Abstract
There have been considerable strides made in the characterization of the dispensability of teichoic acid biosynthesis genes in recent years. A notable omission thus far has been an early gene in teichoic acid synthesis encoding the N-acetylmannosamine transferase (tagA in Bacillus subtilis; tarA in Staphylococcus aureus), which adds N-acetylmannosamine to complete the synthesis of undecaprenol pyrophosphate-linked disaccharide. Here, we show that the N-acetylmannosamine transferases are dispensable for growth in vitro, making this biosynthetic enzyme the last dispensable gene in the pathway, suggesting that tagA (or tarA) encodes the first committed step in wall teichoic acid synthesis.
The cell wall of gram-positive bacteria is composed of not only peptidoglycan, but also a significant proportion of the polyol phosphate polymer known as teichoic acid. Wall teichoic acid has long been held as an essential component of the cell wall architecture (2-5, 19). However, recently, our group has demonstrated a complex pattern of dispensability for wall teichoic acid biosynthetic genes of both Bacillus subtilis and Staphylococcus aureus (9, 10).
The synthesis of wall teichoic acid polymers occurs through the sequential action of several enzymes (14, 17). The action of no less than seven enzymes is thought to synthesize the completed polymer on the cytoplasmic face of the cell membrane for export to the outside of the cell. Once outside, the completed polymer is covalently attached to the C-6 of the N-acetylmuramic acid of peptidoglycan through the action of an uncharacterized transferase. The best-characterized wall teichoic acid biosynthetic machinery is that for polymers composed of glycerol phosphate and ribitol phosphate. In the last several years, biochemical experiments have characterized the activities of nearly all of the enzymes responsible for the synthesis of both glycerol phosphate and ribitol phosphate polymers (6, 11, 18).
Work on the essential nature of wall teichoic acid dates back many years to the discovery and characterization of temperature-sensitive B. subtilis tag mutants for poly(glycerol phosphate) synthesis by D. Karamata's lab (4, 5, 19). That work and follow-up studies by our research group (2, 3, 20) showed convincingly that genetic lesions in several wall teichoic acid biosynthetic steps led to cell death in vitro. Recently, however, we uncovered some remarkable complexity in the dispensability pattern of wall teichoic acid synthetic genes. Working with both B. subtilis and S. aureus, we showed that viable deletions could be generated in the first gene of the pathway, encoding the N-acetylglucosamine-1-phosphate transferase (tagO in B. subtilis; tarO in S. aureus), while deletions could not be made for late-acting genes, including those encoding the glycerol phosphate primase (tagB in B. subtilis; tarB in S. aureus) and downstream enzymes. This apparent paradox was resolved when it was discovered that all of the indispensable genes became dispensable in a tagO (or tarO) deletion background and suggested that lesions in late steps of wall teichoic acid synthesis lead to a premature termination of the pathway, causing a buildup of toxic intermediates or the sequestration of a common and vital precursor molecule (i.e., undecaprenol phosphate).
While extensive investigations have charted the complex genetics of wall teichoic acid synthesis in both B. subtilis 168 (2-5, 9, 15, 16, 19, 21) and S. aureus (10, 23), no experiments have so far been reported to characterize the dispensability phenotype of the N-acetylmannosamine transferase encoded by tagA (B. subtilis) and tarA (S. aureus). Indeed, tagA from B. subtilis was recently shown to catalyze the addition of N-acetylmannosamine to complete the synthesis of undecaprenol pyrophosphate-linked disaccharide, a core component of the “linkage unit” of wall teichoic acid (6, 11, 25). This places TagA (TarA) as an enzyme catalyzing the second step in wall teichoic biosynthesis after TagO (TarO), the N-acetylglucosamine-1-phosphate transferase. Given the dispensable phenotype of tagO (tarO) and the capacity of this deletion for suppression of downstream, essential, late-acting genes, we were motivated to explore the dispensability phenotype of this as-yet-unexplored step of wall teichoic acid synthesis. Here, we analyzed the dispensability of the N-acetylmannosamine transferase genes of both B. subtilis and S. aureus (tagA and tarA, respectively) for growth in vitro.
Gene tarA from S. aureus COL was identified as SACOL0693, using BLAST analysis. Dispensability testing of tarA was done in S. aureus strain SA178RI, using an allelic replacement system developed by us (pSAKO) and described previously (10). Using this methodology (see the supplemental material for detailed methods), we demonstrated that in a wild-type background, S. aureus tarA could be readily replaced with an erythromycin resistance cassette, allowing for mutant generation at a high frequency (Table 1). Thus, our data reveal that this locus is dispensable for growth in vitro. In B. subtilis, we were likewise able to replace the tagA gene with a spectinomycin resistance cassette after the generation and transformation of a PCR product containing the flanking regions of tagA surrounding the resistance cassette. To confirm that the deletion of tagA was not the result of a suppressor mutation elsewhere in the chromosome, we performed an analysis of congression to compare the efficiency of recombination of the Spec resistance determinant (replacing tagA) into wild-type B. subtilis to that of the Erm resistance determinant (replacing tagO). We also compared these with that of a control Chl resistance cassette at the amy locus. The frequencies of recombination for all of these experiments were very similar (data not shown). These findings indicated that the loss of tagA was not the result of a concomitant suppressor mutation. The resulting colonies (ΔtagA) were small, smooth, and very similar in morphology to the tagO mutant that we have described previously (9).
TABLE 1.
Allelic replacement for testing gene dispensability in S. aureus
| Strain | No. of colonies with indicated phenotype
|
||
|---|---|---|---|
| Wild type | Nonexcisant | Mutant | |
| No complementation | |||
| tarA mutant | 65 | 1 | 34 |
| ΔtarA background | |||
| tarB mutant | 75 | 0 | 15 |
| tarF mutant | 50 | 2 | 48 |
| tarIJ mutant | 88 | 0 | 12 |
To confirm that these strains were devoid of teichoic acid polymers, the cell wall phosphate contents for B. subtilis (S. aureus) wild-type, tagO (tarO), and tagA (tarA) null strains were analyzed (Table 2). These results revealed that the cell wall phosphate content of tagA and tarA null strains were approximately 10% that of the wild type and comparable to those found in the cell walls of the tagO and tarO null strains. The generation of tagA (tarA) mutants in conjunction with a significant loss of cell wall phosphate content was consistent with the conclusion that B. subtilis tagA and S. aureus tarA mutants were devoid of wall teichoic acid. While the presence of residual phosphate in this mutant was noteworthy, it was not surprising. Previous analyses by our group and another group have revealed residual phosphate in a tagO mutant (9, 21). We speculate that this phosphate might originate from minor teichoic acid species or other phosphate-containing cellular components.
TABLE 2.
Phosphate content of cell wall isolated from B. subtilis and S. aureus
| Strain | Phosphate content (μmol phosphate/mg cell wall) |
|---|---|
| Bacillus subtilis | |
| Wild type | 1.6 ± 0.4 |
| ΔtarO mutant | 0.09 ± 0.02 |
| ΔtarA mutant | 0.10 ± 0.03 |
| Staphylococcus aureus | |
| Wild type | 1.2 ± 0.1 |
| ΔtarO mutant | 0.140 ± 0.003 |
| ΔtarA mutant | 0.140 ± 0.005 |
As stated above, our group has previously been able to demonstrate, using both B. subtilis tagO and S. aureus tarO, that these deletions were able to suppress the lethality associated with deletion of late-acting gene products (9, 10). Having succeeded in making strains of B. subtilis and S. aureus that lacked the N-acetylmannosamine transferase gene and wall teichoic acid, we were interested in testing for genetic interactions with the late-acting genes in the pathway. Previously, we were able to leverage the capacity of allelic replacement with pSAKO to test the dispensability of late-acting teichoic genes in the presence and absence of a tarO deletion (10). We reasoned that the dispensable phenotype of tarA should provide for a dispensable phenotype of the downstream genes tarB, tarF, and tarIJ just as we have seen for tarO. From Table 1, it is clear that in the absence of tarA, the otherwise essential genes tarB, tarF, and tarIJ become dispensable. These data demonstrate that tarA has the same peculiar genetic interactions previously observed with tarO.
With this work we have established that tagA and tarA are dispensable for in vitro growth in both B. subtilis and S. aureus strains, respectively. Phenotypic characterization of these mutants indicated that the strains were devoid of wall teichoic acid. Furthermore we have shown that the deletion of tarA in S. aureus is able to suppress the essential phenotypes of several late-acting wall teichoic acid synthesis genes. These findings reveal that tagA and tarA are the last dispensable genes in their respective biosynthetic pathways and suggest that the N-acetylmannosamine transferase commits the cell to synthesizing wall teichoic acid. This would mean that TagO (TarO) catalyzes a reversible biosynthetic step. Indeed, the reversibility of enzymes homologous and analogous to TagO (TarO) has been well established (1, 7, 13, 22). With TagO (TarO) catalyzing a reversible step, the reaction controlled by TagA (TarA) represents the first committed step in wall teichoic acid synthesis. Having committed to teichoic acid biosynthesis, the cell must complete polymer assembly to avoid the lethal consequences of blocks in the later steps of this pathway.
To further evaluate the phenotype of the deletion of the N-acetylmannosamine transferase gene in both B. subtilis and S. aureus, growth analysis and transmission electron microscopy were performed. The growth characteristics of the B. subtilis tagA and S. aureus tarA deletion strains with respect to those of the wild-type strains are very different (Fig. 1). Figure 1A shows the growth kinetics of the B. subtilis tagA null strain (EB1494) compared to those of the wild-type (EB6) and tagO deletion (EB1451) strains. The data reveal that the mutant is significantly impaired for growth compared to the wild-type strain, with a growth rate comparable to that of the tagO mutant previously described (9). For S. aureus, the tarA deletion strain grew similarly to both the wild type and the tarO deletion strain (Fig. 1B).
FIG. 1.
Growth kinetics of B. subtilis and S. aureus deletion mutants. (A) Growth curves are depicted for the B. subtilis tagA deletion strain (EB1494 [○]). Growth data for the wild-type (EB6 [▪]) and tagO deletion (EB1451 [•]) strains (9) are shown for comparison. (B) Growth curves are shown for the S. aureus wild-type (SA178RI [▪]), tarO null (EBII44 [•]), and tarA null (EBII58 [○]) strains. All cultures were inoculated to a starting optical density value at 600 nm (OD600) of 0.005, and absorbance measurements were taken every 1 to 2 h.
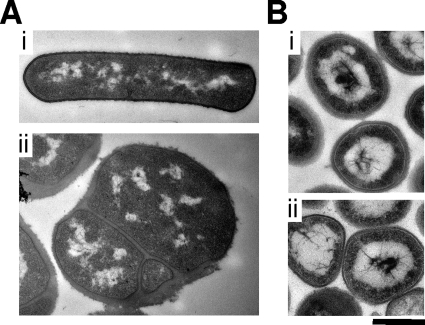
The differences shown in the growth curves were paralleled in the transmission electron micrographs shown in Fig. 2. While the S. aureus tarA mutant did not have any significant morphological defects, the B. subtilis tagA deletion mutant showed abnormalities that were very similar to those seen previously for the tagO deletion mutant (9). These gross morphological defects included loss of its rod shape, aberrant septation, and asymmetrical peptidoglycan architecture. Thus, the loss of wall teichoic acid had a much more profound effect on B. subtilis than it did on S. aureus. Further characterization of the tagA and tarA mutants revealed that N-acetylmannosamine transferase deficiency had dramatically different impacts on the growth and morphology of B. subtilis and S. aureus. Deletion of tagA in B. subtilis resulted in a remarkable impact on ultrastructure including complete loss of rod shape, abberant septation and cell wall asymmetry. These observations were reminiscent of that seen for the B. subtilis tagO deletion described previously (9). In contrast, the S. aureus tarA deletion mutant had growth and ultrastructural characteristics that were not unlike those of the wild type. The gross morphological and growth defect differences between the two organisms is not understood as of yet. We predict that these differences result from the shape of the organism; in particular, there are significantly more-profound effects on B. subtilis, given its rod structure, while the coccoid shape associated with S. aureus resists such defects. Although not yet demonstrated, an interesting hypothesis may be the alternate manners in which these two organisms grow and build their cell walls (8). S. aureus growth occurs only at the septum, while B. subtilis growth occurs both at the septum and along the cell cylinder. It is interesting to speculate that teichoic acid biogenesis plays a role in only the cylinder growth or bacteria, indicating why defects are seen only with the rod-shaped B. subtilis.
FIG. 2.
Ultrastructure of B. subtilis tagA and S. aureus tarA null mutants. Bacteria were harvested at late log phase of growth and embedded in thin sections for examination with transmission electron microscopy as described in the supplemental material. Panel A shows micrographs of (i) the B. subtilis wild type (EB6) and (ii) the tagA null strain (EB1494). Panel B depicts micrographs of (i) the S. aureus wild-type (SA178RI) and (ii) the S. aureus tarA null strain (EBII58). The bar represents 500 nm.
Given the similar phenotypes of the tagO (tarO) and tagA (tarA) deletion strains, we broadened our search for phenotypes in this work to include antibiotic susceptibility. We restricted our investigations for these studies to S. aureus because of the robust growth of the tarO and tarA deletion strains of this organism. MIC determinations to a variety of antimicrobials are largely unchanged relative to those of the wild type. Among the 20 antibiotics tested of various chemical classes and mechanisms (see the supplemental material), tarO and tarA deletion strains showed increased susceptibility (>2-fold compared to the wild types) only to fusidic acid and phosphomycin, 8- and 16-fold reductions in MIC, respectively (data not shown). Interestingly, these two compounds are negatively charged, as are teichoic acid polymers. We posit that the increased susceptibility was due to improved delivery of these compounds to their intracellular targets. Therefore, with the exception of a potential influence of negatively charged molecules, the loss of teichoic acid polymers in the cell wall does not significantly alter the drug susceptibility of S. aureus.
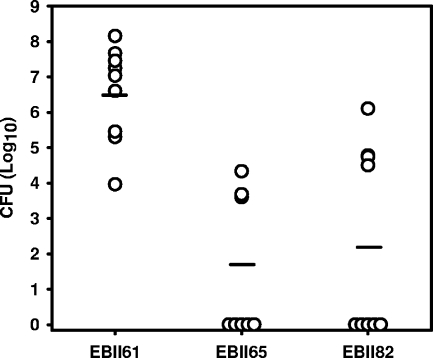
Having shown that B. subtilis tagA and S. aureus tarA deletion mutants were viable and analogous in many respects to the tagO and tarO mutants characterized previously, we were interested in comparing the in vivo phenotypes of the tarO and tarA mutants. Weidenmaier et al. previously showed that the tarO null mutant was compromised for colonization in rat nasopharyngeal and rabbit endocarditis models (23, 24). Here, we tested the hypothesis that the tarA deletion would similarly impair the colonization of S. aureus in a mouse kidney abscess model. Figure 3 charts the colony counts recovered from mouse kidneys 5 days after infection with a wild-type S. aureus Newman strain (EBII61) as well as the tarO and tarA null mutants in the Newman background (EBII65 and EBII82, respectively). At the time of sacrifice, mice infected with the wild-type Newman strain (EBII61) had high bacterial cell numbers in their kidneys (average of 106.5 ± 101.3 CFU). In stark contrast, we were unable to recover viable bacteria from most of the mice infected with either the tarO or tarA mutant strains, while some mice had low but detectable bacterial loads. The average cell number recovered from mice infected with the tarO (EBII65) and tarA (EBII82) null strains was 101.7 ± 102.0 and 102.2 ± 102.7 CFU, respectively. Generally, mice infected with the mutant strains were significantly more healthy than those infected with wild-type bacteria. Clinical scoring through examination of the overall fitness of the mice showed that the ΔtarO mutant had an average score of 0.66 ± 1, the ΔtarA mutant had an average score of 0 ± 0, and the wild-type strain had a significantly higher score of 3.1 ± 1.2. As a further measure of health, we observed that mice infected with mutant strains lost, on average, significantly less weight than mice infected with the wild-type Newman strain, as follows: 21 ± 6% (wild-type Newman strain), 0.6 ± 3.4% (tarO null strain), and 1.4 ± 4.3% (tarA null strain).
FIG. 3.
Teichoic acid mutants are impaired for growth in vivo. The graph shows the CFU recovered from the homogenized kidneys of mice infected with the S. aureus wild-type Newman strain (EBII61) and corresponding tarO (EBII65) and tarA (EBII81) deletion mutants. In these experiments, mice were injected in the tail vein with 107 bacteria, and CFU were determined 5 days postinfection.
Here, we found that the tarO and tarA mutants were compromised similarly to the wild type in a mouse kidney abscess model of infection. It has been well established that teichoic acid polymers play a significant role in the adherence of bacteria, likely the result of the charge associated with the polymer (12, 24). The failure of the tarA null mutant to colonize and persist in the mouse model here provides additional support for the importance of wall teichoic acid to infection and draws further parallels with the tarO mutant in terms of phenotype.
In conclusion, our findings reveal that B. subtilis tagA and S. aureus tarA are dispensable in their respective biosynthetic pathways. Indeed, the encoded N-acetylmannosamine transferases should be considered the first committed step in wall teichoic acid polymer production. In this particular pathway, commitment to wall teichoic acid synthesis marks an obligation to complete polymer assembly and export. The consequence of failing to do so in these organisms is cell death. Thus, despite the dispensability of the polymer for in vitro growth, wall teichoic acid biosynthesis represents an exploitable target for new antibiotic development. Interestingly, results shown here and elsewhere (9, 10) predict that the suppression of lethal phenotypes associated with blocks in late steps of wall teichoic acid synthesis could be accomplished with mutations in the first steps, namely tagO (tarO) and/or tagA (tarA). Nevertheless, the requirement of wall teichoic acid for virulence in various animal models suggests that such suppressor mutations would lead to noninfectious strains. We maintain therefore that wall teichoic acid synthesis may well be an ideal target for new antibacterial drug discovery.
Supplementary Material
Acknowledgments
We thank Bob Harris of the University of Guelph for his technical assistance in preparing samples for electron microscopy.
Microscopy was performed in the NSERC Guelph Regional Integrated Imaging Facility (GRIIF), which is partially funded by an NSERC Major Facility Access granted to T.J.B. This work was supported, in part, by a Canadian Institutes of Health Research (CIHR) operating grant (MOP-15496). M.A.D. is a recipient of a CIHR Canadian Graduate Scholarship. E.D.B. and T.J.B. acknowledge support from the Canada Research Chair program.
We would like to dedicate this work to Terry J. Beveridge, an outstanding scientist and excellent colleague, who passed away during the preparation of the manuscript.
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Barr, K., and P. D. Rick. 1987. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J. Biol. Chem. 2627142-7150. [PubMed] [Google Scholar]
- 2.Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol-3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 1836688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhavsar, A. P., L. K. Erdman, J. W. Schertzer, and E. D. Brown. 2004. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J. Bacteriol. 1867865-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, C., and D. Karamata. 1987. Thermosensitive Bacillus subtilis mutants which lyse at the non-permissive temperature. J. Gen. Microbiol. 1331159-1170. [DOI] [PubMed] [Google Scholar]
- 5.Briehl, M., H. M. Pooley, and D. Karamata. 1989. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid synthesis. J. Gen. Microbiol. 1351325-1334. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S., Y. H. Zhang, and S. Walker. 2008. A revised pathway proposed for Staphylococcus aureus wall teichoic acid biosynthesis based on in vitro reconstitution of the intracellular steps. Chem. Biol. 1512-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartee, R. T., W. T. Forsee, M. H. Bender, K. D. Ambrose, and J. Yother. 2005. CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation. J. Bacteriol. 1877425-7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113767-776. [DOI] [PubMed] [Google Scholar]
- 9.D'Elia, M. A., K. E. Millar, T. J. Beveridge, and E. D. Brown. 2006. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol. 1888313-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Elia, M. A., M. P. Pereira, Y. S. Chung, W. Zhao, A. Chau, T. J. Kenney, M. C. Sulavik, T. A. Black, and E. D. Brown. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 1884183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg, C., Y. H. Zhang, Y. Yuan, and S. Walker. 2006. In vitro reconstitution of two essential steps in wall teichoic acid biosynthesis. ACS Chem. Biol. 125-28. [DOI] [PubMed] [Google Scholar]
- 12.Gross, M., S. E. Cramton, F. Gotz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 693423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. G., and D. B. Wilson. 1977. Role of a sugar-lipid intermediate in colanic acid synthesis by Escherichia coli. J. Bacteriol. 129225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarevic, V., F. X. Abellan, S. B. Moller, D. Karamata, and C. Mauel. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148815-824. [DOI] [PubMed] [Google Scholar]
- 15.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16345-355. [DOI] [PubMed] [Google Scholar]
- 16.Mauël, C., M. Young, P. Margot, and D. Karamata. 1989. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol. Gen. Genet. 215388-394. [DOI] [PubMed] [Google Scholar]
- 17.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira, M. P., J. W. Schertzer, M. A. D'Elia, K. P. Koteva, D. W. Hughes, G. D. Wright, and E. D. Brown. 2008. The wall teichoic acid polymerase TagF efficiently synthesizes poly(glycerol phosphate) on the TagB product lipid III. Chembiochem 91385-1390. [DOI] [PubMed] [Google Scholar]
- 19.Pooley, H. M., F. X. Abellan, and D. Karamata. 1991. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J. Gen. Microbiol. 137921-928. [DOI] [PubMed] [Google Scholar]
- 20.Schertzer, J. W., and E. D. Brown. 2003. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J. Biol. Chem. 27818002-18007. [DOI] [PubMed] [Google Scholar]
- 21.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 1482079-2087. [DOI] [PubMed] [Google Scholar]
- 22.Troy, F. A., F. E. Frerman, and E. C. Heath. 1971. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J. Biol. Chem. 246118-133. [PubMed] [Google Scholar]
- 23.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10243-245. [DOI] [PubMed] [Google Scholar]
- 24.Weidenmaier, C., A. Peschel, Y. Q. Xiong, S. A. Kristian, K. Dietz, M. R. Yeaman, and A. S. Bayer. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 1911771-1777. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Y. H., C. Ginsberg, Y. Yuan, and S. Walker. 2006. Acceptor substrate selectivity and kinetic mechanism of Bacillus subtilis TagA. Biochemistry 4510895-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.