majumdar SS, Winters SJ, Plant TM. A study of the relative roles of follicle-stimulating hormone and luteinizing hormone in the regulation of testicular inhibin secretion in the rhesus monkey (Macaca mulatta) Endocrinology. 1997;138:1363–1373. doi: 10.1210/endo.138.4.5058. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Wickings EJ, Lüdecke DK, Nieschlag E. Stimulation of spermatogenesis in stalk-sectioned rhesus monkeys by testosterone alone. J Clin Endocrinol Metab. 1983;57:152–159. doi: 10.1210/jcem-57-1-152. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Zorub DS, Plant TM. Follicle-stimulating hormone amplifies the population of differentiated spermatogonia in the hypophysectomized testosterone-replaced adult rhesus monkey (Macaca mulatta) Endocrinology. 1995;136:3504–3511. doi: 10.1210/endo.136.8.7628387. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Ramaswamy S, Plant TM. Gonadotropin independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Biol Reprod. 2005;73:222–229. doi: 10.1095/biolreprod.104.038968. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Rec Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- Medhamurthy R, Aravindan GR, Moudgal NR. Hemiorchidectomy leads to dramatic and immediate alterations in pituitary follicle-stimulating hormone secretion and the functional activity of the remaining testis in the adult male bonnet monkey (Macaca radiata) Biol Reprod. 1993;49:743–749. doi: 10.1095/biolreprod49.4.743. [DOI] [PubMed] [Google Scholar]
- Narula A, Gu YQ, O’Donnell L, Stanton PG, Robertson DM, McLachlan RI, Bremner WJ. Variability in sperm suppression during testosterone administration to adult monkeys is related to follicle stimulating hormone suppression and not to intratesticular androgens. J Clin Endocrinol Metab. 2002;87:3399–3406. doi: 10.1210/jcem.87.7.8681. [DOI] [PubMed] [Google Scholar]
- Odell WD, Swerdloff RS, Jacobs HS, Hescox MA. FSH induction of sensitivity to LH: one cause of sexual maturation in the male rat. Endocrinology. 1973;92:160–165. doi: 10.1210/endo-92-1-160. [DOI] [PubMed] [Google Scholar]
- Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light–dark cycle. Biol Reprod. 1981;25:244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology. 2000;a 141:18–27. doi: 10.1210/endo.141.1.7276. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Plant TM, Marshall GR. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious Sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta) Biol Reprod. 2000;b 63:82–88. doi: 10.1095/biolreprod63.1.82. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, Pohl CR, Friedman RI, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta) Endocrinology. 2003;144:1175–1185. doi: 10.1210/en.2002-221078. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Matthiesson KL, Meachem SJ. Gonadotrophins regulate germ cell survival, not proliferation, in normal adult men. Hum Reprod. 2008;23:403–411. doi: 10.1093/humrep/dem376. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Ramaswamy S, Marshall GR, Plant TM. In the adult male rhesus monkey (Macaca mulatta), unilateral orchidectomy in the face of unchanging gonadotropin stimulation results in partial compensation of testosterone secretion by the remaining testis. Endocrinology. 2004;145:5115–5120. doi: 10.1210/en.2004-0824. [DOI] [PubMed] [Google Scholar]
- Simorangkir DR, Marshall GR, Plant TM. A re-examination of proliferation and differentiation of type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Hum Reprod. 2009 doi: 10.1093/humrep/dep051. doi: 10.1093/humrep/dep051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen MMA, van de Kant HJG, de Rooij DG. Follicle-stimulating hormone stimulates spermatogenesis in the adult monkey. Endocrinology. 1988;123:1449–1455. doi: 10.1210/endo-123-3-1449. [DOI] [PubMed] [Google Scholar]
- Vihko KK, La Polt PS, Nishimori K, Hsueh AJW. Stimulatory effects of recombinant follicle-stimulating hormone on Leydig cell function and spermatogenesis in immature hypophysectomized rats. Endocrinology. 1991;129:1926–1932. doi: 10.1210/endo-129-4-1926. [DOI] [PubMed] [Google Scholar]
- Weinbauer GF, Behre HM, Fingscheidt U, Nieschlag E. Human follicle-stimulating hormone exerts a stimulatory effect on spermatogenesis, testicular size, and serum inhibin levels in the gonadotropin-releasing hormone antagonist-treated nonhuman primate (Macaca fascicularis) Endocrinology. 1991;129:1831–1839. doi: 10.1210/endo-129-4-1831. [DOI] [PubMed] [Google Scholar]
- Young J, Couzinet B, Chanson P, Brailly S, Loumaye E, Schaison G. Effects of human recombinant luteinizing hormone and follicle-stimulating hormone in patients with acquired hypogonadotropic hypogonadism: study of Sertoli and Leydig cell secretions and interactions. J Clin Endocrinol Metab. 2000;85:3239–3244. doi: 10.1210/jcem.85.9.6811. [DOI] [PubMed] [Google Scholar]