Abstract
Iron is an essential metal for virtually all organisms. Iron acquisition is well characterized for various organisms, whereas intracellular iron distribution is poorly understood. In contrast to bacteria, plants, and animals, most fungi lack ferritin-mediated iron storage but possess an intracellular siderophore shown to be involved in iron storage. Here we demonstrate that deficiency in the intracellular siderophore ferricrocin causes iron starvation in conidia of Aspergillus fumigatus, demonstrating that ferricrocin is also involved in intra- and transcellular iron distribution. Thus, ferricrocin represents the first intracellular iron transporter identified in any organism.
Virtually all organisms require iron as an indispensable cofactor for various metabolic processes, including electron transport and redox reactions. Excess or incorrect storage of iron, however, is toxic, as this metal has the capacity to reinforce the production of reactive oxygen species. Therefore, organisms have evolved precisely regulated iron acquisition systems, which are well characterized in numerous prokaryotes and eukaryotes. In contrast, mechanisms for intracellular distribution of acquired iron are poorly understood. We investigated these mechanisms in Aspergillus fumigatus, a typical saprophytic ascomycete, which has become the most common airborne fungal pathogen of humans, causing life-threatening invasive disease especially in immunocompromised patients (16). A. fumigatus employs four siderophores (low-molecular-mass ferric iron chelators) for maintenance of iron homeostasis (5): it excretes two siderophores for solubilization and uptake of iron, and it accumulates two structurally different siderophores, ferricrocin (FC) and its hydroxylated derivative hydroxy-FC, within hyphae and conidia, respectively. Both intracellular siderophores are believed to be involved in intracellular iron storage. The siderophore system became a matter of particular interest as it represents an attractive target for antifungal therapy due to its requirement for virulence of A. fumigatus and its lack in mammalian hosts (12, 13). Recently, extra- and intracellular siderophores have also been implicated in the phytopathogenicity of various ascomycetes (6, 11). Here we demonstrate that intracellular siderophores are also involved in the intracellular long-distance distribution of iron.
Extracellular siderophores are utilized by most fungi and bacteria and some plants, whereas intracellular siderophores are found exclusively in fungi, which in contrast to bacteria, plants, and animals lack ferritin-mediated iron storage (5). The function of intracellular siderophores has been studied in the most detail with Aspergillus nidulans and A. fumigatus (5). Several lines of evidence support a role for FC and hydroxy-FC in iron storage in these fungi. (i) FC accumulation increases under conditions of intracellular iron excess (4, 9, 14); (ii) FC deficiency reduces the iron content of conidia by 34% and 76% in A. nidulans and A. fumigatus, respectively (4, 13); (iii) FC deficiency reduces conidial germination efficiency (4, 13); and (iv) FC deficiency concomitantly increases the labile iron pool and decreases the oxidative stress resistance of hyphae (3, 13). Similarly, FC was found to be involved in hyphal and conidial iron storage in Neurospora crassa (8).
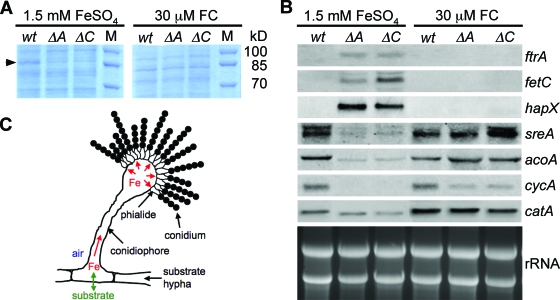
Remarkably, FC deficiency also decreases oxidative stress resistance of conidia in A. nidulans and A. fumigatus (4, 13). In accordance, conidia of FC-deficient A. fumigatus ΔsidA (lacks ornithine monooxygenase, which causes deficiency of all siderophores) and ΔsidC (lacks FC synthetase, which causes deficiency of intracellular siderophores only) mutants display decreased activity of the conidial catalase CatA (13). Further analysis of the conidial proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed decreased intensity of an 85-kDa-protein band in both the ΔsidA and ΔsidC mutants, compared to the wild type, unless the cultures were supplemented with FC during conidiogenesis (Fig. 1A). The protein bands of interest were excised, in-gel digested with trypsin, and analyzed by nanospray-mass spectrometry using an LTQ ion trap instrument (Thermo Finnigan). Protein identification using the SEQUEST algorithm (Thermo-Electron Corp.) revealed that this protein band contains both CatA and aconitase AcoA and that in the FC-deficient mutants CatA and AcoA are reduced in amount to approximately 25% and 15%, respectively, of their levels in the wild type (data not shown). CatA and AcoA are iron-dependent enzymes, the expression of which is transcriptionally repressed during iron starvation via interaction of the CCAAT binding complex with HapX (7, 9, 14). This observation indicates that FC-deficient conidia are iron starved. To further test this hypothesis, we analyzed the expression of previously identified iron-regulated genes by Northern analysis of conidial RNA. Transcript levels of the iron-induced genes catA, acoA, cycA (cytochrome c), and sreA (transcriptional repressor of iron uptake) were substantially decreased in conidia of both the ΔsidA and ΔsidC mutants, compared to the wild type, unless the cultures were supplemented with FC during conidiogenesis (Fig. 1B). On the other hand, transcript levels of the iron-repressed genes ftrA (iron permease involved in reductive iron assimilation), fetC (ferroxidase involved in reductive iron assimilation), and hapX were significantly increased in conidia of both the ΔsidA and ΔsidC mutants, unless supplemented with FC (Fig. 1B).
FIG. 1.
Conidial iron homeostasis of A. fumigatus wild-type (wt), ΔsidA (ΔA), and ΔsidC (ΔC) strains. Conidia were harvested after 5 days of mycelial growth at 37°C on minimal medium plates containing either 1.5 mM FeSO4 or 30 μM FC as the iron source. We have previously shown that FC deficiency causes reduction in production of conidia, which is partially (80%) compensated for by supplementation with large amounts (1.5 mM) of iron and fully cured by small amounts (30 μM) of FC-iron. (A) Analysis of Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel of conidial protein extracts; the 85-kDa proteins reduced in amount in the ΔsidA and ΔsidC mutants are marked by an arrow. (B) Northern analysis of iron-regulated genes in conidia indicating iron starvation in the ΔsidA and ΔsidC mutants. (C) Schematic representation of the role of FC in conidiogenesis. Hyphal iron uptake from the substrate is marked in green; FC-mediated iron transport in aerial cells, which is impaired in the ΔsidA and ΔsidC mutants, is marked in red. Materials and Methods are described in the supplemental material, which includes Table S1.
In contrast to substrate hyphae, which are in close contact with the substrate for nutrient uptake, conidia are produced by conidiophores, which reach into the air and depend on nutrient transport (including iron) from substrate hyphae (Fig. 1C). Taken together, the conidial protein and gene expression patterns (Fig. 1A and B) demonstrate that FC deficiency causes iron starvation in conidia and that FC not only functions in intracellular iron storage but is also responsible for intra- and transcellular transport of iron during conidiogenesis. Consistent with transport of FC from substrate hyphae via conidiophores and phialides to conidia (Fig. 1C), we have previously shown that hydroxy-FC can be isolated from conidia of FC-lacking A. fumigatus mutants when supplemented with FC during conidiogenesis (13). The important role of FC as an intracellular iron carrier is also supported by the fact that FC deficiency causes reduced production of conidia in A. nidulans and A. fumigatus (3, 13) and blocks sexual development in A. nidulans, which also depends on nutritional transport from substrate hyphae (4). Similarly, FC deficiency blocks sexual development in the plant-pathogenic fungi Cochliobolus heterostrophus and Gibberella zeae (10). In contrast to the situation with iron storage molecules, production of iron carriers involved in transport (e.g., bacterial and fungal extracellular siderophores, mammalian transferrin) is upregulated by iron starvation (1, 5, 17). The intracellular iron carrier activity of FC now also explains the upregulation of FC production upon iron starvation found in several fungal species, including A. fumigatus, A. nidulans, and N. crassa (5).
Remarkably, expression analysis of the same iron-affected genes in hyphae from liquid shake cultures did not reveal a significant difference between the wild-type and the FC-deficient ΔsidA and ΔsidC mutants under either iron-replete or iron-depleted conditions (data not shown). Nevertheless, it appears likely that FC also plays a role in the transport of iron in substrate hyphae, as we have shown previously that both FC-deficient mutants have a reduced growth rate under iron-depleted conditions but not under iron-replete conditions (4, 13), which fits better with a role in iron supply than in iron storage. Moreover, under iron-replete conditions, the ΔsidC mutant displayed a slightly increased production of extracellular siderophores and both the ΔsidA and ΔsidC mutants showed an about twofold-increased iron content (4, 13), suggesting that substrate hyphae compensate for the lack of FC by increased iron uptake, which is not possible for aerial cells such as conidiophores and conidia. The slightly increased siderophore production of the ΔsidC mutant indicates involvement of siderophore-mediated iron uptake in the increase of the total iron content. The ΔsidA mutant lacks siderophore biosynthesis, and therefore only reductive or low-affinity iron uptake can account for the increased iron content in this mutant. Remarkably, neither the ΔsidA nor the ΔsidC mutant showed increased expression of genes involved in reductive iron assimilation or siderophore biosynthesis, at least when analyzed by Northern blotting (data not shown). This might be due to the insufficient sensitivity of this technique or to the possibility that a slight increase in the steady-state mRNA level of genes involved in iron acquisition is sufficient for the twofold increase in the total iron content.
FC deficiency results in the reduced virulence of A. fumigatus in a murine aspergillosis model (13) and of Magnaporthae grisea on rice (6). The data presented here suggest that FC-deficient conidia not only lack FC-iron stores but additionally display reduced activity of iron-dependent pathways, which negatively affects their resistance to hostile conditions during pathogenic growth.
In Escherichia coli, the mobile ferrous iron pool was found to be bound to a phosphorylated sugar derivative (2), and recently, human poly(rC)-binding protein 1 was shown to function as a cytosolic iron chaperone in the delivery of iron to ferritin (15). However, it is not known if either of these two iron carriers is involved in long-distance distribution of iron.
Most fungi produce an intracellular siderophore (5), indicating the evolutionary conservation of siderophore-mediated intracellular iron transport. Exceptions include Saccharomyces cerevisiae and Candida albicans, yeast species without aerial hyphae that depend on iron transport from substrate hyphae, which might explain the loss of siderophore biosynthesis in these species.
Supplementary Material
Acknowledgments
This work was supported by Austrian Science Foundation grant FWF-P18606-B11 (to H.H.).
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 2.Böhnke, R., and B. F. Matzanke. 1995. The mobile ferrous iron pool in Escherichia coli is bound to a phosphorylated sugar derivative. Biometals 8:223-230. [DOI] [PubMed] [Google Scholar]
- 3.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 4.Eisendle, M., M. Schrettl, C. Kragl, D. Müller, P. Illmer, and H. Haas. 2006. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 5:1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas, H., M. Eisendle, and G. B. Turgeon. 2008. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 46:149-187. [DOI] [PubMed] [Google Scholar]
- 6.Hof, C., K. Eisfeld, K. Welzel, L. Antelo, A. J. Foster, and H. Anke. 2007. Ferricrocin synthesis in Magnaporthae grisaea and its role in pathogenicity in rice. Mol. Plant Pathol. 8:163-172. [DOI] [PubMed] [Google Scholar]
- 7.Hortschansky, P., M. Eisendle, Q. Al-Abdallah, A. D. Schmidt, S. Bergmann, M. Thon, O. Kniemeyer, B. Abt, B. Seeber, E. R. Werner, M. Kato, A. A. Brakhage, and H. Haas. 2007. Interaction of HapX with the CCAAT-binding complex—a novel mechanism of gene regulation by iron. EMBO J. 26:3157-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzanke, B. F., E. Bill, A. X. Trautwein, and G. Winkelmann. 1988. Ferricrocin functions as the main intracellular iron-storage compound in mycelia of Neurospora crassa. Biol. Met. 1:18-25. [DOI] [PubMed] [Google Scholar]
- 9.Oberegger, H., M. Schoeser, I. Zadra, B. Abt, and H. Haas. 2001. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 41:1077-1089. [DOI] [PubMed] [Google Scholar]
- 10.Oide, S., S. B. Krasnoff, D. M. Gibson, and B. G. Turgeon. 2007. Intracellular siderophores are essential for ascomycete sexual development in heterothallic Cochliobolus heterostrophus and homothallic Gibberella zeae. Eukaryot. Cell 6:1339-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oide, S., W. Moeder, S. Krasnoff, D. Gibson, H. Haas, K. Yoshioka, and B. G. Turgeon. 2006. NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell 18:2836-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrettl, M., E. Bignell, C. Kragl, C. Joechl, T. Rogers, H. N. Arst, Jr., K. Haynes, and H. Haas. 2004. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 200:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrettl, M., E. Bignell, C. Kragl, Y. Sabiha, O. Loss, M. Eisendle, A. Wallner, H. N. Arst, K. Haynes, and H. Haas. 2007. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 3:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrettl, M., H. S. Kim, M. Eisendle, C. Kragl, W. C. Nierman, T. Heinekamp, I. Jacobsen, E. R. Werner, A. A. Brakhage, and H. Haas. 2008. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 70:27-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi, H., K. Z. Bencze, T. L. Stemmler, and C. C. Philpott. 2008. A cytosolic iron chaperone that delivers iron to ferritin. Science 320:1207-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekaia, F., and J. P. Latge. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385-392. [DOI] [PubMed] [Google Scholar]
- 17.Weiss, G., and L. T. Goodnough. 2005. Anemia of chronic disease. N. Engl. J. Med. 352:1011-1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.