Abstract
The induction of hydroxyl radical (OH) production via quinone redox cycling in white-rot fungi was investigated to improve pollutant degradation. In particular, we examined the influence of 4-methoxybenzaldehyde (anisaldehyde), Mn2+, and oxalate on Pleurotus eryngii OH generation. Our standard quinone redox cycling conditions combined mycelium from laccase-producing cultures with 2,6-dimethoxy-1,4-benzoquinone (DBQ) and Fe3+-EDTA. The main reactions involved in OH production under these conditions have been shown to be (i) DBQ reduction to hydroquinone (DBQH2) by cell-bound dehydrogenase activities; (ii) DBQH2 oxidation to semiquinone (DBQ−) by laccase; (iii) DBQ− autoxidation, catalyzed by Fe3+-EDTA, producing superoxide (O2−) and Fe2+-EDTA; (iv) O2− dismutation, generating H2O2; and (v) the Fenton reaction. Compared to standard quinone redox cycling conditions, OH production was increased 1.2- and 3.0-fold by the presence of anisaldehyde and Mn2+, respectively, and 3.1-fold by substituting Fe3+-EDTA with Fe3+-oxalate. A 6.3-fold increase was obtained by combining Mn2+ and Fe3+-oxalate. These increases were due to enhanced production of H2O2 via anisaldehyde redox cycling and O2− reduction by Mn2+. They were also caused by the acceleration of the DBQ redox cycle as a consequence of DBQH2 oxidation by both Fe3+-oxalate and the Mn3+ generated during O2− reduction. Finally, induction of OH production through quinone redox cycling enabled P. eryngii to oxidize phenol and the dye reactive black 5, obtaining a high correlation between the rates of OH production and pollutant oxidation.
The degradation of lignin and pollutants by white-rot fungi is an oxidative and rather nonspecific process based on the production of substrate free radicals (36). These radicals are produced by ligninolytic enzymes, including laccase and three kinds of peroxidases: lignin peroxidase, manganese peroxidase, and versatile peroxidase (VP) (23). The H2O2 required for peroxidase activities is provided by several oxidases, such as glyoxal oxidase and aryl-alcohol oxidase (AAO) (9, 18). This free-radical-based degradative mechanism leads to the production of a broad variety of oxidized compounds. Common lignin depolymerization products are aromatic aldehydes and acids, and quinones (34). In addition to their high extracellular oxidation potential, white-rot fungi show strong ability to reduce these lignin depolymerization products, using different intracellular and membrane-bound systems (4, 25, 39). Since reduced electron acceptors of oxidized compounds are donor substrates for the above-mentioned oxidative enzymes, the simultaneous actions of both systems lead to the establishment of redox cycles (35). Although the function of these redox cycles is not fully understood, they have been hypothesized to be related to further metabolism of lignin depolymerization products that require reduction to be converted in substrates of the ligninolytic enzymes (34). A second function attributed to these redox cycles is the production of reactive oxygen species, i.e., superoxide anion radicals (O2−), H2O2, and hydroxyl radicals (OH), where lignin depolymerization products and fungal metabolites act as electron carriers between intracellular reducing equivalents and extracellular oxygen. This function has been studied in Pleurotus eryngii, whose ligninolytic system is composed of laccase (26), VP (24), and AAO (9). Incubation of this fungus with different aromatic aldehydes has been shown to provide extracellular H2O2 on a constant basis, due to the establishment of a redox cycle catalyzed by an intracellular aryl-alcohol dehydrogenase (AAD) and the extracellular AAO (7, 10). The process was termed aromatic aldehyde redox cycling, and 4-methoxybenzaldehyde (anisaldehyde) serves as the main Pleurotus metabolite acting as a cycle electron carrier (13). A second cyclic system, involving a cell-bound quinone reductase activity (QR) and laccase, was found to produce O2− and H2O2 during incubation of P. eryngii with different quinones (11). The process was described as the cell-bound divalent reduction of quinones (Q) by QR, followed by extracellular laccase oxidation of hydroquinones (QH2) into semiquinones (Q−), which autoxidized to some extent, producing O2− (Q− + O2 ⇆ Q + O2−). H2O2 was formed by O2− dismutation (O2− + HO2 + H+ → O2 + H2O2). In an accompanying paper, we describe the extension of this O2− and H2O2 generation mechanism to OH radical production by the addition of Fe3+-EDTA to incubation mixtures of several white-rot fungi with different quinones (6). Among them, those derived from 4-hydroxyphenyl, guaiacyl, and syringyl lignin units were used: 1,4-benzoquinone (BQ), 2-methoxy-1,4-benzoquinone (MBQ), and 2,6-dimethoxy-1,4-benzoquinone (DBQ), respectively. Semiquinone autoxidation under these conditions was catalyzed by Fe3+-EDTA instead of being a direct electron transfer to O2. The intermediate Fe2+-EDTA reduced not only O2, but also H2O2, leading to OH radical production by the Fenton reaction (H2O2 + Fe2+ → OH + OH− + Fe3+).
Although OH radicals are the strongest oxidants produced by white-rot fungi (2, 14), studies of their involvement in pollutant degradation are quite scarce. In this context, the objectives of this study were to (i) determine possible factors enhancing the production of OH radicals by P. eryngii via quinone redox cycling and (ii) test the validity of this inducible OH production mechanism as a strategy for pollutant degradation. Our selection of possible OH production promoters was guided by two observations (6). First, the redox cycle of benzoquinones working with washed P. eryngii mycelium is rate limited by hydroquinone oxidation, since the amounts of the ligninolytic enzymes that remained bound to the fungus under these conditions were not large. Second, H2O2 is the limiting reagent for OH production by the Fenton reaction.
With these considerations in mind, anisaldehyde and Mn2+ were selected to increase H2O2 production. As mentioned above, anisaldehyde induces H2O2 production in P. eryngii via aromatic aldehyde redox cycling (7). Mn2+ has been shown to enhance H2O2 production during the oxidation of QH2 by P. eryngii laccase by reducing the O2− produced in the semiquinone autoxidation reaction (Mn2+ + O2− → Mn3+ + H2O2 + 2 H+) (26). Mn2+ was also selected to increase the hydroquinone oxidation rate, since this reaction has been shown to be propagated by the Mn3+ generated in the latter reaction (QH2 + Mn3+ → Q− + Mn2+ + 2 H+). The replacement of Fe3+-EDTA by Fe3+-oxalate was also planned in order to increase the QH2 oxidation rate above that resulting from the action of laccase. Oxalate is a common extracellular metabolite of wood-rotting fungi to which the function of chelating iron and manganese has been attributed (16, 45). The use of Fe3+-oxalate and nonchelated Fe3+, both QH2 oxidants, has been proven to enable quinone redox cycling in fungi that do not produce ligninolytic enzymes, such as the brown-rot fungus Gloeophyllum trabeum (17, 40, 41). Finally, phenol and the azo dye reactive black 5 (RB5) were selected as model pollutants.
MATERIALS AND METHODS
Chemicals and enzymes.
H2O2 (Perhydrol 30%) was obtained from Merck. 2-Deoxyribose, 2-thiobarbituric acid, bovine liver catalase, and oxalate oxidase were purchased from Sigma. 2,6-Dimethoxyphenol, 3,4-dimethoxybenzyl (veratryl) alcohol, 3,4,5-trimethoxybenzyl alcohol, 3,4,5-trimethoxybenzaldehyde, anisaldehyde, BQ, DBQ, 1,4-benzohydroquinone (BQH2), 2-methoxy-BQH2, hydroxybenzene (phenol), and 1,2-dihydroxybenzene (catechol) were from Aldrich. RB5 and 1,3-dihydroxybenzene (resorcinol) were from Sigma-Aldrich. 2,6-Dimethoxy-1,4-benzohydroquinone (DBQH2) and MBQ were synthesized as previously reported (6). All other chemicals used were of analytical grade. Laccase isoenzyme I (EC 1.10.3.2) from P. eryngii was produced and purified as described previously (26).
Organism and culture conditions.
P. eryngii IJFM A169 (Fungal Culture Collection of the Centro de Investigaciones Biológicas) (= ATCC 90787 and CBS 613.91) was grown in a glucose-peptone medium supplemented with 50 μM MnSO4 in order to repress VP synthesis by the fungus (6). Oxalate production by the fungus was also investigated in the absence of Mn.
Enzyme activities.
Washed mycelium was used for the determination of cell-bound laccase, AAO, QR, and AAD activities. Laccase and QR activities were estimated as reported in an accompanying paper (6). AAO activity was assayed in 100 mM phosphate buffer, pH 6, as the oxidation of veratryl alcohol to veratraldehyde (extinction coefficient at 310 nm [ɛ310] = 9,300 M−1 cm−1). For the determination of AAD activity, the substrate selected was 3,4,5-trimethoxybenzaldehyde to avoid, as much as possible, underestimations due to the action of AAO on its reduction product (3,4,5-trimethoxybenzyl alcohol). AAO activity on this alcohol has been shown to be quite low (9). QR activity was determined in 50 mM phosphate buffer, pH 5, containing 500 μM 3,4,5-trimethoxybenzaldehyde, as the production of 3,4,5-trimethoxybenzyl alcohol, which was analyzed by high-performance liquid chromatography (HPLC). Samples (20 μl) were injected into a Pharmacia system equipped with a Spherisorb S50DS2 column (Hichrom) and a diode array detector. The analyses were carried out at 40°C with a flow rate of 1 ml min−1 using 10 mM phosphoric acid-methanol (60/40) as the eluent. The alcohol concentrations in samples were calculated using a standard calibration curve. For these cell-bound analyses of enzymatic activities, appropriate amounts of mycelium were incubated at room temperature (22 to 25°C) with 20 ml substrate solutions in shaken 100-ml conical flasks (150 rpm). Samples were taken at 1-min intervals for 5 min. The mycelium was separated from the liquid by filtration. Absorbance was measured immediately after filtration for the analysis of laccase and AAO activities. The pH of samples used for the determination of QR and AAD activities was lowered to 2 with phosphoric acid, and they were kept frozen at −20°C until they were analyzed. International units (μmol min−1) were used.
Redox cycling experiments.
Anisaldehyde, BQ, MBQ, and DBQ (500 μM) redox cycling experiments were performed using 10-day-old pellets (202 ± 14 mg [dry weight]) as described previously (6). For OH production experiments, the complex 100 μM Fe3+-110 μM EDTA and 2.8 mM 2-deoxyribose were added to the incubation mixture. Iron salt (FeCl3) solutions were made up fresh immediately before use. Incubations were performed in the dark at 28°C and 150 rpm in 100-ml conical flasks. In phenol and RB5 degradation studies, initial concentrations of 500 and 50 μM were used, respectively. Samples were taken periodically from three replicate flasks, and the incubation liquid was separated from the mycelium by filtration. In order to inactivate laccase and AAO that could be released to the extracellular solution during the experiments, samples were treated in different ways depending on the kind of analysis to be done. For the analysis of quinone, hydroquinone, 2-thiobarbituric acid-reactive substances (TBARS), and phenol, the pH of samples was lowered to 2 with phosphoric acid. For H2O2 estimation, samples were heated at 80°C for 20 min (a treatment that does not affect H2O2 levels). The rest of the analyses were performed just after samples were taken. Verification of laccase and AAO inactivation by these treatments was carried out using a 10-day-old culture liquid sample.
Analytical techniques.
The Somogyi-Nelson method for the determination of reducing sugars was used to estimate the glucose concentrations in fungal cultures (38).
Levels of DBQ, DBQH2, phenol, catechol, resorcinol, and BQH2 were determined by HPLC, using standard calibration curves for each compound and the same chromatographic conditions described above for AAD activity determination, except for the ratio of the phosphoric acid-methanol used as the eluent (80/20). The compounds were identified by comparing the retention time and spectrum with those of standards. Chemical and enzymatic oxidation of DBQH2 was determined spectrophotometrically as DBQ production (ɛ397 = 562 M−1 cm−1) (12). Oxalate production by P. eryngii was determined by HPLC, using oxalate oxidase to confirm the acid identity, as reported by Kuan and Tien (22).
H2O2 levels were estimated by measuring the production of O2 with a Clark-type electrode after the addition of catalase to samples (6). The production of OH radicals was estimated as the conversion of 2-deoxyribose into TBARS (6). The RB5 concentration was determined spectrophotometrically at 596 nm. The molar extinction coefficient used was calculated using solutions of the dye in 20 mM phosphate buffer, pH 5, and proved to be 43,974 M−1 cm−1.
Statistical analysis.
All the results included in the text and shown in the figures are the means and standard deviations of three replicates (full biological experiments and technical analyses).
RESULTS
Selection of incubation conditions for quinone and aromatic aldehyde redox cycling.
To study the effects of anisaldehyde, Mn2+, and oxalate on the production of extracellular OH by P. eryngii, quinone redox cycling standard conditions were defined as follows: incubation of 10-day-old washed mycelium, grown under conditions expressing laccase as the sole ligninolytic enzyme, with a benzoquinone (usually DBQ) and Fe3+-EDTA. Washed mycelium was used for two reasons: (i) to prevent, as much as possible, the reaction of OH radicals with compounds other than the probe used for their detection and (ii) to decrease the amount of laccase and thus to be able to evaluate better the effects of factors increasing hydroquinone oxidation. VP production was repressed to avoid (i) the consumption of the H2O2 required for OH production (6); (ii) the oxidation of Mn2+ (24), which could mask this reaction during the course of quinone redox cycling; and (iii) the oxidation of RB5 (15), which would hinder any effort made to ascribe a role to OH radicals in the degradation of the dye. Enzyme activity analysis in this mycelium showed that all the enzymes required for the catalysis of the redox cycling of quinones (QR and laccase) and aromatic aldehydes (AAD and AAO) were present. The amounts of the extracellular enzymes laccase and AAO that remained associated with the mycelium after being washed were 10.8 ± 1.5 and 7.3 ± 0.3 mU mg−1 (dry weight), respectively. The activities of intracellular dehydrogenases were shown to be 27.8 ± 3.1 and 1.2 ± 0.1 mU mg−1 for QR and AAD, respectively.
Effect of anisaldehyde on OH production.
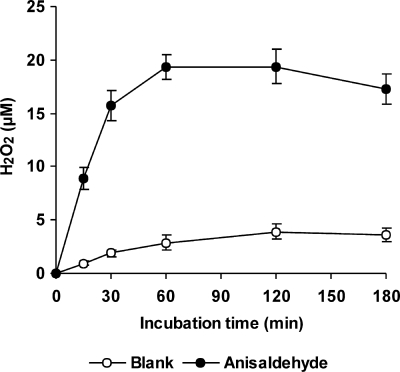
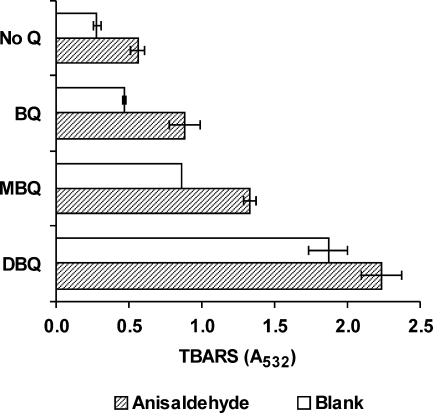
The time course of production of extracellular H2O2 by washed P. eryngii mycelium when incubated with anisaldehyde is shown in Fig. 1. Constant H2O2 levels reached after 60 min of incubation increased from 3.5 to 18.6 μM (average of 60- to 180-min samples). This increase was caused by the concerted actions of AAD reducing anisaldehyde and AAO oxidizing anisyl alcohol. In order to test if this increase had any effect on OH production by the fungus, anisaldehyde was included in quinone redox cycling experiments carried out with BQ, MBQ, and DBQ. In all cases, the presence of anisaldehyde enhanced OH production, which was linear during the 180 min that the experiments lasted (data not shown). Figure 2 shows TBARS levels in 120-min samples. Regardless of anisaldehyde inclusion, the highest values were found in incubations with DBQ, followed by MBQ, BQ, and no Q. In quantitative terms, the increase caused by anisaldehyde followed the opposite order: 2.0-, 1.9-, 1.5-, and 1.2-fold higher in incubations with no Q, BQ, MBQ, and DBQ, respectively. H2O2 production in these incubations without anisaldehyde has been reported to reach constant 4, 8, 72, and 192 μM levels with no Q, BQ, MBQ, and DBQ, respectively (6). Therefore, the lower these levels, the greater the effect of anisaldehyde on OH radical production.
FIG. 1.
Production of H2O2 by P. eryngii via anisaldehyde redox cycling. Ten-day-old washed mycelium was incubated with 1 mM anisaldehyde in 50 ml 20 mM phosphate buffer, pH 5. Anisaldehyde was omitted in blank incubations. The error bars indicate standard deviations.
FIG. 2.
Effect of anisaldehyde on OH production by P. eryngii through the redox cycling of several benzoquinones. TBARS levels produced after 120-min incubation of the fungus with 500 μM BQ, MBQ, DBQ, or no quinone (No Q); 1 mM anisaldehyde; 100 μM Fe3+-110 μM EDTA; and 2.8 mM 2-deoxyribose are shown. Anisaldehyde was absent in incubation blanks. The error bars indicate standard deviations.
Effect of Mn2+ on OH production.
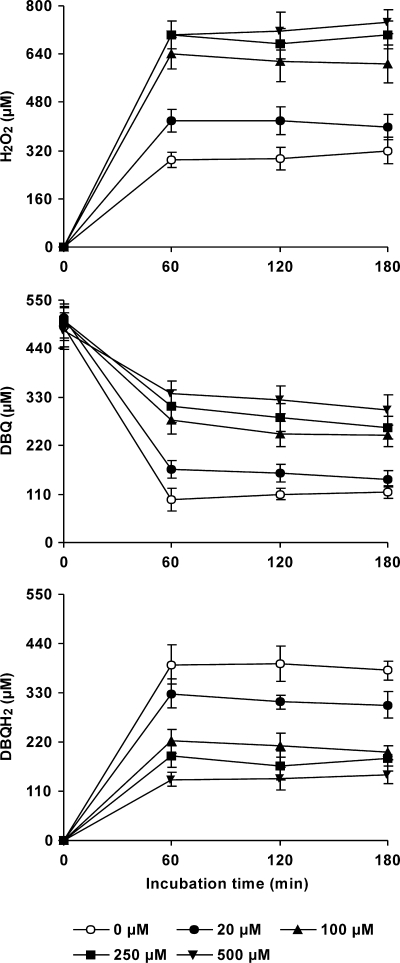
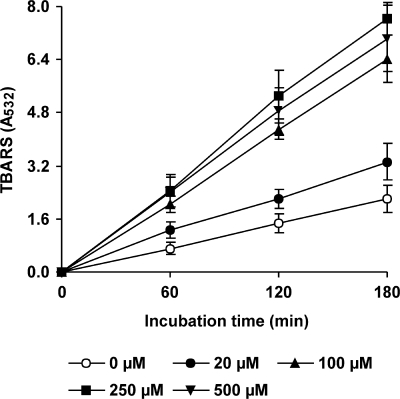
DBQ was selected for experiments evaluating the effect of Mn2+ on OH production by P. eryngii. The two effects expected to be caused by Mn2+ on quinone redox cycling (increased H2O2 production by reduction of O2− and propagation of DBQH2 oxidation by the resulting Mn3+) were first evaluated in vitro. The reactions were carried out in 20 mM phosphate buffer, pH 5, with purified laccase I from P. eryngii and DBQH2. H2O2 levels, estimated after full oxidation of 100 μM DBQH2 in the absence and presence of 100 μM Mn2+, were found to be 9.0 ± 0.3 and 50.2 ± 0.7 μM, respectively. The oxidation rate of DBQH2 (500 μM) increased from 37 ± 1 μM min−1 in reactions without Mn2+ to 296 ± 4 μM min−1 in reactions containing 100 μM Mn2+. These two Mn effects were then tested in vivo during the incubation of P. eryngii with DBQ in the absence of Fe3+-EDTA. The levels of H2O2, DBQ, and DBQH2 at system equilibrium in the absence and presence of different Mn2+ concentrations are shown in Fig. 3. Mn2+ exerted a pronounced effect on the three parameters being analyzed. H2O2 levels increased as the Mn2+ concentration did, with no significant differences observed beyond 100 μM. The presence of Mn2+ also decreased DBQH2/DBQ ratios in samples, showing the propagation of DBQH2 oxidation by Mn3+ and, therefore, the acceleration of the DBQ redox cycle. The global effect of Mn2+ on OH production was evaluated in parallel incubations with DBQ including Fe3+-EDTA (Fig. 4). The TBARS production rate improved from 12 mU A532 min−1 (incubation without Mn2+) to 19 and 36 mU A532 min−1 in incubations with 20 and 100 μM Mn2+, respectively. Similarly to the H2O2 production levels depicted in Fig. 3, a further increase of the Mn2+ concentration to 250 and 500 μM did not lead to any significant increase in the TBARS production rate.
FIG. 3.
Effects of different concentrations of Mn2+ on H2O2 production and DBQ(H2) levels during the incubation of P. eryngii with DBQ. Washed mycelium was incubated with 500 μM DBQ in the absence and presence of 20, 100, 250, and 500 μM Mn2+. The error bars indicate standard deviations.
FIG. 4.
Effects of different concentrations of Mn2+ on OH production by P. eryngii via DBQ redox cycling. The incubation mixtures were as described in the legend to Fig. 3 plus 100 μM Fe3+-110 μM EDTA and 2.8 mM 2-deoxyribose. The error bars indicate standard deviations.
Effect of oxalate on OH production and phenol oxidation.
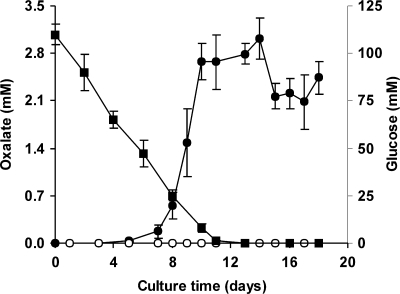
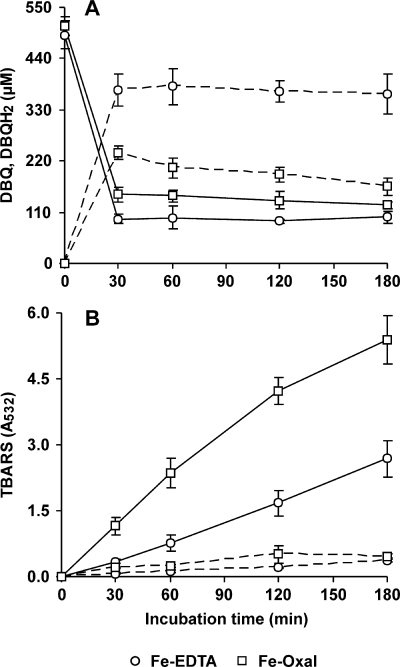
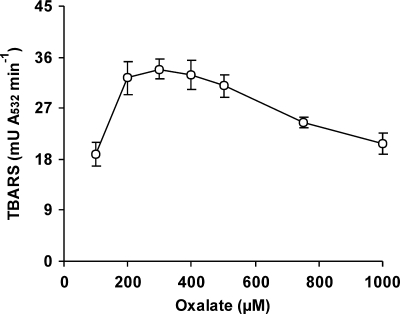
The capability of P. eryngii to produce oxalate is shown in Fig. 5. Oxalate was detected only when the fungus was grown in the absence of Mn2+. The highest levels (around 2.8 mM) were measured after 10 days, when glucose had been nearly depleted (6). The effect of replacing Fe3+-EDTA by Fe3+-oxalate on OH production was also studied during the DBQ redox cycle. Since the aim of this replacement was to increase the DBQH2 oxidation rate, the likelihood of Fe3+-oxalate acting as a DBQH2 oxidant was first tested. Compared with 100 μM Fe3+-110 μM EDTA, which prevented the oxidation of 500 μM DBQH2 (5-min reactions in 20 mM phosphate buffer, pH 5), 100 μM Fe3+-500 μM oxalate oxidized DBQH2 at a rate of 20.6 ± 0.3 μM min−1. Figure 6A and B shows the effect caused by Fe3+-oxalate on the DBQ redox cycle and TBARS production, respectively. The replacement of Fe3+-EDTA by Fe3+-oxalate produced a decrease in the DBQH2/DBQ concentration ratio in all samples. These results indicated that the redox cycle rate was raised, according to the increase seen in vitro to be caused by Fe3+-oxalate in the DBQH2 oxidation rate. As a consequence, the TBARS production rate was about threefold greater with Fe3+-oxalate than with Fe3+-EDTA. TBARS production in the absence of DBQ was also observed in both cases, although at a much lower rate and showing no significant differences between them. We have previously reported that Fe3+-EDTA reduction by an unknown cell-bound system was causing OH production in incubation blanks without quinones (6). The TBARS production rate (Fig. 6) was shown to be constant with Fe3+-EDTA during the whole period of study (14.4 mU A532 min−1). However, with Fe3+-oxalate, it decreased gradually from 39.0 mU A532 min−1 during the first hour to 31.4 and 19.5 mU A532 min−1 during the second and third hours, respectively. Among the possible causes, DBQ consumption was clearly observed. Thus, whereas the sum of the DBQ and DBQH2 levels in samples from Fe3+-EDTA incubations remained steady around 468 μM (average of 30- to 180-min samples), in the case of Fe3+-oxalate, decreases from the initial 500 μM DBQ concentration to 386 μM in the 30-min sample and 290 μM in the 180-min sample were found. Afterward, OH production was optimized in terms of both the Fe3+/oxalate ratio and the concentration of the best ratio obtained. Figure 7 shows an optimal Fe3+/oxalate ratio of 1:3, although no significant differences in the TBARS production rates were observed from the 1:2 to 1:5 ratios. Using the 1:3 Fe3+/oxalate ratio, no significant differences were observed in the TBARS production rates using concentrations ranging from 100:300 to 1,000:3,000 μM (data not shown).
FIG. 5.
Time course of oxalate production (•) and glucose consumption (▪) by P. eryngii in glucose-peptone medium. Cultures were carried out in the absence (filled symbols) and presence (open symbols) of 50 μM MnSO4. Glucose levels without Mn were not significantly different from those with Mn (data not shown). The error bars indicate standard deviations.
FIG. 6.
Effects of oxalate, used as an iron-chelating agent, on DBQ(H2) levels and OH production during the incubation of P. eryngii with DBQ. The incubation mixtures contained 500 μM DBQ, 2.8 mM 2-deoxyribose, and either 100 μM Fe3+-110 μM EDTA or 100 μM Fe3+-500 μM oxalate. (A) DBQ (solid lines) and DBQH2 (dashed lines) levels. (B) TBARS levels in whole incubations (solid lines) and blanks without DBQ (dashed lines). The error bars indicate standard deviations.
FIG. 7.
Effect of the Fe3+/oxalate concentration ratio on OH production by P. eryngii via DBQ redox cycling. Washed mycelium was incubated with 500 μM DBQ, 2.8 mM 2-deoxyribose, and 100 μM Fe3+ chelated with different oxalate concentrations. The error bars indicate standard deviations.
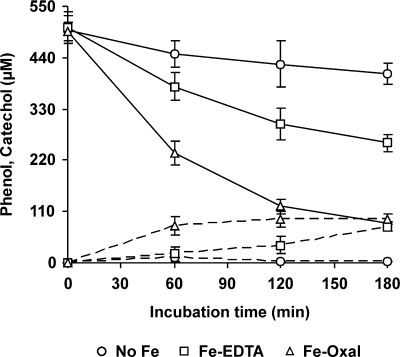
To better ascribe a role to OH radicals in the oxidation of pollutants, two DBQ redox cycling incubation conditions leading to quite different TBARS production rates were used. Thus, phenol oxidation was studied using Fe3+-EDTA and Fe3+-oxalate complexes. Phenol removal was clearly associated with induction of OH radical production (Fig. 8). We have previously reported that phenol is not a substrate of P. eryngii laccase (27). Due to phenol volatility, a gradual decrease in its initial concentration was observed in the different incubation controls used. After subtracting this decrease, the extents of phenol removal in 180-min samples in incubations with Fe3+-EDTA and Fe3+-oxalate were 29 and 64%, respectively. As hydroxylation is one of the main reactions caused by OH radicals when acting on aromatic compounds, samples were analyzed for the production of the three possible dihydroxylated phenol derivatives, i.e., catechol, resorcinol and BQH2. Among them, only catechol was detected in all samples (Fig. 8). BQH2 was sporadically found in some samples (6.2 μM was the highest level detected), and resorcinol was always absent (data not shown).
FIG. 8.
Phenol removal by P. eryngii under conditions producing different levels of OH radicals. Incubations of washed mycelium with 500 μM concentrations of phenol and DBQ were performed in the absence (no Fe) and presence of 100 μM Fe3+-110 μM EDTA or 100 μM Fe3+-300 μM oxalate. The time courses of phenol disappearance and catechol production are shown (solid and dashed lines, respectively). The error bars indicate standard deviations.
Combined effects of oxalic acid and Mn2+ on OH production and RB5 oxidation.
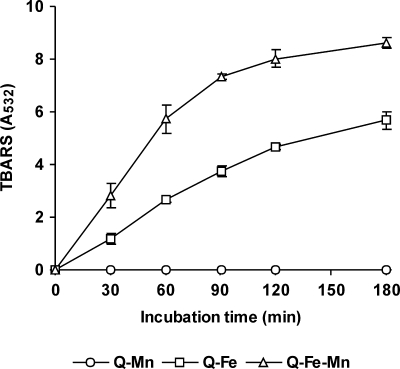
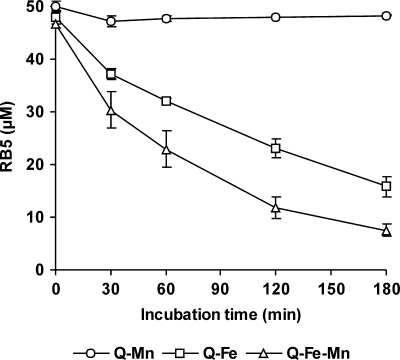
A combination of Fe3+-oxalate and Mn2+ to promote OH radical production was included in RB5 degradation studies. Figure 9 shows the effect of Mn2+ on TBARS production by P. eryngii in incubations with DBQ and Fe3+-oxalate. The TBARS production rate, calculated from the results obtained during the first 60 min, increased from 43.6 to 91.1 mU A532 min−1 by the addition of Mn2+. This represents a 6.3-fold increase relative to the rate observed in incubations with Fe3+-EDTA (Fig. 6B). RB5 oxidation results obtained under the same OH radical induction conditions are shown in Fig. 10. The dye was oxidized only when OH radicals were produced, showing a high correlation with the TBARS produced under each incubation condition (Fig. 9). Thus, the extents of degradation observed after 3 h were 68 and 85% in the absence and presence of Mn2+, respectively. Incubation blanks in these experiments were carried out with DBQ and Mn2+, but in the absence of Fe3+-oxalate, as the Mn3+ produced in the course of the DBQ redox cycle could oxidize RB5. Figure 10 shows that this was not the case. The lack of laccase activity on RB5 was confirmed in in vitro reactions with purified enzyme.
FIG. 9.
Effect of Mn2+ on OH production by P. eryngii in incubations with DBQ and Fe3+-oxalate. The incubation mixtures contained 500 μM DBQ, 100 μM Fe3+-300 μM oxalate, 2.8 mM 2-deoxyribose, and 100 μM Mn2+ (Q-Fe-Mn). Incubation blanks without Mn (Q-Fe) or Fe3+-oxalate (Q-Mn) were performed. The error bars indicate standard deviations.
FIG. 10.
RB5 removal by P. eryngii under conditions producing different levels of OH radicals. The composition of the reaction mixtures was as described in the legend to Fig. 9, except 2-deoxyribose was replaced by 50 μM RB5. The error bars indicate standard deviations.
DISCUSSION
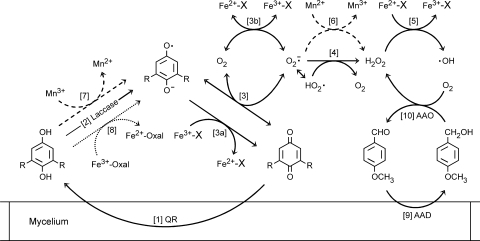
The production of OH radicals by P. eryngii via DBQ redox cycling in the presence of Fe3+-EDTA is illustrated in Fig. 11 by reactions 1 to 5. In the accompanying paper (6), we reported that after DBQ reduction by QR (reaction 1) and DBQH2 oxidation by laccase (reaction 2), the production of O2− by DBQ− autoxidation (reaction 3) is mainly catalyzed by Fe3+-EDTA (reactions 3a and 3b). Fenton's reagent formation is accomplished by O2 − dismutation (reaction 4), and as a result, OH radicals are generated (reaction 5). In the present study, we have identified three approaches to increase OH production by this mechanism. Two are based on the enhancement exerted by adequate metal ions on the DBQH2 oxidation rate and H2O2 production during the course of quinone redox cycling. The third approach implies the induction of aromatic aldehyde redox cycling, which increases H2O2 production further to that caused by quinone redox cycling itself.
FIG. 11.
Scheme showing the main reactions involved in the production of OH radicals by P. eryngii via DBQ redox cycling in the presence of Mn2+, the complex Fe3+-oxalate, and anisaldehyde. R = OCH3, and X = EDTA or oxalate (see Discussion for an explanation).
The addition of Mn2+ to DBQ redox cycling experiments was shown to increase H2O2 levels and the DBQH2 oxidation rate (Fig. 3), improving OH radical production (Fig. 4). The reactions involving Mn2+, shown in Fig. 11, are the reduction of O2− to H2O2 (reaction 6) and the oxidation of DBQH2 by the resulting Mn3+ (reaction 7). Stoichiometrically, the first reaction doubles the amount of H2O2 produced by O2− dismutation. Since DBQ− autoxidation is a reversible reaction, the consumption of O2− by Mn2+ shifts equilibrium toward the right, favoring this reaction over other competing reactions converting DBQ− into DBQ (dismutation and oxidation by laccase) (12). The results supporting the propagation of DBQH2 oxidation by Mn3+ were as follows: (i) the decrease in the DBQH2/DBQ ratios observed in redox cycling experiments carried out in the presence of different concentrations of Mn2+ (Fig. 3) and (ii) the eightfold increase caused by Mn2+ in the rate of DBQH2 oxidation catalyzed by purified laccase (see above). We have previously reported that the addition of superoxide dismutase to laccase reaction mixtures with the same composition increased the rate of DBQH2 oxidation by only 1.1 times (12) (superoxide dismutase shifts DBQ− autoxidation reaction to DBQ production by consuming O2−, as Mn2+ does, but has no effect on the hydroquinone oxidation rate). Demonstration of Mn3+ production through quinone redox cycling (a sequence of reactions 1 to 3 and 6 in Fig. 11) provides in vivo validation of our previous finding of the involvement of laccase in Mn2+ oxidation (11, 26, 33). Furthermore, it implies the cooperation of QR activities by the recycling of laccase reaction products. Therefore, we extend to quinone redox cycling our earlier proposal of hydroquinone oxidation by laccase as an alternative mechanism to manganese peroxidase- and VP-mediated production of Mn3+.
A second successful strategy to improve OH production was the substitution of Fe3+-EDTA by Fe3+-oxalate (Fig. 6). The use of Fe3+-EDTA, preventing hydroquinone oxidation by this metal ion, was shown to be a useful tool to ascribe a role to ligninolytic enzymes in quinone redox cycling (6). However, chemical oxidation of hydroquinones by Fe3+ complexes involving white-rot fungus metabolites, such as oxalate, could also play a role in the process, as reported in G. trabeum (40, 41). The capability of P. eryngii to produce oxalate is shown for the first time in the present study (Fig. 5). Further research is required in order to know if the absence of oxalate in cultures containing Mn2+ was due to repression of its synthesis or its continuous consumption. Although VP production is repressed in the presence of Mn2+ (6), the existence of an alternative mechanism providing Mn3+, which has been shown to oxidize oxalate (22), cannot be excluded. The results shown in Fig. 6 demonstrated that the oxidation of DBQH2 by Fe3+-oxalate makes a good contribution to OH production. This reaction, labeled 8 in Fig. 11, leads to the production of Fe2+-oxalate, whose involvement in the reduction of O2 (reaction 3b) and H2O2 (reaction 5) has been previously reported (28). Compared with Fe3+-EDTA, one distinctive effect of Fe3+-oxalate on the DBQ redox cycle has been the progressive consumption of DBQ(H2) (Fig. 6), which could be attributed to an increase in OH production. It seems unlikely that other possible oxalate side reactions occurring during the course of quinone redox cycling could have a direct effect on DBQ(H2) removal, although they may affect the rate of redox cycling. For instance, the production of the powerful reducing agent formate anion radical (CO2−) through the oxidation of oxalate by OH (OH + oxalate → CO2− + CO2 + H2O) (3, 41) could increase O2− production via autoxidation (CO2− + O2 → CO2 + O2−) (31), as well as the reduction rates of iron (19) and most probably quinone. In terms of the TBARS production rate, the contribution of Fe3+-oxalate (Fig. 6) was similar to that observed with Mn2+ (Fig. 4), i.e., a threefold increase. The combination of Fe3+-oxalate and Mn2+ increased this parameter two more times (Fig. 9). In the presence of oxalate and Mn2+, H2O2 production could be promoted, not only by the reduction of O2− by Mn2+ (Fig. 11, reaction 6), but also by the production of CO2− through the oxidation of oxalate by the resulting Mn3+ (oxalate + Mn3+ → CO2− + CO2 + Mn2+), followed by CO2− autoxidation and either O2− dismutation or reduction by Mn2+ (22). Although the oxidation of oxalate by OH and Mn2+ could have a positive effect on H2O2 production, the occurrence of these reactions would consume oxalate. This consumption, joined to the disappearance of DBQ(H2) (Fig. 6), could explain the decline of the TBARS production rate observed after 60 min under these conditions (Fig. 9). It is also likely that this decline was caused by the uptake of iron by the fungus, which could be facilitated by its binding to oxalate (EDTA has been reported to inhibit this process [20]).
The third approach to improve OH production by P. eryngii consisted of anisaldehyde addition to fungal incubations with ferric iron and different quinones. Our results show that this improvement is due to increased H2O2 production by the fungus via anisaldehyde redox cycling (Fig. 1 and 2). Reactions 9 and 10 in Fig. 11 illustrate this process. From the constant H2O2 levels observed in incubations of P. eryngii with 1 mM anisaldehyde (19 μM) (Fig. 1) and those previously found in incubations with 500 μM BQ, MBQ, and DBQ (8, 72, and 192 μM, respectively) (6), it is noticeable that the redox cycling of methoxyquinones is a much more efficient H2O2 production mechanism than that of aromatic aldehydes. Thus, the increase caused by anisaldehyde in OH production with these quinones, mainly with DBQ (1.2 times), was small (Fig. 2). This increase was nearly insignificant compared with that caused by Mn2+ or Fe3+-oxalate in DBQ incubations (three times) (Fig. 4 and 6B, respectively). This led us to discard anisaldehyde as a factor promoting OH production in pollutant removal studies. Regardless, the results shown in Fig. 2 are of interest, since they confirm the cooperation of laccase and AAO for OH production (8), which we have shown incorporates QR and AAD activities. In other words, quinone and aromatic aldehyde redox cycling acting together for OH production has been demonstrated.
Under the incubation conditions used in the present study (washed mycelium producing laccase as the sole ligninolytic enzyme), the induction of OH production enabled P. eryngii to degrade the two pollutants tested. This has been shown by the high correlation between the rates of phenol and RB5 removal (Fig. 8 and 10, respectively) and those of TBARS production observed in each case (Fig. 6B and 9, respectively). These results support our previous proposal for the use of quinone redox cycling as a useful tool to increase OH production in white-rot fungi and to enable us to ascribe a role to these radicals in pollutant degradation. In brown-rot fungi, whose degradative capability is mainly due to OH radical production, quinone redox cycling studies have provided good evidence of the crucial role these radicals play in the degradation of polyethylene glycol by G. trabeum (17) and 2-fluorophenol by Gloeophyllum striatum (21). The results shown in Fig. 8 and 10 also provide the basis for the use of quinone redox cycling as a strategy to increase the degradative capability of white-rot fungi with regard to pollutants. Although the production of OH radicals by white-rot fungi has not yet been taken advantage of for this purpose, physical-chemical processes based on the formation of these radicals have gained great attention in recent years due to their high efficiency in the degradation and even mineralization of pollutants (29, 30, 44). These processes, some of them based on Fenton's reagent, are referred to as advanced oxidation processes (5). In the present study, catechol and BQH2 have been identified as phenol degradation intermediates (Fig. 8). Degradation studies of phenolic compounds and azo dyes (including phenol and RB5) by different advanced oxidation processes have shown not only the production of hydroquinones as primary intermediates, but also of quinones and oxalate as consecutive intermediates (1, 37, 42, 43).
From these observations, two interesting inferences can be drawn. First, although mineralization of pollutants is possible by OH radical attack, their initial conversion into hydroquinones can facilitate the degradative process, as these intermediates are well-known substrates of the ligninolytic enzymes. For instance, the two hydroquinones derived from phenol hydroxylation (catechol and BQH2) have been shown to be oxidized by P. eryngii laccase (26). Furthermore, catechol is susceptible to ring cleavage by intracellular dioxygenases already characterized in white-rot fungi (32). Second, although the quinone used to induce OH production, and probably oxalate, is consumed during quinone redox cycling (Fig. 6), the production of similar or the same pollutant degradation intermediates can sustain the production of OH radicals. In this respect, we have already shown that BQ and 2-methyl-1,4-naphthoquinone (menadione), which can derive from OH attack on phenol and 2-methylnaphthalene, respectively, support OH radical production (6). More work is needed to corroborate the notion that the induction of OH production in white-rot fungi via quinone redox cycling improves their degradative capabilities. In this regard, our research group is investigating the degradation of a wide range of recalcitrant and toxic compounds by several white-rot fungi using this strategy.
Acknowledgments
This research was funded by two projects of the Comunidad de Madrid (GR/AMB/0812/2004 and S-0505/AMB0100) and one project of the Comunidad de Madrid-Universidad de Alcalá (CAM-UAH2005/065). The stays of V. Gomez-Toribio at the Centro de Investigaciones Biológicas and A. B. García-Martín at the Universidad de Alcalá were supported, respectively, by fellowships from the Comunidad de Madrid and the Universidad de Alcalá.
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Alnaizy, R., and A. Akgerman. 2000. Advanced oxidation of phenolic compounds. Adv. Environ. Res. 4:233-244. [Google Scholar]
- 2.Backa, S., J. Gierer, T. Reitberger, and T. Nilsson. 1993. Hydroxyl radical activity associated with the growth of white-rot fungi. Holzforschung 47:181-187. [Google Scholar]
- 3.Cameron, M. D., and S. D. Aust. 1999. Degradation of chemicals by reactive radicals produced by cellobiose dehydrogenase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 367:115-121. [DOI] [PubMed] [Google Scholar]
- 4.Constam, D., A. Muheim, W. Zimmermann, and A. Fiechter. 1991. Purification and partial characterization of an intracellular NADH:quinone oxidoreductase from Phanerochaete chrysosporium. J. Gen. Microbiol. 137:2209-2214. [Google Scholar]
- 5.Glaze, W. H., J. W. Kang, and D. H. Chapin. 1987. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 9:335-352. [Google Scholar]
- 6.Gómez-Toribio, V., A. B. García-Martín, M. J. Martínez, Á. T. Martínez, and F. Guillén. 2009. Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling. Appl. Environ. Microbiol. 75:3944-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillén, F., and C. S. Evans. 1994. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 60:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillén, F., V. Gómez-Toribio, M. J. Martínez, and A. T. Martínez. 2000. Production of hydroxyl radical by the synergistic action of fungal laccase and aryl alcohol oxidase. Arch. Biochem. Biophys. 383:142-147. [DOI] [PubMed] [Google Scholar]
- 9.Guillén, F., A. T. Martínez, and M. J. Martínez. 1992. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur. J. Biochem. 209:603-611. [DOI] [PubMed] [Google Scholar]
- 10.Guillén, F., A. T. Martínez, M. J. Martínez, and C. S. Evans. 1994. Hydrogen peroxide-producing system of Pleurotus eryngii involving the extracellular enzyme aryl-alcohol oxidase. Appl. Microbiol. Biotechnol. 41:465-470. [Google Scholar]
- 11.Guillén, F., M. J. Martínez, C. Muñoz, and A. T. Martínez. 1997. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch. Biochem. Biophys. 339:190-199. [DOI] [PubMed] [Google Scholar]
- 12.Guillén, F., C. Muñoz, V. Gómez-Toribio, A. T. Martínez, and M. J. Martínez. 2000. Oxygen activation during the oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl. Environ. Microbiol. 66:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez, A., L. Caramelo, A. Prieto, M. J. Martínez, and A. T. Martínez. 1994. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi from the genus Pleurotus. Appl. Environ. Microbiol. 60:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammel, K. E., A. N. Kapich, K. A. Jensen, and Z. C. Ryan. 2002. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb. Technol. 30:445-453. [Google Scholar]
- 15.Heinfling, A., M. J. Martínez, A. T. Martínez, M. Bergbauer, and U. Szewzyk. 1998. Transformation of industrial dyes by manganese peroxidase from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl. Environ. Microbiol. 64:2788-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry, W. P. 2003. Non-enzymatic iron, manganese, and copper chemistry of potential importance in wood decay, p. 175-195. In B. Goodell, D. D. Nicholas, and T. P. Schultz (ed.), Wood deterioration and preservation. Advances in our changing world. Oxford University Press, Washington, DC.
- 17.Kerem, Z., K. A. Jensen, and K. E. Hammel. 1999. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett. 446:49-54. [DOI] [PubMed] [Google Scholar]
- 18.Kersten, P. J., and T. K. Kirk. 1987. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J. Bacteriol. 169:2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khindaria, A., T. A. Grover, and S. D. Aust. 1994. Oxalate-dependent reductive activity of manganese peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 314:301-306. [DOI] [PubMed] [Google Scholar]
- 20.Kosman, D. J. 2003. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 47:1185-1197. [DOI] [PubMed] [Google Scholar]
- 21.Kramer, C., G. Kreisel, K. Fahr, J. Kassbohrer, and D. Schlosser. 2003. Degradation of 2-fluorophenol by the brown-rot fungus Gloeophyllum striatum: evidence for the involvement of extracellular Fenton chemistry. Appl. Microbiol. Biotechnol. 64:387-395. [DOI] [PubMed] [Google Scholar]
- 22.Kuan, I. C., and M. Tien. 1993. Stimulation of Mn-peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc. Natl. Acad. Sci. USA 90:1242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez, A. T., M. Speranza, F. J. Ruiz-Duenas, P. Ferreira, S. Camarero, F. Guillén, M. J. Martínez, A. Gutiérrez, and J. C. del Río. 2005. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 8:195-204. [PubMed] [Google Scholar]
- 24.Martínez, M. J., F. J. Ruiz-Dueñas, F. Guillén, and A. T. Martínez. 1996. Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 237:424-432. [DOI] [PubMed] [Google Scholar]
- 25.Muheim, A., R. Waldner, D. Sanglard, J. Reiser, H. E. Schoemaker, and M. S. A. Leisola. 1991. Purification and properties of an aryl-alcohol dehydrogenase from the white-rot fungus Phanerochaete chrysosporium. Eur. J. Biochem. 195:369-375. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz, C., F. Guillén, A. T. Martínez, and M. J. Martínez. 1997. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties and participation in activation of molecular oxygen and Mn2+ oxidation. Appl. Environ. Microbiol. 63:2166-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz, C., F. Guillén, A. T. Martínez, and M. J. Martínez. 1997. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr. Microbiol. 34:1-5. [DOI] [PubMed] [Google Scholar]
- 28.Park, J. S. B., P. M. Wood, M. J. Davies, B. C. Gilbert, and A. C. Whitwood. 1997. A kinetic and ESR investigation of iron(II) oxalate oxidation by hydrogen peroxide and dioxygen as a source of hydroxyl radicals. Free Radic. Res. 27:447-458. [DOI] [PubMed] [Google Scholar]
- 29.Pera-Titus, M., V. García-Molina, M. A. Baños, J. Giménez, and S. Esplugas. 2004. Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl. Catal. B 47:219-256. [Google Scholar]
- 30.Pignatello, J. J., E. Oliveros, and A. MacKay. 2006. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 36:1-84. [Google Scholar]
- 31.Popp, J. L., B. Kalyanaraman, and T. K. Kirk. 1990. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry 29:10475-10480. [DOI] [PubMed] [Google Scholar]
- 32.Rieble, S., D. K. Joshi, and M. H. Gold. 1994. Purification and characterization of a 1,2,4-trihydroxybenzene 1,2-dioxygenase from the basidiomycete Phanerochaete chrysosporium. J. Bacteriol. 176:4838-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saparrat, M. C. N., F. Guillén, A. M. Arambarri, A. T. Martínez, and M. J. Martínez. 2002. Induction, isolation, and characterization of two laccases from the white-rot basidiomycete Coriolopsis rigida. Appl. Environ. Microbiol. 68:1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoemaker, H. E. 1990. On the chemistry of lignin degradation. Recl. Trav. Chim. Pays-Bas 109:255-272. [Google Scholar]
- 35.Schoemaker, H. E., E. M. Meijer, M. S. A. Leisola, S. D. Haemmerli, R. Waldner, D. Sanglard, and H. W. H. Schmidt. 1989. Oxidation and reduction in lignin biodegradation, p. 454-471. In N. G. Lewis and M. G. Paice (ed.), Plant cell wall polymers: biogenesis and biodegradation. ACS Symposium Series 399. American Chemical Society, Washington, DC.
- 36.Schoemaker, H. E., U. Tuor, A. Muheim, H. W. H. Schmidt, and M. S. A. Leisola. 1991. White-rot degradation of lignin and xenobiotics, p. 157-174. In W. B. Betts (ed.), Biodegradation: natural and synthetic materials. Springer-Verlag, London, United Kingdom.
- 37.Skoumal, M., P. L. Cabot, F. Centellas, C. Arias, R. M. Rodriguez, J. A. Garrido, and E. Brillas. 2006. Mineralization of paracetamol by ozonation catalyzed with Fe2+, Cu2+ and UVA light. Appl. Catal. B 66:228-240. [Google Scholar]
- 38.Somogyi, M. 1945. A new reagent for the determination of sugars. J. Biol. Chem. 160:61-73. [Google Scholar]
- 39.Stahl, J. D., and S. D. Aust. 1995. Properties of a transplasma membrane redox system of Phanerochaete chrysosporium. Arch. Biochem. Biophys. 320:369-374. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki, M. R., C. G. Hunt, C. J. Houtman, Z. D. Dalebroux, and K. E. Hammel. 2006. Fungal hydroquinones contribute to brown rot of wood. Environ. Microbiol. 8:2214-2223. [DOI] [PubMed] [Google Scholar]
- 41.Varela, E., and M. Tien. 2003. Effect of pH and oxalate on hydroquinone-derived hydroxyl radical formation during brown rot wood degradation. Appl. Environ. Microbiol. 69:6025-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinodgopal, K., and J. Peller. 2003. Hydroxyl radical-mediated advanced oxidation processes for textile dyes: a comparison of the radiolytic and sonolytic degradation of the monoazo dye Acid Orange 7. Res. Chem. Intermed. 29:307-316. [Google Scholar]
- 43.Vinodgopal, K., J. Peller, O. Makogon, and P. V. Kamat. 1998. Ultrasonic mineralization of a reactive textile azo dye, Remazol black B. Water Res. 32:3646-3650. [Google Scholar]
- 44.Vogelpohl, A., and S. M. Kim. 2004. Advanced oxidation processes (AOPs) in wastewater treatment. J. Ind. Eng. Chem. 10:33-40. [Google Scholar]
- 45.Zapanta, L. S., and M. Tien. 1997. The roles of veratryl alcohol and oxalate in fungal lignin degradation. J. Biotechnol. 53:93-102. [Google Scholar]