Abstract
In this report we provide evidence that the antimicrobial action of stannous salts and a gold drug, auranofin, against Treponema denticola is mediated through inhibition of the metabolism of selenium for synthesis of selenoproteins.
The biological use of selenium as a catalyst, incorporated into proteins as selenocysteine, is broad. It plays an essential role in energy metabolism, redox balance, and reproduction in a variety of organisms, from bacterial pathogens to eukaryotic parasites to humans. The results of several epidemiological studies indicate that higher levels of selenium in the mammalian diet can have a negative effect on dental health (2, 17-19, 39). Although the impact of selenium is attributed to its influence on the physical properties of the enamel surface (10), the role of selenium in supporting the oral microbial community has not been studied.
The oral cavity is a highly complex microbiome, with a large proportion of its residents uncharacterized due to their fastidious nature and resistance to traditional culture methods (11). Analysis of whole saliva indicates that bacterial metabolism influences the amino acid composition and indicates a role for amino acid fermentation (38). Curtis et al. demonstrated the occurrence of Stickland reactions in dental plaque (9). These reactions were first described in clostridia (35-37). They involve the coupled fermentation of amino acids in which one amino acid is oxidized (Stickland donor) and another (Stickland acceptor) is reduced (29). Treponema denticola, an established resident of the oral cavity, performs Stickland reactions via the selenoprotein glycine reductase (32). Glycine reductase is composed of a multiprotein complex that contains two separate selenoproteins, termed selenoprotein A and selenoprotein B (1, 7, 8, 15, 16). This complex of proteins converts glycine to acetyl phosphate by using inorganic phosphate and the reducing potential from thioredoxin. For the organisms that use this complex, this is a vital source of ATP. Thus far, the requirement for selenocysteine at the active site of this enzyme complex is universally conserved, even though all other selenoproteins that have been identified using computational techniques have a putative cysteine homologue (24).
Treponema denticola is considered one of the primary pathogens responsible for periodontitis, a chronic inflammatory disease that is the major cause of adult tooth loss (11, 27, 33). It is the best-studied oral spirochete, commonly found with other spirochetes within the periodontal pocket. It expresses a variety of virulence factors and is capable of adhering to and penetrating endothelial cell monolayers (31). Its health impact may reach beyond the oral cavity. A recent study linked periodontitis with peripheral arterial disease and detected T. denticola, along with other periodontal pathogens, in atherosclerotic plaque (3). Sequence analysis indicates the presence of several selenoproteins in addition to glycine reductase within the genome of T. denticola (24). This organism exhibits a strict growth requirement for selenium (32).
A significant literature exists that clearly demonstrates the antimicrobial activity of fluoride compounds against microorganisms associated with dental decay and periodontitis. Both sodium fluoride and stannous fluoride, as well as stannous ions alone, inhibit the growth of T. denticola (21). The inhibitory effect of stannous salts on T. denticola's growth is unexplained. It should be noted that toothpastes containing stannous fluoride are more effective in reducing gingivitis and plaque (28, 30).
Tin, as well as several other trace elements, modulates the effects of acute selenium toxicity (20). Conversely, selenium affects the activity of tin in animal models (4-6). In this study, we examine the possibility that stannous ions interfere with selenium metabolism in T. denticola.
T. denticola's growth is inhibited by stannous salts and auranofin.
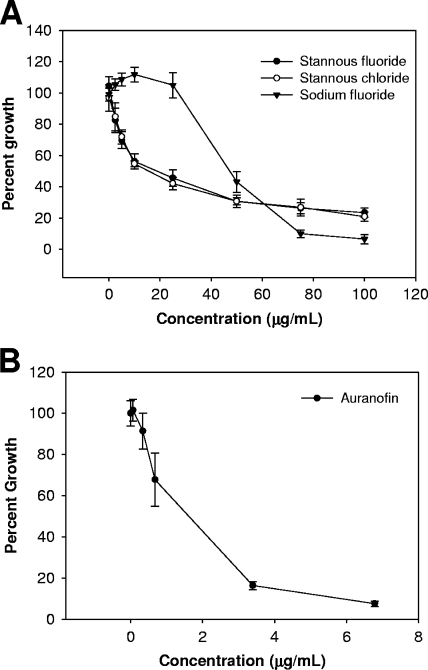
We first aimed to define the inhibitory concentrations of sodium fluoride, stannous fluoride, and stannous chloride against T. denticola (ATCC strain 33520) in new oral spirochete medium (ATCC 1357). We cultivated cells in 96-well polystyrene plates and incubated them at 37°C in multiplate Bio-Bag environmental chambers (type A; Becton Dickinson) (Fig. 1A). Optical density measurements were obtained after 48 h of growth by using a Spectramax 190 UV-visible spectrophotometer at a wavelength of 600 nm (Molecular Devices). Under these conditions, we found that cultures grew steadily over a period of 48 to 72 h, and thus, 48 h represents an appropriate point in the batch growth to assess changes in growth yield. A 50% reduction in growth yield was observed in the presence of 10 μg/ml stannous fluoride, whereas cultures treated with sodium fluoride did not show a similar reduction until a dose of 50 μg/ml. This difference in inhibitory concentrations could be attributed to the concentration of fluoride ions. Cultures treated with stannous chloride, however, exhibited levels of growth inhibition similar to the levels in cultures treated with stannous fluoride, indicating that stannous ions, independent of fluoride, inhibit the growth of T. denticola. This is consistent with the results of a previous study of the inhibition of growth by stannous salts (21).
FIG. 1.
T. denticola growth inhibition by stannous fluoride, stannous chloride, sodium fluoride, and auranofin. Cultures (200 μl) were incubated anaerobically at 37°C in new oral spirochete medium (ATCC 1357) in 96-well polystyrene plates. Optical density at a wavelength of 600 nm was measured after 48 h of growth. (A and B) Stannous salts and sodium fluoride (0, 2.5, 5, 10, 25, 50, 75, and 100 μg/ml, final concentrations) (A) and auranofin (0.068, 0.34, 0.68, 3.4,and 6.8 μg/ml, final concentrations) (B) were added to the culture medium prior to inoculation. Percent growth (growth yield of inhibited cultures versus that of control) is plotted. Data shown are from at least three independent cultures. Error bars indicate standard deviations.
Recent work in our laboratory demonstrated that auranofin [2,3,4,6-tetra-o-acetyl-1-thio-β-d-glucopyranosato-S-(triethyl-phosphine) gold], a known inhibitor of selenocysteine-containing enzymes, can interact with the reduced form of selenium, selenide (Se2−), to form a stable complex and thus prevent its nutritional utilization and incorporation into selenoproteins (22). Given that tin is known to interact with selenium in biological systems, we chose to also examine the impact of auranofin on the growth of T. denticola (Fig. 1B). As with the stannous salts, cultures were incubated in the presence of auranofin for 48 h before the growth yield was analyzed. Auranofin potently inhibits the growth of T. denticola at concentrations of less than 5 μg/ml.
Selenium supplementation modulates the effects of stannous salts but not sodium fluoride.
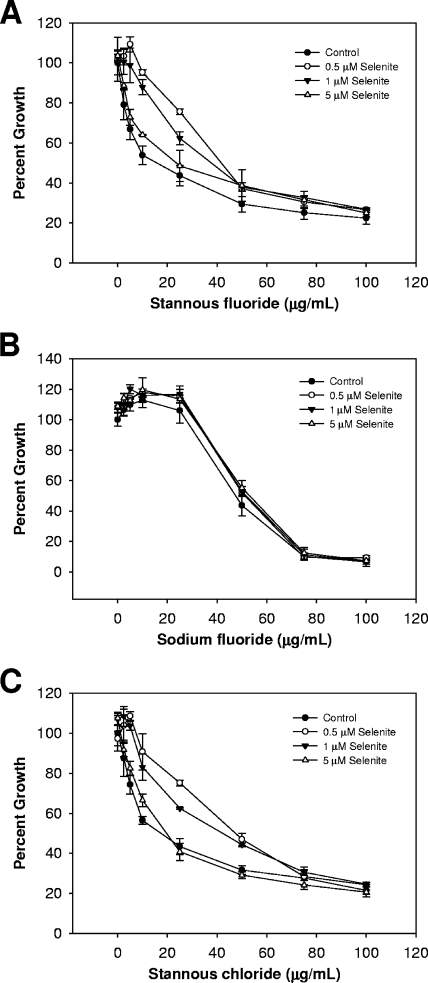
If stannous ions present in the growth medium are interacting with available selenium, then selenium supplementation should alleviate the observed growth inhibition. Selenium was added to the growth medium in the form of sodium selenite (Fig. 2) or l-selenocysteine (Fig. 3) before inoculation. This addition reduced but did not eliminate the effect of stannous fluoride on T. denticola's growth. Similar results were obtained for stannous chloride. It should be noted that the lowest concentration of selenite tested had the most pronounced effect on growth when added to cells alongside either stannous fluoride or stannous chloride, suggesting that a simple titration of selenium and tin cannot fully explain the changes in growth observed. It is possible that an insoluble complex of tin and selenium is formed and that this complex is more toxic at higher concentrations.
FIG. 2.
Supplementation with sodium selenite counteracts the antimicrobial nature of stannous ions but of not fluoride. (A to C) Cultures were grown and optical densities recorded as described in the Fig. 1 legend, in the presence of stannous fluoride (A), sodium fluoride (B), and stannous chloride (C). Sodium selenite was added prior to inoculation. Data shown are from at least three independent cultures. Error bars indicate standard deviations.
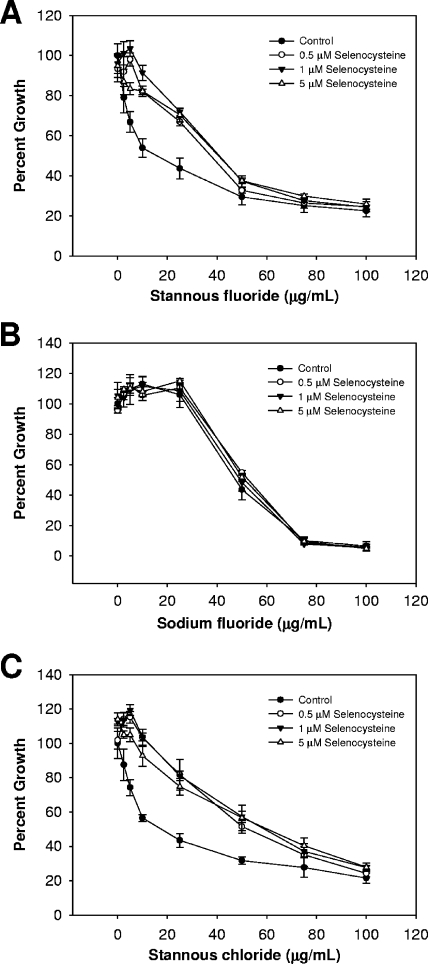
FIG. 3.
Supplementation with selenocysteine also alleviates stannous ion-dependent growth inhibition. (A to C) Cultures were grown and optical densities recorded as described in the Fig. 1 legend, in the presence of stannous fluoride (A), sodium fluoride (B), and stannous chloride (C). Selenocysteine was added prior to inoculation. Data shown are from at least three independent cultures. Error bars indicate standard deviations.
Both selenite and selenocysteine can be utilized for selenoprotein synthesis (25). In either case, the selenium atom must be reduced to Se2−, which is highly reactive and serves as the substrate for selenophosphate synthetase (40). Se2− is the most likely candidate for interaction with stannous ions in T. denticola. For both stannous chloride and stannous fluoride, selenocysteine had a more pronounced effect on the toxicity of tin than sodium selenite. Moreover, there was clearly not an additional toxicity when selenocysteine was present in increasing levels, as was the case with the addition of selenite. Without a further understanding of how T. denticola processes and utilizes selenium, this difference cannot yet be explained.
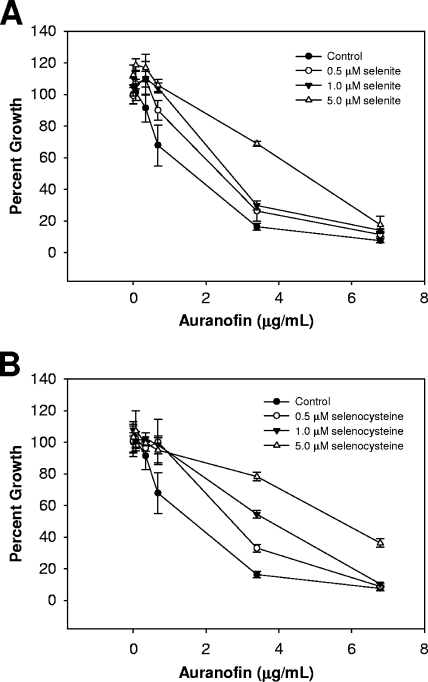
In contrast to the results obtained with the stannous salts, the toxicity of sodium fluoride was unaffected by the addition of selenium to the growth medium (Fig. 2B and 3B). The antimicrobial property of the fluoride ion alone does not appear to be related to T. denticola's dependence on selenoproteins for growth. Taken together, these results suggest that an intersection exists between selenium metabolism and stannous salt toxicity. We used auranofin to further demonstrate this point. The addition of both selenite and selenocysteine in the presence of auranofin induced a protective effect similar to that observed with the stannous salts (Fig. 4). Likewise, selenocysteine was more potent in reducing the toxicity.
FIG. 4.
Selenium supplementation in the presence of auranofin yields an effect similar to that observed with stannous salts. Cultures were grown and optical densities recorded as described in the Fig. 1 legend. Cultures were treated with various concentrations of auranofin (0.068, 0.34, 0.68, 3.4, and 6.8 μg/ml, final concentrations). (A and B) Selenium in the form of sodium selenite (A) or l-selenocysteine (B) were added prior to inoculation. Percent growth (growth yield of inhibited cultures versus the yield of the control) is plotted. Data shown are from at least three independent cultures. Error bars indicate standard deviations.
Stannous salts and auranofin inhibit selenoprotein synthesis.
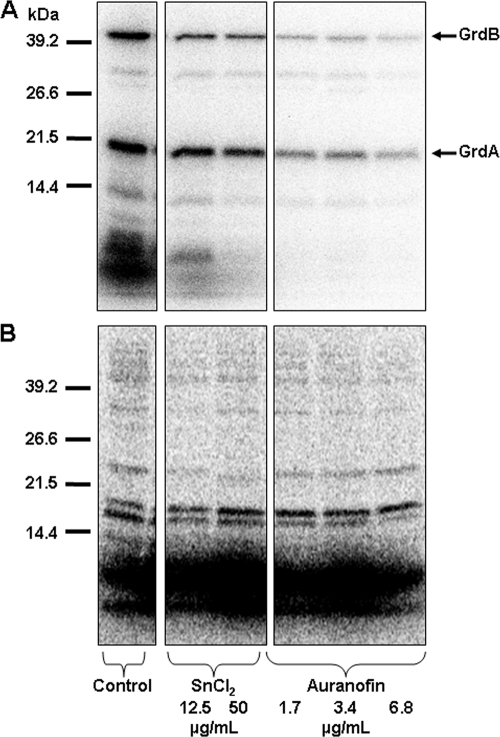
Radiolabeling studies were performed to further examine the relationship between stannous salts and selenium metabolism. Inhibitory concentrations of stannous chloride and auranofin were used to treat actively growing cultures of T. denticola (48 h after inoculation), followed by the addition of the radiolabel, 3 μCi of 75Se in the form of sodium selenite (10 nM). Cultures were incubated for 4 h to allow for de novo protein synthesis. Cells were harvested by centrifugation for 5 min at 5,000 × g and resuspended in lysis buffer (50 mM Tris, pH 7, 1 mM dithiothreitol, 0.5 mM EDTA, 0.1 mM benzamidine). Cells were lysed by sonication using a sonic dismembranator, model 100 (Fisher Scientific), for 10 s at a power output of 12 W, and the resultant crude cell extracts were clarified by centrifugation at 13,500 × g for 10 min. Protein concentration was determined with the Bradford assay, using bovine serum albumin (Pierce) as a standard (5). Selenoproteins and total protein synthesis were analyzed by separation of 20 μg of cell extracts using a 12% sodium dodecyl sulfate-polyacrylamide gel, and radioisotope-labeled proteins were detected by PhosphorImager analysis (Molecular Dynamics). Both stannous chloride and auranofin inhibited 75Se incorporation (Fig. 5A). Interestingly, the most-profound effect was to reduce the presence of low-molecular-weight selenium compounds.
FIG. 5.
Stannous chloride and auranofin inhibit 75Se incorporation into T. denticola. (A and B) Actively growing cultures were treated with stannous chloride (12.5 and 50 μg/ml) or auranofin (1.7, 3.4, and 6.8 μg/ml), followed by the addition of 75Se (sodium selenite) (A) or 35S (methionine-cysteine mixture) (B). Cultures were harvested after 4 h at 37°C, and crude cell extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Radiolabeled proteins were visualized by using a phosphorimager (Molecular Dynamics). Identification of glycine reductase subunits (GrdA and GrdB, 18 and 45 kDa, respectively) is based upon predicted molecular masses (32).
Replicate cultures were labeled with 35S (44 μCi of 35S-labeled methionine-cysteine mixture) to evaluate total protein synthesis. Both stannous chloride and auranofin induced a slight reduction in 35S labeling (Fig. 5B). The relative level of incorporation of 75Se or 35S was determined by quantitative phosphoimage analysis (ImageQuant; Molecular Dynamics). To control for the differences in total protein synthesis, the ratio of 75Se band intensity to that of the major bands labeled with 35S was calculated (Table 1). Despite the decrease in total protein, both stannous chloride and auranofin specifically reduced selenoprotein synthesis compared to the level in the control. While this reduction was expected with auranofin, based on our recently published results from studying selenium metabolism in Clostridium difficile (22), the effect of stannous chloride provides additional evidence that stannous ions interfere with selenium metabolism in this organism.
TABLE 1.
Quantification of selenium incorporation into selenoproteins
| Condition | Concentration (μg/ml) | 75Se/35Se ratioa |
|---|---|---|
| Control | 9.7 | |
| SnCl | 12.5 | 5.3 |
| 50 | 2.9 | |
| Auranofin | 1.7 | 1.4 |
| 3.4 | 1.2 | |
| 6.8 | 0.75 |
Relative incorporation of 75Se or 35S into proteins was determined by quantitative phosphoimage analysis (ImageQuant; Molecular Dynamics).
Stannous chloride prevents the uptake of selenium.
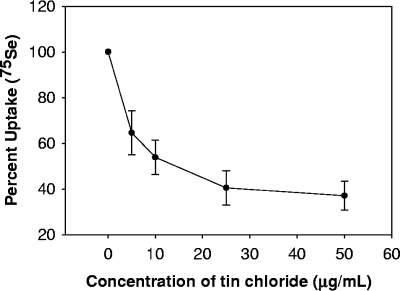
The reduction in the small-molecule selenium compounds and selenoprotein synthesis observed by radiolabeling indicates that stannous ions interfere with the ability of T. denticola to utilize nutritionally available selenium from the growth medium. It is unclear at which point in selenium metabolism this interference occurs. The mechanisms of uptake and processing of nutritional sources of selenium by either prokaryotic or eukaryotic cells are poorly understood. However, we have recently shown that hydrogen selenide is likely the form of selenium taken up by mammalian cells (14) or Clostridium difficile (22). We can determine whether stannous salts inhibit selenium uptake by T. denticola by following the assimilation of 75Se in whole cells. The impact of stannous ions on selenium uptake was determined in actively growing cells. Chloramphenicol (30 μg/ml) was added to inhibit protein synthesis. Stannous chloride was added to the cultures, followed immediately by 4 μCi of [75Se]selenite (2 nM selenium). Cultures were incubated for 20 min at 37°C. Cells were harvested by centrifugation (1 min at 16,200 × g). Cells were washed once in 500 μl PBS and harvested by centrifugation (1 min at 16,200 × g). The supernatant was discarded, and the cells were resuspended in 500 μl of phosphate-buffered saline before the total uptake of 75Se was measured by using a model 1470 gamma counter (Perkin-Elmer, Wellesley, ME). Stannous chloride potently inhibits the uptake of selenium (Fig. 6).
FIG. 6.
Stannous chloride prevents uptake of 75Se by T. denticola. Actively growing cultures were treated with stannous chloride (5, 10, 25, and 50 μg/ml), followed by the addition of 75Se (sodium selenite). Cultures were incubated for 20 min at 37°C. Cells were harvested by centrifugation and washed with phosphate-buffered saline. Total uptake of 75Se was analyzed by using a model 1470 gamma counter (Perkin-Elmer, Wellesley, ME) and is reported as percent uptake (compared to level in control). Data shown are from at least three independent cultures. Error bars indicate standard deviations.
Although we have added selenium in the form of selenite for our labeling study (only form available), it is clear from many chemical and biochemical studies that selenite will react with any available thiols present in the culture medium to form selenotrisulfides and hydrogen selenide (12, 13, 26, 34). Tin selenides are commonly studied in materials science, especially in the formation of solid thin films (23), yet no literature exists on solution complexes or adducts of SnSe or SnSe2 in a biological context. In a recent work, we describe an adduct of auranofin that is a stable Au-Se complex formed upon reaction of the gold drug with hydrogen selenide (22). Hydrogen selenide is unstable under aerobic conditions (quickly oxidizes and forms a red precipitate); however, we found that reaction of auranofin and selenide led to the formation of a stable adduct that did not yield a red precipitate (22). Likewise, we have also mixed tin chloride and tin fluoride with hydrogen selenide and found that a red precipitate was not formed but that a colored precipitate did form (data not shown). This reactivity suggests that we are forming SnSe and SnSe2 insoluble salts, and the chemistry of this reaction will be the subject of ongoing investigation emanating from this work. It should be noted that although we did see a reduction in selenium transport, we are still not sure that any chemical reaction that occurs between tin and selenium is limited to the extracellular milieu.
The impact of stannous salts on the growth of T. denticola was previously established without deriving the mechanism of action. Here we demonstrate that stannous salts impair selenium metabolism in this organism. Given that selenium is required for the synthesis of glycine reductase and, consequently, acetyl phosphate for ATP synthesis, we proposed that that is the root of growth inhibition. Stannous fluoride is widely used in toothpastes and other oral treatments. Understanding the implications of these results requires a further understanding of the role of selenium metabolism and amino acid fermentation in the oral bacterial community.
Acknowledgments
We thank Cassandra Korsvik, Rebecca Tarrien, Janet Dowding, Milan Srivastava, and Sarah Talbot for their assistance in maintaining cultures and radiolabeling studies.
This work was supported in part by a grant from the Florida Department of Health to W.T.S. (05-NIR-10) and a grant from the NIH (ES01434).
Footnotes
Published ahead of print on 10 April 2009.
REFERENCES
- 1.Andreesen, J. R., M. Wagner, D. Sonntag, M. Kohlstock, C. Harms, T. Gursinsky, J. Jager, T. Parther, U. Kabisch, A. Grantzdorffer, A. Pich, and B. Sohling. 1999. Various functions of selenols and thiols in anaerobic gram-positive, amino acids-utilizing bacteria. Biofactors 10263-270. [DOI] [PubMed] [Google Scholar]
- 2.Bowen, W. H. 1994. Food components and caries. Adv. Dent. Res. 8215-220. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y. W., M. Umeda, T. Nagasawa, Y. Takeuchi, Y. Huang, Y. Inoue, T. Iwai, Y. Izumi, and I. Ishikawa. 2008. Periodontitis may increase the risk of peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 35153-158. [DOI] [PubMed] [Google Scholar]
- 4.Chiba, M., N. Fujimoto, and M. Kikuchi. 1985. Protective effect of selenium on the inhibition of erythrocyte 5-aminolevulinate dehydratase activity by tin. Toxicol. Lett. 24235-241. [DOI] [PubMed] [Google Scholar]
- 5.Chiba, M., N. Kamiya, and M. Kikuchi. 1988. Experimental study on interactions between selenium and tin in mice. Biol. Trace Elem. Res. 15289-301. [DOI] [PubMed] [Google Scholar]
- 6.Chiba, M., and A. Shinohara. 1992. Inhibition of erythrocyte 5-aminolaevulinate hydrolase activity by tin and its prevention by selenite. Br. J. Ind. Med. 49355-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone, J. E., R. M. Del Rio, J. N. Davis, and T. C. Stadtman. 1976. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc. Natl. Acad. Sci. USA 732659-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cone, J. E., R. M. del Rio, and T. C. Stadtman. 1977. Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J. Biol. Chem. 2525337-5344. [PubMed] [Google Scholar]
- 9.Curtis, M. A., C. W. Kemp, S. A. Robrish, and W. H. Bowen. 1983. Stickland reactions of dental plaque. Infect. Immun. 42431-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, B. E., and R. J. Anderson. 1987. The epidemiology of dental caries in relation to environmental trace elements. Experientia 4387-92. [DOI] [PubMed] [Google Scholar]
- 11.Ellen, R. P., and V. B. Galimanas. 2005. Spirochetes at the forefront of periodontal infections. Periodontol. 2000. 3813-32. [DOI] [PubMed] [Google Scholar]
- 12.Ganther, H. E. 1999. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis 201657-1666. [DOI] [PubMed] [Google Scholar]
- 13.Ganther, H. E. 1968. Selenotrisulfides. Formation by the reaction of thiols with selenious acid. Biochemistry 72898-2905. [DOI] [PubMed] [Google Scholar]
- 14.Ganyc, D., and W. T. Self. 2008. High affinity selenium uptake in a keratinocyte model. FEBS Lett. 582299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia, G. E., and T. C. Stadtman. 1991. Selenoprotein A component of the glycine reductase complex from Clostridium purinolyticum: nucleotide sequence of the gene shows that selenocysteine is encoded by UGA. J. Bacteriol. 1732093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graentzdoerffer, A., A. Pich, and J. R. Andreesen. 2001. Molecular analysis of the grd operon coding for genes of the glycine reductase and of the thioredoxin system from Clostridium sticklandii. Arch. Microbiol. 1758-18. [DOI] [PubMed] [Google Scholar]
- 17.Hadjimarkos, D. M. 1965. Effect of selenium on dental caries. Arch. Environ. Health 10893-899. [DOI] [PubMed] [Google Scholar]
- 18.Hadjimarkos, D. M. 1969. Selenium: a caries-enhancing trace element. Caries Res. 314-22. [DOI] [PubMed] [Google Scholar]
- 19.Hadjimarkos, D. M., C. A. Storvick, and L. F. Remmert. 1952. Selenium and dental caries; an investigation among school children of Oregon. J. Pediatr. 40451-455. [DOI] [PubMed] [Google Scholar]
- 20.Howell, G. O., and C. H. Hill. 1978. Biological interaction of selenium with other trace elements in chicks. Environ. Health Perspect. 25147-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes, C. A., and W. W. Yotis. 1986. Effect of fluoride on Treponema denticola. Infect. Immun. 52914-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson-Rosario, S., D. Cowart, A. Myers, R. Tarrien, R. L. Levine, R. A. Scott, and W. T. Self. 2009. Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. J. Biol. Inorg. Chem. [DOI] [PMC free article] [PubMed]
- 23.John, K. J., B. Pradeep, and E. Mathai. 1993. Tin selenide (SnSe) thin films prepared by reactive evaporation. J. Mater. Sci. 291581-1583. [Google Scholar]
- 24.Kryukov, G. V., and V. N. Gladyshev. 2004. The prokaryotic selenoproteome. EMBO Rep. 5538-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacourciere, G. M., H. Mihara, T. Kurihara, N. Esaki, and T. C. Stadtman. 2000. Escherichia coli NifS-like proteins provide selenium in the pathway for the biosynthesis of selenophosphate. J. Biol. Chem. 27523769-23773. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann, T., and H. Hintelmann. 2002. Identification of selenium-containing glutathione S-conjugates in a yeast extract by two-dimensional liquid chromatography with inductively coupled plasma MS and nanoelectrospray MS/MS detection. Anal. Chem. 744602-4610. [DOI] [PubMed] [Google Scholar]
- 27.Moter, A., B. Riep, V. Haban, K. Heuner, G. Siebert, M. Berning, C. Wyss, B. Ehmke, T. F. Flemmig, and U. B. Gobel. 2006. Molecular epidemiology of oral treponemes in patients with periodontitis and in periodontitis-resistant subjects. J. Clin. Microbiol. 443078-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niederman, R. 2007. Stannous fluoride toothpastes reduce the gingival index more than sodium fluoride toothpastes. Evid. Based Dent. 874-75. [DOI] [PubMed] [Google Scholar]
- 29.Nisman, B. 1954. The Stickland reaction. Bacteriol. Rev. 1816-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paraskevas, S., and G. A. van der Weijden. 2006. A review of the effects of stannous fluoride on gingivitis. J. Clin. Periodontol. 331-13. [DOI] [PubMed] [Google Scholar]
- 31.Peters, S. R., M. Valdez, G. Riviere, and D. D. Thomas. 1999. Adherence to and penetration through endothelial cells by oral treponemes. Oral Microbiol. Immunol. 14379-383. [DOI] [PubMed] [Google Scholar]
- 32.Rother, M., A. Bock, and C. Wyss. 2001. Selenium-dependent growth of Treponema denticola: evidence for a clostridial-type glycine reductase. Arch. Microbiol. 177113-116. [DOI] [PubMed] [Google Scholar]
- 33.Sela, M. N. 2001. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 12399-413. [DOI] [PubMed] [Google Scholar]
- 34.Self, W. T., L. Tsai, and T. C. Stadtman. 2000. Synthesis and characterization of selenotrisulfide-derivatives of lipoic acid and lipoamide. Proc. Natl. Acad. Sci. USA 9712481-12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stickland, L. H. 1934. Studies in the metabolism of the strict anaerobes (genus Clostridium). I. The chemical reactions by which Cl. sporogenes obtains its energy. Biochem. J. 281746-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stickland, L. H. 1935. Studies in the metabolism of the strict anaerobes (genus Clostridium). III. The oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. Biochem. J. 29889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stickland, L. H. 1935. Studies in the metabolism of the strict anaerobes (Genus Clostridium). II. The reduction of proline by Cl. sporogenes. Biochem. J. 29288-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syrjanen, S. M., L. Alakuijala, P. Alakuijala, S. O. Markkanen, and H. Markkanen. 1990. Free amino acid levels in oral fluids of normal subjects and patients with periodontal disease. Arch. Oral Biol. 35189-193. [DOI] [PubMed] [Google Scholar]
- 39.Tank, G., and C. A. Storvick. 1960. Effect of naturally occurring selenium and vanadium on dental caries. J. Dent. Res. 39473-488. [DOI] [PubMed] [Google Scholar]
- 40.Veres, Z., I. Y. Kim, T. D. Scholz, and T. C. Stadtman. 1994. Selenophosphate synthetase. Enzyme properties and catalytic reaction. J. Biol. Chem. 26910597-10603. [PubMed] [Google Scholar]