Abstract
In cyanobacteria, gene expression is regulated mainly at the level of transcription initiation, which is mediated by the RNA polymerase holoenzyme. The RNA polymerase core is catalytically active, while the σ factor recognizes promoter sequences. Group 2 σ factors are similar to the principal σ factor but are nonessential. Group 2 σ factors SigB and SigD are structurally the most similar σ factors in Synechocystis sp. strain PCC 6803. Under standard growth conditions, simultaneous inactivation of sigB and sigD genes did not affect the growth, but the photosynthesis and growth of the ΔsigBD strain were slower than in the control strain at double light intensity. Light-saturated electron transfer rates and the fluorescence and thermoluminescence measurements showed that photosynthetic light reactions are fully functional in the ΔsigBD strain, but absorption and 77 K emission spectra measurements suggest that the light-harvesting system of the ΔsigBD strain does not acclimate normally to higher light intensity. Furthermore, the ΔsigBD strain is more sensitive to photoinhibition under bright light because impaired upregulation of psbA genes leads to insufficient PSII repair.
Cyanobacteria have an oxygen-evolving photosynthetic apparatus similar to plants, and they are responsible for nearly one-half of the net primary production (3, 7). Light affects the growth and physiology of all photosynthetic organisms, including cyanobacteria. Synechocystis sp. strain PCC 6803 (referred to hereafter strain 6803) is a unicellular cyanobacterium commonly used as a model organism in photosynthesis studies because of its suitability for genetic engineering (16). Cyanobacteria acclimate to changing light conditions by adjustments of antenna composition (23), by state transitions (19, 55), and by varying the expression of genes coding for components of the photosynthetic apparatus (11, 27). In addition to photosynthetic genes, many other genes involved in cellular processes are light regulated (13, 14). Furthermore, cyanobacteria synthesize pigment-protein complexes such as the orange carotenoid protein OCP (57, 58) and IsiA (10, 59) that apparently protect the photosynthetic machinery against the adverse effects of intense light.
Although light is required for photosynthesis, light is also a source of stress. In the light, photosystem II (PSII) of photosynthesis is damaged at a rate proportional to the intensity of the light (47). Simultaneously, an elaborate repair mechanism of PSII operates on the thylakoid membranes (28, 29). The damaged PSII reaction center protein D1 is degraded, most probably by the FtsH protease (39), and replaced with a new copy in order to maintain a functional PSII. In strain 6803 a three-member psbA gene family encodes the D1 protein. The psbA2 and psbA3 genes, encoding identical D1 proteins, are upregulated under high light, while the more divergent psbA1 gene remains virtually silent (26, 37, 52). The psbA1 gene is specifically upregulated under low-oxygen conditions (38, 43). In addition to transcriptional regulation, the expression of the psbA genes in strain 6803 is regulated at the levels of mRNA stability (26, 54) and translation elongation (51). Furthermore, after synthesis of the D1 protein, the PSII complex needs to be reactivated before it is functional again (5, 35).
Transcription initiation, mediated by the RNA polymerase holoenzyme, is an essential stage of gene regulation. The eubacterial RNA polymerase holoenzyme consists of a catalytically active multisubunit core and a σ factor, which is responsible for the specific recognition of promoter sequences (4, 6). Bacterial genomes usually code for several σ factors. The strain 6803 genome encodes nine σ factors, all of them belonging to the σ70 family (20). The essential σ factor (group 1) is SigA (18). Group 2 σ factors SigB, SigC, SigD, and SigE closely resemble the SigA factor in structure, but they are nonessential under optimal growth conditions (18, 33, 46). The SigF, SigG, SigH, and SigI factors of strain 6803 are group 3 σ factors and differ considerably in amino acid sequence from group 1 and group 2 σ factors. SigF has a role in the formation of the pilus structure and hence in cell motility (1, 2). The genes encoding the SigH and SigG factors are stress-inducible, and the sigG gene cannot be inactivated in strain 6803 (15, 18).
Recent results have shown that group 2 σ factors are important for acclimation to various stress conditions in different cyanobacterial species (31). In strain 6803, the SigB (40, 44) and SigC (45) factors are involved in acclimation to high-temperature stress. The SigE factor, in turn, has a role in sugar metabolism and is required for light activated heterotrophic growth (32). All group 2 σ factors affect the acclimation of strain 6803 cells to osmotic stress conditions, SigB being the most important one (33).
Previous studies have shown that the expression of the sigB and sigD genes is light regulated (17, 46). Furthermore, inactivation of either the sigB gene or the sigD gene affects gene expression patterns in light-dark transitions (42), and inactivation of the sigD gene retards growth at 80 μmol of photons m−2 s−1 (33). In the present study, we further investigated the roles of the strain 6803 SigB and SigD factors in high-light stress. Simultaneous inactivation of the sigB and sigD genes makes the cells unable to take full advantage of higher light intensities, since adjustment of the phycobilisome antennae does not function normally. Moreover, we show that the simultaneous inactivation of the sigB and sigD genes causes deficiencies in the PSII repair cycle, which makes the cells more sensitive to light-induced damage.
MATERIALS AND METHODS
Strains and growth conditions.
The glucose-tolerant strain of Synechocystis sp. strain PCC 6803 (56) was used as the control strain (CS). The construction of the ΔsigB, ΔsigD, and ΔsigBD strains has been described previously (44). Cells were grown in BG-11 medium buffered with 20 mM HEPES-NaOH (pH 7.5) at 32°C and ambient CO2 with shaking at 90 rpm under the continuous photosynthetic photon flux density (PPFD) of 40 μmol m−2 s−1. These are referred to as standard growth conditions. For the single inactivation strains the BG-11 plates were supplemented with kanamycin (50 μg/ml), and for the double inactivation strains in addition with streptomycin (20 μg/ml) and spectinomycin (10 μg/ml). No antibiotics were added to liquid cultures. For growth experiments, the optical density of the cell cultures at A730 was set to 0.1 (corresponding to 3.6 × 106 cells in all strains), and growth under standard growth conditions (40 μmol of photons m−2 s−1) and at 80 μmol of photons m−2 s−1 was monitored at A730.
Oxygen evolution measurements.
Photosynthetic oxygen evolution was measured in vivo with an oxygen electrode (Hansatech, Kign's Lynn, United Kingdom). The samples (10 μg of chlorophyll a [chl a]/ml) were supplemented with 10 mM NaHCO3. The measurements were done by using either the same light intensity that was used as a growth light, PPFD of 40 or 80 μmol m−2 s−1, or under the saturating PPFD of 500 μmol m−2 s−1, as indicated. The light-saturated rate of PSII oxygen evolution was measured in vivo in the presence of 0.7 mM 2,6-dichloro-p-benzokinone (DCBQ) as an artificial electron acceptor. We added 0.7 mM ferricyanide to keep the electron acceptor in oxidized form.
Thermoluminescence measurements.
The A730 of the cell culture grown for 2 days under standard conditions was adjusted to 1.0, and the samples were concentrated 300-fold and resuspended in BG-11 medium containing 30% glycerol. Thermoluminescence was measured with a homemade luminometer (49). To measure the Q band, 20 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) was added. Prior to the measurements, the cell suspension was dark incubated for 10 s (B-band measurements) or 200 s (Q-band measurements) at 32°C. The temperature was then lowered to −20°C, and a 4-μs Xenon flash was fired. Heating at the rate of 1°C s−1 was started 30 s after the flash.
Fluorescence relaxation kinetics.
Flash-induced increase and subsequent decay of chl a fluorescence yield was measured with an FL200 fluorometer (P.S. Instruments, Brno, Czech Republic). Cells were grown for 2 days under standard growth conditions. A 2-ml sample of cell suspension (10 μg of chl/ml) was dark adapted for 5 min before measuring QA reoxidation after a saturating flash. Fluorescence relaxation was also measured in the presence of 10 μM DCMU.
77 K emission spectra and in vivo absorption spectra.
Cells were grown for 2 days under standard growth light (PPFD at 40 μmol m−2 s−1) or at the PPFD of 80 μmol of photons m−2 s−1. The cultures were concentrated to 40 μg of chl/ml, and 50-μl samples were used in the measurements. Fluorescence emission spectra were measured at 77 K with an Ocean Optics S2000 spectrometer by exciting the sample with blue or orange light. Blue light was obtained by filtering the output from a slide projector through 450- and 500-nm cutoff filters (Corion, Dunedin, FL) and orange light by using a 580-nm narrow-band filter (Corion). The spectra were corrected by subtracting the background signal, smoothed by a moving median with a 2-nm window, and normalized by dividing by the peak value of PSI emission at 723 nm.
For state transition measurements, cells were grown at the PPFD of 40 or 80 μmol m−2 s−1, concentrated to 40 μg of chl/ml, and then treated in the dark for 5 min at 32°C or illuminated with blue light (450-nm cutoff filter) at 80 μmol of photons m−2 s−1 for 5 min at 32°C. After the treatments, the samples were rapidly frozen with liquid nitrogen, and 77 K fluorescence spectra were measured with orange light excitation as described above.
In vivo absorption spectra were measured with a UV-3000 spectrophotometer (Shimadzu, Japan) from 350 to 800 nm. The phycobilin versus chl a content was calculated by dividing the phycobilin peak at 625 nm by the sum of the two chl a peaks at 438 and 678 nm.
Photoinhibition treatments.
Control, ΔsigB, ΔsigD, and ΔsigBD cell cultures containing 10 μg of chl a/ml were illuminated at the PPFD of 1,500 μmol m−2 s−1 with a slide projector at 32°C in the presence or in the absence of lincomycin (10 mg/ml) as indicated. Samples (1 ml) were drawn for PSII measurements from untreated cultures and after 15, 30, and 45 min of illumination.
l-[35S]methionine labeling and immunodetection of D1 protein.
The control and ΔsigBD strains were grown under standard conditions and concentrated to10 μg of chl a/ml. The cells were pulse-labeled for 10 min with radioactive methionine (l-[35S]methionine, 185 MBq; Perkin-Elmer) under standard conditions or after a 45-min preillumination at 1,500 μmol of photons m−2 s−1, as indicated. Cells were harvested from 20-ml samples by centrifugation at 4°C after the addition of cold l-methionine (0.4 mg/ml). Membrane proteins were isolated, and the chl a concentration was determined according to the method of Tyystjärvi et al. (50). Polypeptides were solubilized for 5 min at 70°C, and samples containing 4 μg of chl a were loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% NEXT GEL (Amresco) according to the manufacturer's instructions. The proteins were transferred onto an Immobilon membrane (Millipore), and equal loading was confirmed by staining the membranes with 0.1% Ponceau S solution. Radioactive proteins were visualized with autoradiography. After visualization of radioactive proteins, immunodetection of the D1 protein was done using the same membrane that was used for the autoradiogram (51), a D1 antibody was purchased from Agrisera, and the CDP Star chemiluminescence kit from New England Biolabs.
Quantitative real-time PCR.
A 50-ml portion of cell culture containing 10 μg of chl a/ml was treated at the PPFD of 1,500 μmol m−2 s−1, and 15-ml samples were drawn after 0, 15, 30, and 45 min of illumination. The cells were harvested by centrifugation at 8,000 × g for 5 min at 4°C, and the total RNA was isolated by the hot phenol method as described by Tyystjärvi et al. (51). The samples were then treated with Turbo DNase (Ambion), and thereafter 1 μg of RNA was used for cDNA synthesis (iScript; Bio-Rad Laboratories). After reverse transcription, the reactions were diluted fivefold with water, and 2-μl aliquots were used as templates in the real-time PCRs. A common antisense primer (5′-TCC GGT TGT TGG TAG AGG TC-3′) was used for both psbA2 and psbA3 genes. The specific sense primers were 5′-TCC AAT CTG AAC ATC GAC AAA-3′ for the psbA2 gene and 5′-CTC TGA GCT TGA GGC CAA AT-3′ for the psbA3 gene. Two reference genes were used. The antisense and sense primers for rrn16Sa (16S rRNA) were 5′-AGC GTC CGT AGG TGG TTA TG-3′ and 5′-CTA CGC ATT TCA CCG CTA CA-3′ and for the rnpB gene were 5′-GTG AGG ACA GTG CCA CAG AA-3′ and 5′-CCT TTG CAC CCT TAC CCT TT-3′. We performed quantitative reverse transcription-PCR on a Bio-Rad iCycler using iQ SYBR green Supermix (Bio-Rad Laboratories) at a final volume of 25 μl. Three independent biological replicates and two technical replicates were performed for each sample. The efficiency of each reaction was estimated by using the LineReg program (34). The changes in the amounts of the psbA transcripts in treated samples were calculated relative to the expression of reference genes with the equation ECTreference gene−CTgene, where E is the amplification efficiency of the PCR and CT the cycle number where fluorescence from the PCR amplicon reached the detection threshold level. The relative abundances of the psbA2 and psbA3 amplicons were summed for an estimation of the total psbA mRNA pool, and the percentage fractions of the transcripts were calculated for both control and ΔsigBD strains.
RESULTS
Simultaneous inactivation of the sigB and sigD genes prevents the cells from taking full advantage of a higher light intensity.
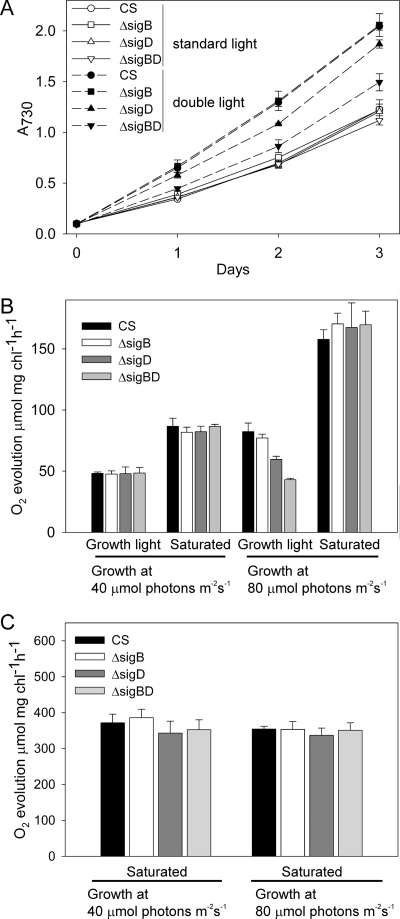
Under standard growth conditions the ΔsigB, ΔsigD, and ΔsigBD strains grew like the control strain, the doubling time of the cells being 13 h during the first day in liquid culture (Fig. 1A). We measured the rate of photosynthetic oxygen evolution after growing the cells for 2 days under standard conditions. Photosynthesis was measured both at the growth light intensity (PPFD of 40 μmol m−2 s−1) and under saturating light. In accordance with similar growth rates, no differences in the photosynthetic activities were observed between the control, ΔsigB, ΔsigD, and ΔsigBD strains grown under standard conditions (Fig. 1B).
FIG. 1.
Growth rate, light-limited and light-saturated photosynthetic rate, and light-saturated PSII oxygen evolution rate measured for cells grown under two light intensities. (A) The A730 of the cultures was set to 0.1, and the control (CS), ΔsigB, ΔsigD, and ΔsigBD cells were grown at the PPFD of 40 μmol m−2 s−1 (open symbols) or 80 μmol m−2 s−1 (solid symbols). (B) Oxygen evolution activity measured from cells grown for 2 days at the PPFDs of 40 or 80 μmol of photons m−2 s−1, as indicated. Oxygen evolution was measured at the same PPFD as the growth light was or under the saturating PPFD of 1,500 μmol m−2 s−1, as indicated. (C) Light-saturated PSII activity measured in the presence of 0.7 mM DCBQ. Each data point represent the mean of three biological replicates with independent liquid cultures, and the error bars denote the standard errors (SE).
At the PPFD of 80 μmol m−2 s−1, the doubling time of the control strain was only 9 h during the first day, indicating that the growth of the control strain improved when the light intensity was doubled (Fig. 1A). The first-day doubling time was 9 h in the ΔsigB strain and 9.5 h in the ΔsigD strain. The ΔsigBD strain, in turn, could not take full advantage of the greater availability of light energy, and the doubling time of ΔsigBD remained as long as 11 h. During the second day of growth at the PPFD of 80 μmol m−2 s−1, doubling times were 24 h for the control and ΔsigB strains and 26 h for the ΔsigD and ΔsigBD strains. On the third day, the doubling times of all strains were longer than 30 h and the ΔsigD and ΔsigBD strains no longer grew more slowly than the control or ΔsigB strain.
Photosynthetic activity was measured after growing the cells for 2 days at the PPFD of 80 μmol m−2 s−1. The photosynthetic activity of the control strain, measured at 80 μmol m−2 s−1, was 1.6 times as high as it was at 40 μmol of photons m−2 s−1. Furthermore, the light-saturated rate of photosynthesis of the control cells almost doubled when the cells were grown under double light intensity. Doubling the growth light intensity caused a similar doubling of the light-saturated photosynthetic rate in all inactivation strains as in the control strain, but differences were detected in photosynthetic activities measured at the PPFD of 80 μmol m−2 s−1. The photosynthetic activity of the ΔsigBD strain, measured at the PPFD of 80 μmol m−2 s−1, was only 60% of that measured in the control strain (Fig. 1B). For the single inactivation strains, photosynthetic activity of the ΔsigD strain at 80 μmol of photons m−2 s−1 was 20% lower than that of the control strain, but in the ΔsigB strain the photosynthetic activity was similar to that of the control strain (Fig. 1B). To find out the reasons for lower photosynthetic activities of the inactivation strains, we further analyzed the photosynthetic electron transfer chain.
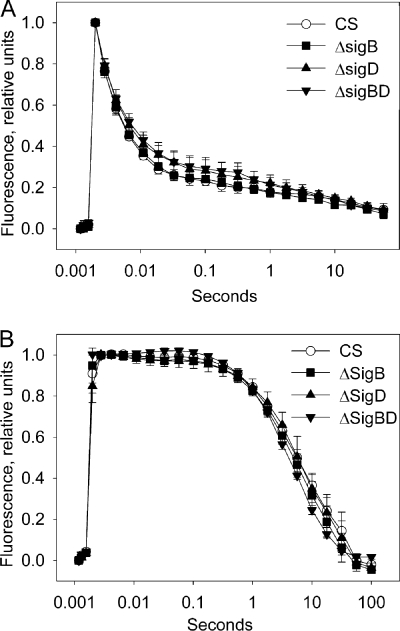
PSII electron transport was investigated by the thermoluminescence method. Cells were grown for 2 days in standard conditions and used to measure the B and Q thermoluminescence bands originating from charge recombination reactions between the S2 state of the oxygen evolving complex and the QA and QB electron acceptors, respectively (see reference 48). No differences were found between the temperatures of the B and Q bands in the control, ΔsigB, ΔsigD, and ΔsigBD strains (Table 1). PSII capacities, measured as the light-saturated oxygen evolution activity from water to the electron acceptor DCBQ, were similar in the control and all inactivation strains (Fig. 1C).
TABLE 1.
Thermoluminescence peak temperatures of the B and Q bands of the control, ΔsigB, ΔsigD, and ΔsigBD strains in standard growth conditions
| Strain | Thermoluminescence peak temp (°C)a
|
|
|---|---|---|
| B band | Q band | |
| Control | 44 | 20 |
| ΔsigB mutant | 44 | 20 |
| ΔsigD mutant | 45 | 20 |
| ΔsigBD mutant | 44 | 20 |
Thermoluminescence was measured with a home-made luminometer from cells grown under standard conditions. DCMU at 20 μM was used in the Q band.
The function of the photosynthetic electron transport chain was examined by measuring the kinetics of the decay of chl a fluorescence yield after a single turnover flash. Cells grown for 2 days in standard conditions were dark adapted for 5 min, and then a saturating flash of light was fired. Flash-induced electron transfer reactions reduce the QA electron acceptor, causing an increase in variable fluorescence. Subsequently, fluorescence yield decreases when QA− is reoxidized by electron transfer to QB. DCMU is a quinone analogue that blocks the QB binding site, thus preventing the transfer of electrons from QA, which results in slow reoxidation of QA− by charge recombination reactions. No significant differences in the shapes of the fluorescence relaxation curves measured either in the absence of DCMU or in the presence of DCMU were found between the control and inactivation strains (Fig. 2). Together, these measurements show that PSII and the photosynthetic electron transfer chain function similarly in the inactivation strains and in the control strain under standard growth conditions.
FIG. 2.
Flash-induced increase and subsequent decay of chl a fluorescence yield in the control, ΔsigB, ΔsigD, and ΔsigBD strains grown under standard conditions. Fluorescence was measured in the absence (A) and in the presence (B) of 10 μM DCMU. A strong flash was fired after 5 min of dark incubation. Initial fluorescence, measured with a weak probe flash, has been subtracted, and fluorescence values have been normalized by dividing by the value obtained with a probe flash fired 180 μs after the strong flash. Each data point represents the mean of three biological replicates with independent liquid cultures, and the error bars denote the SE.
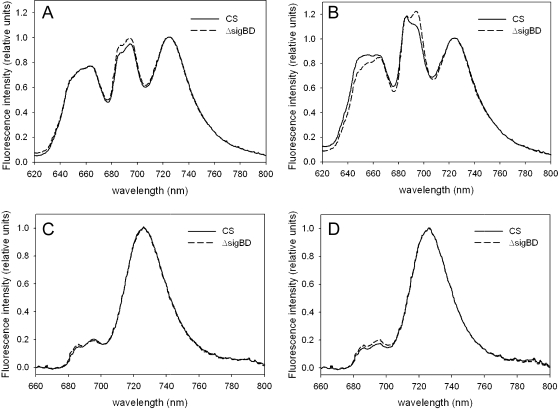
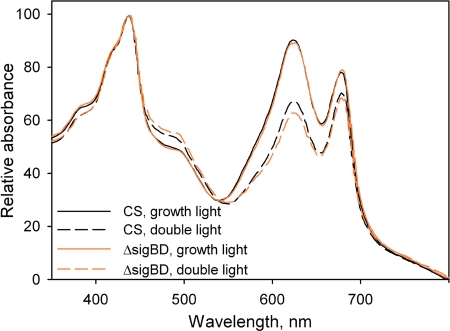
The finding that light-limited photosynthesis was affected in the ΔsigBD strain grown at the PPFD of 80 μmol m−2 s−1 pointed to an effect on either light harvesting or photosystem stoichiometry. To explore these possibilities, we measured in vivo absorption spectra and 77 K fluorescence emission spectra from the control and ΔsigBD strains. Cells grown at 40 and 80 μmol of photons m−2 s−1 were compared. In fluorescence measurements, blue and orange light, respectively, were used to excite preferentially chl a and phycobilisomes. All 77 K spectra were normalized so that the value at 723 nm (PSI peak) was 1.0 (Fig. 3). Emission spectra from cells grown under standard conditions were almost identical in the control and ΔsigBD strains when the samples were excited with blue light (Fig. 3C). When samples were excited with orange light, the PSII peaks at 685 nm, originating from the terminal emitter of phycobilisome and CP43 (55) and at 695 nm (originating from CP47) were slightly higher in the ΔsigBD strain than in the control strain (Fig. 3A). In vivo whole-cell absorption spectra measured from the control and ΔsigBD cells grown at 40 μmol of photons m−2 s−1 (Fig. 4) were virtually identical. These analyses indicate that the ΔsigBD strain has photosystem stoichiometry and antenna functions that are fairly similar to those of the control strain under our standard conditions.
FIG. 3.
Fluorescence emission at 77 K. Orange light-excited spectra from control (solid line) and ΔsigBD (dashed line) cells grown at 40 (A) and 80 (B) μmol of photons m−2 s−1. Blue-light-excited spectra from cells grown at 40 (C) and 80 (D) μmol of photons m−2 s−1 were also determined. The data were normalized by dividing by the PSI emission peak at 723 nm. Each spectrum represents an average of three independent liquid cultures.
FIG. 4.
Absorption spectra of control (black lines) and ΔsigBD (orange lines) strains cells grown under standard light (40 μmol of photons m−2 s−1; solid lines) or double light (80 μmol of photons m−2 s−1; dashed lines) for 2 days. The value measured at 800 nm has been subtracted from each spectrum.
The similarity of the blue-light-excited emission spectra of the control and ΔsigBD strains grown at 40 or 80 μmol of photons m−2 s−1 (Fig. 3C and D) suggests that the PSII to PSI ratio remained similar in both light conditions and in both strains. However, whole-cell absorption spectra measurements showed that the phycobilin to chl ratio was reduced from 0.5 to 0.4 in both control and ΔsigBD strains when the cells were grown for 2 days at 80 μmol of photons m−2 s−1 (Fig. 4). Although the relative amounts of phycobilins decreased, the relative intensities of the phycobilisome peaks at 657 and 663 nm and the PSII peaks at 685 and 695 nm increased in both strains when orange-light-excited emission spectra from samples grown at double light are compared to those grown under standard light (Fig. 3A and B). These data suggest that doubling the light intensity decreases the thermal dissipation of energy absorbed by the phycobilisomes, and therefore phycobilisomes both fluoresce more and deliver more energy to PSII.
The changes in phycobilisome emission caused by doubling the light intensity were larger in the control strain than in the ΔsigBD strain; in particular, phycocyanin emission at 657 nm increased more in the control strain than in the ΔsigBD strain. Furthermore, in the control strain the 685-nm PSII peak increased more than the 695-nm peak, which led to a change in the ratio of the two peaks; in the ΔsigBD strain the ratio of the 685- and 695-nm peaks did not change. The higher rate of light-limited photosynthesis in control cells grown under double light, compared to cells grown in standard conditions (Fig. 1B), may partially result from more efficient function of the phycobilisome antenna of PSII under double light. Apparently, this antenna adjustment does not function as efficiently in the ΔsigBD strain as in the control strain.
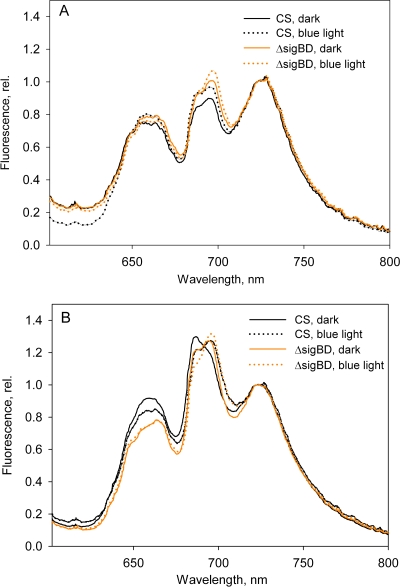
The acclimation of the phycobilisome antennae in the ΔsigBD strain was further investigated by measuring fluorescence emission spectra at 77 K after 5 min of blue light or dark incubation of the cells. The typical pattern of strain 6803 state transitions where more emission from PSII was observed in blue-light-adapted cells (state 1) than in dark-adapted cells (state 2) was seen in the control strain grown under the standard growth conditions (Fig. 5A). The state transition occurred in the ΔsigBD strain, but it was not as prominent as in the control strain (Fig. 5A). In cells were grown at 80 μmol of photons m−2 s−1, clear induction of the PSII peak occurred in both strains, although the 685-nm peak always remained lower in the ΔsigBD strain than in the control strain (Fig. 5B); this feature of the emission spectrum of the ΔsigBD strain can also be seen in Fig. 3B. Upregulation of phycobilisome emission peaks was not seen in the ΔsigBD strain (Fig. 5B).
FIG. 5.
State transitions in the control and ΔsigBD strains. Orange-light-excited spectra from cells grown at 40 (A) and 80 (B) μmol of photons m−2 s−1 were determined. The cells were incubated for 5 min in the dark or illuminated for 5 min with blue light at 80 μmol of photons m−2 s−1, as indicated. The data were normalized by dividing by the PSI emission peak at 723 nm. Each spectrum represents an average of at least three independent measurements.
The ΔsigBD strain is sensitive to photoinhibition.
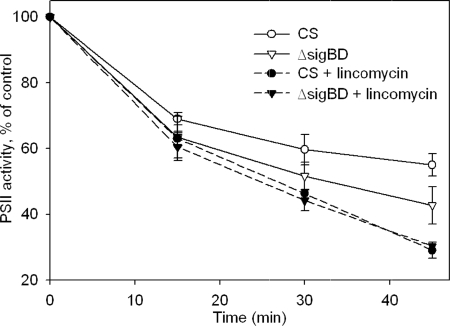
Photoinhibition of PSII was studied by measuring PSII activity from cells illuminated at the PPFD of 1,500 μmol m−2 s−1. At this strong light, the PSII capacity of the ΔsigBD strain decreased faster than that of the control strain, and after 45 min of illumination, 55% of PSII activity was left in the control strain, while only 42% remained in the ΔsigBD strain (Fig. 6). To investigate whether the difference between the control and ΔsigBD strains was due to differences in the reaction that damages PSII in the light or in the efficiency of the PSII repair cycle, we measured the loss in PSII capacity in the presence of lincomycin, an inhibitor of translation. In the presence of lincomycin, PSII capacity declined similarly in the control and ΔsigBD strains so that after 45 min of high-light illumination, PSII activity had decreased to 30% of the original value (Fig. 6).
FIG. 6.
Photoinhibition of the control and ΔsigBD strains. Cell cultures were illuminated at the PPFD of 1,500 μmol m−2 s−1, and the light-saturated PSII activity was measured after 0, 15, 30, and 45 min with a Clark-type oxygen electrode at 32°C using 0.7 mM DCBQ as an artificial electron acceptor. The experiments were done with (open symbols, solid lines) and without (black symbols, dashed lines) lincomycin (10 mg ml−1) in the control (circles) and ΔsigBD (triangles) strains. PSII activity is expressed as the percentage of the activity measured from untreated control samples. Each data point represents an average of three independent experiments and the error bars denote the SE.
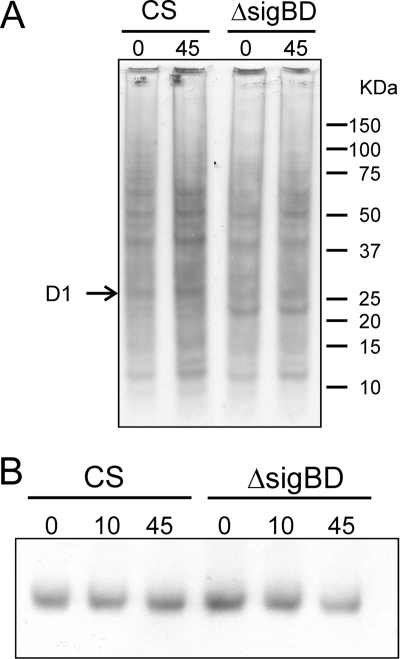
The photoinhibition experiments showed that the ΔsigBD strain is more susceptible to high light than the control strain due to a deficient PSII repair cycle. We continued by studying different steps in the PSII repair cycle. In vivo methionine pulse-labeling experiments indicated a slightly lower overall translation activity in the ΔsigBD strain than in the control strain under high-light conditions (Fig. 7A); in particular, production of the D1 protein was low in the ΔsigBD strain under high-light conditions. We also monitored possible changes in the amount of the D1 protein by Western blot analysis. The amount of the D1 protein remained constant in the control strain during the 45-min illumination under high light but slightly decreased in the ΔsigBD strain (Fig. 7B).
FIG. 7.
Translational activity and amount of the D1 protein in the control and ΔsigBD strains under high light. (A) The cells were pulse-labeled with l-[35S]methionine for 10 min under standard conditions (0 min) and after illumination at a PPFD of 1,500 μmol m−2 s−1 (45 min). Membrane proteins were isolated, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto a membrane for visualization with autoradiography. (B) The amount of the D1 protein was determined by Western blotting with a D1 protein specific antibody.
Effect of high light on the expression of psbA2 and psbA3 genes in control and ΔsigBD strains.
We used quantitative real-time PCR to study whether the simultaneous inactivation of sigB and sigD genes influences the expression of the psbA genes that encode the D1 protein. Since transcripts of the third member of the psbA gene family in strain 6803, psbA1, have been detected only in extremely small amounts under standard or high-light conditions (37) or not at all (25, 26), psbA1 was excluded from the analysis, and only the amounts of psbA2 and psbA3 were measured. The nucleotide sequences of the psbA2 and psbA3 genes are nearly identical in the coding regions. Taking advantage of this, the same reverse primer was used for both psbA genes, while forward primers were designed in the less similar 5′-untranslated region so that only one gene was specifically amplified in each reaction. Originally, two different reference genes, the rrn16Sa and rnpB genes, were used. Calculations with both reference genes gave similar overall outcomes of psbA expression, and the results are shown for rnpB.
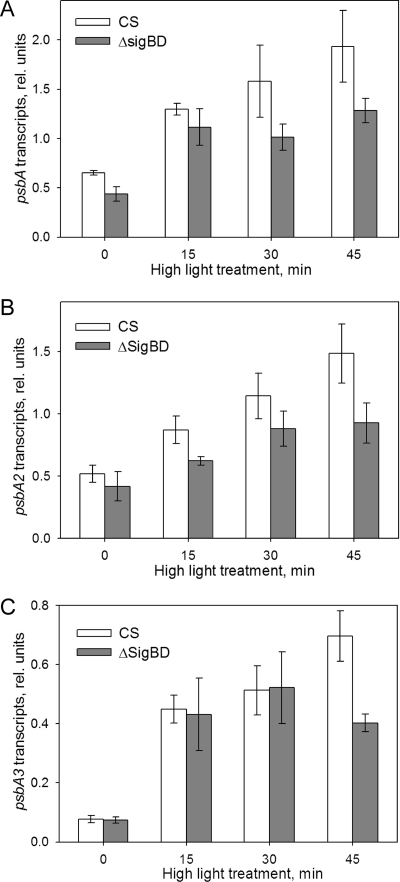
The total psbA transcript pool, which was calculated as the sum of the psbA2 and psbA3 transcripts, doubled in the control strain during the first 15 min of illumination at the PPFD of 1,500 μmol m−2 s−1 (Fig. 8A). The upregulation of psbA transcription continued so that after 45 min of illumination the transcript pool had more than tripled from the size measured under standard growth light. In contrast, in the ΔsigBD strain the total psbA abundance did not increase further after 15 min of illumination. After 45 min the ΔsigBD strain had 34% less psbA transcripts than the control strain (Fig. 8A).
FIG. 8.
Upregulation of the psbA genes in response to high-light irradiation. Quantitative real-time PCR was performed to investigate changes in the amounts of psbA2 and psbA3 transcripts after 0, 15, 30, and 45 min of illumination at a PPFD of 1,500 μmol m−2 s−1. After isolation of total RNA from the samples, 1 μg was used for cDNA synthesis. Reverse transcription-PCR was performed using gene-specific primers. (A) Changes in the size of the total psbA transcript pool in the control and ΔsigBD strains. The size of total psbA transcript pool was calculated by summing the abundances of the psbA2 and psbA3 transcripts. (B and C) Abundance of psbA2 (B) and psbA3 (C) transcripts relative to transcripts of the reference gene (rnpB) in control and ΔsigBD strains. Each column represents the mean of three independent experiments, and the error bars denote the SE.
The psbA2 transcripts exhibited a steady increase under high light in the control strain, while upregulation of psbA2 in the ΔsigBD strain remained lower (Fig. 8B). The activation of psbA3 transcription occurred rapidly after the start of illumination so that the abundance of psbA3 transcripts in both the control and ΔsigBD strains increased fivefold in 15 min and further to sixfold in 30 min. However, after 45 min the amount of psbA3 transcripts further increased in the control strain, while it actually slightly decreased in the ΔsigBD strain (Fig. 8C).
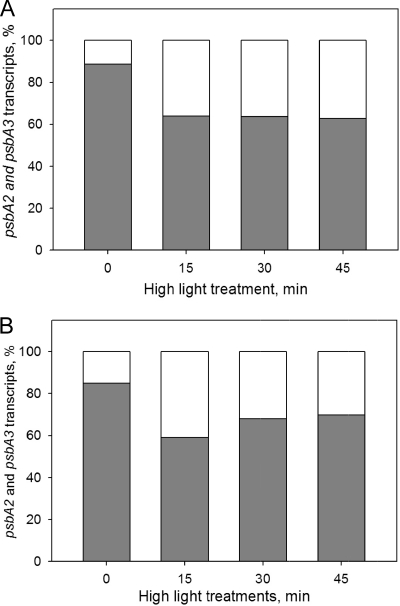
Under standard growth conditions, psbA2 contributed by an overwhelming majority, ca. 90%, to the total psbA transcript pool in the control strain, while psbA3 transcripts made up the remaining 10% (Fig. 9A). In the ΔsigBD strain 85% of psbA transcripts originated from the psbA2 gene and 15% originated from the psbA3 gene (Fig. 9B). Upon illumination under high light, the ratio of psbA2 and psbA3 transcripts changed so that after 15 min of illumination psbA2 contributed ca. 60% and psbA3 contributed ca. 40% to the transcript pool in both control and ΔsigBD strains. After 45 min of high-light treatment the ratio of the two forms in the control strain remained at the respective distributions of 60 and 40% (Fig. 9A). However, at this time point psbA2 transcripts accounted for 70% and psbA3 transcripts accounted for 30% of the total psbA pool in the ΔsigBD strain (Fig. 9B).
FIG. 9.
Distribution of transcripts from the psbA2 and psbA3 genes after high-light treatment. The percent fractions of the psbA2 (gray) and psbA3 (white) transcripts in the control (A) and ΔsigBD (B) strains were calculated from the data presented in Fig. 8.
DISCUSSION
Strain 6803 is routinely grown in different laboratories under very different light conditions ranging from as low a PPFD as 25 μmol m−2 s−1 (24) to as high a PPFD as 250 μmol m−2 s−1 (9). We use the PPFD of 40 μmol m−2 s−1 as the standard condition, and Fig. 1 clearly shows that light availability limits growth under standard conditions, since doubling the light intensity enhanced the growth of the control strain. The ΔsigBD strain grows more slowly than the control strain in liquid culture at the PPFD of 80 μmol m−2 s−1, although no differences in growth rates were detected under our standard growth conditions (Fig. 1). The growth of the ΔsigD strain was also slightly retarded at the PPFD of 80 μmol m−2 s−1. Both SigB and SigD are light regulated themselves. The sigD gene is upregulated in response to high light both at transcript and protein levels (13, 17, 18). Activation of the sigB gene has also been reported under high light (14) and upon transfer of cells from darkness to light (46). Another cyanobacterium, Synechococcus elongatus PCC 7942, encodes a close SigD homolog, RpoD3, which is upregulated in high light, and the inactivation strain is sensitive to high light (36).
Fluorescence and thermoluminescence measurements (Fig. 2 and Table 1) show that photosynthetic electron transfer reactions occur similarly in the ΔsigBD strain as in the control strain. Under double light intensity, however, the ΔsigBD strain was not able to enhance its photosynthetic activity like the control strain. The light-saturated rates of photosynthesis and PSII electron transport, as well as the 77 K fluorescence spectra measured with blue light that excites chl a, were all similar in the ΔsigBD and control strains grown at the double light intensity. This suggests that the PSII/PSI ratio is fairly similar in both strains. Bright light is known to induce an increase in the PSII/PSI ratio in strain 6803, since the amount PSI is more downregulated than that of PSII upon a shift to bright light (for a review, see reference 12). We only doubled the light intensity from 40 to 80 μmol of photons m−2 s−1, and apparently the change was not big enough to induce a measurable change in the PSII/PSI ratio.
Figure 6 shows that under very bright light the PSII repair cycle of the ΔsigBD strain does not function as efficiently as in the control strain. However, deficiencies in the PSII repair cycle are unlikely to explain the low photosynthetic activity of the ΔsigBD strain at the PPFD of 80 μmol m−2 s−1, since the light-saturated PSII activity was similar in the ΔsigBD and control strains at this PPFD, indicating that the cells of the ΔsigBD strain have a normal amount of functional PSII, although the photosynthetic activity is low. Absorption spectra (Fig. 4) and 77 K emission spectra (Fig. 3 and 5) measurements revealed that although the amount of phycobilisome antenna was fairly similar in control and ΔsigBD strains, the functional adjustment of phycobilisome antenna did not occur normally in the ΔsigBD strain, and thus light harvesting at the PPFD of 80 μmol m−2 s−1 less efficiently supports balanced function of the two photosystems in the ΔsigBD strain than in the control strain.
The ΔsigBD strain lost PSII activity faster than the control strain under high-light illumination at the PPFD of 1,500 μmol m−2 s−1. However, when the PSII repair cycle was blocked with the translation inhibitor lincomycin, the loss of PSII activity was similar in both strains. These data indicate that the vulnerability of the ΔsigBD strain under high light is connected to the PSII repair cycle and is not a result of an increased rate of light-induced damage. The loss of PSII activity and upregulation of psbA mRNAs at a PPFD of 1,500 μmol m−2 s−1occurred similarly in ΔsigB and ΔsigD strains as in the control strain (see Fig. S1 in the supplemental material). These results show that the group 2 σ factors SigB and SigD have functional redundancy in light acclimation. Redundancy is not surprising, since the SigB and SigD factors are the most similar pair of strain 6803 group 2 σ factors in both amino acid sequence and three-dimensional structure (33).
Western blot analysis revealed that in the ΔsigBD strain the amount of the D1 protein diminishes under high light, while the amount remains constant in the control strain. Less newly synthesized D1 protein accumulated in the ΔsigBD strain than in the control strain under high-light conditions. The PSII repair cycle has been shown to be sensitive to oxidative stress (30). Oxidative stress inhibits the function of the translational elongation factor G, leading to an overall decrease in translation elongation, including the translation of the D1 protein (21). Intense light is an important source of oxidative stress because it causes the production of reactive oxygen species by the photosynthetic machinery (8, 22, 47). Since the SigD factor has a role in acclimation to mild oxidative stress (33) and the SigB factor participates in the expression of protective chaperones (33, 40, 44), it is not surprising that overall translation activity is slightly lower in the ΔsigBD strain than in the control strain under high light. However, the SigB and SigD factors seem to have an additional, more specific effect on production of new D1 proteins under high-light conditions, since in vivo pulse-labeling clearly shows that the production of the D1 protein is more strongly affected in the inactivation strain than is the production of other proteins. Most probably, this is simply because the amount of psbA mRNAs is lower in the ΔsigBD strain than in the control strain under high-light conditions.
The psbA2 and psbA3 genes encode identical D1 proteins in strain 6803. Using quantitative real-time PCR analysis, we found that under standard conditions the psbA2 gene produced ∼90% and the psbA3 gene produced ∼10% of psbA transcripts in the control and ΔsigBD strains. A similar ratio of psbA2 and psbA3 transcripts was found earlier in the control strain with primer extension analysis (25). Illumination with high light caused upregulation of the expression of both the psbA2 and the psbA3 genes, but the enhancement was more rapid and prominent in the psbA3 gene. Prominent upregulation of the psbA3 gene has earlier been reported under UV light (37). Light-induced upregulation of psbA2 and psbA3 genes was partly impaired in the ΔsigBD strain, and therefore high light induced a smaller increase in the whole psbA transcript pool in the ΔsigBD strain than in the control strain. The similarity of action spectra of photoinhibition and transcription of psbA genes (53) and the correlation between the degradation rate of the D1 protein and the amount of psbA transcripts in D1 mutant strains (52) suggest that photoinhibition activates the transcription of psbA genes. It was recently suggested that degradation products of the D1 protein can bind to the promoter region of the psbAI gene in the cyanobacterium Synechococcus PCC 7942 and upregulate transcription (41). Our results show that in the ΔsigBD strain transcription is not upregulated as efficiently as in the control strain to meet the elevated need for D1 translation in high light, and consequently the repair of photoinactivated PSII centers occurs more slowly in the ΔsigBD strain than in the control strain.
Simultaneous inactivation of SigB and SigD factors influences the transcription of both psbA2 and psbA3 genes. Inactivation strains with only the psbA2 gene or only the psbA3 gene (26) are fully viable. Inactivation of the psbA2 gene induced an eightfold enhancement of the expression of the psbA3 gene, which normally produces only 10% of the transcripts (25). Although UV light mainly induces the psbA3 gene in the wild-type cells (37), no differences were detected in the amount of psbA transcripts between the wild-type strain and a strain containing psbA2 gene as the only functional psbA gene when the cyanobacteria were illuminated with UV light (53). These results suggest that the same factors regulate the expression of the psbA2 and psbA3 genes.
In summary, we show here that the SigB and SigD factors show functional redundancy in light regulation. In the absence of the SigB and SigD factors, cells cannot take full advantage of an increase in light intensity because the adjustment of the light harvesting system does not occur as efficiently as in the control cells. Furthermore, the ΔsigBD strain was sensitive to bright light because low expression of the psbA genes led to insufficient PSII repair.
Supplementary Material
Acknowledgments
We thank Emilia Helminen for helping with the oxygen evolution measurements, Jussi Meriluoto from Åbo Academy University for help with the absorption spectra measurements, and Paula Mulo for helpful discussions.
This study was financially supported by the Academy of Finland and the Finnish Cultural Foundation.
Footnotes
Published ahead of print on 10 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Asayama, M., and S. Imamura. 2008. Stringent promoter recognition and autoregulation by the group 3 sigma-factor SigF in the cyanobacterium Synechocystis sp. strain PCC 6803. Nucleic Acids Res. 365297-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaya, D., N. Watanabe, T. Ogawa, and A. R. Grossman. 1999. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC 6803. Proc. Natl. Acad. Sci. USA 963188-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant, D. A. 2003. The beauty in small things revealed. Proc. Natl. Acad. Sci. USA 1009647-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess, R. R., A. A. Travers, J. J. Dunn, and E. K. F. Bautz. 1969. Factor stimulating transcription by RNA polymerase. Nature 22143-46. [DOI] [PubMed] [Google Scholar]
- 5.Constant, S., I. Perewoska, M. Alfonso, and D. Kirilovsky. 1997. Expression of the psbA gene during photoinhibition and recovery in Synechocystis PCC 6714: inhibition and damage of transcriptional and translational machinery prevent the restoration of photosystem II activity. Plant Mol. Biol. 341-13. [DOI] [PubMed] [Google Scholar]
- 6.Dombroski, A. J., W. A. Walter, M. T. Record, D. A. Siegele, and C. A. Gross. 1992. Polypeptides containing highly conserved regions of transcription initiation factor sigma-70 exhibit specificity of binding to promoter DNA. Cell 70501-512. [DOI] [PubMed] [Google Scholar]
- 7.Field, C. B., M. J. Behrenfeld, J. T. Randerson, and P. Falkowski. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281237-240. [DOI] [PubMed] [Google Scholar]
- 8.Foyer, C. H., and G. Noctor. 2000. Oxygen processing in photosynthesis: regulation and signalling. New Phytol. 146359-388. [Google Scholar]
- 9.Gill, R. T., E. Katsoulakis, W. Schmitt, G. Taroncher-Oldenburg, J. Misra, and G. Stephanopoulos. 2002. Genome-wide dynamic transcriptional profiling of the light-to-dark transition in Synechocystis sp. strain PCC 6803. J. Bacteriol. 1843671-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havaux, M., G. Guedeney, M. Hagemann, N. Yeremenko, H. C. P. Matthijs, and R. Jeanjean. 2005. The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett. 5792289-2293. [DOI] [PubMed] [Google Scholar]
- 11.Herranen, M., E.-M. Aro, and T. Tyystjärvi. 1999. Two distinct mechanisms regulate the transcription of photosystem II genes in Synechocystis sp. PCC 6803. Physiol. Plant. 112531-539. [DOI] [PubMed] [Google Scholar]
- 12.Hihara, Y. 1999. The molecular mechanism for acclimation to high light in cyanobacteria. Curr. Top. Plant Biol. 137-50. [Google Scholar]
- 13.Hihara, Y., A. Kamei, M. Kanehisa, A. Kaplan, and M. Ikeuchi. 2001. DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13793-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, L., M. P. McCluskey, H. Ni, and R. A. LaRossa. 2002. Global gene expression profiles of the cyanobacterium Synechocystis sp. strain PCC6803 in response to irradiation with UV-B and white light. J. Bacteriol. 1846845-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huckauf, J., C. Nomura, K. Forchhammer, and M. Hagemann. 2000. Stress responses of Synechocystis sp. strain PCC 6803 mutants impaired in genes encoding putative alternative sigma factors. Microbiology 1462877-2889. [DOI] [PubMed] [Google Scholar]
- 16.Ikeuchi, M., and S. Tabata. 2001. Synechocystis sp. PCC 6803: a useful tool in the study of genetics in cyanobacteria. Photosynth. Res. 7073-83. [DOI] [PubMed] [Google Scholar]
- 17.Imamura, S., M. Asayama, H. Takahashi, K. Tanaka, H. Takahashi, and M. Shirai. 2003. Antagonistic dark/light-induced SigB/SigD, group 2 sigma factors, expression through redox potential and their roles in cyanobacteria. FEBS Lett. 554357-362. [DOI] [PubMed] [Google Scholar]
- 18.Imamura, S., S. Yoshihara, S. Nakano, N. Shiozaki, A. Yamada, K. Tanaka, H. Takahashi, M. Asayama, and M. Shirai. 2003. Purification, characterization, and gene expression of all sigma factors of RNA polymerase in a cyanobacterium. J. Mol. Biol. 325857-872. [DOI] [PubMed] [Google Scholar]
- 19.Joshua, S., and C. W. Mullineaux. 2004. Phycobilisome diffusion is required for light-state transitions in cyanobacteria. Plant Physiol. 1352112-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3109-136. [DOI] [PubMed] [Google Scholar]
- 21.Kojima, K., M. Oshita, Y. Nanjo, K. Kasai, Y. Tozawa, H. Hayashi, and Y. Nishiyama. 2007. Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol. Microbiol. 65936-947. [DOI] [PubMed] [Google Scholar]
- 22.Krieger-Liszkay, A. 2005. Singlet oxygen production in photosynthesis, J. Exp. Bot. 56337-346. [DOI] [PubMed] [Google Scholar]
- 23.Li, L., R. M. Alvey, R. P. Bezy, and D. M. Kehoe. 2008. Inverse transcriptional activities during complementary chromatic adaptation are controlled by the response regulator RcaC binding to red and green light-responsive promoters. Mol. Microbiol. 68286-297. [DOI] [PubMed] [Google Scholar]
- 24.Marin, K., M. Stirnberg, M. Eisenhut, R. Krämer, and M. Hagemann. 2006. Osmotic stress in Synechocystis sp. PSS 6803: low tolerance toward nonionic osmotic stress results from lacking activation of glucosylglycerol accumulation. Microbiology 1522023-2030. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed, A., J. Eriksson, H. D. Osiewacz, and C. Jansson. 1993. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol. Gen. Genet. 238161-168. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed, A., and C. Jansson. 1989. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol. Biol. 13693-700. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu, M., and Y. Hihara. 2007. Coordinated high-light response of genes encoding subunits of photosystem I is achieved by AT-rich upstream sequences in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 1892750-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata, N., S. Takahashi, Y. Nishiyama, and S. I. Allakhverdiev. 2007. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 17671414-1421. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama, Y., S. I. Allakhverdiev, and N. Murata. 2005. Inhibition of the repair of PSII by oxidative stress in cyanobacteria. Photosynth. Res. 841-7. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama, Y., H. Yamamoto, S. I. Allakhverdiev, M. Inaba, A. Yokota, and N. Murata. 2001. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 205587-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osanai, T., M. Ikeuchi, and K. Tanaka. 2008. Group 2 sigma factors in cyanobacteria. Physiol. Plant 133490-506. [DOI] [PubMed] [Google Scholar]
- 32.Osanai, T., Y. Kanesaki, T. Nakano, H. Takahashi, M. Asayama, M. Shirai, M. Kanehisa, I. Suzuki, N. Murata, and K. Tanaka. 2005. Positive regulation of sugar catabolic pathways in the cyanobacterium Synechocystis sp. PCC 6803 by the group 2 σ factor SigE. J. Biol. Chem. 28030653-30659. [DOI] [PubMed] [Google Scholar]
- 33.Pollari, M., L. Gunnelius, I. Tuominen, V. Ruotsalainen, E. Tyystjärvi, T. Salminen, and T. Tyystjärvi. 2008. Characterization of single and double inactivation strains reveals new physiological roles for group 2 sigma factors of the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 1471994-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramakers, C. J., M. Ruijter, R. H. L. Deprez, and A. F. M. Moorman. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 33962-66. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai, I., M. Hagio, Z. Gombos, T. Tyystjärvi, V. Paakkarinen, E.-M. Aro, and H. Wada. 2003. Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol. 1331376-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki, A., M. Hanaoka, Y. Akimoto, S. Masuda, H. Iwasaki, and K. Tanaka. 2007. Induction of a group 2 σ factor, RPOD3 by high light and the underlying mechanism in Synechococcus elongatus PCC 7942. J. Biol. Chem. 28236887-36894. [DOI] [PubMed] [Google Scholar]
- 37.Sicora, C. I., S. E. Appleton, C. M. Brown, J. Chung, J. Chandler, A. M. Cockshutt, I. Vass, and D. A. Campbell. 2006. Cyanobacterial psbA families in Anabaena and Synechocystis encode trace, constitutive and UVB-induced D1 isoforms. Biochim. Biophys. Acta 175747-56. [DOI] [PubMed] [Google Scholar]
- 38.Sicora, C. I., F. M. Ho, T. Salminen, S. Styring, and E.-M. Aro. 2009. Transcription of a “silent” cyanobacterial psbA gene is induced by microaerobic conditions. Biochim. Biophys. Acta 1787105-112. [DOI] [PubMed] [Google Scholar]
- 39.Silva, P., E. Thompson, S. Bailey, O. Kruse, C. W. Mullineaux, C. Robinson, N. H. Mann, and P. Nixon. 2003. FtsH is involved in the early stages of repair of photosystem II in Synechocystis sp. PCC 6803. Plant Cell 152152-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, A. K., T. C. Summerfield, H. Li, and L. A. Sherman. 2006. The heat shock response in the cyanobacterium Synechocystis sp. strain PCC 6803 and regulation of gene expression by HrcA and SigB. Arch. Microbiol. 186273-286. [DOI] [PubMed] [Google Scholar]
- 41.Stelljes, C., and F. Koenig. 2007. Specific binding of D1 protein degradation products to the psbAI promoter in Synechococcus sp. strain PCC 7942. J. Bacteriol. 1891722-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summerfield, T. C., and L. A. Sherman. 2007. Role of sigma factors in controlling global gene expression in light/dark transitions in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 1897829-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summerfield, T. C., J. Toepel, and L. A. Sherman. 2008. Low-oxygen induction of normally cryptic psbA genes is cyanobacteria. Biochemistry 4712939-12941. [DOI] [PubMed] [Google Scholar]
- 44.Tuominen, I., M. Pollari, E. Tyystjärvi, and T. Tyystjärvi. 2006. The SigB sigma factor mediates high-temperature responses in the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett. 580319-323. [DOI] [PubMed] [Google Scholar]
- 45.Tuominen, I., M. Pollari, E. A. von Wobeser, E. Tyystjärvi, B. W. Ibelings, H. C. P. Matthijs, and T. Tyystjärvi. 2008. Sigma factor SigC is required for heat acclimation of the cyanobacterium Synechocystis sp. strain PCC 6803. FEBS Lett. 582346-350. [DOI] [PubMed] [Google Scholar]
- 46.Tuominen, I., E. Tyystjärvi, and T. Tyystjärvi. 2003. Expression of primary sigma factor (PSF) and PSF-like sigma factors in the cyanobacterium Synechocystis sp. PCC 6803. J. Bacteriol. 1851116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyystjärvi, E. 2008. Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 252361-376. [Google Scholar]
- 48.Tyystjärvi, E., and I. Vass. 2004. Light emission as a probe of charge separation and recombination in the photosynthetic apparatus: relation of prompt fluorescence to delayed light emission and thermoluminescence, p. 363-388. In G. C. Papageorgiou and Govindjee (ed.), Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 49.Tyystjärvi, E., S. Rantamäki, and J. Tyystjärvi. Connectivity of photosystem II is the physical basis of retrapping in photosynthetic thermoluminescence. Biophys. J., in press. [DOI] [PMC free article] [PubMed]
- 50.Tyystjärvi, T., E.-M. Aro, C. Jansson, and P. Mäenpää. 1994. Changes of amino acid sequence in PEST-like area and QEEET motif affect degradation rate of D1 polypeptide in photosystem II. Plant Mol. Biol. 25517-526. [DOI] [PubMed] [Google Scholar]
- 51.Tyystjärvi, T., M. Herranen, and E.-M. Aro. 2001. Regulation of translation elongation in cyanobacteria: membrane targeting of the ribosome nascent-chain complexes controls the synthesis of D1 protein. Mol. Microbiol. 40476-484. [DOI] [PubMed] [Google Scholar]
- 52.Tyystjärvi, T., P. Mulo, P. Mäenpää, and E.-M. Aro. 1996. D1 polypeptide degradation may regulate psbA gene expression at transcriptional and translational levels in Synechocystis sp. PCC6803. Photosynth. Res. 47111-120. [DOI] [PubMed] [Google Scholar]
- 53.Tyystjärvi, T., I. Tuominen, M. Herranen, E.-M. Aro, and E. Tyystjärvi. 2002. Action spectrum of psbA transcription is similar to that of photoinhibition in Synechocystis sp. PCC 6803. FEBS Lett. 516167-171. [DOI] [PubMed] [Google Scholar]
- 54.Tyystjärvi, T., E. Tyystjärvi, I. Ohad, and E.-M. Aro. 1998. Exposure of Synechocystis 6803 cells to series of single turnover flashes increase the psbA transcript level by activating transcription and down-regulating psbA mRNA degradation. FEBS Lett. 436483-487. [DOI] [PubMed] [Google Scholar]
- 55.van Thor, J. J., C. W. Mullineaux, H. C. P. Matthijs, and K. J. Hellingwerf. 1998. Light Harvesting and state transitions in cyanobacteria. Bot. Acta 111430-443. [Google Scholar]
- 56.Williams, J. K. G. 1988. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 167766-778. [Google Scholar]
- 57.Wilson, A., G. Ajlani, J. Z. Verbavatz, I. Vass, C. A. Kerfeld, and D. Kirilovsky. 2006. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18992-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, A., C. Punginelli, A. Gall, C. Bonettit, M. Alexandre, J. M. Routaboul, C. A. Kerfeld, R. van Grondelle, B. Robert, J. T. M. Kennis, and D. Kirilovsky. 2008. A photoactive carotenoid protein acting as light intensity sensor. Proc. Natl. Acad. Sci. USA 10512075-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeremenko, N., R. Kouril, J. A. Ihalainen, S. D'Haene, N. van Oosterwijk, E. G. Andrizhiyevskaya, W. Keegstra, H. L. Dekker, M. Hagemann, E. J. Boekema, H. C. P. Matthijs, and J. P. Dekker. 2004. Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 4310308-10313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.