Abstract
There are few appropriate single-copy genetic tools for most Burkholderia species, and the high level of antibiotic resistance in this genus further complicates the development of genetic tools. In addition, the utilization of resistance genes for clinically important antibiotics is prohibited for the bioterrorism agents Burkholderia pseudomallei and Burkholderia mallei, necessitating the development of additional nonantibiotic-based genetic tools. Three single-copy systems devoid of antibiotic selection based on two nonantibiotic selectable markers, tellurite resistance (Telr) and Escherichia coli aspartate-semialdehyde dehydrogenase (asdEc), were developed to facilitate genetic manipulation in Burkholderia species. These systems include one mariner transposon, a mini-Tn7-derived site-specific transposon, and six FRT reporter fusion vectors based on the lacZ, gfp, and luxCDABE reporter genes. Initially, we showed that the random mariner transposon pBT20-Δbla-Telr-FRT efficiently transposed within Burkholderia cenocepacia, Burkholderia thailandensis, B. pseudomallei, and B. mallei. We then utilized the mini-Tn7-Telr-based transposon vector (mini-Tn7-Telr-betBA) and a transposase-containing helper plasmid (pTNS3-asdEc) to complement the B. thailandensis ΔbetBA mutation. Next, one of the FRT-lacZ fusion vectors (pFRT1-lacZ-Telr) was integrated by Flp (encoded on a helper plasmid, pCD13SK-Flp-oriT-asdEc) to construct the B. thailandensis ΔbetBA::FRT-lacZ-Telr reporter fusion strain. The betBA operon was shown to be induced in the presence of choline and under osmotic stress conditions by performing β-galactosidase assays on the B. thailandensis ΔbetBA::FRT-lacZ-Telr fusion strain. Finally, we engineered B. thailandensis ΔbetBA::FRT-gfp-Telr and ΔbetBA::FRT-lux-Telr fusion strains by utilizing fusion vectors pFRT1-gfp-Telr and pFRT1-lux-Telr, respectively. The induction of the betBA operon by choline and osmotic stress was confirmed by performing fluorescent microscopy and bioluminescent imaging analyses.
The genus Burkholderia, consisting of more than 40 different species, occupies diverse ecological niches ranging from the soil rhizosphere to the human respiratory tract (39). Within this genus, members exhibit considerable genetic diversity and broad metabolic capabilities (26, 39), facilitating their adaptation to a variety of environmental conditions including nutrient limitation, the presence of antibiotics and toxic compounds, and pH fluctuations. Many Burkholderia species are known plant pathogens, including Burkholderia caryophylli, B. plantarri, and B. glumae, while others (e.g., B. cepacia complex) cause opportunistic infections (39). In addition, Burkholderia pseudomallei and B. mallei are primary pathogens for humans and animals and are listed as category B select agents in the United States.
To best exploit the genomic information available for several Burkholderia species, a wide array of tools is required for molecular genetic and pathogenesis studies of these bacteria. For Burkholderia species not classified as select agents, antibiotic-resistance-based tools could be used for genetic manipulation. However, the Centers for Disease Control and Prevention restricts the introduction of markers conferring resistance against clinically important antibiotics into the two select agents B. mallei and B. pseudomallei. At present, only gentamicin, kanamycin, and zeocin resistance markers are approved for limited use for B. pseudomallei, while only the kanamycin and zeocin resistance markers are approved for B. mallei (35). However, most wild-type strains of B. mallei and B. pseudomallei have high levels of resistance to all three antibiotics (7, 29, 36), and even at high concentrations, the selection is not tight, and spontaneous resistance still arises (10, 15, 32). Consequently, there is still a need to expand universal genetic tools based on nonantibiotic selectable markers, allowing broader applications in various Burkholderia species.
Several nonantibiotic selection schemes have been used in bacteria including, but not limited to, resistance to various compounds (e.g., arsenate; bialaphos or its degradation product, phosphinothricin; mercury; and tellurite [Tel]) and metabolic markers (e.g., lactose utilization and purine and amino acid biosynthesis). Potential drawbacks to using arsenate and mercury are high toxicity levels and narrow selective concentration ranges (4, 16). Bialaphos and its degradation product, phosphinothricin, have been shown to be ineffective for Burkholderia select agents, requiring concentrations greater than 1,000 μg/ml, whereas these bacteria have been shown to be sensitive to Tel concentrations of less than 1 μg/ml (M. Frazier, K. Choi, A. Kumar, C. Lopez, R. R. Karkhoff-Schweizer, and H. P. Schweizer, presented at the American Society for Microbiology Biodefense and Emerging Diseases Research Meeting, Washington, DC, 2007). Therefore, the nonantibiotic selectable marker based on Tel resistance (Telr) could be useful for genetic manipulation in various Burkholderia species, particularly B. mallei and B. pseudomallei. The Telr marker, consisting of three genes (kilA, telA, and telB) (38), has been successfully employed as a nonantibiotic selectable marker originally in Pseudomonas putida (34), in several other gram-negative bacteria (25), and, more recently, in B. thailandensis (2). Additionally, the asd gene (a metabolic marker encoding aspartate-semialdehyde dehydrogenase for amino acid biosynthesis) has been used as a nonantibiotic selectable marker in Δasd backgrounds (2, 30). Combining the Telr marker and the asd gene may expand the repertoire of genetic tools available for Burkholderia species.
Strategies and tools for the manipulation of genetic elements as a single copy on the chromosome have been developed, such as Himar1-based mariner transposons (22, 32), the mini-Tn7 site-specific transposition system (1, 9), and FRT-lacZ fusion vectors (12, 37). The random Himar1-based mariner transposon plasmid pBT20 was successfully used for mutant library construction in Pseudomonas aeruginosa (6, 19, 22) and has also been proven useful for transposition in a broad range of gram-negative bacteria (20). Similarly, the Himar1-based transposons carrying the Kmr cassette were proven to be useful in B. pseudomallei (32). The second single-copy system based on the mini-Tn7 site-specific transposon, when used in conjunction with the transposase-encoding helper plasmid, has broad applications for the introduction of single-copy chromosomal elements into gram-negative bacteria (9) and the select agent B. mallei (8). Lastly, after mutant construction with an FRT-flanked selectable marker and Flp excision, the introduction of an Flp-containing helper plasmid and an FRT-lacZ fusion vector allows for simple Flp-catalyzed recombination to the “FRT scar” at the target gene downstream of the native promoter, facilitating regulation studies without prior knowledge of the promoter sequence (12, 37). Nevertheless, there are disadvantages to these existing systems when used in Burkholderia species, particularly in the select agents B. pseudomallei and B. mallei, due to the antibiotic resistance markers used (e.g., gentamicin, kanamycin, ampicillin, and streptomycin) and the occurrence of spontaneously resistant mutants (10, 15, 32). Moreover, to our knowledge, no FRT-reporter fusion vectors based on reporter genes other than lacZ have been developed.
In this study, genetic tools using the Telr marker for selection were developed for single-copy analyses of chromosomally targeted genetic elements. These include a Himar1-based random mariner transposon plasmid and a mini-Tn7 site-specific transposon vector. We also engineered FRT-reporter fusion vectors based on three common reporters, lacZ, gfp, and the luxCDABE operon, allowing for Flp-catalyzed recombination. These systems expand upon our previously published nonantibiotic selectable marker approach for allelic replacement (2) and will aid in routine genetic manipulations including transposon mutagenesis, complementation studies, and promoter regulation studies of Burkholderia species. Most importantly, all genetic tools presented here are completely devoid of antibiotic resistance selection and are in compliance with select-agent regulations. We utilized these tools to characterize the B. thailandensis betBA operon, encoding betaine aldehyde dehydrogenase (BetB) and choline dehydrogenase (BetA).
MATERIALS AND METHODS
Bacterial strains, media, and culturing conditions.
All the strains and plasmids involved in this study are listed in Tables 1 and 2. Escherichia coli strain EPMax10B-pir116 was routinely used as a cloning strain. E. coli strain DH5α-pir was used for the cloning of pBT20-Δbla-Telr-FRT. E. coli strain E1345 was used to clone E. coli asd (asdEc)-containing vectors. The E. coli conjugal and suicidal strain E1354 was routinely used for introducing plasmids into Burkholderia species through conjugation. An alternative E. coli conjugal donor, E463, was used for the conjugal transfer of transposon plasmid pBT20-Δbla-Telr-FRT. Luria-Bertani (LB) medium (Difco) was used to culture all E. coli, Burkholderia cenocepacia, B. pseudomallei, and B. mallei strains. B. thailandensis wild-type strain E264 and its derivatives were cultured in LB medium or 1× M9 minimal medium supplemented with 20 mM glucose (MG). For the single-copy complementation study (see Fig. 3), B. thailandensis strains were grown in 1× M9 minimal medium plus 1% Brij 58 (Sigma) and 20 mM glucose or 30 mM choline chloride (Sigma). One percent Brij 58 was added to prevent bacterial clumping during growth. To study betBA regulation, B. thailandensis strains were grown in MG plus 1% Brij 58 along with different concentrations of choline chloride (see Fig. 5) or in no-salt LB medium (LS medium; Teknova) supplemented with different NaCl concentrations (see Fig. 6). Antibiotics and nonantibiotic bactericidal compounds were added to the media utilized for both selection and plasmid maintenance as follows: 110 μg/ml ampicillin (Ap), 25 μg/ml chloramphenicol (Cm), 15 μg/ml gentamicin (Gm), 35 μg/ml kanamycin (Km), 25 μg/ml, streptomycin (Sp), and 20 μg/ml potassium Tel (Teknova) for E. coli; 125 μg/ml Tel for B. cenocepacia strain K56-2 and B. thailandensis; 200 μg/ml Tel for B. cenocepacia strain J2315; and 25 μg/ml Tel for B. pseudomallei strains K96243 and 1026b and B. mallei strain ATCC 23344. For the growth of E. coli Δasd strains E463, E1345, and E1354, without asdEc-containing plasmids, 100 μg/ml of diaminopimelic acid (Sigma) was supplied. All manipulations of B. pseudomallei and B. mallei were conducted in a CDC/USDA-approved and -registered biosafety level 3 facility at the University of Hawaii at Manoa. All experiments with these two select agents were performed with biosafety level 3 practices according to recommendations described previously (32a).
TABLE 1.
Bacterial strains utilized in this study
| Strain | Lab IDa | Relevant characteristic(s) | Source or reference |
|---|---|---|---|
| E. coli | |||
| EPMax10B-pir116 | E1249 | F− λ−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK rpsL nupG Tn-pir116-FRT2 | Laboratory collection |
| DH5α-pir | E0175 | Tcr; F− λ− φ80dlacZΔM15 (lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 uidA::pir zdg-232::Tn10 | 31 |
| HPS1-mob-Δasd-pir | E0463 | Tcr Kmr Cmr; e14− (mcrA) recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 Δ(lac-proAB) rif zxx::mini-Tn5-Lac4 (lacIq+lacZ M15) Δasd::FRT uidA::pir zdg-232::Tn10 recA::RP4-2 Tc::Mu Kmr | —b |
| EPMax10B-pir116-Δasd::Gmr | E1345 | Gmr; F− λ−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK rpsL nupG Tn-pir116-FRT2 Δasd::Gmr | —b |
| EPMax10B-pir116-Δasd-mob-Kmr-Δtrp::Gmr | E1354 | Kmr Gmr; F− λ−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK rpsL nupG Tn-pir116-FRT2 Δasd::FRT recA::RP4-2 Tc::Mu Kmr Δtrp::Gmr | —b |
| DH5α-λattB::pCD13SK-Flp | E0982 | Spr; F− φ80dlacZΔM15 (lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 λattB::pCD13SK-Flp | —b |
| B. thailandensis | |||
| E264 | E1298 | Prototroph | 5 |
| E264-ΔbetBA::FRT | E1671 | B. thailandensis ΔbetBA::FRT mutant | 2 |
| E264-ΔbetBA::FRT/attTn7::Telr | E1709 | Telr; B. thailandensis ΔbetBA::FRT mutant with empty vector mini-Tn7-Telr integrated at the attTn7 site | This study |
| E264-ΔbetBA::FRT/attTn7::Telr-betBA | E1711 | Telr; B. thailandensis ΔbetBA::FRT mutant with mini-Tn7-Telr-betBA integrated at the attTn7 site | This study |
| E264-ΔbetBA::FRT-lacZ-Telr | E1731 | Telr; B. thailandensis ΔbetBA::FRT mutant with FRT-lacZ-Telr fusion | This study |
| E264-ΔbetBA::FRT-gfp-Telr | E2045 | Telr; B. thailandensis ΔbetBA::FRT mutant with FRT-gfp-Telr fusion | This study |
| E264-ΔbetBA::FRT-lux-Telr | E2047 | Telr; B. thailandensis ΔbetBA::FRT mutant with FRT-lux-Telr fusion | This study |
| E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA | E1849 | Telr; E264-ΔbetBA::FRT/attTn7::betBA with ΔbetBA-lacZ-Telr fusion | This study |
| E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA | E2046 | Telr; E264-ΔbetBA::FRT/attTn7::betBA with ΔbetBA-gfp-Telr fusion | This study |
| E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA | E2048 | Telr; E264-ΔbetBA::FRT/attTn7::betBA with ΔbetBA-lux-Telr fusion | This study |
| B. cenocepacia | |||
| K56-2 | E1554 | Prototroph; cystic fibrosis isolate | P. Sokol |
| J2315 | E1553 | Prototroph | J. Goldberg |
| B. pseudomallei | |||
| K96243 | B0005 | Prototroph; clinical isolate | 18 |
| 1026b | B0003 | Prototroph; clinical isolate | 11 |
| B. mallei ATCC 23344 | B0001 | Prototroph; clinical isolate | 40 |
For strains constructed in this study, please see the text for further details. Please use the laboratory identification (Lab ID) number to request strains.
—, details on the engineering of these strains will be published elsewhere.
TABLE 2.
Bacterial plasmids utilized in this study
| Plasmid | Lab IDa | Relevant properties | Reference or source |
|---|---|---|---|
| pBT20-Δbla | E1029 | Gmr; mariner transposon plasmid | 20 |
| pwFRT-PS12-Telr | E1584 | Telr; PS12-Telr cassette flanked by wild-type FRT sequences | 2 |
| pBT20-Δbla-Telr-FRT | E1727 | Telr; mariner transposon plasmid based on Telr | This study |
| pCD11-Gmr-pir116-oriT | E1254 | Cmr Gmr; conjugation vector | Laboratory collection |
| pUC18R6KT-mini-Tn7 | E1190 | Apr; Tn7-based broad-host-range transposon vector | 9 |
| mini-Tn7-Telr-bla | E1645 | Apr Telr; Telr cassette cloned into pUC18R6K-mini-Tn7 | This study |
| mini-Tn7-Telr | E1825 | Telr; mini-Tn7-bla-Telr with bla gene deleted | This study |
| mini-Tn7-Telr-betBA | E1829 | Telr; mini-Tn7-Telr with betBA operon cloned | This study |
| pTNS3 | E1189 | Apr; helper plasmid for Tn7 transposition system | 9 |
| pTNS3-asdEc | E1831 | pTNS3 with bla replaced by the E. coli asd gene | This study |
| pFRT1-lacZ | E0790 | Gmr; FRT1-lacZ fusion containing suicidal vector | 37 |
| pFRT1-lacZ-Telr | E1707 | Telr; pFRT1-lacZ with Gmr cassette replaced by Telr cassette | This study |
| pFRT2-lacZ | E0787 | Gmr; FRT2-lacZ fusion containing suicidal vector | 37 |
| pFRT2-lacZ-Telr | E1708 | Telr; pFRT2-lacZ with Gmr cassette replaced by Telr cassette | This study |
| pPS856-ΔXbas | E1044 | Gmr Apr; Gmr cassette flanked by wild-type FRT sequences | —b |
| pPS747 | E0042 | Apr; gfp-containing vector | 17 |
| pAKlux2 | E1863 | Apr; luxCDABE bioluminescence operon-containing vector | 21 |
| pFRT1-Gmr-lacZ-Telr | E2049 | Gmr Telr; pFRT1-lacZ-Telr with FRT1 replaced by the FRT1-Gmr-FRT1 fragment | This study |
| pFRT2-Gmr-lacZ-Telr | E2050 | Gmr Telr; pFRT1-lacZ-Telr with FRT1 replaced by the FRT2-Gmr-FRT2 fragment | This study |
| pFRT1-lacZ-Telr-ΔBam | E2051 | Telr; pFRT1-Gmr-lacZ-Telr with Flp-excised FRT1-Gmr | This study |
| pFRT2-lacZ-Telr-ΔBam | E2052 | Telr; pFRT2-Gmr-lacZ-Telr with Flp-excised FRT2-Gmr | This study |
| pFRT1-gfp-Telr | E2053 | Telr; pFRT1-lacZ-Telr-ΔBam with gfp replacing lacZ | This study |
| pFRT2-gfp-Telr | E2055 | Telr; pFRT2-lacZ-Telr-ΔBam with gfp replacing lacZ | This study |
| pFRT1-lux-Telr | E2064 | Telr; pFRT1-lacZ-Telr-ΔBam with luxCDABE replacing lacZ | This study |
| pFRT2-lux-Telr | E2066 | Telr; pFRT2-lacZ-Telr-ΔBam with luxCDABE replacing lacZ | This study |
| pCD13SK-Flp-oriT | E0803 | Spr; Flp-containing suicidal vector | 37 |
| pCD13SK-Flp-oriT-asdEc | E1827 | pCD13SK-Flp-oriT with asdEc replaced by the Spr cassette | This study |
| pFlpAB-5 | E1662 | Tpr; broad-host-range Flp-containing vector | 2 |
For plasmids constructed in this study, please see the text for further details. Please use the laboratory identification (Lab ID) number when requesting plasmids.
—, details on the engineering of this plasmid are to be published elsewhere.
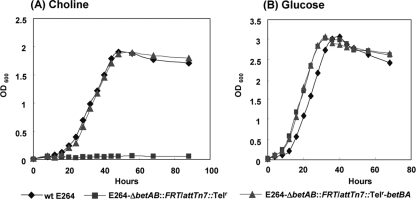
FIG. 3.
Growth analyses of the B. thailandensis ΔbetBA mutant and its complement on choline and glucose. Wild-type strain E264, the E264-ΔbetBA::FRT/attTn7::Telr mutant, and the E264-ΔbetBA::FRT/attTn7::Telr-betBA complement were grown in 1× M9 minimal medium supplemented with 30 mM choline (A) or 20 mM glucose (B). All three strains exhibited similar growth rates and overall cell densities when grown in glucose. The complemented strain, E264-ΔbetBA::FRT/attTn7::Telr-betBA, displayed the same growth rate as the wild-type (wt) strain on choline. However, the betBA mutant strain containing the empty Tn7 transposon control, E264-ΔbetBA::FRT/attTn7::Telr, was not able to grow on choline as a sole carbon source. OD600, optical density at 600 nm.
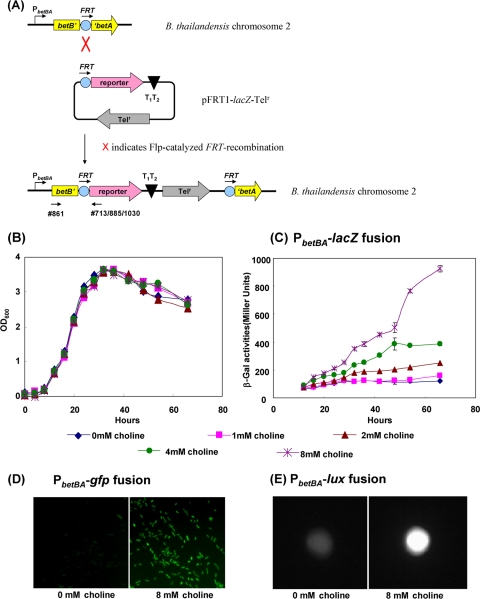
FIG. 5.
Induction of the betBA operon by choline. (A) Flp-catalyzed recombination of the promoterless FRT-lacZ fusion into the B. thailandensis chromosome at the ΔbetBA::FRT loci. Oligonucleotides 713 and 861 were used to screen for correct integration and are indicated by arrows. Oligonucleotides 885 and 1030 were used along with oligonucleotide 861 to screen gfp and lux reporters, respectively. (B) E264-ΔbetBA::FRT-lacZ was grown in 1× M9 medium plus 20 mM glucose supplemented with 0, 1, 2, 4, or 8 mM choline. (C) β-Galactosidase assays were performed in triplicate for all of the growth cultures shown above (B) at various time points, indicating that betBA is inducible by choline. Two alternative fusion strains, E264-ΔbetBA::FRT-gfp-Telr (D) and E264-ΔbetBA::FRT-lux-Telr (E), were grown in MG medium or MG medium plus 8 mM choline. (D and E) The expressions of GFP (D) and bioluminescent proteins (E) were significantly induced in the presence of choline. Images in D are representative of multiple fields for the same samples. OD600, optical density at 600 nm.
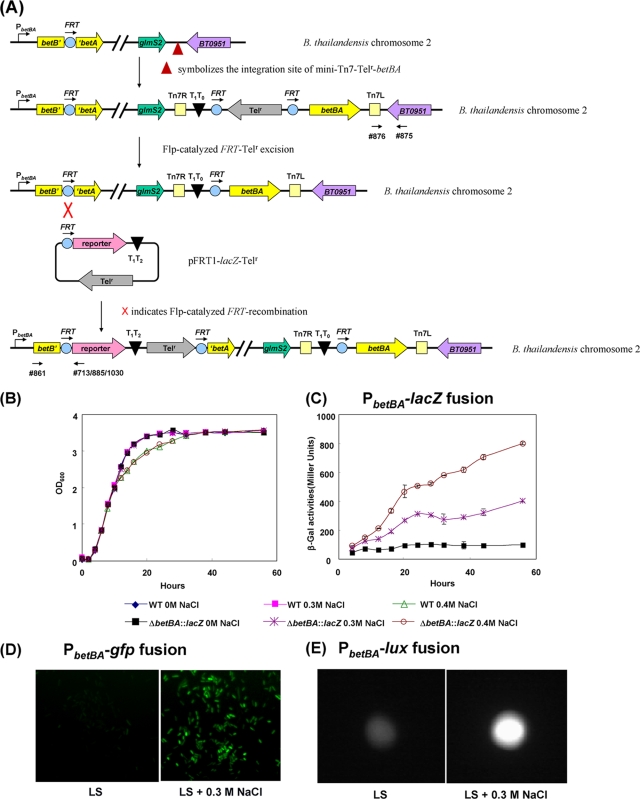
FIG. 6.
Induction of the betBA operon by osmotic stress. (A) Strategy for constructing fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA. Oligonucleotide 861 was used along with oligonucleotide 713, 885, or 1030 for screening, and they are indicated by arrows. Parallel diagonal lines indicate a large distance of separation on the chromosome. (B) The resulting strain, E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA, and wild-type (WT) strain E264 were grown in LS (0 M NaCl) medium supplemented with 0.3 and 0.4 M NaCl. Both the wild-type and fusion strains produced identical growth characteristics in LS medium and LS medium plus 0.3 M NaCl yet displayed slightly decreased levels of growth in 0.4 M NaCl after mid-log phase. (C) At each time point, β-galactosidase assays were performed in triplicate on fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA grown in LS medium with 0, 0.3, or 0.4 M NaCl. When the complemented fusion strain was grown under osmotically stressed conditions (0.3 and 0.4 M NaCl), the betBA operon was significantly induced, compared to conditions with no osmotic stress (no salt). (D and E) Two alternative fusion strains, E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA (D) and E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA (E), were grown in LS medium or LS medium plus 0.3 M NaCl. The expressions of GFP (D) and luxCDABE (E) were significantly induced in the presence of NaCl. Images in D are representative of multiple fields in the same sample. OD600, optical density at 600 nm.
Molecular methods and reagents.
All restriction enzymes, deoxynucleoside triphosphates, T4 DNA polymerase, T4 polynucleotide kinase, and T4 DNA ligase were purchased from New England Biolabs and used as recommended by the supplier. Plasmids and DNA gel bands were isolated using the Zyppy plasmid miniprep kit I and Zymoclean gel DNA recovery kit, respectively (Zymo Research Corporation). Competent cells were prepared as previously described (2). All other molecular techniques were conducted according to methods described previously by Sambrook and Russell (33). Oligonucleotide primers (Table 3) were synthesized by Integrated DNA Technology. Pfu polymerase was purchased from Stratagene. Generally, the various PCRs were performed by an initial denaturation step for 1 min at 94°C and 34 cycles of 45 s at 94°C, 30 s at 60°C, and 1 min kb−1 at 72°C, and a final step for 10 min at 72°C was included.
TABLE 3.
Oligonucleotide primers utilized in this study
| Primer (name) | Sequencea |
|---|---|
| 89 (asdEc-up) | 5′-CGGTTGAATTCTACTCCGGTGCGCAAATGGC-3′ |
| 91 (asdEc-down) | 5′-TACTGAATTCCGCCAAAATGGCCTGCAATTA-3′ |
| 696 (oriT-ClaI-1) | 5′-TGGGTATCGATTCCTTAAGGTATACTTT-3′ |
| 702 (R6K) | 5′-TGTCAGCCGTTAAGTGTTCC-3′ |
| 713 (lacZα) | 5′-TGTTGGGAAGGGCGATC-3′ |
| 827 (telA-SmaI) | 5′-GGGAACGACCCTGGCCGCGTGCA-3′ |
| 831 (tel-kilA) | 5′-AGCTAAAATGGAAGAACAAA-3′ |
| 834 (telB-XhoI) | 5′-CCTCCTCGAGCAGAAAGTCAAAAGCCTC-3′ |
| 854 (telB-down) | 5′-TACCAGCAGGAATGGAAC-3′ |
| 861 (Bt-betBA-HindIII) | 5′-CCCGCAAGCTTGCCGGCAA-3′ |
| 862 (Bt-betBA-KpnI) | 5′-GACCGGTACCCGGCGGGCGGGGATAT-3′ |
| 874 (glmS1-DN)b | 5′-GTTCGTCGTCCACTGGGATCA-3′ |
| 875 (glmS2-DN)b | 5′-AGATCGGATGGAATTCGTGGAG-3′ |
| 876 (Tn7L)b | 5′-ATTAGCTTACGACGCTACACCC-3′ |
| 885 (gfp-BspHI-down) | 5′-CAGGTCATGACACCTCTCTTTATTTGTATAGTTC-3′ |
| 1030 (lux-rev) | 5′-GGATTGCACTAAATCATC-3′ |
| 1066 (kilA-rev) | 5′-TCGGCTTCGTCCAGCAAC-3′ |
| 1067 (telA-rev) | 5′-GCATTGCGCTTCATCAGG-3′ |
Restriction enzyme sites utilized in this study are underlined.
Oligonucleotides were synthesized as previously described (9).
Conjugal transfer of vectors into Burkholderia species.
E. coli strain E463 was used as the conjugal donor to introduce transposon plasmid pBT20-Δbla-Telr-FRT into all Burkholderia species. Another E. coli conjugal strain, E1354, was used to introduce the mini-Tn7 and FRT-lacZ vectors and their respective helper plasmids into B. thailandensis strains. Conjugation of non-select-agent Burkholderia species was carried out as follows. The donor and recipients were grown to log phase for conjugation. One milliliter of each culture was harvested separately by centrifugation at 9,000 × g for 1 min at room temperature and washed twice with 1 ml of LB medium. The cell pellets of the donor and recipients were then resuspended together in 30 μl of LB medium. The 30-μl cell suspension was spotted onto cellulose acetate filters (Satorius) on LB agar plates and incubated at 37°C for 8 h. Filters were then vortexed in 1 ml of 1× M9 minimal medium, and 100 μl of this cell suspension and 100 μl of 10× dilutions were plated onto LB or MG plates with appropriate concentrations of Tel. Conjugations into B. pseudomallei or B. mallei cells were performed directly on LB plates without filters. Bacteria were gently scraped off the LB plates with disposable inoculation loops and resuspended in 1× M9 medium, and plating was done similarly as described above. Plates were usually incubated for 2 to 3 days at 37°C until single Telr colonies were observed.
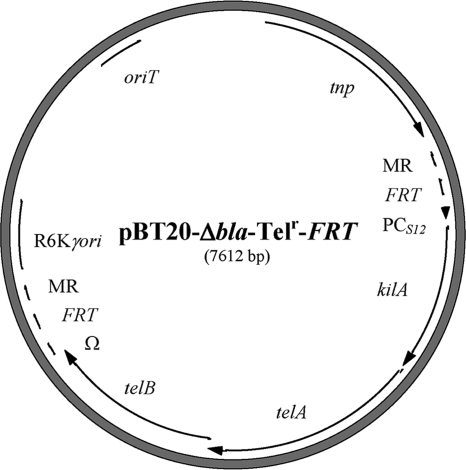
Construction and testing of pBT20-Δbla-Telr-FRT.
A new mariner transposon vector was constructed based on the Telr marker (Fig. 1). pwFRT-PCS12-Telr was digested with SmaI, and the 3.2-kb PCS12-Telr fragment was cloned into the pBT20-Δbla backbone (∼4.3 kb) following BsaI digestion and blunt ending. This replaced the Gmr cassette with the Telr marker, resulting in transposon vector pBT20-Δbla-Telr-FRT.
FIG. 1.
Plasmid map of mariner transposon vector pBT20-Δbla-Telr-FRT based on Telr. The Telr cassette, consisting of the kilA, telA, and telB genes, is flanked by two identical FRT sequences. A B. cenocepacia PCS12 promoter was included upstream of the Telr cassette. Abbreviations: Ω, tonB transcriptional terminator; FRT, Flip recombination target; oriT, conjugal origin of transfer; MR, mariner repeats; PCS12, B. cenocepacia rpsL promoter; R6Kγori, suicidal R6K origin of replication; tnp, mariner transposase gene.
The transposition frequencies of pBT20-Δbla-Telr-FRT were determined by conjugation into several different Burkholderia species and strains: B. cenocepacia strains K56-2 and J2315, B. thailandensis strain E264, B. pseudomallei strains K96243 and 1026b, and B. mallei strain ATCC 23344. Following conjugation as described above, the mating mixtures were diluted and plated onto LB plates and LB plates supplemented with the appropriate concentration of Tel. The transposition frequencies for individual conjugation experiments were calculated based on the ratio of the number of colonies counted that were grown on LB medium plus Tel to the number of colonies that were grown on LB medium. Three independent conjugation experiments were carried out to obtain the average transposition frequency and standard error of the mean for each strain. Similar control conjugation experiments, omitting the E. coli conjugal donor harboring pBT20-Δbla-Telr-FRT, were performed on all recipient strains to ensure that no spontaneous mutants arose from Tel selection. For B. thailandensis and B. cenocepacia, 15 random Telr colonies were purified on LB plates with Tel and PCR screened using telB-specific oligonucleotides 834 and 854 (Table 3). For B. pseudomallei and B. mallei, five random Telr colonies were purified on LB plates with Tel and PCR screened using kilA oligonucleotides 831 and 1066, telA oligonucleotides 827 and 1067, and telB oligonucleotides 834 and 854 (Table 3). Southern hybridization analysis was also performed, as described previously (17), for the B. pseudomallei and B. mallei Telr colonies using a telB-specific probe after the digestion of chromosomal DNA with XhoI.
Construction of mini-Tn7 site-specific transposon vectors and their helper plasmid.
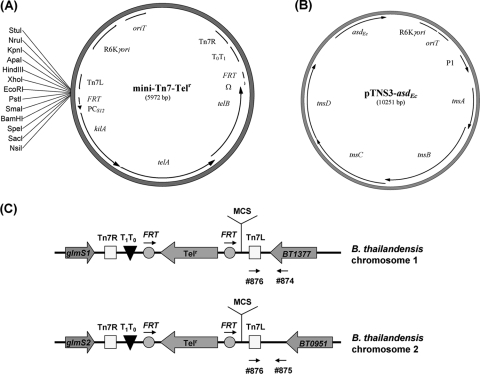
The mini-Tn7-Telr site-specific transposon, based on Telr, was constructed as described below. pwFRT-PCS12-Telr was digested with SacI and blunt ended, and the PCS12-Telr fragment (3.2 kb) was cloned into pUC18R6KT-mini-Tn7 (EcoRV digested), resulting in mini-Tn7-Telr-bla. The R6Kγori-oriT region, amplified from pCD11-Gmr-pir116-oriT using oligonucleotides 696 and 702, was ligated with the mini-Tn7-Telr-bla backbone (including the Telr cassette) following AflII and NarI digestion and blunt ending. This resulted in a mini-Tn7-Telr vector (Fig. 2A).
FIG. 2.
The mini-Tn7-Telr integration vector and its pTNS3-asdEc helper plasmid. (A) mini-Tn7-Telr, a Tn7-based suicidal vector with the Telr marker. (B) pTNS3-asdEc suicidal helper plasmid encoding the transposase, which catalyzes Tn7 transposition. (C) Chromosomally inserted mini-Tn7-Telr elements at two different B. thailandensis attTn7 sites as previously described (6). Oligonucleotides 874, 875, and 876, indicated by arrows, were used to screen for the location of transposition. glmS1 and glmS2 encode glucosamine-6-phosphate synthetases; MCS, multiple-cloning site; P1, P1 integron promoter; Tn7L, Tn7 transposase left recognition sequence; Tn7R, Tn7 transposase right recognition sequence; T0T1, transcriptional terminators; tnsABCD, Tn7 transposases.
Helper plasmid pTNS3-asdEc (Fig. 2B), containing the Tn7 transposase genes, was also constructed based on asdEc. The asdEc fragment was amplified from E. coli K-12 chromosomal DNA using oligonucleotides 89 and 91. The 2.5-kb PCR product was digested with EcoRI and PstI and blunt ended, and the 1.6-kb asdEc gene was ligated into the pTNS3 backbone (digested with BglI and blunt ended), yielding pTNS3-asdEc.
Single-copy complementation of E264-ΔbetBA::FRT.
The mini-Tn7-Telr and the betBA operon fragment (amplified from E264 with oligonucleotides 861 and 862) were digested with HindIII and KpnI and ligated together, yielding mini-Tn7-Telr-betBA. The B. thailandensis E264-ΔbetBA::FRT mutant was complemented using the constructed mini-Tn7-Telr-betBA. Two E1354 strains, each harboring either mini-Tn7-Telr-betBA or helper plasmid pTNS3-asdEc, were used to perform triparental matings with the B. thailandensis recipient E264-ΔbetBA::FRT, creating the E264-ΔbetBA::FRT/attTn7::Telr-betBA complemented strain. As a control, an empty mini-Tn7-Telr vector was conjugated into the E264-ΔbetBA::FRT strain, resulting in E264-ΔbetBA::FRT/attTn7::Telr. B. thailandensis transconjugants with the mini-Tn7 transposon inserted at the attTn7 site were selected on LB plates with Tel and screened by PCR using oligonucleotides 874 and 876 or oligonucleotides 875 and 876 (Fig. 2C).
Characterization of growth of E264-ΔbetBA::FRT and the complemented strain.
Growth curve experiments were performed on three B. thailandensis strains: wild-type E264, the E264-ΔbetBA::FRT/attTn7::Telr control, and the E264-ΔbetBA::FRT/attTn7::Telr-betBA complement. These strains were grown overnight at 37°C in LB medium. Cultures grown overnight were washed twice with 1 volume of 1× M9 buffer and resuspended in an equal volume of the same buffer. Resuspended cultures were then diluted 100-fold into a solution containing fresh 1× M9 medium, 1% Brij 58, and 20 mM glucose or 30 mM choline chloride, and growth was initiated by shaking at 250 rpm and 37°C. At all time points, aliquots of each culture were taken, and optical densities were measured at 600 nm.
Construction of FRT-lacZ, FRT-gfp, and FRT-lux fusion vectors.
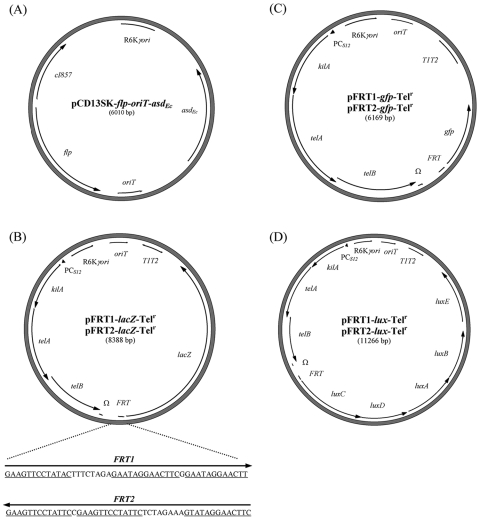
An flp-carrying helper plasmid containing a nonantibiotic resistance marker was first created for the recombination of the various reporter fusion vectors. pCD13SK-Flp-oriT was digested with NdeI and SalI, blunt ended, and ligated with the above-mentioned 1.6-kb asdEc fragment. The resulting helper plasmid, pCD13SK-Flp-oriT-asdEc (see Fig. 4A), contains the asdEc marker in place of the Spr cassette.
FIG. 4.
Plasmid maps of the FRT-reporter fusion vectors and their helper plasmids. (A) pCD13SK-Flp-oriT-asdEc is a flp-containing helper plasmid for the recombination of all FRT-reporter fusions. (B to D) Various fusion vectors were constructed based on the reporters lacZ (B), gfp (C), and the luxCDABE operon (D). With the exception of the FRT oriented relative to the reporter genes, all of the paired pFRT1-Telr and pFRT2-Telr vectors have the same sequence. Depending on the orientation of FRT on the chromosome, either pFRT1-Telr or pFRT2-Telr would be used to orient the promoterless reporter fusion in the same direction as the promoter of interest. Abbreviations: cI857, temperature-sensitive repressor; gfp, green fluorescent protein gene; lacZ, β-galactosidase gene; luxCDABE, genes encoding the bacterial bioluminescent operon; T1T2, transcriptional terminators.
Two pFRT-lacZ-Telr vectors, pFRT1-lacZ-Telr and pFRT2-lacZ-Telr (Fig. 4B), were constructed in this study by replacing the Gmr cassette with the Telr cassette. To create pFRT1-lacZ-Telr, pwFRT-PCS12-Telr was digested with EcoRV and XhoI and blunt ended, and the resulting PCS12-Telr fragment was cloned into pFRT1-lacZ (digested with BsrGI and SacII and blunt ended to remove the Gmr cassette). Similarly, pFRT2-lacZ-Telr was constructed by cloning the PCS12-Telr fragment into the pFRT2-lacZ backbone.
Four different fusion vectors were constructed based on the gfp reporter gene and the luxCDABE operon. In order to replace the lacZ gene with the promoterless gfp reporter gene or the lux operon, several cloning steps were carried out to eliminate one of the BamHI sites flanking the FRT sequence, leaving a unique BamHI site downstream of the FRT sequence. First, pPS856-ΔXbas was digested with SmaI to recover the FRT-Gmr-FRT fragment, which was cloned into the pFRT1-lacZ-Telr backbone following digestion with the same enzyme. This cloning step resulted in the creation of pFRT1-Gmr-lacZ-Telr (with the Gmr cassette in the same orientation as the lacZ gene) and pFRT2-Gmr-lacZ-Telr (with the Gmr cassette in the opposite orientation of the lacZ gene). To Flp excise the Gmr-FRT fragment, both pFRT1-Gmr-lacZ-Telr and pFRT2-Gmr-lacZ-Telr were introduced into Flp-containing strain DH5α-λattB::pCD13SK-Flp. The resulting constructs, pFRT1-lacZ-Telr-ΔBam and pFRT2-lacZ-Telr-ΔBam, were digested with NdeI, blunt ended, and then digested with BamHI. These Telr cassette-containing vectors were ligated with the gfp gene from pPS747 (digested with HindIII, blunt ended, and then digested with BamHI), yielding pFRT1-gfp-Telr and pFRT2-gfp-Telr, respectively (Fig. 4C). Finally, pFRT1-lacZ-Telr-ΔBam and pFRT2-lacZ-Telr-ΔBam were digested with BamHI and NdeI and blunt ended, and the luxCDABE operon, obtained from pAKlux2 (Addgene plasmid 14080) following EcoRI digestion and blunt ending, was cloned to yield pFRT1-lux-Telr and pFRT2-lux-Telr (Fig. 4D).
Engineering of B. thailandensis E264-ΔbetBA::FRT reporter fusion strains.
To construct the E264-ΔbetBA::FRT-lacZ-Telr reporter strain for the betBA promoter study, pFRT1-lacZ-Telr and the helper plasmid (pCD13SK-Flp-oriT-asdEc) were conjugated from E1354 into E264-ΔbetBA::FRT in a triparental mating experiment. Colonies on MG plates with 125 μg/ml Tel were screened by PCR using oligonucleotides 713 and 861 to confirm the correct orientation of the lacZ gene relative to the betBA promoter region. This strain was then used in the choline induction study (see Fig. 5B and C).
The E264-ΔbetBA::FRT-lacZ-Telr fusion strain was complemented by engineering the E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA strain in several steps (see Fig. 6A). First, the Telr cassette in strain E264-ΔbetBA::FRT/attTn7::Telr-betBA (described above) was Flp excised using pFLP-AB5 according to a previously described procedure (2). The resulting strain, E264-ΔbetBA::FRT/attTn7::betBA, was conjugated with two E1354 donor strains harboring either pFRT1-lacZ-Telr or pCD13SK-Flp-oriT-asdEc in a triparental mating experiment. This triparental mating mixture was then plated onto MG plates with 125 μg/ml Tel to select for fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA, harboring a lacZ reporter driven by the native betBA promoter. Colonies were screened by PCR using oligonucleotides 713 and 861 (Fig. 6A). Isolates with lacZ integrated at the betBA locus were purified once on LB medium and used in the osmotic regulation study (see Fig. 6B and C).
Fusion vector pFRT1-gfp-Telr was also integrated into the ΔbetBA::FRT mutant and complemented strains as described above. The resulting fusion strains, E264-ΔbetBA::FRT-gfp-Telr and E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA, were screened by PCR using oligonucleotides 861 and 885 (Fig. 6A). Similarly, vector pFRT1-lux-Telr was used to construct fusion strains E264-ΔbetBA::FRT-lux-Telr and E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA, which were screened by PCR using oligonucleotides 861 and 1030 (see Fig. 6A).
Choline and osmotic regulation studies of the betBA operon.
β-Galactosidase activity of the integrated betBA::FRT-lacZ-Telr fusion was measured under various growth conditions. To study choline induction of the betBA operon, fusion strain E264-ΔbetBA::FRT-lacZ-Telr was grown overnight in LB medium. Cultures grown overnight were washed twice with 1 volume of 1× M9 medium and resuspended in an equal volume of the same medium. Resuspended cultures were then diluted 100-fold into a solution containing fresh 1× M9 medium, 1% Brij 58, 20 mM glucose, and 0, 1, 2, 4, or 8 mM choline chloride. Growth curve experiments were performed on each culture by diluting sample aliquots twice in 4% Brij 58 and measuring the optical density at 600 nm (see Fig. 5B). Additional 1-ml cell culture aliquots were taken at each time point during the growth curve experiments to assay for β-galactosidase activity. These assays were done in triplicate and are displayed as average Miller units (28), with standard errors of the means (see Fig. 5C). gfp fusion strain E264-ΔbetBA::FRT-gfp-Telr was grown in a solution containing 1× M9 medium, 1% Brij 58, and 20 mM glucose with or without 8 mM choline chloride to early stationary phase (∼36 h), at which point wet mounts were prepared and examined under an Olympus BX51 fluorescent microscope to assay fluorescent activity in the presence or absence of choline (Fig. 5D). Fusion strain E264-ΔbetBA::FRT-lux-Telr was grown in 1× M9 medium with 1% Brij 58 and 20 mM glucose with or without 8 mM choline chloride to early stationary phase, at which point 1 ml of each culture was centrifuged, and the cell pellet was resuspended with 20 μl of 1× M9 medium. The resuspended cells were then spotted onto an MG plate, and images were obtained immediately using a Bio-Rad biochemiluminescent imaging system.
To study the NaCl-mediated osmotic regulation of the betBA operon, we complemented the ΔbetBA mutant because NaCl significantly affected the growth in the absence of the betBA operon. Growth curve experiments were conducted on the complemented fusion strain (E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA) and the wild-type strain (E264) by first growing cultures overnight in LS medium. Cultures grown overnight were washed with 1 volume of LS medium, resuspended in an equal volume of the same medium, and diluted 100-fold into fresh LS medium with 0, 0.3, and 0.4 M NaCl. Growth curve experiments were performed for each culture by taking optical density measurements at 600 nm (see Fig. 6B). Additional 1-ml cell culture aliquots of E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA were taken at each time point during the growth curve experiments to assay for β-galactosidase activity (Fig. 6C). Two other fusion strains, E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA and E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA, were grown in LS medium with or without 0.3 M NaCl to late log phase (∼30 h), at which time fluorescent microscopy and bioluminescence imaging analyses (see Fig. 6D and E) were performed as described above.
Nucleotide sequence accession numbers.
All sequences of vectors presented in Fig. 1, 2, and 4 were submitted to the GenBank database. The accession numbers are as follows: EU626135 for pBT20-Δbla-Telr-FRT, EU626136 for mini-Tn7-Telr, FJ797680 for pTNS3-asdEc, EU626138 for pCD13SK-Flp-oriT-asdEc, EU626139 for pFRT1-lacZ-Telr, EU626140 for pFRT2-lacZ-Telr, FJ455408 for pFRT1-gfp-Telr, FJ455409 for pFRT2-gfp-Telr, FJ455410 for pFRT1-lux-Telr, and FJ455411 for pFRT2-lux-Telr.
RESULTS
Engineering and utilization of a random Telr transposon in Burkholderia species.
A Himar1-based mariner transposon carrying a Kmr marker has been used successfully in B. pseudomallei although with some reported leakiness (32). To further develop and test a mariner transposon based on the alternative nonantibiotic Telr marker for a broader range of Burkholderia spp., we replaced the Gmr marker on mariner transposon plasmid pBT20. pBT20, originally based on the Gmr marker with a bla gene in its plasmid backbone, is not appropriate for selection in Burkholderia species due to their high level of Gmr and may be inappropriate for use in the select-agent species B. pseudomallei and B. mallei. In this study, we constructed a mariner transposon, pBT20-Δbla-Telr-FRT, based on the nonantibiotic Telr marker (Fig. 1) previously shown to be effective in B. thailandensis (2). We eliminated the bla gene from pBT20 and replaced the Gmr cassette on the transposon with the Telr marker for selection in both E. coli and Burkholderia. To demonstrate the effectiveness of this transposon, we conjugated pBT20-Δbla-Telr-FRT from a suicidal Δasd E. coli strain into four different Burkholderia species: B. cenocepacia (two strains), B. thailandensis, B. pseudomallei (two strains), and B. mallei. For each species, three independent mating experiments were conducted, and the average transposition frequencies were determined and are shown in Table 4. We determined the effective Tel concentrations for the four Burkholderia species, and no spontaneous Telr mutants were detected when 109 CFU were plated alone on LB medium with Tel as controls (see Materials and Methods). On average, conjugation mixtures were resuspended in 1 ml of LB medium and diluted 10×, where 100-μl volumes were plated onto LB medium with Tel, yielding 50 to 200 colonies depending on the species. Fifteen random Telr colonies from B. cenocepacia (J2315 and K56-2) and B. thailandensis were screened by PCR using telB-specific oligonucleotides, and five random colonies from B. pseudomallei (K96243 and 1026b) and B. mallei were positively screened by PCR using kilA-, telA-, and telB-specific oligonucleotides (see Fig. S1A to S1F in the supplemental material). Southern blot analysis was also performed on the 15 Telr isolates of B. pseudomallei and B. mallei using a telB-specific probe (see Fig. S1G in the supplemental material). Single bands with different sizes were obtained in all isolates, suggesting random transposition into the B. pseudomallei and B. mallei genomes (see Fig. S1G in the supplemental material). All Burkholderia species tested displayed similar transposition frequencies, ranging from 2.08 × 10−6 ± 0.16 × 10−6 to 1.51 × 10−5 ± 0.23 × 10−5 (Table 4), which are comparable to the frequencies obtained when using a Kmr-based transposon in B. pseudomallei (32). However, analyses of 5 to 15 Telr colonies showed that 100% of the Telr colonies contained the transposon, demonstrating the effectiveness of Telr selection (see Fig. S1 in the supplemental material). No spontaneous resistance was observed when 109 CFU were plated, indicating that the spontaneous resistance frequency is <10−9. If required, the transposon insertion sites could easily be determined by sequencing the flanking region of the transposon using semirandom PCR methods as previously described (19, 24). The Telr cassette in our transposon, flanked by FRT sequences (17), could then be excised by Flp recombinase for subsequent recycling of the Telr cassette or integration of FRT-reporter fusions (below) at the transposed loci for immediate gene regulation studies.
TABLE 4.
Transposition frequencies of pBT20-Δbla-Telr-FRT in Burkholderia species
| Species (strain) | Avg frequency of transposition ± SEMa |
|---|---|
| B. cenocepacia (K56-2) | (1.22 ± 0.21) × 10−5 |
| B. cenocepacia (J2315) | (2.34 ± 0.33) × 10−6 |
| B. thailandensis (E264) | (4.29 ± 0.42) × 10−6 |
| B. pseudomallei (K96243) | (1.51 ± 0.23) × 10−5 |
| B. pseudomallei (1026b) | (1.07 ± 0.10) × 10−5 |
| B. mallei (ATCC 23344) | (2.08 ± 0.16) × 10−6 |
All experiments were performed in triplicate, and averages are shown with the standard error of the mean.
Engineering and testing of the single-copy mini-Tn7-Telr site-specific transposon by complementing the B. thailandensis ΔbetBA mutant.
The mini-Tn7 site-specific transposon and helper plasmid (carrying the Tn7 transposase genes) were utilized in various species (1, 3, 8, 23). This system could be used for single-copy complementation studies, promoter-reporter fusion integration, and reporter gene (e.g., fluorescence and bioluminescence proteins) tagging in Burkholderia species (1, 3, 8, 23). However, all these systems contain antibiotic resistance markers for selection, requiring the need for reengineering with nonantibiotic selectable markers. In this study, we developed a mini-Tn7-Telr site-specific transposon and a helper plasmid for Burkholderia species based on two nonantibiotic selectable markers, the Telr cassette and the asdEc gene, respectively (Fig. 2A and B). This mini-Tn7-Telr vector contains a multiple-cloning site for conveniently cloning genes of interest for subsequent site-specific transposition, which is catalyzed by the transposase encoded on the pTNS3-asdEc helper plasmid. The location of the chromosomally inserted transposon could be determined by PCR with site-specific and transposon-specific oligonucleotides (9) (Fig. 2C). The FRT-flanked Telr cassette allows Flp-catalyzed excision of the Telr marker while maintaining the introduced gene of interest at the specific transposition site.
As a proof of concept, the mini-Tn7-Telr system was used to complement the betBA mutation in B. thailandensis. Previously, we engineered a B. thailandensis ΔbetBA mutant that exhibits a growth defect when grown in choline as a sole carbon source (2). A wild-type copy of the betBA operon was cloned into the multiple-cloning site of mini-Tn7-Telr (Fig. 2A), resulting in the mini-Tn7-Telr-betBA vector. The helper plasmid pTNS3-asdEc and the mini-Tn7Telr-betBA vector were simultaneously conjugated by triparental mating into strain E264-ΔbetBA::FRT. Site-specific transposition of the betBA-Telr complement was confirmed by PCR as previously described (9). In the majority of Telr isolates (8 out of 10 screened), the mini-Tn7 transposon was inserted downstream of the glmS2 gene on the second chromosome, while two transpositions occurred downstream of the glmS1 gene on the first chromosome (9). None of the isolates displayed integration on both chromosomes (see Fig. S2 in the supplemental material).
To show the complementation of the ΔbetBA mutant, the constructed strain E264-ΔbetBA::FRT/attTn7::Telr-betBA was tested for its ability to grow on choline as a sole carbon source. As shown in Fig. 3A, this chromosomally integrated copy of the betBA operon recovered the growth ability of the ΔbetBA mutant strain on choline as a sole carbon source, displaying a growth rate and an overall cell density comparable to those of wild-type strain E264. The transposition of the empty mini-Tn7-Telr control into the ΔbetBA mutant yielded no complementation of the ΔbetBA mutation, and it was unable to grow with choline as a sole carbon source (Fig. 3A). Growth curve studies for these three strains on glucose as a sole carbon source were also conducted (Fig. 3B) to show that the integrated mini-Tn7 system did not alter any other growth phenotypes of mutant strain E264-ΔbetBA::FRT.
Engineering of reporter gene constructs and regulation studies of the betBA operon.
Recombination of the FRT-lacZ reporter fusion with the single chromosomally located “FRT scar” aided by the Flp-encoding helper plasmid, following mutant construction with an FRT-flanked antibiotic resistance cassette and Flp excision, has facilitated regulation studies of target genes at their native chromosomal loci (12, 37). In our experience, when coupled with FRT-based resistant-marker approaches for chromosomal mutagenesis (e.g., allelic replacement or FRT-based transposon) (32), these fusion vectors were found to be simple and powerful tools for studying gene regulation without promoter mapping or prior knowledge of promoter sequence or location. The disadvantages of previously reported fusion vectors and helper plasmids (12, 37) are the use of antibiotic resistance markers (Apr, Cmr, Gmr, Kmr, and Spr) and the limitation of a single reporter gene (lacZ). Here, the Telr cassette replaced the Gmr cassette in the previously reported vectors pFRT1-lacZ and pFRT2-lacZ (37), resulting in pFRT1-lacZ-Telr and pFRT2-lacZ-Telr (Fig. 4B). The difference between these two new vectors is the orientation of the FRT sequence relative to that of the reporter gene, accounting for the selection of the appropriate fusion vector relative to the orientation of the “FRT scar” on the chromosome, thus aligning the reporter gene in the same direction as the promoter. To provide more reporter gene options, four other fusion vectors (pFRT1-gfp-Telr, pFRT2-gfp-Telr, pFRT1-lux-Telr, and pFRT2-lux-Telr) were constructed based on the gfp and lux operon reporters (Fig. 4C and D). We constructed an Flp-encoding helper plasmid (pCD13SK-Flp-oriT-asdEc) based on the asdEc nonantibiotic selectable marker for plasmid maintenance in E. coli Δasd strains (e.g., E463 and E1354) (Table 1 and Fig. 4A).
The regulation mechanisms of the betBA operon have been widely studied in a variety of organisms such as E. coli (13), Pseudomonas putida (14), and Sinorhizobium meliloti (27). Extensive characterization of the betBA operon in E. coli (13) has shown that this operon is regulated by osmolarity, temperature, oxygen, choline, and glycine betaine. However, little is known about the regulation of the betBA operon in B. thailandensis. As a proof of concept, we used one of the reporter fusion vectors to determine the regulatory mechanism of the betBA operon in B. thailandensis by choline and osmotic stress. B. thailandensis strain E264-ΔbetBA::FRT-lacZ-Telr was engineered using fusion vector pFRT1-lacZ-Telr and helper plasmid pCD13SK-Flp-oriT-asdEc (Fig. 4A and B and 5A). Flp-catalyzed recombination and the orientation of the FRT-lacZ fusion at the “FRT scar” within the chromosome of the ΔbetBA mutant, a location at which the lacZ reporter gene is controlled by an unknown betBA promoter, were verified by PCR using oligonucleotides 713 and 861 (Fig. 5A). Choline induction of the betBA operon was studied by growing fusion strain E264-ΔbetBA::FRT-lacZ-Telr in 1× M9 medium with glucose and supplemented with different concentrations of choline. These conditions resulted in similar growth rates and overall cell densities (Fig. 5B). β-Galactosidase assays were performed to compare the expression levels of the betBA operon in different choline concentrations (Fig. 5C). As choline concentrations increased, corresponding increases in β-galactosidase activities were observed, indicating that the betBA operon was responsive to choline. Utilizing two alternative gene fusion vectors (pFRT1-gfp-Telr and pFRT1-lux-Telr), fusion strains E264-ΔbetBA::FRT-gfp-Telr and E264-ΔbetBA::FRT-lux-Telr were also constructed. By comparing the fluorescent and luminescent intensities, the induction of the betBA operon by choline was observed using the gfp or lux reporter, respectively (Fig. 5D and E).
A previously reported study has shown that the E. coli betBA operon was involved in osmotic regulation and was induced by osmotic stress (13). Here, we demonstrated that osmotic stress (e.g., NaCl) induces the B. thailandensis betBA operon by utilizing the mini-Tn7-Telr-based system and the reporter fusion vectors described above. To compare the level of expression of the betBA operon in the presence of osmotic stress to that in the absence of osmotic stress, it was necessary to obtain the same growth rates and overall cell densities through complementation of the ΔbetBA mutation (Fig. 6B). Consequently, fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA was constructed in the E264-ΔbetBA::FRT/attTn7::betBA background (Fig. 6A) because we found that the growth of the ΔbetBA mutant was significantly affected by NaCl without complementing the ΔbetBA mutation. The constructed strain, E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA, and wild-type strain E264 were grown in LS (0 M NaCl) medium supplemented with 0.3 and 0.4 M NaCl. These strains produced identical growth characteristics in LS medium and LS medium with 0.3 M NaCl, but both strains displayed slightly decreased growth rates in 0.4 M NaCl after the mid-log growth phase (Fig. 6B). The presence of 0.3 M NaCl significantly induced the betBA operon as determined by β-galactosidase assays (Fig. 6C). Despite the increased induction of the betBA operon in the presence of 0.4 M NaCl relative to that in the absence of NaCl, this comparison was unreliable due to differing growth rates. These data indicated that the betBA operon in B. thailandensis was induced by osmotic stress. Similarly, two fusion strains (E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA and E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA) were constructed to show the induction of the betBA operon by observing the increased fluorescence and bioluminescence levels under conditions of osmotic stress (Fig. 6D and E).
DISCUSSION
In this study, the nonantibiotic Telr cassette was utilized to construct three genetic systems: a random mariner transposon, a mini-Tn7 site-specific transposon vector, and six FRT-reporter fusion vectors based on three different reporters. A constitutive promoter, PCS12 (B. cenocepacia rpsL gene) (41), was included upstream of the Telr cassettes in all these tools to ensure the efficient expression of this resistance marker. First, the constructed Himar1-based random mariner transposon was successfully tested in four different Burkholderia spp.: B. cenocepacia, B. thailandensis, B. pseudomallei, and B. mallei. PCR screening with Tel-specific oligonucleotides revealed that 100% of Telr colonies harbored the Telr cassette. Next, the mini-Tn7 system was utilized successfully to complement the ΔbetBA mutant. Finally, three different FRT-reporter fusion vectors were used to study the regulation of the B. thailandensis betBA operon. Results showed that the betBA operon, which is essential for B. thailandensis choline degradation, was induced significantly by choline and osmotic stress (NaCl).
There are several advantages to including FRT-flanked resistance cassettes in random transposon mutagenesis and allelic replacement (2, 32). First, unmarked mutations can be obtained subsequent to allelic replacement or transposon mutagenesis by Flp excision of FRT-flanked resistance selection cassettes. The use of FRT-flanked resistance cassettes in allelic replacement allows for easier selection, resulting in higher mutation frequencies than that reported for a recently published approach to obtain unmarked mutations where there was a lack of positive selection for the second homologous recombination (15). Furthermore, the lack of positive selection requires laborious screening, and mutating essential genes may not be possible. In addition, the remaining “FRT scar” adds flexibility to subsequent fusion integrations, aiding in the construction of fusion strains for regulation studies of nonessential genes without prior knowledge of the identity and location of promoter sequences. For essential genes, mutant fusion strains can be complemented with the mini-Tn7-Telr system presented here, and gene regulation studies can be performed. Because single copies are more representative of the natural genetic regulation mechanism, as opposed to multicopy plasmids, single-copy tools could ameliorate the difficulties of complementation and promoter studies. The six FRT-reporter fusion vectors, based on three different reporters (lacZ, gfp, and the luxCDABE operon), add further flexibility and provide simplified visualization and quantification of gene expression during regulation studies. For example, by fusing the gfp reporter downstream of a target gene with pFRT-gfp-Telr vectors, it is possible to measure gene expression via detecting the bacterial green fluorescent protein (GFP) signal under different growth conditions or during eukaryotic cell infections. Similarly, by utilizing the pFRT-lux-Telr vectors, the regulation of target genes during animal model infections can be studied by measuring the bacterial bioluminescence intensity.
The genetic tools described in this paper will aid in elucidating the physiology, environmental behavior, and pathogenic mechanisms of Burkholderia species. Although the model organism B. thailandensis was utilized primarily to demonstrate the efficacy of these tools, Telr selection was successfully tested in the select agents B. pseudomallei and B. mallei by using the transposon pBT20-Δbla-Telr-FRT. Thus, we believe that these tools could be particularly useful for various studies of B. pseudomallei and B. mallei. The alternative nonantibiotic asdEc selectable marker in helper plasmids pTNS3-asdEc and pCD13SK-Flp-oriT-asdEc may allow their use in other select-agent species. The genetic tools presented here could be further developed by substituting the Telr cassette with other nonantibiotic selectable markers. Finally, because these tools are completely devoid of any antibiotic resistance markers, they are in full compliance with CDC select-agent regulations.
Supplementary Material
Acknowledgments
This work was supported by an NIH grant (R21-AI074608) to T.T.H. A graduate stipend to M.H.N. was supported by NSF IGERT award 0549514 to B.A.W. Salary support for A.R.B. was provided by grant P20RR018727 from the National Center for Research Resources, a component of the NIH.
The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or the NIH.
We thank Herbert Schweizer (Colorado State University) for the gifts of pUC18R6KT-mini-Tn7 and pTNS3 and Attila Karsi (Mississippi State University) for the gift of pAKlux2.
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bao, Y., D. P. Lies, H. Fud, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, A. R., Y. Kang, K. S. Inamasu, M. S. Son, J. M. Vukovich, and T. T. Hoang. 2008. Genetic tools for allelic replacement in Burkholderia species. Appl. Environ. Microbiol. 74:4498-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, G. F. 1986. Permanent insertion of foreign genes into the chromosomes of soil bacteria. Nat. Biotechnol. 4:446-449. [Google Scholar]
- 4.Baulard, A., V. Escuyer, N. Haddad, L. Kremer, C. Locht, and P. Berche. 1995. Mercury resistant as a selective marker for recombinant mycobacteria. Microbiology 141:1045-1050. [DOI] [PubMed] [Google Scholar]
- 5.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., description of Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 6.Caiazza, N. C., R. M. Q. Shanks, and G. A. O'Toole. 2005. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol. 187:7351-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, Y. Y., T. M. C. Tan, Y. M. Ong, and K. L. Chua. 2004. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 48:1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, K. H., D. DeShazer, and H. P. Schweizer. 2006. Mini-Tn7 insertion in bacteria with multiple glmS-linked attTn7 sites: example Burkholderia mallei ATCC 23344. Nat. Protoc. 1:162-169. [DOI] [PubMed] [Google Scholar]
- 9.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2:443-448. [DOI] [PubMed] [Google Scholar]
- 10.Choi, K. H., T. Mima, Y. Casart, D. Rholl, A. Kumar, I. R. Beacham, and H. P. Schweizer. 2008. Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei. Appl. Environ. Microbiol. 74:1064-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeShazer, D., P. J. Brett, R. Carlyon, and D. E. Woods. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J. Bacteriol. 179:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and Flp-mediated site-specific recombination in bacteria. Gene 290:153-161. [DOI] [PubMed] [Google Scholar]
- 13.Eshoo, M. W. 1988. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J. Bacteriol. 170:5208-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvão, T. C., V. de Lorenzo, and D. Cánovas. 2006. Uncoupling of choline-O-sulphate utilization from osmoprotection in Pseudomonas putida. Mol. Microbiol. 62:1643-1654. [DOI] [PubMed] [Google Scholar]
- 15.Hamad, M. A., S. L. Zajdowicz, R. K. Holmes, and M. I. Voskuil. 2009. An allelic exchange system for compliant genetic manipulation of the select agents Burkholderia pseudomallei and Burkholderia mallei. Gene 430:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero, M., V. De Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 18.Holden, M. T., R. W. Titball, S. J. Peacock, A. M. Cerdeno-Tarraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 101:14240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, Y., D. T. Nguyen, M. S. Son, and T. T. Hoang. 2008. The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 beta-oxidation operon. Microbiology 154:1584-1598. [DOI] [PubMed] [Google Scholar]
- 20.Kang, Y., M. S. Son, and T. T. Hoang. 2007. One step engineering of T7-expression strains for protein production: increasing the host-range of the T7-expression system. Protein Expr. Purif. 55:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsi, A., and M. L. Lawrencea. 2007. Broad host range fluorescence and bioluminescence expression vectors for gram-negative bacteria. Plasmid 57:286-295. [DOI] [PubMed] [Google Scholar]
- 22.Kulasekara, H. D., I. Ventre, B. R. Kulasekara, A. Lazdunski, A. Filloux, and S. Lory. 2005. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 55:368-380. [DOI] [PubMed] [Google Scholar]
- 23.Lambertsen, L., C. Sternberg, and S. Molin. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6:726-732. [DOI] [PubMed] [Google Scholar]
- 24.Levano-Garcia, J., S. Verjovski-Almeida, and A. C. R. da Silva. 2005. Mapping transposon insertion sites by touchdown PCR and hybrid degenerate primers. BioTechniques 38:225-229. [DOI] [PubMed] [Google Scholar]
- 25.Lynch, M. D., and R. T. Gill. 2006. Broad host range vectors for stable genomic library construction. Biotechnol. Bioeng. 94:151-158. [DOI] [PubMed] [Google Scholar]
- 26.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144-156. [DOI] [PubMed] [Google Scholar]
- 27.Mandon, K., M. Osterås, E. Boncompagni, J. C. Trinchant, G. Spennato, M. C. Poggi, and D. Le Rudulier. 2003. The Sinorhizobium meliloti glycine betaine biosynthetic genes (betlCBA) are induced by choline and highly expressed in bacteroids. Mol. Plant-Microbe Interact. 16:709-719. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Moore, R. A., D. DeShazer, S. Reckseidler, A. Weissman, and D. E. Woods. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama, K., M. S. Kelly, and R. Curtiss III. 1988. Construction of an asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat. Biotechnol. 6:693-697. [Google Scholar]
- 31.Platt, R., C. Drescher, S.-K. Park, and G. J. Phillips. 2000. Genetic systems for reversible integration of DNA constructs and lacZ gene fusions into the Escherichia coli chromosome. Plasmid 43:12-23. [DOI] [PubMed] [Google Scholar]
- 32.Rholl, D. A., L. A. Trunck, and H. P. Schweizer. 2008. Himar1 in vivo transposon mutagenesis of Burkholderia pseudomallei. Appl. Environ. Micrbiol. 74:7529-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Richmond, J. Y., and R. W. McKinney. 2007. Biosafety in microbiological and biomedical laboratories, 5th ed. Centers for Disease Control and Prevention, Atlanta, GA.
- 33.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Sanchez-Romero, J. M., R. Diaz-Orejas, and V. De Lorenzo. 1998. Resistance to tellurite as a selection marker for genetic manipulations of Pseudomonas strains. Appl. Environ. Microbiol. 64:4040-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweizer, H. P., and S. J. Peacock. 2008. Antimicrobial drug-selection markers for Burkholderia pseudomallei and B. mallei. Emerg. Infect. Dis. 14:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson, A. J., N. J. White, and V. Wuthiekanun. 1999. Aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43:2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son, M. S., D. T. Nguyen, Y. Kang, and T. T. Hoang. 2008. Engineering of FRT-lacZ fusion constructs: induction of the Pseudomonas aeruginosa fadAB1 operon by medium and long chain-length fatty acids. Plasmid 59:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 39.Vandamme, P., J. Govan, and J. LiPuma. 2007. Diversity and role of Burkholderia spp., p. 1-28. In T. Coenye and P. Vandamme (ed.), Burkholderia: molecular microbiology and genomics. Horizon Scientific, Wymondham, United Kingdom.
- 40.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]
- 41.Yu, M., and J. S. H. Tsang. 2006. Use of ribosomal promoters from Burkholderia cenocepacia and Burkholderia cepacia for improved expression of transporter protein in Escherichia coli. Protein Expr. Purif. 49:219-227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.