Abstract
Magnetotactic bacteria have the unique capacity of synthesizing intracellular single-domain magnetic particles called magnetosomes. The magnetosomes are usually organized in a chain that allows the bacteria to align and swim along geomagnetic field lines, a behavior called magnetotaxis. Two mechanisms of magnetotaxis have been described. Axial magnetotactic cells swim in both directions along magnetic field lines. In contrast, polar magnetotactic cells swim either parallel to the geomagnetic field lines toward the North Pole (north seeking) or antiparallel toward the South Pole (south seeking). In this study, we used a magnetospectrophotometry (MSP) assay to characterize both the axial magnetotaxis of “Magnetospirillum magneticum” strain AMB-1 and the polar magnetotaxis of magneto-ovoid strain MO-1. Two pairs of Helmholtz coils were mounted onto the cuvette holder of a common laboratory spectrophotometer to generate two mutually perpendicular homogeneous magnetic fields parallel or perpendicular to the light beam. The application of magnetic fields allowed measurements of the change in light scattering resulting from cell alignment in a magnetic field or in absorbance due to bacteria swimming across the light beam. Our results showed that MSP is a powerful tool for the determination of bacterial magnetism and the analysis of alignment and swimming of magnetotactic bacteria in magnetic fields. Moreover, this assay allowed us to characterize south-seeking derivatives and non-magnetosome-bearing strains obtained from north-seeking MO-1 cultures. Our results suggest that oxygen is a determinant factor that controls magnetotactic behavior.
Magnetotactic bacteria are morphologically, metabolically, and phylogenetically diverse prokaryotes (1, 11). They synthesize unique intracellular organelles, the magnetosomes, which are single-domain magnetic crystals of the mineral magnetite or greigite enveloped by membranes. Magnetosomes are usually organized in a chain(s) within the cell and cause the cell to align along geomagnetic field lines while it swims. The highest numbers of magnetotactic bacteria are generally found at, or just below, the oxic-anoxic transition zone (OATZ) or redoxocline in aquatic habitats (1). Early studies showed that Northern Hemisphere magnetotactic bacteria swim preferentially northward in parallel with the geomagnetic field lines (north seeking [NS]) (2) and that those from the Southern Hemisphere swim preferentially antiparallel to the geomagnetic field lines to the magnetic South Pole (south seeking [SS]) (4). The geomagnetic field is inclined downward from horizontal in the Northern Hemisphere and upward in the Southern Hemisphere, with the inclination magnitude increasing from the equator to the poles. Therefore, magnetotaxis might guide cells in each hemisphere downward to less-oxygenated regions of aquatic habitats, where they would presumably stop swimming until conditions change (1). A recent study reported the coexistence of both NS and SS magnetotactic bacteria in the Northern Hemisphere, which conflicts with the prevalent model of the adaptive value of magnetotaxis (14).
Under laboratory conditions, magnetotactic bacteria form microaerophilic bands of cells in oxygen-gradient medium. In fact, magnetotaxis and aerotaxis work together in these bacteria, and the behavior observed has been referred to as “magnetoaerotaxis.” Two different magnetoaerotactic mechanisms, termed polar and axial, are found in different bacterial species (6). The magnetotactic bacteria, principally the magnetotactic cocci, that swim persistently in one direction along the magnetic field (NS or SS) are polar magnetoaerotactic. Magnetotactic bacteria, especially the freshwater spirilla, that swim in either direction along the magnetic field lines with frequent, spontaneous reversals of swimming direction without turning around are axial magnetoaerotactic. For polar magnetotactic bacteria, the magnetic field provides an axis and a direction for motility, whereas for axial magnetotactic bacteria, the magnetic field provides only an axis of motility. The two mechanisms can best be seen in flattened capillary tubes containing suspensions of cells in reduced medium in a magnetic field oriented parallel to the capillary. An oxygen gradient forms along the tube, beginning at the ends of the capillary, with one oriented parallel and the other antiparallel to the magnetic field (1). Band formation by axial magnetoaerotactic cells, such as Magnetospirillum magnetotacticum cells, occurs at both ends of the capillary. Rotation of the magnetic field by 180° after the formation of the bands causes the cells in both bands to rotate 180°, but the bands remain intact. In contrast, band formation by polar magnetoaerotactic cells, such as the marine cocci, occurs only at the end of the capillary for which the magnetic field and the oxygen concentration gradient are oriented opposite to each other. Rotation of the magnetic field by 180° after the formation of the band causes the cells in the band to rotate 180° and swim away, resulting in the dispersal of the band (1). In this study, we developed a magnetospectrophotometry (MSP) assay that provides an alternative method for the quantitative and versatile characterization of the two magnetotactic mechanisms. Using this assay, we demonstrated the effect of artificial magnetic fields on the generation of homogeneous NS or SS magnetotactic bacterial populations.
MATERIALS AND METHODS
Growth and sequence analysis of magnetotactic bacteria.
In this study, the magnetotactic bacterial strains used were “Magnetospirillum magneticum” AMB-1 (ATCC 700264) and magneto-ovoid strain MO-1 (8). The AMB-1 cells were routinely incubated in EMSGM medium (ATCC 1653), whereas MO-1 cells were grown in EMS2 medium (8). Under microaerobic growth conditions, screw-cap glass culture tubes were filled with medium generally up to four-fifths of their volume and sealed with rubber stoppers. Cultures were incubated at 24°C to 28°C.
To identify non-magnetosome-bearing cells, the 16S rRNA gene was amplified using primers Eu5b-F (5′-AGAGTTTGATCATGGCTCAGA-3′) and Eu3-R (5′-AAGGAGGTGATCCAGCC-3′). PCR was carried out as follows: template DNA was initially denatured at 94°C for 2 min, followed by 30 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C and a final extension step of 10 min at 72°C. The PCR product was sequenced.
Spectrophotometer-based magnetotaxis assay.
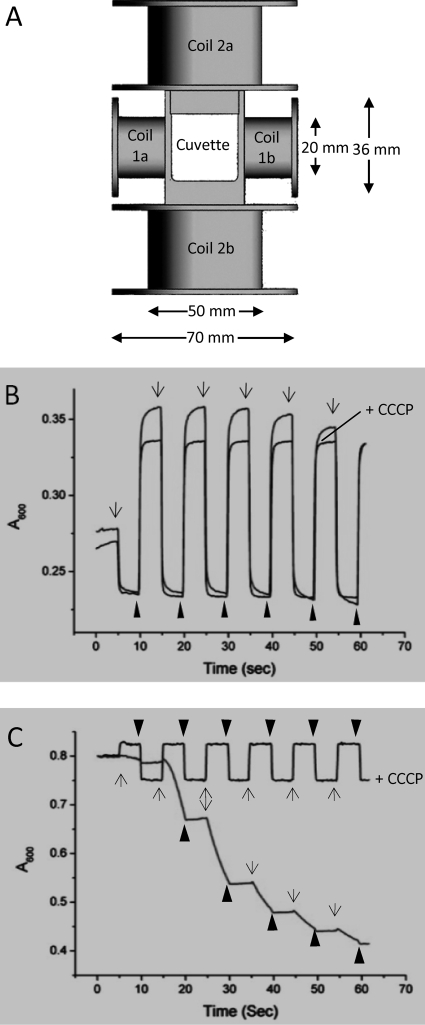
Recently, Zhao et al. developed a simple apparatus for measuring the magnetism of magnetotactic bacteria. Two pairs of Helmholtz coils were mounted onto the cuvette holder of a common laboratory spectrophotometer to generate two mutually perpendicular homogeneous magnetic fields, which allow measurements of the change in light scattering resulting from cell alignment in a magnetic field (15). To extend the utility of this method, we adapted this device to a Varian Cary 50 UV-visible spectrophotometer, which incorporates a xenon flash lamp (Fig. 1A). The coils parallel to the light beam were built with 0.4-mm-diameter copper wire for 2 × 472 turns with a resistance of 12.0 Ω, whereas the perpendicular coils were built with 0.65-mm-diameter copper wire for 2 × 218 turns with a resistance of 4.3 Ω. At 0.5 V with the power supply used, it generates currents of 0.042 A and 0.116 A, with current densities of 0.33 A/mm2 and 0.35 A/mm2, in the parallel and perpendicular coils, respectively. The magnetic flux densities of the corresponding static magnetic fields were both 1.9 mT at 20°C. This device can generate magnetic fields with reliable magnetic flux densities ranging from 0 mT to up to 6 mT by controlling the supplied current density. The earth's magnetic field is not cancelled because of the limited available space around the cuvette holder. The geomagnetic field (50 μT) is negligible compared to the applied magnetic fields generated by copper coils.
FIG. 1.
Characterization of axial and polar magnetotactic behaviors by using an MSP assay. (A) Schematic presentation (not in scale) of two pairs of Helmholtz coils mounted onto the cuvette holder to create homogeneous magnetic fields parallel (coils 1a and 1b) or perpendicular (coils 2a and 2b) to the light beam. (B and C) Typical MSP profiles of axial magnetotactic cells of M. magneticum strain AMB-1 and polar magnetotactic MO-1 cells, respectively. The MSP profiles obtained with the flagellar motor inhibitor CCCP (25 μM) are indicated as +CCCP. The application of perpendicular (arrows) and parallel (arrowheads) magnetic fields is indicated.
Optic and electron microscopy.
The swimming behavior of magnetotactic bacteria was investigated by the “hanging-drop” method (12) by using a phase-contrast microscope (Axiostar Plus; Zeiss). A Canon Powershot G6 camera was used for photographing the bacteria and recording the swimming behavior. The bacteria, deposited on Formvar-carbon-coated grids (5), were examined with a Zeiss EM9 microscope at 80 kV for characterization of magnetosomes.
RESULTS AND DISCUSSION
Characterization of axial and polar magnetotaxis by using the MSP assay.
Light-scattering methods have been developed to assay the magnetism of Magnetospirillum spp. (9, 13). Magnetic spirilla are aspherical inhomogeneous objects that can be modeled as optically anisotropic cylinders. Their scattering depends on the magnetic alignment of individual cells with respect to the direction of the incident light beam. Maximum scattering occurs if the applied magnetic field is directed parallel to the light beam, whereas a perpendicular orientation with respect to the light results in minimal scattering (9, 13). Based on the same principle, Zhao et al. developed a simple apparatus to be used with common laboratory spectrophotometers for measuring the magnetism of magnetotactic bacteria (15). We adapted this device to a Varian Cary 50 UV-visible spectrophotometer. As the xenon lamp beam of the Cary 50 apparatus is narrow and very intense, this spectrophotometer has an excellent signal-to-noise ratio and is highly sensitive to the quantity of cells across the beam. Moreover, the change in light absorbance with time (MSP profile) reflects the real-time reaction of bacteria to the application of a magnetic field. We chose spiral AMB-1 cells and ovoid MO-1 cells as models to develop the MSP assay, as their morphological differences result in distinct optically anisotropic properties. In addition, the former strain displays axial magnetotaxis, whereas the latter strain is a polar magnetotactic bacterium.
We first performed the MSP assay with axial magnetotactic Magnetospirillum strain AMB-1 cells. As anticipated, the application of a homogenous magnetic field (1.9 mT) perpendicular to the light beam decreased absorbance, whereas changing the magnetic field orientation parallel to the light beam increased absorbance (Fig. 1B). Similar profiles with the highest and lowest absorbance values were observed during the first four cycles of the magnetic field reversal (Fig. 1B). The addition of the flagellum motor inhibitor carbonyl cyanide m-chloro phenyl hydrazone (CCCP) had no effect on the MSP profile except that the maximal absorbance decreased and remained more stable during the repeated alternation of the direction of the magnetic field (Fig. 1B). Therefore, the application of the homogeneous magnetic fields affected mainly the alignment of the axial magnetotactic spirilla that remained within the region detectable by the light beam. Notably, it took about 5 s for the swimming cells to completely align within the field (reaching a stable absorbance), whereas it took only about 1 s when the swimming was abolished by CCCP.
The ovoid, polar, magnetotactic MO-1 cells exhibited different MSP profiles from those of the spirilla. The application of a perpendicular magnetic field decreased the absorbance because the cells swam out of the light beam path toward the north pole (Fig. 1C). The addition of CCCP abolished flagellum-propelled swimming and led to an MSP profile similar to that of Magnetospirillum strain AMB-1. However, the difference between the maximal and minimal absorbances for the ovoid MO-1 cells (Fig. 1C) is smaller than that for the elongated helical AMB-1 cells (Fig. 1B) due to their optically anisotropic properties. Moreover, the application of a parallel magnetic field decreased the absorbance of the MO-1 cells but increased that of the AMB-1 cells (Fig. 1C versus B, respectively). This is probably because the MO-1 cells possess several lipid storage globules that lead to a negative optical index. Together, these results showed that the MSP assay easily distinguishes axial magnetotactic bacteria from polar magnetotactic bacteria.
Potential of the MSP assay for deciphering the magnetotaxis mechanism.
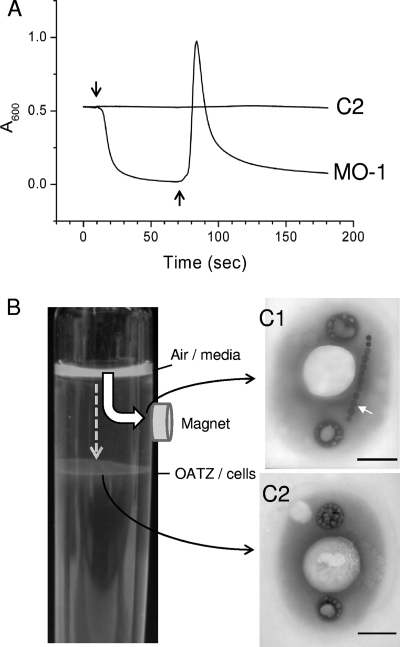
Due to the polar magnetotactic property, the absorbance of the MO-1 cell suspension decreased rapidly upon the application of a perpendicular homogeneous magnetic field (Fig. 2A). The subsequent reversal of the magnetic field, defined as being antiperpendicular (Fig. 2A), led to the appearance of an asymmetric peak with a sharp increase in the maximum and a lagging decrease in absorbance. We interpret this MSP profile as follows. The application of a homogeneous magnetic field perpendicular to the light beam guides the MO-1 cells to swim out of the light path and accumulate at the north side of the cuvette. The subsequent reversal of the magnetic field direction triggers the cells to swim from one side of the cuvette to the new north pole; the passage of the cell swarm across the narrow light beam results in a rapid increase in the absorbance to the maximum and then a decrease to the background level. The width of the peak reflects how synchronously the population reacts to the reversal of the magnetic field. This interpretation was confirmed by direct observations of the cuvette (data not shown).
FIG. 2.
Detection of non-magnetosome-bearing bacteria by MSP assay. (A) An MSP assay was performed with strain MO-1 and non-magnetosome-bearing cells (C2) collected from the OATZ as indicated in B. Downward and upward arrows indicate the application of uniform magnetic fields of 0.19 mT perpendicular and antiperpendicular to the light beam, respectively. (B) A permanent magnet (0.4 T) was fixed between the OATZ and the medium surface on the culture tube. MO-1 cells were inoculated on the surface of the medium (Air/media) and swam downward for the optimal OATZ position. Most cells were attracted to the magnet (wide arrow), and few cells could reach the OATZ and grew at this position (dashed-line arrow). Samples were taken at the position beneath the magnet (C1) or at the OATZ (C2) and inspected by TEM. Scale bars, 0.5 μm.
We sought to corroborate this hypothesis by using non-magnetosome-bearing cells. To isolate such cells, we fixed a magnet (0.4 T) between the OATZ and the medium surface on the culture tube. We reasoned that only the nonmagnetic bacteria could reach the OATZ, whereas magnetic cells should be attracted to the magnet before reaching the OATZ (Fig. 2B). This procedure should allow us to select non-magnetosome-bearing cells. An MO-1 culture was inoculated onto the surface of the medium, and cells swam downward toward the OATZ position optimal for their growth. Most cells were attracted to the magnet, and only few cells reached the OATZ and grew at this position. In addition, it took 10 days for cells to form the growth halo, compared to a few hours in the absence of the magnet. The cells at the OATZ were removed and inoculated into fresh medium. The process was repeated 10 times; fewer and fewer cells were recovered beneath the magnet, and more and more cells were located at the OATZ. The cells collected at the position beneath the magnet possessed magnetosomes (Fig. 2C1) and displayed an MSP profile similar to that of the MO-1 cells. In contrast, the cells located at the OATZ did not have magnetosomes (Fig. 2C2) and displayed a flat MSP profile (Fig. 2A and C2). The 16S rRNA genes were amplified from non-magnetosome-bearing cells and shared 99.87% (1,491/1,493) sequence identity with MO-1. At present, we do not know whether non-magnetosome-bearing cells, called MO-2 cells, belong to a species closely related to MO-1 or are mutant derivatives of MO-1. Nevertheless, the imposed growth conditions allowed the isolation of non-magnetosome-bearing cells that validate the usefulness of the MSP assay in the distinction of magnetic cells from nonmagnetic cells.
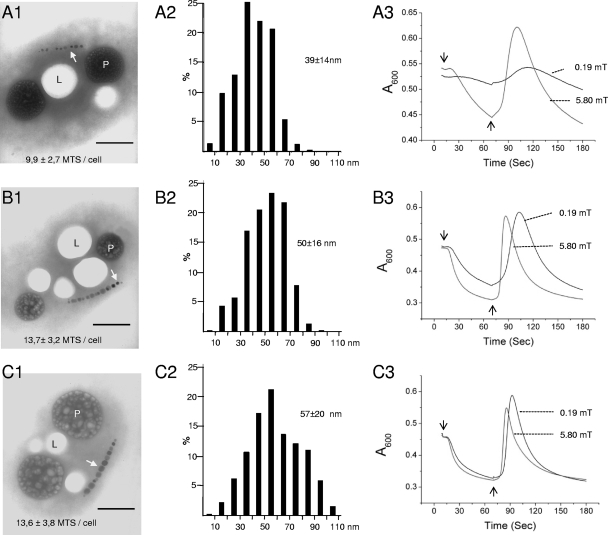
We then analyzed the influence of magnetosome number and size on magnetotaxis. In general, iron concentration influences the number and size of magnetosomes (7, 10). As anticipated, the incubation of MO-1 cells without the addition of ferric quinate resulted in reduced numbers and sizes of magnetosomes (Fig. 3A1 and 3A2) compared to the cells grown with 5 μM (Fig. 3B1 and B2) or 20 μM (Fig. 3C1 and C2) ferric quinate. The decreased number and size of magnetosomes seemed to correlate with a flattened MSP curve when the cells were examined under a 0.19-mT uniform magnetic field (Fig. 3A3). Increasing the magnetic flux density to 5.80 mT substantially changed the MSP profile: an accelerated negative absorbance slope and a reduced time for the appearance of the reaction peak (Fig. 3A3). The cells grown with 5 μM or 20 μM ferric quinate showed similar MSP profiles measured with different magnetic flux densities (Fig. 3B3 and C3). Together, these results show that the MSP assay allows a quick analysis of the cell magnetism of polar magnetotactic bacteria.
FIG. 3.
Characterization of MO-1 magnetotaxis by MSP assay. MO-1 cells were incubated in EMS2 medium without the addition of ferric quinate (A) or with 5 μM (B) or 20 μM (C) ferric quinate for 3 days at 24°C. The cells were inspected by TEM, and average numbers of magnetosomes per cell, with standard deviations, were obtained from analyses of 64 (A1), 73 (B1), and 73 (C1) cells. Bars, 0.5 μm. L and P indicate lipid storage granules and phosphorus-rich inclusions, respectively, as previously described (8). White arrows indicate magnetosomes. The size distributions of 426 (A2), 447 (B2), and 462 (C2) magnetosomes are shown as mean sizes with standard deviations. An MSP assay (A3, B3, and C3) was performed with the application of uniform magnetic fields of either 0.19 mT or 5.80 mT perpendicular (downward arrows) or antiperpendicular (upward arrows) to the light beam.
The time gap from the reversal of the magnetic field to reaching the maximal absorbance implies the time needed for cells to swim from the cuvette wall to the light-beam path. This time gap is influenced by two parameters, the speed of cells to align along the magnetic field lines and the bacterial swimming velocity. The magnetic torque, T, is the key factor that influences bacterial reactivity to the change of magnetic field. It can be expressed as follows: T = P × B × sinθ, where B is the applied magnetic flux density, P is the magnetic moment of a magnetotactic cell, and θ is the angle between the magnetic moment of the magnetotactic cell and the applied magnetic field. Therefore, the magnetic torque, T, increases proportionally with the augmentation of applied magnetic flux density. As a consequence, the reversal of a stronger applied field will cause a faster reversal of the swimming direction.
We estimated the MSP-assessed swimming velocities by dividing the distance between the cuvette wall and the light beam by the time gap and compared them with the swimming velocities measured directly using light microscopy. As shown in Table 1, the swimming velocities were about 170 μm/s for cells grown with different concentrations of ferric quinate. The application of an artificial magnetic field affected only swimming direction but had no effect on the swimming velocity. In contrast, the MSP-assessed velocity depended on the cellular magnetosome number and size. The higher concentration of ferric quinate added to the growth medium resulted in a higher MSP-assessed velocity of the cells (Table 1). In addition, increasing the magnetic flux density augmented the MSP-assessed velocity. We anticipate that the MSP assay will be a powerful means for studying the magnetotaxis mechanism, as it is potentially feasible to quantify the reaction time needed for magnetotactic bacteria to align in magnetic fields.
TABLE 1.
Motilities of MO-1 cells under different conditions
| Ferric quinate concn (μM)a | Mean motility (μm/s) ± SD determined by microscopeb
|
MSP-assessed velocity (μm/s)c at:
|
||
|---|---|---|---|---|
| Without magnet | With magnet | 0.19 mT | 8.79 mT | |
| 0 | 166 ± 39 | 168 ± 40 | 60 | 104 |
| 2.5 | 174 ± 40 | 174 ± 42 | 73 | 108 |
| 5.0 | 169 ± 39 | 173 ± 44 | 79 | 159 |
| 7.5 | 172 ± 44 | 170 ± 39 | 93 | 159 |
| 20.0 | 180 ± 46 | 177 ± 45 | 117 | 169 |
MO-1 cells were incubated at 24°C for 3 days in EMS2 medium without or with ferric quinate at the indicated concentration.
Motility (μm/s) was measured under an optical microscope without or with a 1,080-mT neodymium iron boron (NdFeB) magnet (20 mm by 10 mm by 5 mm) placed about 8 cm from the droplet of the cell suspension. The magnetic moment of the magnet is 0.86 A·m2. Its created field is about 0.34 mT.
A uniform electromagnetic field was applied to the cuvette holder at the magnetic flux density as indicated. The MSP-assessed velocity (μm/s) was calculated by dividing the distance between the cuvette wall and the light beam by the time needed for the appearance of the absorbance peak after the reversal of the direction of the magnetic field.
Distinction between NS and SS populations.
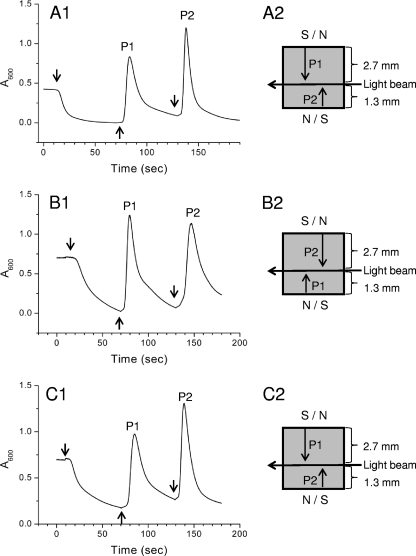
The light beam of our spectrophotometer is asymmetrically positioned with respect to the width of the cuvette (Fig. 4). Therefore, the time needed for cells to swim from one side of the cuvette to the light beam is different for the same cells to swim from the other side to the light beam. As shown in Fig. 4A, the absorbance of the MO-1 cell suspension at 600 nm decreased upon the application of a perpendicular, homogeneous magnetic field (1.9 mT) (Fig. 4A1). The cells accumulated at the north side of the cuvette. The reversal of the magnetic field, defined as being antiperpendicular (Fig. 4A1), led to the appearance of an asymmetric peak (P1) (Fig. 4A1). The subsequent reversal of the magnetic field back to the perpendicular orientation resulted in the appearance of a second peak, P2 (Fig. 4A1). Notably, the second peak (P2) is higher and sharper than the first peak. In fact, in reaction to the first reversal of the magnetic field, the cells must travel 2.7 mm from the cuvette wall to the light beam (P1) (Fig. 4A2). In contrast, the second reversal of the magnetic field guided the cells swimming 1.3 mm from the cuvette wall to the light path (P2) (Fig. 4A2). In the second case, the distance for cells to reach the light path was shorter, and the cell swarm remained better grouped. As a consequence, the second peak, P2, is higher and sharper than the first peak, P1.
FIG. 4.
Detection of NS and SS magnetotactic bacteria. An MSP assay was performed with cells incubated in culture tubes in a geomagnetic field (A1) or in artificial magnetic fields opposite to (B1) or parallel to (C1) the geomagnetic field. Downward and upward arrows indicate that the applied 1.9-mT uniform electromagnetic field is perpendicular or antiperpendicular to the light beam of the spectrophotometer, respectively. P1 and P2 indicate the first and second absorbance peaks of cells swarming across the light beam in reaction to the reversal of the magnetic field. (A2, B2, and C2) Trajectories of the cell swarm leaving the cuvette wall to reach the light beam, leading to P1 and P2 in A1, B1, and C1. The reversal of the magnetic field is indicated by S/N or N/S, and the distances from the cuvette wall to the light beam are indicated on the right.
To corroborate this hypothesis and to validate the use of the MSP assay to distinguish NS from SS cells, we needed SS cells. Changing the magnetic polarity of magnetotactic bacteria was previously reported by Blakemore (3). That author observed that SS bacteria progressively increased in number and became predominant after 3 weeks when a magnetic field opposite to the geomagnetic field was applied to an NS bacterial culture. We sought to generate an SS population from the MO-1 culture by fixing a 0.4-T magnet on the culture tube a few millimeters below the OATZ with the north end in contact with the tube. In this case, the artificial magnetic field had the orientation opposite to the geomagnetic field. Therefore, the NS cells were imposed to swim toward the surface of the medium, away from the OATZ located in the growth medium. The cells grew as a diffuse zone, with the highest cellular density beneath the medium-air surface. They were reinoculated into fresh medium and incubated under the same conditions. After 10 subsequent inoculations, the cells obtained displayed SS behavior when analyzed in droplets using light microscopy. The first peak of their MSP profile was higher and sharper than the second peak when a parallel-antiparallel-parallel sequential alternative magnetic field was applied in the MSP assay (Fig. 4B1). Intriguingly, we obtained NS dominant cells when the SS derivatives were repeatedly incubated under similar conditions but with the magnet fixed in the opposite direction (Fig. 4C). These results suggest that when the cells were “guided” to the surface of the medium, the exposure to a high oxygen level forced the population of cells to change their polarity in order to continue to swim downward to the position with the lower oxygen level. More specifically, it seems that the cells with the polarity that causes them to swim downward toward the OATZ are selected for and that those with the opposite polarity, which causes them to swim upward toward high concentrations of oxygen, are selected against and presumably die. Therefore, aerotaxis might dominate over magnetotaxis.
The linearly arranged magnetosomes impart a net magnetic dipole moment to the bacterium. The magnetic dipoles must be systematically oriented, with the north pole forward for the NS magnetotactic bacteria and vice versa for the SS cells. It should be noted that significant numbers of SS cells coexisted and sometimes outnumbered NS cells of one particular species of magnetotactic bacteria (14). The coexistence of magnetotactic bacteria with north and south polarities in the same chemical environment contradicts the prevalent model of magnetotaxis. Although the existence of SS magnetotactic bacteria in the Northern Hemisphere appears to be an enigma, a significant relationship between the oxidation reduction potential (ORP) and the percentage of magnetotactic bacteria with south polarity was determined (14). At a given sampling site and in a given season, total numbers of magnetotactic bacteria peaked between ORP values of −50 and −200 mV. The average percentage of SS bacteria is higher under more-oxidizing conditions (ORP of around −50) than under more-reducing conditions (ORP of around −200). More in situ studies are required to provide an explanation to account for the existence of SS bacteria in the Northern Hemisphere. Pinpoint adjustment of the MSP assay (light beam position and bacterial concentration in samples) may allow a quantitative characterization of SS and NS populations in a sample with minimal interference with their natural magnetic polarity. Taken together, the MSP assay allows a quantitative and versatile characterization of the axial and polar magnetotactic mechanisms and quick analysis of the magnetism of magnetotactic bacterial cultures.
Acknowledgments
This work was supported by HFSP RGP0035/2004-C.
We are grateful to Olivier Maloberti for designing and constructing Helmholtz coils, Chuanfan Chen for assistance in the initial MSP assay, Wei-Jia Zhang for 16S rRNA gene analysis, and Kui Yu-Zhang and Alain Bernadac for advice and assistance in the characterization of magnetosome crystals. We thank Y. Fukumori, N. Pradel, C.-L. Santini, and N. Philippe for discussions and suggestions and D. A. Bazylinski for critical reading and English correction of the manuscript. MO-1 is available from the Service Partenariat et Valorisation upon request (spv@dr12.cnrs.fr).
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Bazylinski, D. A., and R. B. Frankel. 2004. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2:217-230. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore, R. P. 1975. Magnetotactic bacteria. Science 190:377-379. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore, R. P. 1982. Magnetotactic bacteria. Annu. Rev. Microbiol. 36:217-238. [DOI] [PubMed] [Google Scholar]
- 4.Blakemore, R. P., R. B. Frankel, and A. J. Kalmijn. 1980. South-seeking magnetotactic bacteria in the Southern Hemisphere. Nature 286:384-385. [Google Scholar]
- 5.Epstein, M. A. 1956. A technique for transferring cells prepared on Formvar coated slides to electron microscope specimen grids. J. R. Microsc. Soc. 75:100-102. [DOI] [PubMed] [Google Scholar]
- 6.Frankel, R. B., D. A. Bazylinski, M. S. Johnson, and B. L. Taylor. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 73:994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komeili, A., H. Vali, T. J. Beveridge, and D. K. Newman. 2004. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc. Natl. Acad. Sci. USA 101:3839-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefèvre, C. T., A. Bernadac, K. Yu-Zhang, N. Pradel, and L.-F. Wu. 10 February 2009, posting date. Isolation and characterization of a magnetotactic bacterium from the Mediterranean Sea. Environ. Microbiol. doi: 10.1111/j.1462-2920.2009.01887.x. [DOI] [PubMed]
- 9.Rosenblatt, C., F. F. Torres de Araujo, and R. B. Frankel. 1982. Light scattering determination of magnetic moments of magnetotactic bacteria. J. Appl. Phys. 53:2727-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheffel, A., A. Gardes, K. Grunberg, G. Wanner, and D. Schuler. 2008. The major magnetosome proteins MamGFDC are not essential for magnetite biomineralization in Magnetospirillum gryphiswaldense but regulate the size of magnetosome crystals. J. Bacteriol. 190:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schüler, D. 2008. Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol. Rev. 32:654-672. [DOI] [PubMed] [Google Scholar]
- 12.Schüler, D. 2002. The biomineralization of magnetosomes in Magnetospirillum gryphiswaldense. Int. Microbiol. 5:209-214. [DOI] [PubMed] [Google Scholar]
- 13.Schüler, D., R. Uhl, and E. Baeuerlein. 1995. A simple light scattering method to assay magnetism in Magnetospirillum gryphiswaldense. FEMS Microbiol. Lett. 132:139-145. [Google Scholar]
- 14.Simmons, S. L., D. A. Bazylinski, and K. J. Edwards. 2006. South-seeking magnetotactic bacteria in the Northern Hemisphere. Science 311:371-374. [DOI] [PubMed] [Google Scholar]
- 15.Zhao, L., D. Wu, L.-F. Wu, and T. Song. 2007. A simple and accurate method for quantification of magnetosomes in magnetotactic bacteria by common spectrophotometer. J. Biochem. Biophys. Methods 70:377-383. [DOI] [PubMed] [Google Scholar]