Volume 190, no. 14, p. 4880-4887, 2008.
We have discovered the existence of a minor contaminating protein in our preparations of Mycobacterium tuberculosis SecA1 and SecA2 proteins. This minor contaminant is an acetate-stimulated ATPase, which we were able to remove from our protein preparations by the addition of a Q-Sepharose column. The affinity of M. tuberculosis wild-type SecA1, wild-type SecA2, and SecA2 K115R for ATP did not change after the additional purification step. The ATPase activity for M. tuberculosis SecA2 is significantly lower than first reported, and the substitution K115R in SecA2 essentially eliminates the ATPase activity. Nevertheless, the central conclusions of the paper are unchanged.
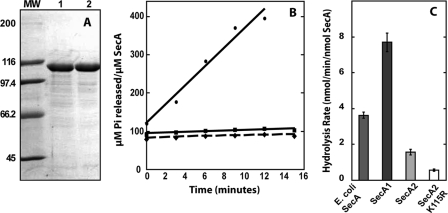
The contaminating ATPase influenced the results of the ATPase assays shown in Fig. 3B and 4C. In the figure below, panel A shows an SDS gel of a representative purification of SecA2 K115R before (lane 1) and after (lane 2) additional purification with the Q-Sepharose column. Panel B shows ATP hydrolysis with time of the SecA2 K115R variant before the Q-Sepharose column in the presence of magnesium acetate (circles) and after the Q-Sepharose column with magnesium acetate (diamonds) and magnesium chloride (squares). The ATPase activity of the proteins was determined by quantifying the amount of inorganic phosphate released from γ-32P-labeled ATP with time. These data verify that the acetate-stimulated ATPase was eliminated from our protein. In panel C, the ATP hydrolysis rates of Escherichia coli SecA and Q-Sepharose-purified M. tuberculosis SecA1, SecA2, and SecA2 K115R proteins are shown with 5 mM magnesium chloride used in the reaction buffer.