Abstract
Pathogenic bacteria have evolved numerous mechanisms to evade the human immune system and have developed widespread resistance to traditional antibiotics. We studied the human pathogen Neisseria meningitidis and present evidence of novel mechanisms of resistance to the human antimicrobial peptide LL-37. We found that bacteria attached to host epithelial cells are resistant to 10 μM LL-37 whereas bacteria in solution or attached to plastic are killed, indicating that the cell microenvironment protects bacteria. The bacterial endotoxin lipooligosaccharide and the polysaccharide capsule contribute to LL-37 resistance, probably by preventing LL-37 from reaching the bacterial membrane, as more LL-37 reaches the bacterial membrane on both lipooligosaccharide-deficient and capsule-deficient mutants whereas both mutants are also more susceptible to LL-37 killing than the wild-type strain. N. meningitidis bacteria respond to sublethal doses of LL-37 and upregulate two of their capsule genes, siaC and siaD, which further results in upregulation of capsule biosynthesis.
Neisseria meningitidis (meningococci) is a gram-negative, aerobic diplococci that is an obligate human pathogen. Infections caused by N. meningitidis are an important cause of morbidity and mortality worldwide. Meningococci colonize the nasopharyngeal mucosa of approximately 10% of healthy individuals but can cross epithelial and endothelial cell barriers and enter the bloodstream, causing septicemia, with mortality rates of 20 to 50% (4). Meningitis occurs when bacteria transverse the blood cerebrospinal fluid, causing a fatal outcome in 15 to 20% of infected patients. Bacterial adherence is initially mediated by type IV pili with host cell receptors. PilT is the molecular motor responsible for pili retraction, which mediates a tight interaction. An important virulence factor of N. meningitidis is the endotoxin lipooligosaccharide (LOS), which is located in the bacterial outer cell membrane. Meningococcal LOS is composed of a conserved inner core of membrane-associated lipid A (16) to which variable α- and β-chains attach (13).
As one of many first lines of defense against invading pathogens like Neisseria bacteria, epithelial cells produce antimicrobial peptides (AMPs). These peptides are effector molecules for the innate immune response, with both direct antimicrobial activity and a broad spectrum of immunomodulatory functions (18, 22). LL-37 is the single known human cathelicidin and is expressed in various immune cells as well as in epithelial cells of inflamed skin, mouth, tongue, esophagus, and lungs. It has been shown that LL-37 interacts with bacterial membranes through both electrostatic and hydrophobic effects. It remains unknown whether LL-37 ultimately kills bacteria by formation of torroidal pores as described by Henzler Wildman et al. (11) or by detergent-like disintegration of the membrane via the carpet model as described by Shai (24), but increasing membrane permeability, osmotic swelling, and loss of the vital proton gradient are important characteristics of the killing process (21). Membrane interactions of LL-37 (and other AMPs) appear to be highly selective for the negative surface charge on prokaryotic membranes. However, it has been shown by Tzeng et al. (28) that meningococci regulate AMP attack via mechanisms that include lipid A modification and an efflux pump. LL-37 toxicity for eukaryotic cells remains low, probably because eukaryotic cell membranes do not have a negative net charge (31).
In order to further investigate the bactericidal activity of LL-37, various Neisseria strains were examined for their susceptibility to LL-37. Our results show that LL-37 exhibits potent killing activity against N. meningitidis, whereas adhesion to host cells, LOS, and the capsule was found to contribute to resistance to LL-37. Neisseria bacteria can respond to sublethal doses of LL-37 to increase capsule production.
MATERIALS AND METHODS
Synthetic LL-37.
Synthetic LL-37 (NH2-LLG DFF RKS KEK IGK EFK RIV QRI KDF LRN LVP RTE S-COOH) was synthesized by Innovagen, Lund, Sweden. PR39 was a kind gift from Dan Andersson, IMBIM, Uppsala University, Sweden.
Bacterial strains.
N. meningitidis serogroup C strain FAM20 (20) and a LOS-deficient FAM20 (lpxA) mutant (1) have been previously described. The MC58 serogroup B strain was sequenced in 2000 (27) and was used in the microarray analysis for this study. All strains were grown on GC agar (Acumedia) plates containing Kelloggs' supplement (14) at 37°C and 5% CO2 for approximately 18 h before use. All plating was performed in modified GC liquid (1.5% Bacto protease peptone no. 3, 3 mM soluble starch, 23 mM K2HPO4, 7 mM KH2PO4, 150 mM NaCl).
Cell lines.
FaDu (ATCC HTB-43) cells, a human-derived pharyngeal epithelial cell line, were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) with Glutamax and pyruvate, supplemented with 10% fetal calf serum (DMEM/FCS) and 0.1 mM nonessential amino acids.
Bactericidal assay.
Bacteria were resuspended to 105 CFU/ml in GC liquid supplemented with 5% FCS, mixed with 16 μM LL-37 or PR39 (a proline- and arginine-rich AMP originally isolated from a pig intestine), and diluted in 0.1% trifluoroacetic acid to promote the alpha helical structure or mixed with trifluoroacetic acid alone in triplicate experiments (under conditions of 37°C and 5% CO2) for 3 h before survival was analyzed by plating serial dilutions. Colonies were counted the following day, and colony counts are expressed as CFU. The effect of LL-37 on different bacterial concentrations of FAM20 was analyzed by incubation of 103 to 108 CFU/ml with LL-37 (at 0 and 10 μM) for 3 h. Time experiments were performed by incubating bacteria (105 CFU/ml) with LL-37 (at 0, 2, and 10 μM) in triplicate experiments and plating serial dilutions after 0 to 180 min. For these experiments, colonies were counted the following day and colony counts are expressed as survival ratios (numbers of CFU after peptide incubation/numbers of CFU after control incubation).
Adhesion assay.
FaDu cells were grown in 48-well plates in DMEM/FCS to be 90% confluent. The ability of LL-37 to affect recovery of bacteria from host cells was monitored by incubating FaDu cells and FAM20 cells at a multiplicity of infection of 5 in DMEM/FCS with LL-37 (at 0 and 10 μM) (known hereafter as the middle stage). In some experiments, epithelial cells were treated with an early dose of LL-37 (at 0 and 10 μM) for 30 min and were thoroughly washed with phosphate-buffered saline (PBS) to remove all unbound LL-37 before bacteria were added for a further 2 h. In other experiments, bacteria were added to epithelial cells for 1 h and thoroughly washed with PBS before LL-37 (at 0 and 10 μM) was added for a further 2 h (late stage). Adhered bacteria were quantified by lysing cells for 5 min in 1% saponin and plating serial dilutions. For the plastic adhesion experiments, bacteria resuspended to 108 CFU/ml were incubated with LL-37 (at 0 and 10 μM) for 2 h (under conditions of 37°C and 5% CO2) in plastic microtiter plates (middle stage). In some experiments, bacteria were incubated for 1 h with cell culture medium, washed extensively, and subsequently incubated with LL-37 (at 0 and 10 μM) for a further 2 h (late stage). Adhered bacteria were then quantified as described previously. All experiments were performed in triplicate at least two times.
Flow cytometry.
Bacteria resuspended to 108 CFU/ml were incubated with LL-37 for 30 min (under conditions of 37°C and 5% CO2) and fixed with 4% paraformaldehyde. Samples were then stained with biotinylated anti-LL-37 polyclonal antibody (Innovagen) (20 μg/ml) and streptavidin-R-phycoerythrin (Caltag Laboratories) (1:1,000). FaDu cells were incubated with 10 μM LL-37 diluted in 0.1% bovine serum albumin-PBS at 4°C for 30 min, followed by LL-37 staining as described above. All samples were read in a FACScan cytometer (BD Biosciences), and the resultant data were analyzed with Cell Quest Pro software (BD Biosciences).
ELISA.
The level of capsule expression was measured by whole-cell enzyme-linked immunosorbent assay (ELISA) analysis with 95/678 monoclonal antibody. Wells were coated with 100 μl of bacteria solution (optical density at 595 nm, 0.1) overnight at 4°C, blocked with 2% bovine serum albumin (2 h), incubated with 95/678 monoclonal antibody (1:400) overnight (4°C), and incubated with horseradish peroxidase-goat anti-mouse immunoglobulin G (1:5,000) for 1 h. Plate-bound peroxidase was detected using TMB (3,3′,5,5′-tetramethylbenzidine) and stop solution, and the absorbance was measured at 450 nm (reference wavelength, 490 nm).
Generation of a capsule-deficient N. meningitidis mutant.
The capsule synthesis operon was inactivated with a chloramphenicol resistance gene by transformation of wild-type N. meningitidis strain FAM20. The chloramphenicol resistance gene was amplified from pACYC184 with the primers cm-fwd (5′-GGT ACC AGA GCC GCA GGC GTT TAA GGG CAC CAA TA-3′) and cm-rev1 (5′-GGT ACC CCT GCA ATC AGT AAG TTG GCA GCA TC-3′). The end of the ctrA gene from the FAM20 genome was amplified with the primers ctrA-fwd (5′-ATG CCG TCT GAA ACC AAC TGC TCT GGC AAC TT-3′) and ctrA-rev (5′-CCT TAA ACG CCT GCG GCT CTG GTA CCT GTA ATG CAA A-3′), and the end of the siaC gene from the FAM20 genome was amplified with the primers siaC-fwd (5′-CAA CTT ACT GAT TGC AGG GGT ACC TTA TGC TTT GCT T-3′) and siaC-rev (5′-TTC AGA CGG CAT ATT GCC TGG GCG TTT AAC CCA T-3′). The primers were designed to have overlapping ends. The three different PCR fragments were ligated in a PCR done in two steps: (i) 7 cycles without any primers added followed by (ii) 35 cycles with the primers ctrA-fwd and siaC-rev. The fragment obtained was incorporated into TOPO pCR2.1 cloning vector. FAM20 bacteria were resuspended to 108 CFU/ml in GC liquid, and 20 μl of bacterial suspension was added to 200 μl of GC liquid containing 1 μg of linearized plasmid and incubated at 37°C for 30 min before dilution in 2 ml of GC liquid supplemented with Kellogg's supplement and incubation for 4 h (under conditions of 37°C and 5% CO2) with constant shaking. The transformation mixture was diluted and plated on GC agar plates containing 10 μg of chloramphenicol/ml. Positive transformants were confirmed by PCR using the primers ctrA-upstream (5′-TTC TGC ACC AAA CGC ACC ATC A-3′) and cm-rev2 (5′-AAT CAG TAA GTT GGC AGC ATC-3′).
Generation of a PilT-deficient N. meningitidis mutant.
The pilT gene was insertionally inactivated by transformation of wild-type N. meningitidis strain FAM20 with a plasmid containing the pilT gene disrupted with a kanamycin resistance marker. An internal fragment of the pilT gene was amplified by PCR using the primers pilT-fwd (5′-GCC GTC TGA AAC AAA GCA TCC GAC CTT CAC CT-3′) and pilT-rev (5′-ACC AGC GAT TGC AGC GAT T-3′). Primer pilT-fwd contains a DNA uptake sequence (GCC GTC TGA A) at the 5′ end, which is required for efficient DNA transformation in Neisseria bacteria (10). This fragment was cloned into pUC19 (Invitrogen) to produce pUC19/PilT. An 886-bp kanamycin resistance gene was inserted into a unique AgeI site of the pilT fragment carried by pUC19/PilT to produce the transformation plasmid pUC19/PilT::kan. The wild-type N. meningitidis FAM20 strain was transformed with HindIII-linearized pUC19/PilT::kan. Linearized vector was transformed in FAM20 bacteria as described for the capsule mutant. The transformation mixture was diluted and plated on GC agar plates containing 50 μg of kanamycin/ml. Positive transformants were confirmed by PCR.
Sublethal LL-37 treatment.
For microarray and real-time PCR experiments, MC58 bacteria that had been resuspended in DMEM/FCS were grown to the log phase. Aliquots (1 ml) were mixed with LL-37 to give final concentrations of 0 and 5 μM and were incubated for 30 or 60 min (under conditions of 37°C and 5% CO2) with constant shaking. A 5 μM concentration was found to be sublethal by performing a killing assay (data not shown).
RNA isolation.
Bacterial suspensions (1 ml) were immediately mixed with 2 ml of RNA Protect (Qiagen), and RNA was isolated using an RNEasy kit (Qiagen), including DNase I on-column digestion (Qiagen). Cells were lyzed with Tris-EDTA buffer[ens]lysozyme (5 mg/ml) and eluted in 100 μl of water. The concentration and purity were analyzed using a NanoDrop spectrophotometer and a Bioanalyzer.
Microarray analysis.
An N. meningitidis MC58 antisense Affymetrix NIMBLE genome array was used. RNA (10 μg) was reverse transcribed to cDNA by the use of random hexamer primers, fragmented by DNaseI, and labeled with terminal transferase and biotinylated Gene Chip DNA labeling reagent at the 3′ terminus. Each RNA sample was hybridized with one gene array. Gene Chip operating software (version 1.4) was used to analyze the gene expression data. With this software, the detection algorithm determines whether a measured transcript is detected or not detected on a single array according to a detection P value that is computed by applying the one-sided Wilcoxon's signed rank test to test discrimination (R) scores against a predefined adjustable threshold.
Real-time RT-PCR analysis.
RNA (150 ng) isolated from independent samples of bacteria treated with sublethal concentrations of LL-37 was reverse transcribed into cDNA with random hexamers by the use of a Superscript III first-strand synthesis Supermix kit (Invitrogen). For real-time PCR amplification, Power SYBR green PCR master mix (ABI) was used with an ABI Prism 7300 sequence detector (ABI) according to the manufacturer's guidelines. The ribosomal protein coded by gene NMB0140 was chosen as an internal control, since it was not transcriptionally regulated in the MC58 microarray studies. The primers used were NMB0140 fwd (5′-GTG CAG TTG TAA AAG GCC CGA TTC-3′), NMB0140 rev (5′-TGG GTG CGG ATT TCC AAT TGC T-3′), NMB0067 fwd (5′-GAA GGG ACT GGA ACA TAT GCT C-3′), NMB0067 rev (5′-TCA GGA AAG GGG ACA TGC AA-3′), NMB0068 fwd (5′-TCT GAA GCC TTT CCA GAC GCA ATC-3′), and NMB0068 rev (5′-GCG GTC AGT AAA GTG ACG CTC TAA-3′). The data were analyzed using the relative standard curve method and are presented as relative copy numbers of mRNA levels normalized to NMB0140. For each time point, 0 μM LL-37 was further normalized to give a relative copy number of 1. Each PCR experiment was performed two times in triplicate.
Statistical analysis.
Student's t test and Microsoft Excel were used to analyze differences between groups for statistical significance. Two-tailed homoscedastic tests were used with a p level of significance of less than 0.01 unless otherwise indicated.
RESULTS
LL-37 kills N. meningitidis in a dose- and time-dependent manner.
The human LL-37 AMP is present in human plasma at a concentration of around 0.3 μM and on unstimulated epithelial surfaces at 0.5 μM and has been detected at highly elevated concentrations (11 μM) on inflamed epithelial cells and in wound tissue (25). Some reports claim that the killing capacity of LL-37 is reduced under conditions of physiological salt concentrations (5). Our experiments thus aimed to mimic human physiological salt conditions (150 mM) to gain insight into the in vivo interaction between the pathogen and AMP. After 3 h of treatment, LL-37 (16 μM) was able to kill almost all N. meningitidis FAM20 bacteria at 105 CFU/ml, while PR39, a porcine AMP rich in proline and arginine, was unable to significantly reduce the bacterial load at the same concentration, highlighting the different specificities of AMP-mediated killing (Fig. 1A).
FIG. 1.

AMP-mediated killing of N. meningitidis FAM20. (A) Bacteria at 105 CFU/ml were incubated with LL-37 and PR39 (at 0 and 16 μM) for 3 h. (B) Bacteria at 103 to 108 CFU/ml were incubated with LL-37 (at 0 and 10 μM) for 3 h. (C) Bacteria at 105 CFU/ml were incubated with LL-37 (at 0, 2, and 10 μM) for 0 to 180 min. Viable counts were determined, with data presented as CFU per milliliter or mean survival ratios ± standard errors (n = 3). * represents a statistically significant difference (P ≤ 0.01).
The kinetics of LL-37-mediated killing was investigated by incubating N. meningitidis serogroup C strain FAM20 (resuspended to 103 to 108 CFU/ml) with LL-37 (at 0 and 10 μM) for 3 h. These data show that LL-37 has the capacity to kill high numbers of bacteria at micromolar concentrations. A 10 μM concentration of LL-37 was able to reduce bacterial viability by more than 99% at 103 to 105 CFU/ml (P ≤ 0.01) and by at least 90% at 106 to 107 CFU/ml (P ≤ 0.01; Fig. 1B). Bacteria (at 105 CFU/ml) were incubated with LL-37 for increasing time periods. No growth inhibition or bactericidal activity could be observed at 2 μM LL-37; however, use of 10 μM LL-37 resulted in killing that occurred in a time-dependent manner (Fig. 1C), with approximately 20% bacterial survival after 30 min (P ≤ 0.01) and less than 1% survival after 3 h (P ≤ 0.01).
LOS protects Neisseria meningitidis from LL-37-mediated killing.
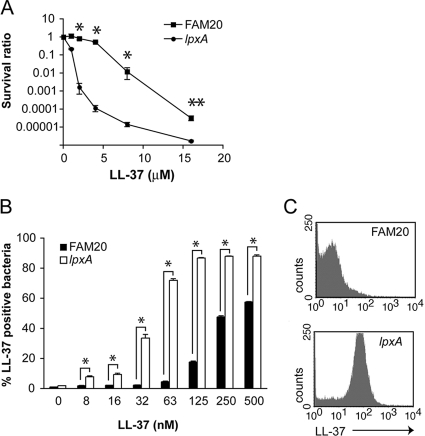
The lpxA mutant strain does not express the surface endotoxin LOS, since it lacks the gene encoding an enzyme responsible for the first step of the lipid A biosynthesis pathway (1). LL-37 was more efficient at killing the lpxA-deficient mutant, killing significantly more lpxA mutants than FAM20 bacteria at between 2 and 16 μM (P ≤ 0.01; Fig. 2A). Interestingly, more than 99% of lpxA mutant bacteria were killed at 2.0 μM LL-37 whereas only 20% of FAM20 bacteria were killed (P ≤ 0.01) (Fig. 2A). We also confirmed that FAM20 and lpxA bacteria had identical growth rates in the growth medium (data not shown).
FIG. 2.
LOS-deficient N. meningitidis bacteria are more susceptible to LL-37-mediated killing. (A) N. meningitidis strain FAM20 (serogroup C) and the isogenic lpxA mutant strain lacking LOS, after being resuspended to form 105 CFU/ml, were incubated with LL-37 (0 to 16 μΜ) for 3 h. Viable counts were determined, with data presented as mean survival ratios ± standard errors (n = 3). (B) The FAM20 strain and the isogenic lpxA mutant strain, after being resuspended to 108 CFU/ml, were treated with LL-37 (0 to 500 nM) for 30 min at 22°C and subsequently fixed and stained using a biotinylated anti-LL-37 antibody before analysis by flow cytometry. The numbers of LL-37-binding bacteria are expressed as percentages of the total number of bacteria. Data are presented as mean percentages + standard errors (n = 3). (C) Representative histogram plots showing LL-37 binding to the FAM20 strain and the lpxA mutant strain after incubation with 125 nM LL-37. * and ** represent statistically significant differences (P ≤ 0.01 and P ≤ 0.05, respectively).
To further understand why the lpxA mutant was more susceptible, we analyzed LL-37 binding to the surface of bacteria. FAM20 wild-type and lpxA mutant bacteria were incubated with LL-37 (at 0 to 500 nM) for 30 min. Figure 2B shows that lpxA mutant bacteria accumulated LL-37 on the surface after exposure to concentrations of LL-37 lower than those required to produce the same effect on FAM20 bacteria. For example, following the incubation of FAM20 and lpxA mutant bacteria with 125 nM LL-37, 18% of FAM20 bacteria exhibited LL-37 binding, whereas significantly (P ≤ 0.01) more lpxA mutant bacteria (87%) were positive for LL-37 binding (Fig. 2C).
The neisserial capsule protects bacteria from LL-37.
It has previously been shown that capsule polysaccharides from gram-negative bacteria contribute to resistance to AMP (3, 26). In particular, Spinosa et al. (26) demonstrated that the capsule plays a key defensive role, protecting intracellular serogroup B N. meningitidis from various AMPs. Killing assays (Fig. 3A) demonstrated that N. gonorrhoeae was significantly more susceptible to LL-37 than encapsulated N. meningitidis. Most neisserial capsules associated with invasive diseases contain sialic acid, with serogroup B α2-8-linked N-acetylneuraminic acid and serogroup C α2-9-linked partially O-acetylneuraminic acid. We wanted to test the serogroup C capsule and constructed a capsule-deficient mutant of the serogroup C FAM20 strain by insertional inactivation of the sia operon. The absence of the capsule was confirmed by ELISA (Fig. 3B) and PCR (data not shown). The growth rate and pilus expression of the capsule mutant were verified to be identical to those of the wild-type FAM20 strain (data not shown). The capsule mutant was compared to the isogenic wild-type FAM20 strain in an LL-37 killing assay and found to be significantly more susceptible to LL-37 (Fig. 3C). We analyzed LL-37 binding to the surface of wild-type and capsule-deficient bacteria. Figure 3D shows that capsule-deficient bacteria accumulated LL-37 on the surface after exposure to lower concentrations of LL-37 compared to wild-type strain FAM20 results. Following incubation of bacteria with 250 nM LL-37, 30% of FAM20 bacteria exhibited LL-37 binding, whereas significantly (P ≤ 0.01) more of the capsule-deficient bacteria (58%) were positive for LL-37 binding (Fig. 3D).
FIG. 3.
The neisserial capsule protects bacteria from LL-37. (A) The encapsulated N. meningitidis FAM20 strain and N. gonorrhoeae strain MS11 were incubated with LL-37 (0 to 3.2 μΜ) for 3 h. Viable counts were determined, with data presented as mean percentages of survival + standard errors (n = 3). (B) ELISA verification of the capsule-deficient strain FAM20ΔCapsule compared to wild-type FAM20. OD405, optical density at 405 nm. (C) FAM20 and FAM20ΔCapsule, after being resuspended to form 105 CFU/ml, were incubated with LL-37 (0 to 8 μM). Viable counts were determined after 3 h. Data are presented as mean survival ratios ± standard errors (n = 3). (D) FAM20 bacteria and the isogenic capsule-deficient mutant, after being resuspended to form 108 CFU/ml, were treated with LL-37 (0 to 500 nM) for 30 min at 22°C and subsequently fixed and stained using a biotinylated anti-LL-37 antibody before analysis by flow cytometry. The numbers of LL-37-binding bacteria are expressed as percentages of the total number of bacteria. Data are presented as mean percentages + standard errors (n = 3). * and ** represent statistically significant differences (P < 0.01 and P < 0.05, respectively).
Neisseria bacteria respond to LL-37 by upregulating the capsule.
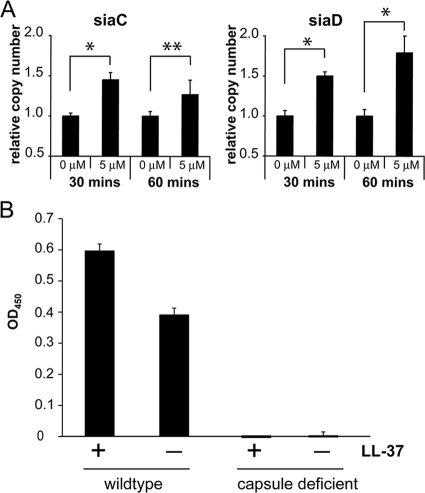
To determine whether LL-37 regulates the transcription of neisserial genes, a microarray study was performed, with bacteria exposed to sublethal doses of LL-37 (0 and 5 μM) for 30 min. The sublethal dose of LL-37 was chosen after killing assays were performed with strain MC58 (data not shown). Affymetrix oligonucleotide Nimble arrays were used with strain MC58 (serogroup B). Numerous genes were regulated; 12 of the most completely regulated genes are listed in Table 1. (The complete list of genes exhibiting >1.5-fold regulation is presented online in Table S1 and Table S2 in the supplemental material.) In particular, two capsule genes, NMB0068 (siaC) and NMB0067 (siaD), were found to have been upregulated 2.57-fold and 2.20-fold, respectively, after 30 min of LL-37 treatment, while lipA (encoding a capsule polysaccharide modification protein) was upregulated 1.3-fold after 30 min but not after 60 min. Spinosa et al. (26) also found that lipA was upregulated 1.6-fold in response to treatment with sublethal concentrations of LL-37. We verified the altered gene transcription data by performing real-time RT-PCR using bacteria treated with sublethal concentrations of LL-37. NMB0140, encoding a ribosomal protein, was chosen as an internal housekeeping gene. Real-time PCR data revealed that NMB0068 (siaC) and NMB0067 (siaD) were significantly upregulated at all time points (Fig. 4A). In order to test whether the two- to threefold upregulation of capsule genes was physiologically relevant, we measured neisserial capsule content by ELISA after exposure of bacteria to sublethal LL-37 concentrations. Indeed, capsule production was upregulated approximately 1.5-fold (P < 0.01) in response to the LL-37 treatment (Fig. 4B).
TABLE 1.
A selection of genes regulated in response to a sublethal LL-37 dose
| Category | ORFa | Description | Fold changeb (time [min])c |
|---|---|---|---|
| Resistance | NMB1955 | Cadmium resistance | 3.59 (30) |
| NMB1715 | mtrD | 2.12 (30) | |
| NMB1763 | Codes for putative toxin-activating protein | 2.24 (30), 2.04 (60) | |
| Capsule | NMB0068 | siaC | 2.57 (30) |
| NMB0067 | siaD | 2.20 (30) | |
| NMB0082 | lipA | 1.33 (30) | |
| Other | NMB0360 | Codes for ampG-related protein | 2.02 (30) |
| NMB0663 | nspA | 0.48 (60) | |
| NMB1628 | Codes for putative tspB protein | 2.14 (30), 1.86 (60) | |
| Hypothetical | NMB1404 | Hypothetical | 3.86 (30), 2.63 (60) |
| NMB0660 | Hypothetical | 3.76 (30), 3.17 (60) | |
| NMB1746 | Hypothetical | 3.28 (30), 3.37 (60) | |
| Housekeeping | NMB0140 | 30S (S10) ribosomal | 1.06 (30), 1.03 (60) |
Designations represent N. meningitidis Serogroup B genome sequence open reading frames (ORF) (27).
Fold change values represent the ratios of mRNA transcript levels in LL-37-treated strain MC58 to those detected in untreated strain MC58.
Values indicate whether the induced regulation occurred after 30 or 60 min of exposure to sublethal LL-37 doses.
FIG. 4.
The neisserial capsule is upregulated in response to treatment with sublethal concentrations of LL-37. (A) Verification of transcription induced in N. meningitidis serogroup B strain MC58 by treatment with sublethal concentrations of LL-37 as quantified by real-time RT-PCR. MC58 bacteria were grown to the log phase and then exposed to LL-37 (0 and 5 μM) for 30 or 60 min before RNA was immediately harvested and reverse transcribed to cDNA. Real-time PCR using SYBR green was performed on the capsule genes siaC (NMB0068) and siaD (NMB0067). Expression levels were normalized to that of the housekeeping gene NMB0140 and are expressed as relative copy numbers, with 0 μM LL-37 further normalized to give a relative copy number of 1.0. (B) Strain FAM20 and the capsule-deficient mutant strain were grown to the log phase and then exposed to 0 (−) or 5 (+) μM LL-37 for 60 min before analysis by ELISA to determine the presence of the capsule. * and ** represent statistically significant differences (P ≤ 0.01 and P ≤ 0.05, respectively). OD450, optical density at 450 nm.
N. meningitidis bacteria are protected from LL-37 when they are attached to an epithelial cell surface.
LL-37 is expressed on epithelial surfaces and can be induced (7) or downregulated (2, 12) in response to infection. N. meningitidis infects humans via the nasopharyngeal tract and attaches to epithelial cells before invading and transversing the subepithelium and entering the bloodstream. We aimed to test the feasibility of using exogenously delivered LL-37 to prevent colonization. For these studies, we chose the FaDu human nasopharyngeal cell line. We investigated the effect of LL-37 on bacterial adhesion by adding LL-37 at early, middle, and late stages throughout the infection process (Fig. 5A). For early treatment, we precoated epithelial cells with LL-37 by adding the peptide (10 μM) to cells for 30 min, washing away unbound LL-37, and then adding bacteria for a further 2 h, which is long enough for bacteria to attach to the cell surface but not long enough for bacteria to invade the cells. Our data showed that early treatment of the cells with LL-37 had no effect on the recovery of viable bacteria (Fig. 5B), despite high levels of LL-37 binding to the epithelial cell surface (Fig. 5C). Middle-stage treatment involved adding bacteria and LL-37 (10 μM) to the cells at the same time. Here, we found that the recovery of viable bacteria from the cell-associated population was reduced to 12.5% ± 6% due to the presence of LL-37 (Fig. 5B). Interestingly, late-stage treatment of LL-37, which involved adding LL-37 (10 μM) after bacteria had been allowed to attach to epithelial cells for 1 h, did not kill the bacteria (Fig. 5B).
FIG. 5.
N. meningitidis bacteria attached to host cells are protected from LL-37. (A) Schematic representing early-, mid-, and late-stage treatments with LL-37 for the adhesion assay. (B) FaDu cells were infected with strain FAM20 (multiplicity of infection = 5) and treated with LL-37 (at 0 and 10 μM). control, no LL-37 treatment; early, epithelial cells were treated with LL-37 for 30 min and then thoroughly washed before addition of bacteria for 2 h; mid, bacteria and LL-37 were added to epithelial cells at the same time for 2 h; late, bacteria were added to epithelial cells to attach for 1 h and thoroughly washed before LL-37 was added for an additional 2 h. Cells were thoroughly washed and viable counts of adherent bacteria determined. Data represent mean percentages of recovery (treated) compared to control results (untreated) ± standard errors (n = 3). (C) FaDu cells were incubated with LL-37 (at 0 and 10 μM) for 30 min at 4°C, washed, stained with a biotinylated anti-LL-37 antibody, and analyzed by flow cytometry. Representative histogram shows LL-37 binding to FaDu cells after incubation with LL-37. (D) FAM20 and LL-37 (at 0 and 10 μM) were added to plastic microtiter plates. control, no LL-37 treatment; mid, bacteria and LL-37 were added to plates at the same time for 2 h; late, bacteria were added to plates to attach for 1 h and thoroughly washed before LL-37 was added for an additional 2 h. Plates were thoroughly washed to remove unbound bacteria and viable counts of adherent bacteria determined. Data represent mean percentages of recovery (treated) compared to control results (untreated) ± standard errors (n = 3). (E) Strain FAM20 and PilT mutant bacteria, after being resuspended to form 105 CFU/ml, were incubated with 0 to 16 μM LL-37, and viable counts were determined after 3 h. Data represent mean survival ratios ± standard errors (n = 3). (F) FaDu cells were infected with FAM20ΔPilT as described for panel B. * and ** represent statistically significant differences (P ≤ 0.01 and P ≤ 0.05, respectively).
We wanted to investigate the mechanism underlying the protection from LL-37 that bacteria receive when attached to host cells. We hypothesized that the host cell surface microenvironment played a key role in mediating the observed protection. To test this theory, we designed another adhesion assay that allowed bacteria to adhere to plastic microtiter plates. There was no significant difference between middle-stage (LL-37 and bacteria added at the same time) and late-stage (10 μM LL-37 added after bacteria had already been attached to the plastic for 1 h) treatment of LL-37 (Fig. 5D). To test whether the resistance can be explained by the “sink effect,” LL-37 (at 0 and 10 μM) was added to a confluent monolayer of FaDu cells or, as a control, was added to wells containing medium only (no cells) for 30 min. The supernatant from FaDu cells preincubated with LL-37 or medium alone was then added to 5 × 105 FAM20 bacteria and incubated with gentle shaking for 2 h. The potency of supernatant containing LL-37 with respect to killing FAM20 bacteria was equal to that of LL-37 (data not shown), indicating that the cell membrane does not act in a sink-effect-like manner to mop up LL-37. Overall, these data show that Neisseria bacteria attached to host cells were approximately 10-fold more resistant to 10 μM LL-37 than bacteria in solution or bacteria attached to plastic.
Thus, our data reveal that the cell microenvironment is an important factor mediating the protection of bacteria from LL-37, since bacteria attached to plastic do not receive the same protection from LL-37 as bacteria attached to cells. We hypothesized that bacteria probably need to have very close contact with the host cell surface to receive the cell-mediated protection. The initial attachment of bacteria to the cell surface is mediated through binding of pili to cell surface receptors. Pili retract and bring the bacteria into close proximity to the host cell membrane, where an intimate interaction is mediated though binding interactions between cell CEACAM receptors and bacterial Opa proteins. PilT is a molecular motor responsible for pilus retraction (20). Mutants lacking PilT are unable to retract their pili and thus cannot make intimate contact with host cells.
To further define the mechanism of adhesion-mediated protection, we constructed an isogenic mutant lacking PilT. Loss of PilT protein expression was confirmed by Western blot analysis using rabbit polyclonal serum raised against recombinant PilT (data not shown). The PilT mutant was verified to have growth rates in cell culture medium similar to those of wild-type FAM20 bacteria (data not shown). We utilized the PilT mutant in the adhesion assay and found again that late-stage treatment of LL-37 was ineffective in reducing the recovery of viable bacteria from host cells (Fig. 5F). Evaluating the importance of retractile pili for cell-mediated protection from LL-37 was complicated by the fact that the PilT mutant was significantly more resistant to LL-37 than wild-type meningococci in the absence of cells (Fig. 5E). However, our results show that PilT is not required for the increased resistance shown by adherent bacteria. The evidence of protection from AMPs mediated by adhesion to host cells reveals a previously undescribed method by which N. meningitidis bacteria evade the immune system.
DISCUSSION
N. meningitidis is an obligate human pathogen that normally first encounters human epithelial cells in the nasopharyngeal tract. In fact, meningococci colonize the nasopharyngeal mucosa of 10% of healthy individuals (4). AMPs are important components of the innate immune system; as a first line of defense, they protect various epithelial surfaces against invading pathogens (30). Bacteria have evolved numerous effective mechanisms to evade the human innate and adaptive immune systems, including secretion of toxins, modulation of bacterial surfaces, and inhibition of complement components and efflux pumps (for a thorough review, see reference 6). Neisseria bacteria have an energy-dependent multiple transferable resistance efflux pump that contributes resistance to hydrophobic agents such as LL-37 (23). Nonetheless, bacterial resistance to the host repertoire of AMPs remains surprisingly low, since AMPs target the bacterial membrane, the microbial weak point, which is distinct in this respect from eukaryotic cell membranes (31). LL-37 is widely expressed in human epithelial cells, including the respiratory tract epithelia targeted by pathogenic N. meningitidis (8). Among the key findings of our study were the novel data showing that N. meningitidis is protected from 10 μM LL-37 when bacteria are attached to host epithelial cells. Recovery of viable bacteria is reduced to 12.5% when LL-37 is added simultaneously with bacteria, under which conditions LL-37 presumably kills bacteria before they have a chance to attach to host cells. However, when bacteria are given the chance to attach to host cells for 1 h prior to LL-37 treatment, bacteria survive in the presence of LL-37. We were interested to further investigate this fascinating and novel form of bacterial protection. Bacteria were resistant to 10 μM LL-37 after 1 h of attachment to host cells, which is long enough for bacteria to attach to cells but not long enough for bacteria to invade. We speculated that bacteria obtain protection from host cells by being closely associated with the host cell membrane. To test this theory, we constructed a PilT-deficient mutant strain of FAM20 bacteria, which were unable to retract their pili and thus could not make an intimate attachment to host cells. We anticipated that PilT mutant bacteria would be more susceptible to LL-37 when loosely attached to host cells via their pili; however, we were surprised to find that the attached PilT mutant bacteria exhibited a small increase in resistance compared to the wild-type strain. This phenomenon may have been due to the PilT-deficient mutant bacteria forming larger microcolonies. Adhesion studies using both PilT mutant and wild-type strains revealed that intimate cell contact was not necessary to mediate the protection that N. meningitidis bacteria experience when attached to host cells. To determine whether a cell environment was necessary at all for mediation of the attachment protection, we performed adhesion assays using plastic microtiter plates and demonstrated that bacteria attached to plastic do not receive the same protection as bacteria that adhere, either loosely or tightly, to host cells. Thus, it became apparent that the cell microenvironment does indeed mediate the adhesion protection. One explanation may be that attached bacteria are physically shielded from the bactericidal activity of LL-37 via their association with the host cell membrane and associated proteins. Alternatively, protection may be mediated by the neutralization of negatively charged bacterial membranes when bacteria associate with the host cell membrane (21). In addition, prophylactic treatment of LL-37 (at the early stage) did not protect host cells from bacterial colonization, despite LL-37 binding avidly to host cells. It is also important to consider that N. gonorrhoeae has been shown to downregulate LL-37 expression in endocervical ME-180 cells after 6 h of infection (2). Our experimental system utilized an exogenous source of LL-37, so any bacterially induced downregulation of LL-37 in host epithelial cells would not influence the adherence-mediated bacterial protection.
Another interesting finding from our study was that LOS contributes to resistance to LL-37. LOS is the bacterial endotoxin of N. meningitidis and the major cause of fulminate meningococcal sepsis due to overstimulation of the cellular immune response. We demonstrated that a LOS-deficient mutant is more sensitive to LL-37 than the wild-type strain and binds more LL-37 that the wild-type strain. These data strongly suggest that LOS limits the interaction of LL-37 with the bacterial surface, either by providing a protective charge for the bacteria or by physically inhibiting LL-37, i.e., halting LL-37 at the outer surface and blocking access to the vulnerable inner membrane. LL-37 has been previously shown to neutralize lipopolysaccharide (9), while surface modifications in the LOS molecule are considered important mechanisms regulating AMP resistance (19). Spinosa et al. (26) recently used a strain of N. meningitidis deficient in both the capsule and LOS outer core to demonstrate that LOS may contribute to AMP resistance. We also show that LOS mediates neisserial resistance to LL-37, possibly by preventing binding of LL-37 to the bacteria. It is important to point out that the LOS-deficient mutant was susceptible to the human cathelicidin LL-37, which is expressed in nasopharyngeal epithelial cells (where Neisseria bacteria commonly colonize) at concentrations within the reported range (25), thereby demonstrating the physiological relevance of our work.
Another major finding from our study of the interaction between LL-37 and Neisseria bacteria was the confirmation that the polysaccharide capsule is also involved in resistance to LL-37. Spinosa et al. (26) previously demonstrated that the serogroup B polysaccharide meningococcal capsule (alpha-2,8-linked sialic acid) is important for intracellular survival in human cells (26), while others have shown that the Klebsiella pneumoniae capsule (consisting of complex acidic polysaccharides) confers resistance to various AMPs (3). Llobet et al. (17) recently showed that anionic bacterial capsule polysaccharides block bactericidal activity of AMPs via binding, thus reducing the amount of peptide reaching the bacterial surface, but that cationic or uncharged ones did not have the same effect. Conversely, other groups have found that the capsule of K. pneumoniae and K1 capsular polysaccharides of Escherichia coli do not protect against AMPs (15, 29). Our data confirm that the serogroup C polysaccharide capsule contributes to neisserial resistance to LL-37. We demonstrated that an N. meningitidis capsule-deficient mutant is more sensitive to LL-37 than the wild-type strain and that the capsule-deficient mutant binds more LL-37, as shown in the fluorescence-activated cell sorter study. One of the most striking findings of our capsule study was that the presence of sublethal concentrations of LL-37 upregulated the transcription of the siaC and siaD capsule genes. These data are in agreement with the studies by Spinosa et al. (26), who found that bacteria upregulated transcription of the capsule lipA gene after exposure to sublethal concentrations of AMP. We provide further evidence that sublethal LL-37 treatment results in an upregulated N. meningitidis capsule. We are currently studying how N. meningitidis senses the presence of AMPs and how the signal is transduced to upregulate production of the capsule.
We found that N. meningitidis bacteria were killed by LL-37 in a time- and dose-dependent manner. LL-37 begins to kill N. meningitidis within 30 min but is more effective after 3 h. As expected, the effectiveness of LL-37 potency was directly proportional to the bacterial load, with 10 μM LL-37 effectively killing more than 99% of bacteria at 103 to 105 CFU/ml, 90% effective against bacteria at 106 to 107 CFU/ml, and not very effective against 108 CFU/ml (P ≤ 0.01).
Our investigations of the interactions between LL-37 and pathogenic Neisseria bacteria have shown that LL-37 exhibits potent bactericidal activity against N. meningitidis. Physiologically relevant concentrations of LL-37 effectively prevent adhesion of bacteria to host cells; interestingly, though, bacteria that are attached to host cells remain protected from the peptide. Both the capsule and the endotoxin LOS also contribute to the resistance of Neisseria bacteria to LL-37. N. meningitidis bacteria also respond to LL-37 by upregulating capsule production. We believe that our findings highlight novel directions in which to aim research for designing new antibacterial agents that could be useful in future treatment of gram-negative bacterial infections. The use of compounds that inhibit adhesion or capsule or LOS production may make bacteria more sensitive to this branch of the innate immune system.
Supplementary Material
Acknowledgments
A.J. is supported by a National Health and Medical Research Council Biomedical C. J. Martin Fellowship (366796), by the Swedish Research Council (grant Dnr 2005-5701), and by Åke Winbergs Stiftelse. A.-B.J. is supported by the Swedish Research Council (grants Dnr 2002-6240, 2004-4831, 2006-4112, 2006-5073, and 2007-3369), the Swedish Cancer Society, Torsten and Rognar Söderbergs Stiftelse, the Laerdal Foundation for Acute Medicine, and Knut and Alice Wallenbergs Stiftelse.
Kind thanks to Jens Eriksson for producing the PilT antiserum and Björn Lindström for performing the real-time PCR experiments.
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Albiger, B., L. Johansson, and A. B. Jonsson. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman, P., L. Johansson, V. Asp, L. Plant, G. H. Gudmundsson, A. B. Jonsson, and B. Agerberth. 2005. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell Microbiol. 71009-1017. [DOI] [PubMed] [Google Scholar]
- 3.Campos, M. A., M. A. Vargas, V. Regueiro, C. M. Llompart, S. Alberti, and J. A. Bengoechea. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 727107-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 1911263-1271. [DOI] [PubMed] [Google Scholar]
- 5.Finlay, B. B., and R. E. Hancock. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2497-504. [DOI] [PubMed] [Google Scholar]
- 6.Finlay, B. B., and G. McFadden. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124767-782. [DOI] [PubMed] [Google Scholar]
- 7.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 27215258-15263. [DOI] [PubMed] [Google Scholar]
- 8.Frohm Nilsson, M., B. Sandstedt, O. Sørensen, G. Weber, N. Borregaard, and M. Ståhle-Bäckdahl. 1999. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 672561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golec, M. 2007. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann. Agric. Environ. Med. 141-4. [PubMed] [Google Scholar]
- 10.Graves, J. F., G. D. Biswas, and P. F. Sparling. 1982. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J. Bacteriol. 1521071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henzler Wildman, K. A., D. K. Lee, and A. Ramamoorthy. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 426545-6558. [DOI] [PubMed] [Google Scholar]
- 12.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7180-185. [DOI] [PubMed] [Google Scholar]
- 13.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24281-334. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohashi, O., T. Ono, K. Ohki, T. Soejima, T. Moriya, A. Umeda, Y. Meno, K. Amako, S. Funakosi, M. Masuda, et al. 1992. Bactericidal activities of rat defensins and synthetic rabbit defensins on staphylococci, Klebsiella pneumoniae (Chedid, 277, and 8N3), Pseudomonas aeruginosa (mucoid and nonmucoid strains), Salmonella typhimurium (Ra, Rc, Rd, and Re of LPS mutants) and Escherichia coli. Microbiol. Immunol. 36369-380. [DOI] [PubMed] [Google Scholar]
- 16.Kulshin, V. A., U. Zahringer, B. Lindner, C. E. Frasch, C. M. Tsai, B. A. Dmitriev, and E. T. Rietschel. 1992. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 1741793-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llobet, E., J. M. Tomas, and J. A. Bengoechea. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 1543877-3886. [DOI] [PubMed] [Google Scholar]
- 18.McPhee, J. B., and R. E. Hancock. 2005. Function and therapeutic potential of host defence peptides. J. Pept. Sci. 11677-687. [DOI] [PubMed] [Google Scholar]
- 19.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10179-186. [DOI] [PubMed] [Google Scholar]
- 20.Rahman, M., H. Kallstrom, S. Normark, and A. B. Jonsson. 1997. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol. Microbiol. 2511-25. [DOI] [PubMed] [Google Scholar]
- 21.Sato, H., and J. B. Feix. 2006. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim. Biophys. Acta 17581245-1256. [DOI] [PubMed] [Google Scholar]
- 22.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 1693883-3891. [DOI] [PubMed] [Google Scholar]
- 23.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 951829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shai, Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers 66236-248. [DOI] [PubMed] [Google Scholar]
- 25.Sørensen, O., J. B. Cowland, J. Askaa, and N. Borregaard. 1997. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods 20653-59. [DOI] [PubMed] [Google Scholar]
- 26.Spinosa, M. R., C. Progida, A. Tala, L. Cogli, P. Alifano, and C. Bucci. 2007. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 753594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 2871809-1815. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 1875387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss, J., M. Victor, A. S. Cross, and P. Elsbach. 1982. Sensitivity of K1-encapsulated Escherichia coli to killing by the bactericidal/permeability-increasing protein of rabbit and human neutrophils. Infect. Immun. 381149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 7539-48. [DOI] [PubMed] [Google Scholar]
- 31.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415389-395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.