Abstract
The genome of the pathogen Clostridium perfringens encodes two proteins, GerO and GerQ, homologous to monovalent cation transporters suggested to have roles in the germination of spores of some Bacillus species. GerO and GerQ were able to transport monovalent cations (K+ and/or Na+) in Escherichia coli, and gerO and gerQ were expressed only in the mother cell compartment during C. perfringens sporulation. C. perfringens spores lacking GerO were defective in germination with a rich medium, KCl, l-asparagine, and a 1:1 chelate of Ca2+ and dipicolinic acid (DPA), but not with dodecylamine, and the defect was prior to DPA release in germination. All defects in gerO spores were complemented by ectopic expression of wild-type gerO. Loss of GerQ had much smaller effects on spore germination, and these effects were most evident in spores also lacking GerO. A modeled structure of GerO was similar to that of the E. coli Na+/H+ antiporter NhaA, and GerO, but not GerQ contained two adjacent Asp residues thought to be important in the function of this group of cation transporters. Replacement of these adjacent Asp residues in GerO with Asn reduced the protein's ability to complement the germination defect in gerO spores but not the ability to restore cation transport to E. coli cells defective in K+ uptake. Together, these data suggest that monovalent cation transporters play some role in C. perfringens spore germination. However, it is not clear whether this role is directly in germination or perhaps in spore formation.
Clostridium perfringens is a gram-positive, spore-forming anaerobic pathogen that causes diseases in animals and humans (13). C. perfringens spores are metabolically dormant, are resistant to many environmental insults, and can survive for long periods. Once conditions are favorable, these spores can germinate, outgrow, return to vegetative growth, and then release toxins and cause disease (14).
Bacterial spores initiate germination when they sense a variety of compounds termed germinants, which include nutrients, a 1:1 chelate of Ca2+ and pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) (Ca-DPA) and cationic surfactants (21, 31). In spores of Bacillus species, nutrient germinants are sensed by specific germinant receptors located in the spore's inner membrane, each generally encoded by tricistronic operons of the gerA family. In Bacillus megaterium spores, the interaction of nutrient germinants with their cognate receptors leads to an energy independent efflux of ∼80% of the spore's depot of Na+ and K+, as well as much H+ efflux causing a rise of the spore core's pH, all within the first 5 min of germination; this efflux is followed by reuptake of K+ by an energy-dependent system (33). The spores' large depot of Ca-DPA is also released shortly after monovalent cation release. The mechanism of release of monovalent cations during spore germination is not known, but monovalent cation antiporters could be involved somehow in this event. Indeed, a member of the CPA-2 monovalent cation-proton antiporter family of membrane transport proteins (27), GrmA, is essential for germination of B. megaterium ATCC 12872 spores (34), since grmA inactivation makes spores unable to release their DPA and complete germination with a variety of germinants. Similarly, in Bacillus cereus ATCC 10876, a GrmA-type homologue, GerN, is essential for spore germination with inosine but not l-alanine (35), and studies with everted vesicles have shown that GerN possesses electrogenic Na+/H+-K+ antiporter activity (32). The GerN homolog, GerT, also plays a minor role in B. cereus spore germination with inosine, as well as a major role in spore outgrowth under some conditions (29). However, in contrast to these latter results, GrmA-like antiporters appear to have no role in the germination of spores of B. megaterium QM B1551 and Bacillus subtilis (3).
In C. perfringens, there is no intact tricistronic gerA-like operon, and the only locus that encodes the three proteins (A, B, and C) of a likely germinant receptor is the gerK locus, comprising a bicistronic gerKA-gerKC operon, and a gerKB gene located just upstream of gerKA-gerKC but in the opposite orientation (16). However, GerKA and GerKC appear able to function in spore germination in the absence of GerKB (23). The lack of a classical GerA-type germinant receptor and the fact that C. perfringens spores germinate with K+ ions alone (21), raises the possibility that GrmA-like antiporters might also play some role in C. perfringens spore germination. The genome of C. perfringens strain SM101 has two genes encoding putative GrmA-like antiporters (see Fig. S1 in the supplemental material) that we have termed gerO (CPR0227) and gerQ (CPR1038). Orthologs of the gerO and gerQ genes are also present in the genomes of nine additional C. perfringens strains (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi). In present study we have constructed gerO, gerQ, and gerO gerQ strains of C. perfringens and have examined the roles of GerO and GerQ in spore germination. The results show that GerO is essential for normal germination of C. perfringens spores, whereas GerQ plays at most only a minor role.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The C. perfringens and Escherichia coli strains, and plasmids used in the present study are described in Table S1 in the supplemental material.
Construction of gusA fusion plasmids and β-glucuronidase assay.
DNA fragments (300 to 400 bp) upstream of gerO and gerQ from C. perfringens SM101, which include the 290- and 29-bp intergenic regions between gerO and CPR0226 and between gerQ and CPR1039, respectively, which most likely contain these ger gene promoters, were PCR amplified using primer pairs CPR383-CPR386 and CPR380-CPR385. The forward and reverse primers (the sequences of all primers used in the present study are given in Table S2 in the supplemental material) had SalI and PstI cleavage sites, respectively, at the 5′ ends. These PCR fragments were digested with SalI and PstI and cloned between the SalI and PstI sites in plasmid pMRS127 in E. coli DH5α, the host for all plasmid construction, as described previously (20, 25) to create gerO- and gerQ-gusA fusions, giving plasmids pDP81 and pDP82 (see Table S1 in the supplemental material). These plasmids were introduced by electroporation (4) into C. perfringens SM101, and erythromycin-resistant (Emr) transformants were selected. Transformants carrying plasmids with the gerO-and gerQ-gusA fusions were grown in TGY vegetative medium (3% Trypticase soy, 2% glucose, 1% yeast extract, 0.1% l-cysteine) (10) and in Duncan-Strong (DS) (5) sporulation medium, and cells were extracted and assayed for β-glucuronidase (GUS) activity as described previously (38). GUS-specific activity was expressed in Miller units that were calculated as described previously (25). Note that lysozyme was present in the cell extraction prior to the GUS assays and, although lysozyme treatment will likely not extract enzymes from intact dormant spores, it does allow extraction of enzymes from chemically decoated spores.
Decoating treatment of sporulating cultures.
Cell pellets from 1 ml of DS sporulating cultures were treated to chemically decoat any spores present in 1 ml of 50 mM Tris-HCl (pH 8.0)-8 M urea-1% (wt/vol) sodium dodecyl sulfate-50 mM dithiothreitol for 90 min at 37°C and remaining spores were washed three times with 150 mM NaCl and twice with water (24). The decoated samples were then extracted and assayed for GUS activity as described above. Note that the decoating treatment will inactivate and/or remove any GUS not in dormant spores.
Construction of gerO- and gerQ-gfp-fusion plasmids and green fluorescent protein (GFP) visualization.
Plasmids carrying the gerO or gerQ promoters fused to gfp were constructed as follows. An ∼715-bp fragment was PCR amplified from plasmid pEGFP (Clontech, Mountain View, CA) with Phusion high-fidelity DNA polymerase (New England Biolabs, Ipswich, MA) using the primer pair CPP602-CPP603 (the forward primer had XbaI, PstI, and SpeI sites, and the reverse primer had two extra Ts and a BamHI site at the 5′ end). The PCR fragment was cloned into plasmid Zero-Blunt-TOPO (Invitrogen, Carlsbad, CA), yielding plasmid pDP149. A 720-bp XbaI-BamHI fragment from plasmid pDP149 was cloned between the XbaI and BamHI sites of plasmid pET16b (Novagen, Gibbstown, NJ), upstream of a strong transcription terminator, yielding plasmid pDP151. A 1,023-bp PstI-HindIII fragment from plasmid pDP151 that probably contains the gerO promoter (Fig. 1B) was cloned between the PstI and HindIII sites in plasmid pDP81, yielding plasmid pDP152. A 433-bp PCR fragment amplified from C. perfringens SM101 DNA using Phusion high-fidelity DNA polymerase and the primer pair CPP380-CPP676, which carries 427 bp upstream and 6 bp from the N-terminal coding region of gerQ, was digested with SalI and PstI and cloned between the SalI and PstI sites in plasmid pDP152, replacing the gerO promoter with the gerQ promoter and giving plasmid pDP182. As shown by assays of GUS activity (Fig. 1C), the 434-bp region upstream of gerQ contains a sporulation-specific promoter.
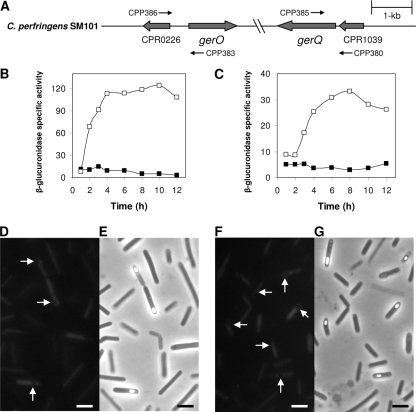
FIG. 1.
A-G. Arrangement and expression of gerO and gerQ genes in C. perfringens SM101. (A) The arrangement of gerO and gerQ in C. perfringens SM101 and the location of the primers used to amplify the upstream regions of each gene are indicated, including the intergenic regions between gerO and CPR0226 and between gerQ and CPR1039. (B and C) GUS-specific activity from gerO-gusA (B) and gerQ-gusA (C) fusions in C. perfringens SM101 grown in TGY vegetative (▪) and DS sporulation (□) media were determined as described in Materials and Methods. The data represent an average of three independent experiments, and time zero denotes the time of inoculation of cells into either TGY or DS media. (D to G) Fluorescence microscopy (D and F) and phase-contrast microscopy (E and G) of gerO-gfp (D and E) and gerQ-gfp (F and G) fusions in C. perfringens SM101 grown for 6 h in DS sporulation medium as described in Materials and Methods. Arrows indicate that there is no green fluorescence in the forespore compartment of the sporulating cell. Bar, 5 μm.
Plasmids pDP152 and pDP182 were introduced by electroporation (4) into C. perfringens strain SM101; Emr transformants were selected, and the presence of the plasmids pDP152 and pDP182 in SM101(pDP152) and SM101(pDP182) was confirmed by PCR (data not shown). Six-hour TGY-vegetative and six-hour DS-sporulating cultures and untreated and decoated purified spores of strains SM101(pDP152) and SM101(pDP182) prepared at 37°C were examined on a DM4008B fluorescence microscope (Leica, Wetzlar, Germany). For visualization of GFP, 5 μl of culture was applied to a poly-l-lysine-coated glass microscope slide, and a dichroic mirror cube unit with a narrow-band-pass (450- to 490-nm) excitation filter and a narrow-band-pass (500- to 550-nm) barrier emission filter were used. Photomicrographs were prepared with Adobe Photoshop and Microsoft Picture Manager Software.
Construction of E. coli strains carrying gerO and gerQ.
E. coli strains TK2420 and KNabc carrying plasmids with C. perfringens gerO and gerQ were constructed as follows. An ∼1.4-kb promoterless fragment carrying 28 bp upstream, the coding region, and 182 bp downstream of gerO was PCR amplified from C. perfringens SM101 DNA with Phusion high-fidelity DNA polymerase by using the primers CPP523 and CPP524 (the forward and reverse primers had KpnI and PstI sites at the 5′ ends, respectively). The ∼1.4-kb PCR fragment was cloned into plasmid Zero-Blunt-TOPO, giving plasmid pDP141. An ∼1.4-kb KpnI-PstI fragment from plasmid pDP141 was then cloned between the KpnI and PstI sites of plasmid pGEM3zf(+) (Promega, Madison, WI) behind the T7 promoter, giving plasmid pDP144. Expression of genes inserted in plasmid pGEM3zf(+) constructs in E. coli, even without concomitant expression of T7 RNA polymerase, results in levels of gene products sufficient for phenotypic effects of membrane transport proteins without the toxicity that often results from overexpression of such proteins (2, 6, 32). An ∼1.6-kb promoterless fragment carrying the coding region and 111 bp downstream of gerQ was PCR amplified from C. perfringens SM101 DNA with Phusion high-fidelity DNA polymerase by using the primers CPP533 and CPP526 (the forward primer had a KpnI site at the 5′ end, and bases TTTATT at positions −7 to −2 relative to the start codon of gerQ were substituted for GGAGGGGA, to provide a better ribosome-binding site [RBS] for gerQ mRNA as reported earlier [32]; the reverse primer had a PstI site at the 5′ end) or with the primers CPP525 and CPP526 (the forward and reverse primers had KpnI and PstI sites, respectively, at their 5′ ends). Both ∼1.6-kb PCR fragments were cloned into plasmid pGEM3zf(+), giving plasmids pDP147 and pDP148, respectively. Plasmids pDP144, pDP147, and pDP148 were transformed into E. coli strains TK2420 and KNabc, and ampicillin-resistant transformants were selected.
The K+ uptake-deficient E. coli strain TK2420 has a normal complement of Na+(K+)/H+ antiporters, and the effects of increasing KCl concentration on the growth of this strain and its derivatives were determined as described previously (12) in a defined medium containing Na+. The Na+/H+ antiporter-deficient KNabc strain has a normal complement of K+ uptake proteins but reduced levels of K+/H+ antiporters mediating K+ efflux; this strain is unable to grow at Na+ concentrations of >75 mM but will grow in low-sodium medium, LBK (6). The effects of increasing the Na+ concentration on the growth of E. coli KNabc and its derivatives were measured using LBK with various concentrations of NaCl (6).
Construction of a C. perfringens gerQ deletion mutant.
To isolate a derivative of C. perfringens SM101 with a deletion of gerQ, a ΔgerQ suicide vector was constructed as follows. An 832-bp DNA fragment carrying 583 bp upstream and 249 bp of the N-terminal coding region of gerQ was PCR amplified with the primers CPP415 and CPP417 (the forward and reverse primers had KpnI and SpeI sites, respectively, at the 5′ ends). A 1,312-bp DNA fragment containing 80 bp of the C-terminal coding region and 1,232 bp downstream of gerQ was PCR amplified with the primers CPP411 and CPP410 (the forward and reverse primers contained PstI and XhoI sites, respectively, at their 5′ ends). These fragments were cloned into plasmid pCR-XL-TOPO (Invitrogen), giving plasmids pDP101 and pDP102. An ∼0.8-kb KpnI-SpeI fragment from pDP101 was then cloned between the KpnI and SpeI sites just upstream of catP in plasmid pDP25 (22), giving plasmid pDP103. An ∼1.3-kb PstI-XhoI fragment from pDP102 was cloned between the PstI and XhoI sites downstream of catP in plasmid pDP103, giving plasmid pDP104. A 3.5-kb KpnI-XhoI fragment from plasmid pDP104, carrying the ΔgerQ::catP construct, was then cloned between the KpnI and SalI sites of plasmid pMRS104 (7), giving plasmid pDP105, which cannot replicate in C. perfringens. Plasmid pDP105 was introduced into C. perfringens SM101 by electroporation (4), and a chloramphenicol-resistant gerQ mutant was isolated as described previously (28). The identity of the gerQ strain DPS113 was confirmed by PCR and Southern blot analyses (data not shown).
Construction of a C. perfringens gerO deletion mutant.
To isolate a derivative of C. perfringens SM101 with a deletion of gerO, a ΔgerO suicide vector was constructed as follows. A 1,070-bp DNA fragment carrying 865 bp upstream and 205 bp of the N-terminal coding region of gerO was PCR amplified with the primers CPP406 and CPP408 (the forward and reverse primers had KpnI and SpeI sites, respectively, at their 5′ ends). A 1,227-bp DNA fragment carrying 246 bp of the C-terminal coding region and 981 bp downstream of gerO was PCR amplified with the primers CPP424 and CPP430 (the forward and reverse primers had PstI and XhoI sites, respectively, at their 5′ ends). These PCR fragments were cloned into plasmid pCR-XL-TOPO, giving plasmids pDP96 and pDP97, respectively. A 1.1-kb KpnI-SpeI fragment from plasmid pDP96 was then cloned between the KpnI and SpeI sites upstream of catP in plasmid pDP25, giving plasmid pDP98, and an ∼1.2-kb PstI-XhoI fragment from plasmid pDP97 was cloned between the PstI and XhoI sites downstream of catP in plasmid pDP98, giving plasmid pDP99. An ∼3.7-kb KpnI-XhoI fragment from plasmid pDP99, carrying the ΔgerO::catP construct, was then cloned between the KpnI and SalI sites in plasmid pMRS104, giving plasmid pDP100, which cannot replicate in C. perfringens. Finally, an ∼3.2-kb SpeI-PstI fragment from plasmid pDP35, carrying tetM, was cloned between the PstI and SpeI sites in plasmid pDP100, replacing the catP gene with tetM and giving plasmid pDP112. Plasmid pDP112 (carrying ΔgerO::tetM) was introduced into C. perfringens SM101 by electroporation, and a tetracycline-resistant (Tetr) gerO mutant was isolated as described previously (28). The identity of the gerO strain DPS116 was confirmed by PCR and Southern blot analyses (data not shown).
To isolate a derivative of C. perfringens SM101 with deletions of both gerO and gerQ, plasmid pDP112 was introduced into C. perfringens DPS113 (gerQ) by electroporation, and a Cmr Tetr gerO gerQ mutant was isolated as described previously (28). The identity of the gerO gerQ strain DPS115 was confirmed by PCR and Southern blot analyses (data not shown).
Construction of a ΔgerO strain complemented with gerO.
To construct a gerO strain complemented with wild-type gerO, a suicide-complementing plasmid targeted to the plc locus was constructed as follows. A 1.8-kb DNA fragment carrying 396 bp upstream and the coding region of gerO was PCR amplified with Phusion high-fidelity DNA polymerase by using the primers CPP599 and CPP600 (the forward and reverse primers had KpnI and SalI sites, respectively, at their 5′ ends). As shown by assays of GUS activity (Fig. 1B), the 396-bp region upstream of gerO contains a sporulation-specific promoter. This PCR fragment was digested with KpnI and SalI and cloned between the KpnI and SalI sites of plasmid pDP129 (a suicide plasmid containing ca. 1.7 and 1.3 kb upstream and downstream of the plc locus) (22), giving plasmid pDP150, which cannot replicate in C. perfringens. Plasmid pDP150 was introduced into the C. perfringens gerO strain DPS116 by electroporation (4), and Emr Tetr transformants of strain DPS116(pDP150) were selected. The presence of both plasmid pDP150 and the original gerO deletion in the latter strain were confirmed by PCR and Southern blot analyses (data not shown).
Construction of a gerO strain containing gerO(D161N, D162N).
The gerO strain containing gerO(D161N, D162N), with Asp161 and Asp162 replaced by two Asn residues, was constructed as follows. A 905-bp DNA fragment carrying 396 bp upstream and 509 bp of the N-terminal coding region of gerO was PCR amplified from C. perfringens SM101 DNA with Phusion high-fidelity DNA polymerase by using the primers CPP599 and CPP662 (the forward and reverse primers contained KpnI and HpaI sites, respectively, at their 5′ ends). To produce the desired mutations, bases at positions 481 and 484 of gerO were altered to A residues in primer CPP662 (see Table S2 in the supplemental material). An 879-bp DNA fragment carrying 697 bp of the C-terminal coding region and 182 bp downstream of gerO was PCR amplified from C. perfringens SM101 DNA with Phusion high-fidelity DNA polymerase by using the primers CPP663 and CPP524 (the forward and reverse primers had HpaI and PstI sites, respectively, at their 5′ ends). These PCR fragments were cloned into plasmid Zero-Blunt-TOPO, giving plasmids pDP169 and pDP167, respectively, and these plasmids were sequenced to confirm the presence of the mutations. A 0.9-kb KpnI-HpaI fragment from plasmid pDP169 was cloned between the KpnI and HpaI sites in plasmid pDP25, giving plasmid pDP170; a 0.8-kb HpaI-PstI fragment from plasmid pDP167 was cloned between the HpaI and PstI sites in plasmid pDP170, giving plasmid pDP171; and a 1.6-kb KpnI-XhoI fragment from plasmid pDP171 was cloned between the KpnI and SalI sites in plasmid pDP129, giving plasmid pDP172. Plasmid pDP172 was sequenced to confirm that the construct was in-frame and contained the desired mutations (data not shown) and was introduced into C. perfringens strain DPS116 (gerO) by electroporation (4), and an Emr Tetr transformant [strain DPS116(pDP172)] was selected. The presence of plasmid pDP172 in strain DPS116(pDP172) was confirmed by PCR and Southern blot analyses (data not shown).
To attempt complementation of E. coli mutants with gerO(D161N, D162N), an ∼1.2-kb DNA fragment was PCR amplified from plasmid pDP172 by using the primers CPP523 and CPP524 (see Table S2 in the supplemental material) and cloned between the KpnI and PstI sites in plasmid pGEM3zf(+), giving plasmid pDP173. Plasmid pDP173 was sequenced to confirm that the construct was in-frame and contained the desired mutations (data not shown) and was introduced into E. coli strains.
Spore preparation and purification.
Spores of C. perfringens isolates were prepared and purified as described previously (20, 21). Briefly, C. perfringens sporulating cultures were prepared by inoculating 0.2 ml of an overnight culture grown at 37°C in fluid thioglycolate broth (Difco) into 10 ml of DS sporulation medium (5), followed by incubation for 24 h at 37°C, and the presence of spores was confirmed by phase-contrast microscopy. Large amounts of spores were prepared by scaling up the latter procedure as described previously (21). Clean spore preparations were obtained by repeated centrifugation; washing with sterile distilled water until spore suspensions were >99% free of sporulating cells, cell debris, and germinated spores; suspended in distilled water at a final optical density at 600 nm (OD600) of ∼6; and stored at −20°C. All mutant strains used in the present study sporulated like the parental wild-type strain, as observed by phase-contrast microscopy (data not shown).
Spore germination.
With the exception of dodecylamine germination, spore suspensions in water were heat activated (80°C, 10 min) prior to germination, cooled in water at ambient temperature for 5 min, and incubated at 40°C for 10 min prior to the addition of buffer and germinants as described previously (20, 21). Except for germination with dodecylamine (see below), spore germination was routinely measured by monitoring the OD600 of spore cultures (Smartspec 3000 spectrophotometer; Bio-Rad Laboratories, Hercules, CA), which falls ∼60% upon complete spore germination, and levels of germination were confirmed by phase-contrast microscopy. The extent of spore germination was calculated from the percentage decrease in OD600 after 1 h, with 60% decrease set at 100% germination. All values reported are averages of two experiments performed on at least two independent spore preparations, and individual values varied by <10% from the average values shown. In some experiments, the maximum rates of spore germination were determined by measuring the OD600 of germinating cultures every 2.5 min, the maximum slopes were calculated, and the maximum rates of germination were expressed as the maximum rate of loss in the OD600 of the spore suspension relative to the initial OD600 of the culture.
Assessment of spore colony-forming efficiency.
The colony-forming efficiency of spores of various strains was assessed by plating aliquots of dilutions of heat activated spores on brain heart infusion (BHI) agar, incubating the plates anaerobically at 37°C for 24 h, and the colonies were counted. Outgrowth experiments were in TGY vegetative medium since this medium allows faster growth and maintains better anaerobiosis. Briefly, 300-μl spore suspensions at an OD600 of 1.0 were heat activated, cooled in water at room temperature, and inoculated into 10 ml of TGY medium at 37°C; the cultures were incubated at 37°C, and the OD600 was measured.
DPA release.
DPA release during spore germination was measured as described previously (20, 21). Briefly, heat-activated spore suspensions (OD600 of 1.5) were cooled and incubated at 40°C in BHI broth or in 25 mM sodium phosphate buffer with or without various germinants. After 60 min or 24 h, aliquots (1 ml) were centrifuged in a microcentrifuge (13,200 rpm, 3 min), and the spore pellets were washed four times with 1 ml of distilled water and suspended in 1 ml of distilled water. The amount of spore DPA remaining was determined by boiling the samples for 60 min, cooling them on ice for 5 min, centrifuging them in a microcentrifuge for 5 min, and measuring the OD270 of the supernatant fluid as described previously (1, 30). The DPA content of the initial dormant spores was measured by boiling 1-ml aliquots for 60 min, centrifuging them in a microcentrifuge for 5 min, and measuring the OD270 of the supernatant fluid as described previously (1, 21). Control experiments were done for each experiment to account for losses due to the multiple centrifugations, and corrections for such losses were made accordingly. In C. perfringens spores, ∼90% of the material absorbing at 270 nm released from spores by boiling is DPA (22). In a few experiments the total DPA content of the spores of various strains was measured by a colorimetric assay as described previously (20, 26).
Dodecylamine germination was assessed by measuring only DPA release by incubating spores (OD600 of 1.5) that had not been heat activated with 1 mM dodecylamine in 25 mM Tris-HCl (pH 7.4) at 60°C; aliquots (1 ml) of germinating cultures were centrifuged for 3 min in a microcentrifuge, and DPA in the supernatant fluid was measured based on the OD270 as described previously (1, 21). Initial DPA levels in dormant spores were measured as described above. No significant DPA release was observed when spores were incubated in 25 mM Tris-HCl (pH 7.4) at 60°C for 1 h (data not shown).
RESULTS
C. perfringens gerO and gerQ are expressed in the mother cell compartment during sporulation.
To evaluate whether the C. perfringens gerO and gerQ genes encoding putative antiporters are expressed during sporulation, upstream DNA from each gene, including the intergenic regions between these genes and the ones preceding them (Fig. 1A) that most likely contain these genes' promoters, was fused to E. coli gusA, and the GUS activity was measured after these fusions were introduced into C. perfringens SM101. No significant GUS activity was observed in vegetative cultures of SM101 carrying gerO- and gerQ-gusA, but significant GUS activity was detected in sporulating cultures carrying these gusA fusions (Fig. 1B and C), indicating that sporulation-specific promoters are located upstream of gerO and gerQ. Note, however, that we cannot rule out the possibility that gerQ is also transcribed with the immediately upstream open reading frame CPR1039 (Fig. 1A), perhaps even from a vegetative promoter (Fig. 1A). GUS expression from the gerO- and gerQ-gusA fusions began ca. 2 and 3 h after the start of sporulation, respectively, and reached maxima at ca. 4 and 8 h (Fig. 1B and C). When sporulating cultures were treated with a decoating regimen (24) that inactivates mother cell enzyme activity but not enzymes within spores, no GUS activity was detected throughout sporulation of strains carrying gerO- and gerQ-gusA fusions or from purified dormant spores (data not shown), suggesting that gerO and gerQ are expressed only in the mother cell compartment of the sporulating cell.
To further confirm the site of expression of gerO and gerQ, transcriptional gerO- and gerQ-gfp fusions were constructed and introduced into C. perfringens SM101. As with the two gusA fusions, no significant GFP fluorescence was detected in vegetative cultures of strain SM101 carrying gerO- and gerQ-gfp fusions (data not shown). However, GFP fluorescence was detected after 4 h of sporulation in DS medium of strains carrying gerO- and gerQ-gfp fusions (data not shown), and this fluorescence was readily seen after 6 h of sporulation (Fig. 1D, E, F, and G). No fluorescence was observed in a 6-h sporulating culture of strain SM101 (data not shown). Strikingly, GFP fluorescence from gerO- and gerQ-gfp was exclusively in the mother cell compartment of the sporulating cell, with no fluorescence observed in forespore compartments that were not fully refractile, developing refractile spores, or in purified dormant spores (Fig. 1D to G and data not shown). An analysis of the intergenic region between CPR1039 and gerQ revealed a sequence very similar to those of sporulation-specific promoters dependent on SigK at positions −146 to −179 relative to the gerQ translation start site, although no similarity to sporulation-specific SigK- and SigE-dependent promoters was found in the intergenic region between CPR0226 and gerO (data not shown). Collectively, these results suggest that GerO and GerQ are synthesized exclusively during sporulation and only in the mother cell compartment of the sporulating cell and that GerQ might be under direct control of SigK.
Complementation of K+ uptake and Na+ sensitivity phenotypes in E. coli by C. perfringens GerO and GerQ.
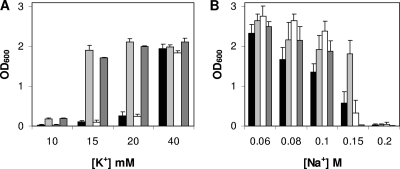
The sporulation-specific synthesis of GerO and GerQ was consistent with these proteins playing some role in spore germination, and the homology between these proteins and known antiporters (see Fig. S1 in the supplemental material) suggested that GerO and GerQ might also be antiporters. To test this latter possibility directly, we examined whether plasmids carrying C. perfringens gerO or gerQ could complement the phenotype of E. coli strain TK2420 that is defective in K+ uptake due to the absence of Kdp, TrkD1, and TrkA; TK2420 cells cannot grow in a defined medium (6) containing a low K+ concentration. As expected, E. coli TK2420 cells carrying the control plasmid pGEM3zf(+) could not grow in defined medium containing less than 40 mM KCl (Fig. 2A). However, TK2420 strains carrying GerO grew in defined medium containing 15 and 20 mM KCl (Fig. 2A), suggesting that GerO is capable of promoting an inward flux of K+ ions. In contrast, E. coli strain TK2420 cells expressing GerQ via either the native gerQ mRNA's RBS or a stronger RBS could only grow with 40 mM KCl (Fig. 2A and data not shown).
FIG. 2.
Effects of KCl and NaCl concentrations on the growth of E. coli strains TK2420 and KNacb carrying C. perfringens gerO and gerQ. The growth of E. coli strains TK2420 (A) and KNabc (B) transformed with the control plasmid (black bars), plasmid pDP144 (gerO) (light gray bars), plasmid pDP147 (gerQ) (white bars), or plasmid pDP173 [gerO(D161N, D162N)] (dark gray bars) was measured after incubation for 15 h at 37°C in defined medium with various KCl concentrations (A) and in LBK medium with increasing NaCl concentrations (B) as described in Materials and Methods.
We also examined the ability of GerO and GerQ to complement the Na+-sensitive phenotype of E. coli strain KNabc that cannot grow in medium containing ≥200 mM NaCl due to absence of Cha, NhaA, and NhaB (9). As expected, growth of KNabc cells carrying the control plasmid decreased as the NaCl concentration of the LBK medium was increased from 0.06 to 0.15 M, and no growth was observed with 0.2 M NaCl (Fig. 2B). However, KNabc cells expressing GerO exhibited significantly (P < 0.0001) higher growth than KNabc cells in LBK medium with 0.1 and 0.15 M NaCl, although no growth was observed with 0.2 M NaCl (Fig. 2B). In addition, KNabc cells expressing GerQ via the native or an even stronger RBS grew significantly (P < 0.0001) more than KNabc(pGEM3zf+) cells in LBK medium with 0.08 and 0.1 M NaCl, although little and no growth were observed with 0.15 and 0.2 M NaCl, respectively (Fig. 2B and data not shown). These results suggest that although both putative antiporters catalyze Na+ efflux, GerO can translocate Na+ against a greater Na+ gradient. Collectively, these findings suggest that while GerO is capable of translocating K+ and Na+, GerQ is only capable of translocating Na+, and perhaps only to a small extent. Note that while the actual levels of GerO and GerQ in E. coli are unknown, these complementation experiments were conducted as done successfully with the B. cereus gerN gene (32).
GerO, but not GerQ, is essential for normal germination of C. perfringens spores.
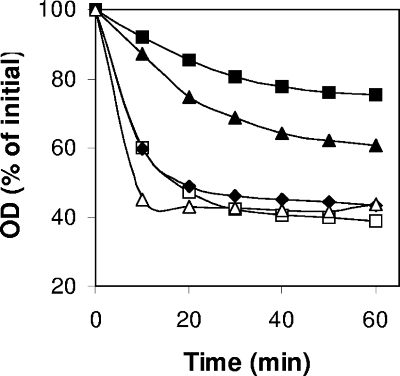
Given that GerO can transport K+ and Na+, with GerQ perhaps having weak activity with Na+, and the role of antiporters in at least some spore germination, it was of obvious interest to examine the function of GerO and GerQ in C. perfringens spore germination. Consequently, we constructed strains with deletions of gerO (strain DPS116), gerQ (DPS113), and both gerO and gerQ (strain DPS115). Wild-type and gerQ spores germinated with similar kinetics and to similar extents in BHI broth, as measured by changes in the OD600 (Fig. 3). Phase-contrast microscopy also showed that >99% of wild-type and gerQ spores had become phase dark after 60 min of incubation in BHI broth (data not shown). However, gerO spores exhibited much poorer germination in BHI broth than wild-type and gerQ spores, with gerO gerQ spores germinating even more poorly (P ≤ 0.01) (Fig. 3). Phase-contrast microscopy further indicated that after 60 min of incubation in BHI broth, only ca. 30 and 20% of gerO and gerO gerQ spores, respectively, had become phase dark (data not shown). These results suggest that GerO plays a major role in spore germination in BHI broth, while significant effects of GerQ are seen only in the absence of GerO.
FIG. 3.
Germination of mutant spores in BHI broth. Heat activated spores of C. perfringens strains SM101 (wild-type) (□), DPS113 (gerQ) (⧫), DPS116 (gerO) (▴), DPS115 (gerO gerQ) (▪), and DPS116(pDP150) (gerO mutant complemented with wild-type gerO) (▵) were germinated at 40°C in BHI broth, and the OD600 was measured as described in Materials and Methods.
In B. cereus spores, GerN is involved in inosine-mediated germination but not in l-alanine-mediated germination (35). Therefore, to gain further understanding of the roles of GerO and GerQ in C. perfringens spore germination, assays were conducted with specific nutrient and nonnutrient germinants (21). The gerQ spores germinated slightly poorer than wild-type spores with KCl, while gerO spores germinated even more poorly (P < 0.0001), and gerO gerQ spores germinated most poorly of all (Fig. 4A). These results were consistent with those from phase-contrast microscopy, where more than 99, 90, 50, and 30% of the wild-type, gerQ, gerO and gerO gerQ spores, respectively, had become phase dark after 60 min of incubation with KCl (data not shown). In contrast to the results with KCl, gerQ spores germinated like wild-type spores with l-asparagine, although gerO spores germinated more poorly than wild-type spores, while gerO gerQ spores germinated the most poorly (Fig. 4B). Phase-contrast microscopy showed that while ∼70% of wild-type and gerQ spores had become phase dark after 60 min of incubation with l-asparagine, only ca. 20 and 10% of gerO and gerO gerQ spores, respectively, had become phase dark. When a mixture of KCl and l-asparagine (AK) was used (Fig. 4C), the germination phenotypes observed were similar to those obtained with KCl (Fig. 4A). Finally, the germination defects observed with gerO spores were eliminated when the gerO mutant spores also carried an ectopic wild-type gerO, indicating that the germination defects in strain DPS116 were due to specific inactivation of gerO (Fig. 4A to C). Indeed, the gerO spores carrying an ectopic wild-type gerO actually germinated better than wild-type spores with l-asparagine (Fig. 4B). All of these results suggest that GerO has a significant role in germination of C. perfringens spores, whereas the role of GerQ is secondary and notable primarily in the absence of GerO.
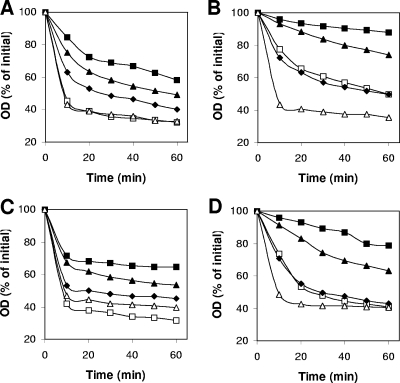
FIG. 4.
Germination of C. perfringens spores with various germinants. Heat-activated C. perfringens spores of strains SM101 (wild-type) (□), DPS113 (gerQ) (⧫), DPS116 (gerO) (▴), DPS115 (gerO gerQ) (▪), and DPS116(pDP150) (gerO mutant complemented with wild-type gerO) (▵) were germinated at 40°C in 25 mM sodium phosphate buffer (pH 7.4) with 100 mM KCl (A), 100 mM l-asparagine (B), or 100 mM l-asparagine and 100 mM KCl (AK) (C) or at 40°C without sodium phosphate buffer in 50 mM Ca-DPA made to pH 8.0 with Tris base (D). At various times, the OD600 was measured as described in Materials and Methods. Spores of various C. perfringens strains incubated in 25 mM sodium phosphate buffer (pH 7.0) at 40°C exhibited less than a 10% decrease in OD600 in 60 min (data not shown).
In contrast to the situation in B. subtilis spores where Ca-DPA triggers spore germination likely by activation of the cortex-lytic enzyme, CwlJ, and bypasses the germinant receptors (18), in C. perfringens spores Ca-DPA likely acts through the germinant receptors as do KCl and l-asparagine (21, 22). As expected, wild-type and gerQ spores germinated similarly with Ca-DPA (Fig. 4D), as confirmed by phase-contrast microscopy (data not shown). However, gerO spores germinated more poorly than wild-type and gerQ spores with Ca-DPA, and gerO gerQ spores germinated even more poorly (Fig. 4D). Phase-contrast microscopy showed that ca. 30 and 20% of gerO and gerO gerQ spores, respectively, had become phase dark after 60 min of incubation with Ca-DPA, while ∼90% of wild-type and gerQ spores had become phase dark (data not shown). Again, the Ca-DPA germination defect of gerO spores was more than complemented by wild-type gerO (Fig. 4D). These results are consistent with Ca-DPA acting on a germinant receptor and indicate that GerO, but GerQ only minimally in the absence of GerO, is required for normal Ca-DPA germination.
To more rigorously compare the effects of gerO and gerQ deletions on spore germination with various germinants, we measured the maximum rates of spore germination by monitoring the fall in OD600 every 2.5 min. As expected, gerQ spores exhibited a maximum germination rate similar to that of wild-type spores with l-asparagine and Ca-DPA and a slightly lower maximum rate than wild-type spores with KCl and AK (Table 1). However, gerO spores had a significantly lower maximum germination rate than wild-type spores with all germinants, and gerO gerQ spores had the lowest maximum germination rates (Table 1).
TABLE 1.
Maximum germination rates of spores of various C. perfringens strains
| Strain (genotype) | Maximum rate of spore germination with the indicated germinanta
|
|||
|---|---|---|---|---|
| KCl | l-Asparagine | AK | Ca-DPA | |
| SM101 (wild-type) | 100 | 100 | 100 | 100 |
| DPS113 (gerQ) | 85 | 97 | 71 | 101 |
| DPS116 (gerO) | 46 | 25 | 40 | 27 |
| DPS115 (gerO gerQ) | 37 | 25 | 40 | 21 |
| DPS116 [ΔgerO gerO(D161N, D162N)] | 67 | 70 | 82 | 52 |
Spores were germinated at 40°C with 100 mM KCl, 100 mM l-asparagine, or 100 mM KCl-100 mM l-asparagine (AK) each in 25 mM sodium phosphate (pH 7.0) or in 50 mM Ca-DPA made to pH 8.0 with Tris base, and maximum rates of spore germination were determined as the percent change in OD600/min. All values are given relative to the value for SM101 spores with the respective germinant, and this latter value was set at 100.
DPA release during germination of spores of various C. perfringens strains.
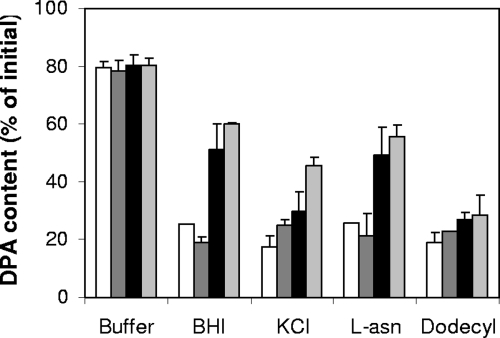
The results noted above suggest that GerO and, to a significantly lesser extent, GerQ are essential for normal spore germination and further that GerO and GerQ are likely involved in transport of Na+ and/or K+. With B. megaterium spores, the release of H+, Na+, and K+ from the spore core is a very early event in spore germination, preceding release of Ca-DPA (33). However, it is unclear whether monovalent cation release is necessary for Ca-DPA efflux in germination. Since we found that dormant gerO, gerQ, and gerO gerQ spores have similar levels of DPA (data not shown) and to measure how far the germination of these spores can progress, we assayed DPA release during germination with various spores. As expected, spores of all strains released only a small amount of DPA when incubated for 60 min in sodium phosphate buffer (Fig. 5). However, wild-type and gerQ spores released the majority of their DPA when germinated for 60 min with BHI broth, KCl, or l-asparagine (Fig. 5). In contrast, while gerO spores released the majority of their DPA when germinated with KCl, only about half of their DPA was released upon germination with BHI broth or l-asparagine, and gerO gerQ spores generally released even less DPA than gerO spores upon incubation for 60 min with BHI broth, KCl, or l-asparagine (Fig. 5). No significant further release of DPA was observed when spores of these strains were incubated for 24 h with BHI broth, KCl, or l-asparagine (data not shown). However, spores of all four strains released 75 to 80% of their DPA when germinated with the cationic surfactant, dodecylamine (Fig. 5). The amount of DPA released is consistent with the extent of germination observed above, and this suggests that a significant fraction of gerO and gerO gerQ spores, but not gerQ spores, cannot progress through stage I of germination with BHI broth or l-asparagine.
FIG. 5.
DPA release from C. perfringens spores incubated with various germinants. Spores of C. perfringens strain SM101 (wild-type) (white bars), DPS113 (gerQ) (dark-gray bars), DPS116 (gerO) (black bars), and DPS115 (gerO gerQ) (light gray bars) were germinated at 40°C for 60 min with 25 mM sodium phosphate buffer (pH 7.0) (Buffer), BHI broth (BHI), 100 mM KCl-25 mM sodium phosphate (pH 7.4) (KCl), or 100 mM l-asparagine-25 mM sodium phosphate (pH 7.4) (L-asn) or at 60°C for 60 min with 1 mM dodecylamine-25 mM Tris-HCl (pH 7.4) (Dodecyl), and DPA release was measured as described in Materials and Methods.
Effects of gerO and gerQ mutations on C. perfringens spore outgrowth and colony-forming efficiency.
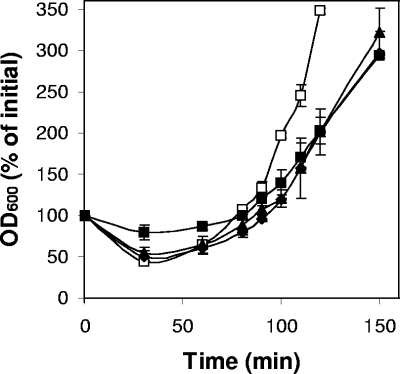
The severe germination defects of gerO and gerO gerQ spores suggested that outgrowth and colony-forming efficiency of these spores might be lower than that of wild-type spores, since only a minority of the mutant spores became phase dark upon germination with BHI broth. As expected, wild-type (3.8 × 107 CFU/ml/OD600) and gerQ (3.3 × 107 CFU/ml/OD600) spores exhibited similar colony-forming efficiencies. However, the colony-forming efficiency of gerO (2.0 × 107 CFU/ml/OD600) and gerO gerQ (2.1 × 107 CFU/ml/OD600) spores was significantly (P < 0.001) lower than that of wild-type spores, in agreement with results on spore germination in BHI broth by phase-contrast microscopy. The rate of increase in the OD600 of wild-type spores incubated in TGY medium was also significantly faster than from all of the mutant spores (Fig. 6), and this was also the case when fewer wild-type spores were used to compensate for the decreased viability of the gerO and gerO gerQ spores (data not shown). However, the gerO, gerQ, and gerO gerQ strains grew vegetatively like the wild-type strain in TGY medium (data not shown). These results suggest that GerO and GerQ may also play roles in spore outgrowth and perhaps early growth of nascent vegetative cells released from germinated spores.
FIG. 6.
Outgrowth of spores of C. perfringens strains. Heat activated spores of strains SM101 (wild-type) (□), DPS113 (gerQ) (⧫), DPS116 (gerO) (▴), and DPS115 (gerO gerQ) (▪) were incubated anaerobically in TGY vegetative medium at 37°C, and the OD600 was monitored as described in Materials and Methods.
Effect of D161N and D162N mutations on GerO function.
The results described above indicated that GerO (i) has cation transport activity and (ii) plays a significant role in C. perfringens spore germination. In order to potentially provide a direct connection between these two observations, we sought to generate a site-directed gerO mutant that might be defective in cation transport. A cluster of amino acids (Asp164, Asp163, Asp133, and Thr132) that forms the putative Na+-binding site in NhaA (8) is highly conserved among members of the NhaA protein family (17). Asp163 and Asp164 are essential for cation translocation by E. coli and Helicobacter pylori NhaA, and replacement of these Asp residues with Asn residues in E. coli NhaA leads to cells unable to grow in LBK broth with 0.6 M NaCl (9, 11). The alignment of amino acid sequences of GerO and GerQ with various other bacterial cation antiporters, as well as a homology structural model (see Fig. S1 and S2 in the supplemental material) of GerO suggested that Asp161 and Asp162 in GerO might be involved in cation-translocation and thus important in germination of C. perfringens spores. To test this hypothesis, we changed these Asp residues to Asn residues, and gerO(D161N, D162N) was expressed in E. coli strains defective in monovalent cation transport. Surprisingly, GerO(D161N, D162N) complemented the K+ uptake defect of E. coli strain TK2420, as well as wild-type GerO (Fig. 2A). In addition, Na+-sensitive E. coli KNabc cells expressing gerO(D161N, D162N) exhibited similar growth to that of this E. coli strain expressing wild-type gerO in LBK medium containing 0.1 M NaCl or less (Fig. 2B) but were unable to grow in LBK medium with 0.15 M NaCl (Fig. 2B). These results indicate that Asp161 and Asp162 are not essential for K+ uptake by C. perfringens GerO but are required at least in part for Na+ translocation.
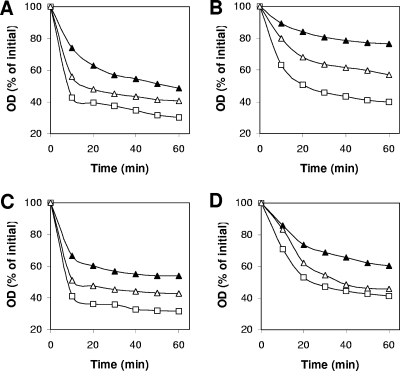
The gerO(D161N, D162N) gene was also expressed in C. perfringens DPS116 (gerO) to evaluate the importance of Asp161 and Asp162 in C. perfringens spore germination. As noted above, gerO spores germinated slower and to a lesser extent than wild-type spores with BHI broth, l-asparagine, KCl, or Ca-DPA (Fig. 7). However, spores of strain DPS116 [gerO(D161N, D162N)] germinated faster and to a higher extent than gerO spores but significantly slower and to a lesser extent than wild-type spores (Fig. 7A to D). Determination of maximum rates of spore germination (Table 1) revealed that while spores of strain DPS116 [ΔgerO gerO(D161N, D162N)] exhibited higher maximum germination rates than gerO and gerO gerQ spores, these rates were significantly lower than those of wild-type and gerQ spores (Table 1).
FIG. 7.
Effects of D161N and D162N substitutions on GerO function during C. perfringens spore germination. Heat-activated spores of strains SM101 (wild-type) (□), DPS116 (gerO) (▴), and DPS116(pDP172) [gerO mutant complemented with gerO(D161N, D162N)] (▵) were germinated at 40°C with 100 mM KCl-25 mM sodium phosphate (pH 7.4) (A), 100 mM l-asparagine-25 mM sodium phosphate (pH 7.4) (B), AK-25 mM sodium phosphate (pH 7.4) (C), and 50 mM Ca-DPA made to pH 8.0 with Tris base (D), and the OD600 was measured as described in Materials and Methods.
DISCUSSION
This findings presented here lead to a number of new conclusions, including a few less notable or minor conclusions and several more notable ones. Four minor conclusions include the following. (i) Although both gerO and gerQ were expressed only in C. perfringens sporulation, gerQ expression began ∼1 h later than that of gerO. This suggests that the regulation of the expression of these two genes during sporulation is not identical and that, while transcription of gerQ might be under the control of the RNA polymerase sigma factor, SigK, transcription of gerO might be under the control of a different RNA polymerase sigma factor and/or that DNA-binding proteins are involved in gerO and gerQ transcription. (ii) GerO and GerQ appear to play no role in C. perfringens spore germination by dodecylamine, although they are involved to at least some degree in spore germination by BHI broth, KCl, l-asparagine, and Ca-DPA. This is similar to the effects of the only enzyme, SleC, essential for cortex hydrolysis during C. perfringens spore germination (22). Unfortunately, the mechanism of spore germination with dodecylamine is not known, although it is possible that this cationic surfactant somehow opens a new or preexisting DPA channel in the spore's inner membrane (20-23, 30). In contrast, the other germinants noted above trigger C. perfringens spore germination by activation of at least the spore's GerK receptor (21, 23), and perhaps such different germination mechanisms have different requirements for cation transport proteins. Indeed, with B. cereus spores, inosine germination requires the antiporter GerN, whereas l-alanine germination does not (35). (iii) GerO has two conserved adjacent Asp residues thought to be important at least in Na+ transport by this class of proteins (9, 11). Conversion of these Asp residues in GerO to Asn residues greatly reduced the ability of ectopic expression of the gerO gene variant's ability to complement the germination defects of spores lacking a normal gerO gene. This result suggests that GerO exerts its effects in spore germination through its ability to transport cations. However, the change of the Asp residues to Asn residues had little to no effect on cation transport by GerO in E. coli. The reason for the apparent contradiction between the results in E. coli and C. perfringens is not clear, but there are many unknowns in these experiments, in particular the effects of the double mutation on protein stability and, indeed, whether these Asp residues are even involved directly in cation transport by these proteins. (iv) The final minor conclusion is that GerQ and GerO play roles not only in C. perfringens spore germination but also in spore outgrowth. It is not clear whether the role in spore outgrowth is a direct or indirect one, since it is not clear that either of these proteins is actually present in dormant spores (see below). However, a requirement for the likely cation transporter, GerT, in spore outgrowth has been observed with B. cereus spores (29). It is also known that germinating and outgrowing spores of at least Bacillus species are resistant to very high salt concentrations and are much more resistant than are vegetative cells (36). Perhaps GerO and/or GerQ play some role in this resistance by transporting appropriate cations in spore outgrowth. It is also possible that GerO and GerQ are actually synthesized during spore outgrowth, perhaps in response to salt stress, although this has not been studied.
In addition to the minor conclusions noted above, there are three more notable conclusions. (i) The C. perfringens GerO and GerQ proteins exhibit sequence and predicted structural homology to monovalent cation antiporters, including proteins that appear to be involved in germination of spores of at least some Bacillus species and with at least some germinants. (ii) GerO clearly can function in the transport of K+ and, to a lesser extent, the transport of Na+ in E. coli, and GerQ appears to have at least weak Na+ transport activity in E. coli. (iii) Finally, the loss of GerO and to a lesser extent of GerQ results in defects in C. perfringens spore germination. These results suggesting roles for one or more cation transport proteins in C. perfringens spore germination are similar to those suggested for spores of some Bacillus species, although not with all strains and/or species and not with all germinants (3, 29, 34, 35). The major questions provoked by these observations are how GerO and, likely to a lesser degree, GerQ function in spore germination and/or outgrowth and whether the effects of these proteins are direct or indirect.
The loss of GerO and/or GerQ has no obvious effects on C. perfringens growth or sporulation. Thus, the sporulation-specific expression of gerO and gerQ suggests that GerO and GerQ might be spore-specific proteins. There is also evidence suggesting that the spore germination-associated likely monovalent cation antiporters GerN and GerT of B. cereus are encoded by sporulation-specific genes (12, 29). While the latter results suggest that all of these cation transport proteins are present in spores, gerO and gerQ appear to be expressed only in the mother cell compartment of sporulating C. perfringens cells, although this analysis has not been carried out with gerN and gerT. Synthesis of GerO and GerQ only in the mother cell compartment suggests that these proteins are most likely not present in the spore's inner membrane, since at least in spores of Bacillus species and probably C. perfringens spores as well, spore-specific inner membrane proteins such as germinant receptors and SpoVA proteins are synthesized in the developing forespore (19-21, 23, 31, 37). The likely absence of GerO and GerQ from the C. perfringens spore's inner membrane leaves the spore's outer membrane as the only likely spore-specific location for these proteins (although it is also possible that these proteins are located only in the mother cell's plasma membrane). An outer membrane location for GerO and GerQ further means that these proteins could not be involved directly in cation transport across the spore's inner membrane in spore germination and also makes it unlikely that these proteins could interact with the spore's germinant receptors. In addition, a decoating treatment applied to C. perfringens spores that likely removes much outer membrane protein (although this has not been shown directly) does not abolish spores' ability to release DPA in response to the activation of germinant receptors by germinants, although cortex hydrolysis by the decoated spores is abolished due to extraction of SleC (15, 20). These findings suggest, although by no means prove, that the effects of GerO and GerQ on C. perfringens spore germination and outgrowth are not exerted directly during spore germination or outgrowth but rather during spore formation in some way.
Supplementary Material
Acknowledgments
This study was supported by a grant from the N. L. Tartar Foundation of Oregon State University, by a grant from the Agricultural Research Foundation of Oregon State University (to M.R.S.), by a grant from the Army Research Office (to M.R.S. and P.S.), by NIH grant GM19698 (to P.S.), and by a fellowship from MIDEPLAN (Chile) to D.P.-S.
We thank Terry Krulwich, Mount Sinai School of Medicine, New York, NY, for providing E. coli strains TK2420 and KNabc.
Footnotes
Published ahead of print on 10 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Cabrera-Martinez, R. M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 1852457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, J., A. A. Guffanti, and T. A. Krulwich. 1994. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J. Biol. Chem. 26927365-27371. [PubMed] [Google Scholar]
- 3.Christie, G., and C. R. Lowe. 2007. Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J. Bacteriol. 1894375-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeczulin, J. R., R. E. Collie, and B. A. McClane. 1996. Regulated expression of Clostridium perfringens enterotoxin in naturally cpe-negative type A, B, and C isolates of C. perfringens. Infect. Immun. 643301-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan, C. L., and D. H. Strong. 1968. Improved medium for sporulation of Clostridium perfringens. Appl. Microbiol. 1682-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guffanti, A. A., J. Cheng, and T. A. Krulwich. 1998. Electrogenic antiport activities of the gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J. Biol. Chem. 27326447-26454. [DOI] [PubMed] [Google Scholar]
- 7.Huang, I. H., M. Waters, R. R. Grau, and M. R. Sarker. 2004. Disruption of the gene (spo0A) encoding sporulation transcription factor blocks endospore formation and enterotoxin production in enterotoxigenic Clostridium perfringens type A. FEMS Microbiol. Lett. 233233-240. [DOI] [PubMed] [Google Scholar]
- 8.Hunte, C., E. Screpanti, M. Venturi, A. Rimon, E. Padan, and H. Michel. 2005. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 4351197-1202. [DOI] [PubMed] [Google Scholar]
- 9.Inoue, H., T. Noumi, T. Tsuchiya, and H. Kanazawa. 1995. Essential aspartic acid residues, Asp-133, Asp-163 and Asp-164, in the transmembrane helices of a Na+/H+ antiporter (NhaA) from Escherichia coli. FEBS Lett. 363264-268. [DOI] [PubMed] [Google Scholar]
- 10.Kokai-Kun, J. F., J. G. Songer, J. R. Czeczulin, F. Chen, and B. A. McClane. 1994. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J. Clin. Microbiol. 322533-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwabara, N., H. Inoue, Y. Tsuboi, K. Mitsui, M. Matsushita, and H. Kanazawa. 2006. Structure-function relationship of the fifth transmembrane domain in the Na+/H+ antiporter of Helicobacter pylori: topology and function of the residues, including two consecutive essential aspartate residues. Biochemistry 4514834-14842. [DOI] [PubMed] [Google Scholar]
- 12.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. A. Rasko, J. Ravel, T. D. Read, S. N. Peterson, J. Yates III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClane, B. A. 2007. Clostridium perfringens, p. 423-444. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 14.McDonnell, J. L. 1986. Toxins of Clostridium perfringens type A, B, C, D, and E, p. 477-517. In F. Dorner and J. Drews (ed.), Pharmacology of bacterial toxins. Pergamon Press, Oxford, England.
- 15.Miyata, S., S. Kozuka, Y. Yasuda, Y. Chen, R. Moriyama, K. Tochikubo, and S. Makino. 1997. Localization of germination-specific spore-lytic enzymes in Clostridium perfringens S40 spores detected by immunoelectron microscopy. FEMS Microbiol. Lett. 152243-247. [DOI] [PubMed] [Google Scholar]
- 16.Myers, G. S., D. A. Rasko, J. K. Cheung, J. Ravel, R. Seshadri, R. T. DeBoy, Q. Ren, J. Varga, M. M. Awad, L. M. Brinkac, S. C. Daugherty, D. H. Haft, R. J. Dodson, R. Madupu, W. C. Nelson, M. J. Rosovitz, S. A. Sullivan, H. Khouri, G. I. Dimitrov, K. L. Watkins, S. Mulligan, J. Benton, D. Radune, D. J. Fisher, H. S. Atkins, T. Hiscox, B. H. Jost, S. J. Billington, J. G. Songer, B. A. McClane, R. W. Titball, J. I. Rood, S. B. Melville, and I. T. Paulsen. 2006. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 161031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padan, E. 2008. The enlightening encounter between structure and function in the NhaA Na+-H+ antiporter. Trends Biochem. Sci. 33435-443. [DOI] [PubMed] [Google Scholar]
- 18.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 1834886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 1833982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paredes-Sabja, D., B. Setlow, P. Setlow, and M. R. Sarker. 2008. Characterization of Clostridium perfringens spores that lack SpoVA proteins and dipicolinic acid. J. Bacteriol. 1904648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paredes-Sabja, D., J. A. Torres, P. Setlow, and M. R. Sarker. 2008. Clostridium perfringens spore germination: characterization of germinants and their receptors. J. Bacteriol. 1901190-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paredes-Sabja, D., P. Setlow, and M. R. Sarker. 2009. SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. J. Bacteriol. 1912711-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paredes-Sabja, D., P. Setlow, and M. R. Sarker. 2009. Role of GerKB in germination and outgrowth of Clostridium perfringens spores. Appl. Environ. Microbiol., 753813-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popham, D. L., S. Sengupta, and P. Setlow. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl. Environ. Microbiol. 613633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju, D., M. Waters, P. Setlow, and M. R. Sarker. 2006. Investigating the role of small, acid-soluble spore proteins (SASPs) in the resistance of Clostridium perfringens spores to heat. BMC Microbiol. 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotman, Y., and M. L. Fields. 1967. A modified reagent for dipicolinic acid analysis. Anal. Biochem. 22168. [DOI] [PubMed] [Google Scholar]
- 27.Saier, M. H., Jr., B. H. Eng, S. Fard, J. Garg, D. A. Haggerty, W. J. Hutchinson, D. L. Jack, E. C. Lai, H. J. Liu, D. P. Nusinew, A. M. Omar, S. S. Pao, I. T. Paulsen, J. A. Quan, M. Sliwinski, T. T. Tseng, S. Wachi, and G. B. Young. 1999. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta 14221-56. [DOI] [PubMed] [Google Scholar]
- 28.Sarker, M. R., R. J. Carman, and B. A. McClane. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33946-958. [DOI] [PubMed] [Google Scholar]
- 29.Senior, A., and A. Moir. 2008. The Bacillus cereus GerN and GerT protein homologs have distinct roles, in spore germination and outgrowth, respectively. J. Bacteriol. 1906148-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95637-648. [DOI] [PubMed] [Google Scholar]
- 31.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 32.Southworth, T. W., A. A. Guffanti, A. Moir, and T. A. Krulwich. 2001. GerN, an endospore germination protein of Bacillus cereus, is an Na+/H+-K+ antiporter. J. Bacteriol. 1835896-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J. Bacteriol. 14820-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tani, K., T. Watanabe, H. Matsuda, M. Nasu, and M. Kondo. 1996. Cloning and sequencing of the spore germination gene of Bacillus megaterium ATCC 12872: similarities to the NaH-antiporter gene of Enterococcus hirae. Microbiol. Immunol. 4099-105. [DOI] [PubMed] [Google Scholar]
- 35.Thackray, P. D., J. Behravan, T. W. Southworth, and A. Moir. 2001. GerN, an antiporter homologue important in germination of Bacillus cereus endospores. J. Bacteriol. 183476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tovar-Rojo, F., R. M. Cabrera-Martinez, B. Setlow, and P. Setlow. 2003. Studies on the mechanism of the osmoresistance of spores of Bacillus subtilis. J. Appl. Microbiol. 95167-179. [DOI] [PubMed] [Google Scholar]
- 37.Vepachedu, V. R., and P. Setlow. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 1875677-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao, Y., and S. B. Melville. 1998. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J. Bacteriol. 180136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.