Abstract
Summary: Infections by human papillomaviruses (HPVs) are the most frequently occurring sexually transmitted diseases. The crucial role of genital oncogenic HPV in cervical carcinoma development is now well established. In contrast, the role of cutaneous HPV in skin cancer development remains a matter of debate. Cutaneous beta-HPV strains show an amazing ubiquity. The fact that a few oncogenic genotypes cause cancers in patients suffering from epidermodysplasia verruciformis is in sharp contrast to the unapparent course of infection in the general population. Our recent investigations revealed that a natural barrier exists in humans, which protects them against infection with these papillomaviruses. A central role in the function of this HPV-specific barrier is played by a complex of the zinc-transporting proteins EVER1, EVER2, and ZnT-1, which maintain cellular zinc homeostasis. Apparently, the deregulation of the cellular zinc balance emerges as an important step in the life cycles not only of cutaneous but also of genital HPVs, although the latter viruses have developed a mechanism by which they can break the barrier and impose a zinc imbalance. Herein, we present a previously unpublished list of the cellular partners of EVER proteins, which points to future directions concerning investigations of the mechanisms of action of the EVER/ZnT-1 complex. We also present a general overview of the pathogenesis of HPV infections, taking into account the latest discoveries regarding the role of cellular zinc homeostasis in the HPV life cycle. We propose a potential model for the mechanism of function of the anti-HPV barrier.
INTRODUCTION
Among the human cancers induced by pathogens, as many as 35% result from infection by human papillomaviruses (HPVs) (49), giving an eminent place to this group of viruses in human pathologies. HPVs are a heterogeneous group of widespread DNA viruses, and they differ largely in their tropisms and oncogenic potentials (38, 189, 236). HPVs are the agents of skin lesions (different clinical types of warts) or mucous membrane lesions (genital warts or condylomas and cervical, anal, vulvar, or vaginal intraepithelial neoplasias), which in general are spontaneously cured but can evolve toward invasive cancer under certain conditions.
Basic Features and Classification of Papillomaviruses
The papillomaviruses are part of the family Papillomaviridae (18). These ubiquitous viruses are detected in numerous animal species including humans and are generally specific to their respective hosts. HPVs are small, nonenveloped viruses that are 55 nm in diameter and are very resistant (54). A circular double-stranded DNA molecule of about 8,000 nucleotide base pairs that contains 7 to 9 open reading frames (ORFs), depending on the genotype (235), constitutes the HPV genome. The viral DNA is associated with cellular histones to form a “minichromosome” (51) and is enclosed in an icosahedral capsid consisting of 72 capsomers (39, 54).
The classification of papillomaviruses is based on a comparison of the nucleotide sequence of the major capsid L1 ORF (37). A genotype is defined by sequence homology of below 90%. Subtypes or variants of the same genotype correspond to a nucleotide identity of between 90 and 98% or above 98%, respectively. An alternative classification system based on the nucleotide sequence of the E1 and E2 ORFs has recently been proposed (23). Apart from that, HPVs are also classified according to their tissue tropisms (cutaneous or mucosal) on the one hand and according to their oncogenic potentials on the other hand (190, 236).
A high level of genetic heterogeneity of HPV genotypes has been described (24, 65, 77). Up until now, more than 200 HPV genotypes have been identified, 112 (HPV1 to HPV112) of which were characterized after cloning and sequencing of their genomes (23, 37, 56, 57). The phylogeny of papillomaviruses indicates that these viruses have evolved by multiple mechanisms including recombination events but not exclusively recombination between the virus and the corresponding host (71). Papillomaviruses are classified into 16 distinct genera, and most HPVs belong to either the alpha or beta genus. The alpha genus comprises genital/mucosal HPVs and some cutaneous genotypes such as HPV2, HPV3, and HPV10, which cause skin warts. The beta genus is associated mainly with unapparent skin infections, but beta-HPV can induce lesions in immunocompromised individuals. The remaining HPVs are classified into three genera (gamma, mu, and nu) and induce cutaneous papillomas or warts (37).
Genetic Organization
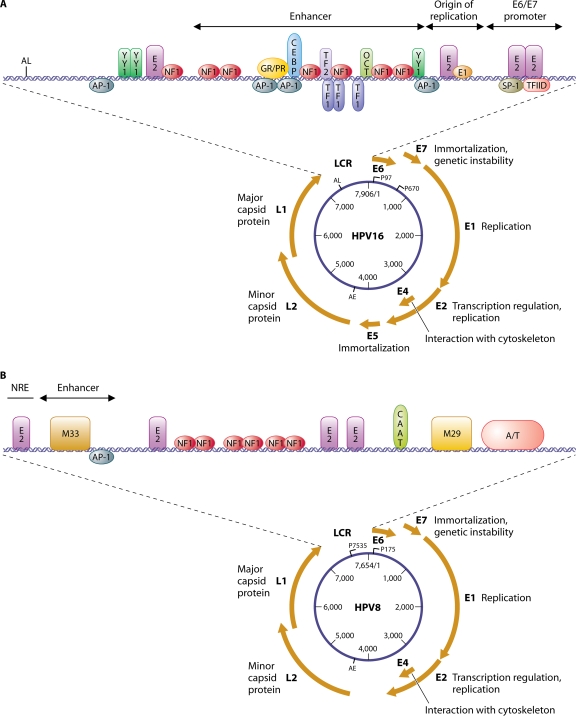
All genotypes share the same pattern of genetic organization, with only one coding strand. The HPV genome can be divided into a coding region and a long control region (LCR) (41, 54, 235). The former encodes early (E) and late (L) proteins (Fig. 1A and B). The early proteins are involved in the regulation of viral genome replication and expression as well as in the adaptation of the cell to the demands of the virus (see below). The late proteins are structural constituents of the viral capsid. The LCR is placed upstream of the E region, and this LCR comprises approximately 10 to 15% of the viral genome. Many potential binding sites for numerous cellular transcription factors have been identified within the HPV LCR. The transcription factors in question are AP1, cEBP NF1, YY1, Oct1, TEF1 (TF1), TFIID, Sp1, and glucocorticoid receptor and progesterone receptor (GR/PR), and the potential binding sites might be biologically important for viral genome expression (19) (Fig. 1A and B). In addition, the positioning of E2-binding sites in the mucosal HPV LCR most probably reflects common features for the regulation of viral genome expression. The 5′ part of the LCR contains transcription termination and polyadenylation sites (AL) for the late transcripts. The central region functions as an epithelium-specific transcriptional enhancer. It is believed that the tissue tropism displayed by HPV is related to this region. The 3′ part contains a single E1-binding site that corresponds to the origin of replication and sequences recognized by the viral E2 protein and transcription factors that define the E6 and E7 promoter. The binding of E2 to this promoter leads to the repression of viral E6 and E7 genes (207).
FIG. 1.
Genetic organization of the HPV16 (A) and HPV8 (B) genomes. The main functions of the early (E) and late (L) genes are mentioned. Promoters (P97 and P670) and early (AE) and late (AL) polyadenylation sites are indicated for HPV16. Early (P175) and late (P7535) sites are mentioned for HPV8. A schematic representation of the LCR is shown at the top of the figure, comprising an enhancer region, the origin of viral replication, and the E6/E7 promoter, as well as some binding sites for viral (E2) or cellular (YY1, NF1, AP1, GR/PR, CEBP, TEF1, TEF2, OCT-1, Sp1, and TFIID) transcription factors. Specific conserved motifs (M33 and M29) among the beta-HPVs are shown in the LCR of HPV8.
Cutaneous HPVs differ from other HPVs in that they have a less easily identifiable binding site for the E1 replication protein, and consequently, the origin of replication is not shown for HPV8 (Fig. 1B). HPVs belonging to the beta genus have a short LCR and different organization of regulatory sequences compared to those of the other genera (180). These viruses also share specific conserved motifs, designated M33 and M29. The M33 motif is followed by a unique AP1-binding site, and these sequences have been shown to correspond to a constitutive transcriptional enhancer (86) for the HPV8 late promoter (P7535). In contrast, the 38-bp sequence that contains an E2-binding site corresponds to a negative regulatory element for this promoter (134). The cellular transcription factor IRF5.2 has been found to interact with the NRE and M29 motifs, leading to the downregulation of the HPV8 early promoter (P175). In addition, IRF5.2 has been demonstrated to be a transcriptional repressor of beta-HPV genomes such as HPV5, -8, -14, and -25 (1). It must be stressed that E2-binding sites observed in the early promoter of HPV16 (Fig. 1A) and other alpha-HPVs are not detected in the beta-HPVs. This suggests a different regulation mechanism of the early promoter of mucosal and beta-HPV.
The Papillomavirus Life Cycle
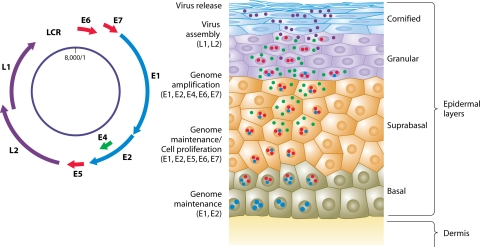
HPVs infect stratified epithelia, and their whole life cycle is inextricably linked to keratinocyte differentiation (recently reviewed in references 41 and 125). Upon entering the host cell, papillomaviruses might initiate long-term productive lesions. However, in order to establish a persistent infection, an HPV has to overcome a few “obstacles.” First, as differentiation of the stratified epithelia is associated with continuous cellular turnover and desquamation of the terminally differentiated keratinocytes, maintenance of the papillomavirus within the tissue requires the infection of basal epithelial cells. This is thought to take place when these cells are directly exposed to the virus during microinjuries (47). Infection of epithelial stem cells, which have the capacity for self-renewal, can ensure long-term maintenance of the viral genome. Although the exact entry receptor has not yet been unequivocally determined, the role of heparin sulfate in this process has been described (66, 94). In the basal epithelial layer, the viral genome is initially replicated at a relatively low copy number. The HPV E1 and E2 proteins are expressed in these cells and bind to the viral origin of replication, letting cellular polymerases replicate episomal papillomavirus genomes (59, 60, 139). The E2 protein is also involved in the segregation of viral genomes during stem cell or basal cell division (40, 227, 233). Second, the virus has to cope with the loss of the proliferation potential of differentiating cells. After they exit the basal layer, epithelial cells stop proliferating and initiate a terminal differentiation program. Papillomaviruses using the cellular machinery for their replication might need the environment of actively dividing cells. On the other hand, the expression of some of the viral genes requires transcription factors, which emerge only in differentiated keratinocytes. Therefore, while HPV may need to maintain cell division, it could require delayed differentiation but not the entire inhibition of this process. These conditions could ensure HPV DNA replication in the suprabasal and upper granular layers (Fig. 2). It seems that the E7 protein is crucial for the maintenance of proliferation competence in the upper layers of the epithelium (28), but cooperation with another viral protein, E6, seems to be essential. E7 promotes S-phase progression in differentiated cells, whereas the E6 protein is involved in the prevention of apoptosis, which can be induced in response to such an unscheduled S-phase entry (39, 41). As a consequence, in HPV-induced lesions, the dividing cells can also be found in suprabasal layers. Nevertheless, during normal productive infection, proliferating cells in upper layers remain relatively rare. This probably results from an equilibrium between cellular (cyclin-dependent kinase inhibitors) and viral (E7) cell cycle-controlling factors. Apparently, the balance between these factors is maintained in the majority of cells, and cell division can be initiated in only a few of them. In contrast to such benign lesions, along with progression to cancer, dividing cells become common in the suprabasal layers, which might represent a consequence of the deregulation of the normal HPV life cycle, particularly the loss of a “negative regulator,” such as E2, and increased levels of expression of E6 and E7. As a result, the normal cell stratification becomes disturbed, with nucleated and dividing cells found in both suprabasal and upper layers.
FIG. 2.
The HPV life cycle. (Adapted from reference 39 with permission of the publisher.)
Subsequently, amplification of the previously synthesized viral DNA takes place, and genomes are packed into viral particles during the late stages of viral multiplication in the uppermost layers of the epithelium. In the middle and upper layers, other viral proteins, E4 and E5, are expressed, and these proteins participate in viral DNA amplification in concert with E1/E2. Once the viral DNA has been amplified, the expression of the capsid proteins L1 and L2 begins, and this is followed by assembly of the complete virions. The viruses thereafter leave the epithelium, together with the shed cornified squames. How the virus escapes the terminally cornified cells is not entirely clear, but the E4 protein is supposed to contribute to viral egress by binding keratin filaments and disrupting their structure (42).
Altogether, this simplistic view of the papillomavirus life cycle clearly demonstrates that the expression of the HPV genes is tightly regulated and strictly linked to epithelial differentiation (Fig. 2). From a molecular point of view, an important role in the control of viral genome expression is played by both the viral proteins and host factors (19, 124, 235). Among the multiple cellular transcription factors whose role in the HPV genome expression has been discussed, AP-1 seems to play a central position, although binding sites for multiple other factors, such as Sp-1, NF-1, TEF-1, TEF-2, Oct-1, AP-2, KRF-1, and YY1, have also been found, and their roles in the HPV life cycle have been postulated (Fig. 1).
Pathogenicity
Cutaneous HPV.
Most HPVs target skin and provoke benign proliferations (warts and papillomas) that regress spontaneously in 1 to 5 years. The malignant transformation of these lesions is observed only in patients suffering from epidermodysplasia verruciformis (EV) (see below) (132, 151, 152). Among the beta-HPVs, HPV5 and HPV8 are classified as high-risk genotypes since they are associated with skin carcinomas occurring in EV patients (151, 152). It is worth stressing that beta-HPVs have been detected in trace amounts in a large proportion of nonmelanoma skin carcinomas (NMSCs) of non-EV patients, particularly those carcinomas that develop in immunocompromised individuals (57, 153, 165, 171, 202). Moreover, it has been suggested that these viruses may contribute to skin tumors in immunocompetent individuals (75), and a possible role of beta-HPV in skin carcinogenesis constitutes a hot research topic (35, 76, 153). Recent epidemiological and molecular studies of beta-HPV have provided some insights into the mechanism of their contribution to skin carcinogenesis (20, 224).
Mucosal HPV.
HPVs with mucous tropism are at the origin of the most frequently identified sexually transmitted infections in the world (9, 210). These viruses are responsible for genital condylomas in 1 to 2% of the sexually active population and for cervical intraepithelial neoplasias in approximately 5% of women. The number of women having an HPV infection of the cervix (the presence of HPV DNA) is estimated to be 291 million. Most women would be predicted to be infected at least once in their life by the one of the genotypes that are together responsible for 70% of cervical cancers (HPV16 and -18) (26, 196, 229).
More than 40 genotypes that are capable of infecting genital mucous membranes have been identified and characterized (38, 46, 142, 157, 211). These HPV genotypes are usually divided into two groups according to their oncogenic potentials (236). HPV genotypes detected mostly in anogenital condylomas and in certain low-grade cervical intraepithelial Malpighian damages are considered to be low-risk HPVs, whereas HPVs preferentially found in cervical and anogenital cancers constitute the high-risk group. Among the mucocutaneous viruses belonging to the alpha genus, 18 HPVs were classified as being high risk (HPV16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59) or probably high risk (HPV26, -53, -66, -68, -73, and -82), 12 HPVs were classified as being low risk (HPV6, -11, -40, -42, -43, -44, -54, -55, -61, -72, -81, and -89), and 24 were classified as being of undetermined risk (HPV2, -3, -7, -10, -27, -28, -29, -30, -32, -34, -57, -62, -67, -69, -71, -74, -77, -83, -84, -85, -86, -87, -90, and -91) (31, 142, 143).
Persistent lesions induced by potentially oncogenic HPV (mainly HPV16 and -18) may, in rare cases, evolve into invasive cervical carcinomas (107, 189, 190). HPV16 and HPV18 are together responsible for about 70% of cervical carcinomas, the second leading cause of death from cancer in women worldwide (31). In addition, viral DNA sequences are detected in the vast majority of adenocarcinomas, adenosquamous carcinomas, and squamous cell carcinomas, which are preceded by dysplatic lesions corresponding to cervical intraepithelial neoplasia grades 2 and 3 and adenocarcinoma in situ (142, 189). High-risk mucosal HPVs are also thought to contribute to the pathogenesis of other anogenital and upper aerodigestive tract cancers. Effectively, about 85% of anal cancers (61, 157); 50% of cancers of the vulva, vagina, and penis (89, 93, 96, 198, 204, 217); 20% of oropharyngeal cancers (21, 44, 178); and 10% of laryngeal (237) and esophageal cancers (126) are attributable to HPV.
Epidemiology of Genital HPV
HPV infection occurs early at the beginning of sexual activity. The risk of infection is correlated with the age at which the first sexual intercourse takes place as well as with the number of sexual partners (85, 228). The peak of HPV prevalence, which varies from 20 to 30% of women, is observed between 16 and 25 years of age (67, 229), and it diminishes after the age of 25 years, reaching a level of 5% in women above 45 years old. The prevalence of HPV infection in men is highly variable, ranging between 3.5 and 45%. Multiple-HPV infection is observed in 20 to 30% of cases.
It has been reported that HPV16 has a significantly lower clearance rate than infections with low-risk HPV genotypes. HPV types related to HPV16 (types 31, 33, 35, 52, and 58) show intermediate clearance rates, whereas other high-risk types do not show evidence of slow clearance compared to that of infection with low-risk types (140). In a recent study, it was shown that the median time for clearance of any HPV infection in men was about 6 months, with comparable clearance times for oncogenic and nononcogenic infections (67).
Molecular Mechanisms of Carcinogenesis Induced by High-Risk Genital HPV
Role of the E2 protein.
In productive lesions, the viral genome in infected cells remains in the episomal form irrespective of the oncogenic potential of the virus (41). It is well recognized that the integration of viral DNA sequences into the host cell genome plays an essential role in malignant transformation. Integrated sequences are detected in most genital cancers and in cell lines derived from HPV-induced carcinomas. The integration of high-risk HPV DNA into the host cell genome arises at early stages of carcinogenesis (161, 163, 187, 191), and the frequency of this event increases with the progression of cervical intraepithelial neoplasia to cervical carcinoma (220). Interestingly, the integration of HPV DNA into the host genome interrupts the E1 or E2 ORF, while the E6 and E7 ORFs remain intact. Since the E2 protein represses the expression of the E6 and E7 genes (38, 207, 235), the loss of the E2 ORF upon the integration of viral DNA promotes the synthesis of the E6 and E7 proteins, most probably contributing in this way to cancer development (9, 190, 230). In this context, HPV genome integration represents a rather rare and paradoxical phenomenon of viral replication (235).
Deregulation of the cell cycle.
Three HPV proteins can affect cell proliferation: E5, E6, and E7. Although it has been reported that HPV E5 displays some (rather weak) transforming potential, the exact molecular basis of its action is not fully elucidated (199, 201), and our current knowledge concerning this subject will be presented in the following sections.
Oncogenic genital HPVs differ from low-risk HPVs mainly by the biological properties of the E6 and E7 proteins (38, 45), which, working synergistically, play a central role in the process of malignant transformation. It is believed that their persistent expression is necessary for the maintenance of a transformed phenotype (39, 141). Numerous cellular partners of high-risk HPV-derived E6 and E7 proteins have been identified (38, 141, 144, 189). Among them, the two major factors are p53 and pRB, which participate in the preservation of cellular genome integrity and the control of the transition from the G1 into the S phase of the cell cycle, respectively (38, 45, 79, 235). The identification of these interactions implies that E6 and E7 proteins cooperate to promote the cell cycle, inhibit apoptosis, and immortalize the cell. All these phenomena can support the accumulation of mutations in the cell genome.
Biological properties of the E6 and E7 proteins of high-risk HPV.
It has been shown that the E6 protein of high-risk HPV interferes with p53 function, displaying antiapoptotic activity (231). Cell stress induced by viral infection or other physical or chemical stress events and/or factors increases p53 synthesis. This leads either to the activation of the p21/cip1 transcription factor and the block of the cell cycle in G1 phase or to apoptosis after the activation of the transcription of the bax gene (141). The E6 protein binds to p53 and recruits it to the E6 AP protein, an E3 ubiquitin-protein ligase (231). This facilitates the ubiquitination of p53 and leads to its degradation, significantly decreasing the half-life of p53.
The E7 protein of high-risk HPV binds to pRb with high affinity and eliminates the regulation of the cell cycle driven by this factor (87). Thus, the E7-induced release of E2F transcription factors from their complex with pRb results in the synthesis of cellular genes involved in the replication of the cellular DNA and finally in the progression toward S phase. The E7 protein also interacts with p16, p21, and other proteins that inhibit cell cycle progression (141). Altogether, the E6 and E7 proteins ensure the replication of the HPV genome in differentiated postmitotic epithelial cells, and this deregulation of the natural differentiation program is proposed to be responsible for HPV-related cancerogenesis. In addition, the E6 and E7 proteins of high-risk HPV also play a role in an abnormal chromosomal segregation that possibly lies at the origin of genetic instability. The HPV16 E7 protein can rapidly modify the normal control of centrosome duplication, resulting in the formation of abnormal numbers of centrosomes and centrioles, which leads to aberrations in the number of chromosomes, in particular to aneuploidy (45).
It is worth stressing that the biochemical properties of the E6 and E7 proteins differ between high-risk and low-risk HPVs and that this is believed to determine the differences in their transforming potentials. For instance, in contrast to high-risk HPVs, the E6 protein encoded by low-risk HPVs does not have the capacity to inactivate p53. Furthermore, p53-independent mechanisms of E6 function might differ in high- and low-risk papillomaviruses. The E6 protein of high-risk HPVs contains a motif that binds the PDZ domain of numerous cellular proteins, possibly conferring their proteasomal degradation (141). Some of these PDZ-containing proteins serve as cell cycle negative regulators, and therefore, it has been proposed that the lack of a PDZ domain-binding motif in low-risk HPV E6 proteins could also account for their low transforming potential. Similarly, the E7 protein from low-risk HPV displays a lower transformation efficacy than that from high-risk HPV, and this could result from a single-amino-acid substitution in the pRB-binding site (80).
Molecular Mechanisms of Skin Carcinogenesis Associated with Beta-HPV
Our knowledge concerning the transforming properties of beta-HPV-derived proteins is much more limited. The transforming activity of HPV8 early proteins was supported by the development of skin tumors in transgenic mice expressing the early region under the control of the keratin K14 promoter (188). However, in contrast to high-risk genital HPV, beta-HPV DNA does not integrate into the cellular genome (151). In addition, beta-HPV E6 proteins do not target the p53 protein for degradation (214). This points to distinct transformation mechanisms between the different groups of viruses. It has been reported that the E6 protein of beta-HPV is able to lead the proapoptotic Bak protein, induced by UV, into degradation (91, 92, 118). It has also been reported that the expression of the E6 and E7 proteins of beta-HPV increases the life span of primary human epithelial cells and that the HPV38 E7 protein transforms rodent fibroblasts (25). Moreover, it has been reported that the HPV8 E2 protein has transforming activities in transgenic mice and that UV radiation dramatically accelerates the formation of skin tumors (164). Altogether, the available data suggest that beta-HPV infection might cooperate with UV exposure in the context of skin cancer development.
Recent studies have shown that the E6 proteins of HPV5 and HPV8 inhibit the transforming growth factor beta (TGF-beta) signaling pathway by the degradation of the SMAD3 transcription factor (137) (J. Mendoza and Y. Jacob, unpublished data). This degradation was not detected with E6 proteins of other cutaneous and mucosal HPV genotypes. It is worth stressing that the TGF-beta-triggered pathways lead to the synthesis of inhibitors (p16, p17, p21, and p27) of the cyclin-dependent kinases that play a crucial role in the cell cycle. It can be speculated that the specific degradation of SMAD3 could negatively regulate inhibitors of the cell cycle and favor cell transition from the G1 to the S phase, allowing viral DNA vegetative replication and, as a side effect, cell transformation.
GENETIC PREDISPOSITION TO HPV INFECTION
It is widely accepted that infection with high-risk HPV is not sufficient to cause skin, or even cervical, cancers (154, 189, 236), strongly implying the involvement of other factors or particular conditions. Definitely, one such important condition is the persistence of the infection. It seems that the host genetic background can alter the sensitivity to HPV and/or clearance of already-established papillomavirus infections. In fact, a high interindividual variability in the final clinical outcome of cutaneous and mucosal HPV infections has been observed. Genetic host factors controlling asymptomatic (latent) infection, transition from latent to clinical infection, regression or persistence of lesions, and malignant conversion are still poorly defined. However, several examples of rare genetic diseases associated with unusual sensitivity to papillomavirus infections can provide some important clues as to the mechanisms that protect the host against HPV.
Heck's Disease
Focal epithelial hyperplasia (FEH), also known as Heck's disease, is a distinct clinical entity, with the symptoms confined to the oral cavity and perioral skin, that lasts for several months or sometimes years (78, 166). FEH is characterized by multiple flat, sessile, and elevated papules that may be solitary but are usually multiple. It can be spontaneously cured or, conversely, permanent and resistant to treatment. Histologically, there is a hyperplasia of the epithelium with anastomosing rete ridges.
FEH results from an abnormal predisposition to the infection by two specific HPV genotypes (HPV13 and -32) related to genital alpha-HPV (16, 33, 138). These two viruses have been found in 75 to 100% of the reported cases of FEH (166). The disease has been observed in a significant proportion of Eskimos from Greenland and Canada as well as in the Amerindians of both North and South America. On the other hand, the disease is rare in Europe and generally concerns individuals coming from North Africa, where the rate of consanguinity is high. Thus, the ethnic background and the geographical distribution of FEH imply genetic factors as underlying the predisposition to infection with these two particular HPV genotypes. A recessive autosomal mode of transmission has been suggested on the basis of the analysis of familiar cases of FEH (169).
A few reports of FEH associated with human immunodeficiency virus infection have also been reported. Thus, FEH is now regarded as an oral manifestation of a human immunodeficiency virus-related opportunistic infection (138). These observations point to the fact that immunodeficiency and host genetic factors that could possibly also be related to an inherited (highly restricted and specific) immune defect confer sensitivity to HPV, manifested as FEH.
Severe Combined Immunodeficiency
Severe combined immunodeficiency (SCID) is a rare disease characterized by a lack of T lymphocytes associated, in certain cases, with a lack of natural killer cells (NK) and/or of B lymphocytes. Although hematopoietic stem cell transplantation is a life-saving treatment for SCID, severe cutaneous papillomavirus disease is frequently detected in patients 10 to 21 years after stem cell transplantation (63, 112, 117). It has been reported that patients displayed common warts or lesions similar to those induced by beta-HPV in EV patients. All HPV-positive patients had an NK− B+ immunophenotype of SCID associated with mutations in JAK3 or IL2RG (common gamma c receptor G) genes. No lesions were observed in NK+ patients, suggesting a possible role of NK cells and/or the JAK3/IL2RG-dependent signaling pathway in a predisposition to infection with cutaneous HPV (112). A similar X-linked SCID due to mutations in the IL2RG gene in dogs has been described (70). Also, in this model, severe papillomavirus infection that progressed to metastatic squamous cell carcinoma was observed in bone marrow-transplanted dogs.
WHIM Syndrome
WHIM is an acronym for wart, hypogammaglobulinemia, infection, and myelokathexis. WHIM syndrome is a rare, congenital immunodeficiency disorder characterized by chronic neutropenia and increased susceptibility to bacterial and viral infections (226). Patients exhibit HPV-induced common warts on their hands and feet and condylomas as well as cervical and vulval premalignant lesions. They are characterized by the retention of neutrophils in the bone marrow (myelokathexis) and a deficiency of lymphocytes and antibody levels.
WHIM syndrome results from autosomal dominant mutations in the CXCR4 chemokine receptor gene (82), leading to the deletion of 10 to 19 amino acids in the carboxy terminus of the CXCR4 protein. CXCR4 gene mutations in WHIM patients do not affect cell surface expression of the chemokine receptor and have been described not to change the receptor internalization upon stimulation with its ligand, CXCL12 (73). In contrast, it has been reported by other authors that cell trafficking abnormalities in some patients suffering from WHIM syndrome could be due to a decreased internalization of mutated CXCR4 that may lead to the prolongation and enhancement of signaling in response to CXCL12 (100). However, the refractoriness of CXCR4 to be desensitized and internalized in response to the CXCL12 chemokine constitutes a common feature of WHIM syndrome, leading to an enhancement of G-protein-dependent responses. This suggests that the high sensitivity of patients to develop warts and condylomas is linked to a constant activation of CXCR4 (12).
A recent study pointed to a role of G-protein-coupled receptor kinase 3 (GRK3) in WHIM syndrome, indicating the genetic heterogeneity of the disorder. The results revealed a pivotal role for GRK3 in regulating CXCR4 attenuation and provided a mechanistic link between the GRK3 pathway and WHIM syndrome in patients without CXCR4 mutations (13). It thus appears that the constant activation of CXCR4 in patients' cells accounts for a higher sensitivity to develop warts and other HPV-associated lesions.
Fanconi anemia, an inherited disease, may represent another example of predisposition to HPV infection, since it has been reported that patients develop HPV16-induced squamous cell carcinomas of the head, neck, as well as the anogenital region (110). However, a recent study failed to detect viral sequences in patients with this disease, and it was concluded that HPV does not play a major role in carcinogenesis in these patients (218). In contrast, it is well accepted that cervical carcinoma is a multifactorial disease, where HPV16 and HPV18 constitute the major environmental risk factor in association with host genetic factors that also influence the outcome of the disease (154).
From the data described above, it appears that distinct genetic defects are able to confer sensitivity to some pathogens including HPV. It might be suggested that there is a natural barrier protecting the host from HPV, not surprisingly at least in part founded on the grounds of the immune system. Nevertheless, in all the disorders presented, with the exception of Heck's disease and cervical cancer, the defect confers a relatively broad susceptibility not restricted to human papillomaviruses. Thus, in these cases, susceptibility to HPV constitutes solely one of the manifestations of a more general immune insufficiency, and on this basis, the existence of an HPV-directed barrier cannot be proposed. However, there is another human disease, EV, which is associated with a selective susceptibility to cutaneous HPV infections. Importantly, this susceptibility is absolutely specific and concerns solely this group of viruses. This suggests that a natural anti-HPV barrier truly exists and that a disruption of this barrier sensitizes the host to HPV-mediated diseases. Studies of EV allow not only a better understanding of the pathogenesis of HPV-mediated diseases, in particular cancers, but also offer a unique opportunity to investigate the molecular background of the natural anti-HPV barrier.
PREDISPOSITION TO BETA-HPV INFECTIONS: MODEL OF EPIDERMODYSPLASIA VERRUCIFORMIS
General Features
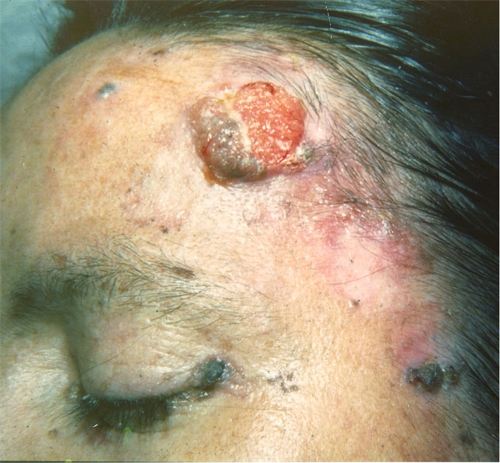
EV is a rare, lifelong autosomal recessive skin disease (OMIM [Online Mendelian Inheritance in Man] number 226400), recently classified as a primary immunodeficiency (145), which is associated with an unusual susceptibility to infections with ubiquitous beta-HPV but not with other pathogens including cutaneous or genital HPVs of the alpha, gamma, mu, and nu genera. EV was first described by Lewandowsky and Lutz in 1922 and was considered to be a potential model for a viral role in skin carcinogenesis (90). The disease appears in childhood and is characterized by disseminated polymorphic cutaneous lesions, including flat warts and pityriasis versicolor-like macules that can undergo malignant transformation in more than half of the patients, corresponding to Bowen's-type carcinoma in situ and invasive squamous cell carcinoma (Fig. 3). Tumors occur in the fourth or fifth decade and are localized mainly in sun-exposed areas of the skin, which indicates a preponderant role for environmental factors (notably, UV irradiation) (64, 132, 151, 152).
FIG. 3.
Patient suffering from epidermodysplasia verruciformis. Note the presence of a squamous cell carcinoma on the forehead.
Whereas patients are usually infected with more than one beta-HPV, only some of the genotypes (mainly HPV5 and HPV8) are detected in their skin carcinomas. HPV5 was the first HPV recognized to be involved in a human cancer (151, 155). EV carcinomas harbor a high copy number of HPV genomes and express high levels of E6 and E7 transcripts. In contrast to genital oncogenic HPV, the viral genome is maintained as episomes in EV cancers, highlighting different cell transformation mechanisms (discussed below).
The unusual sensitivity of EV patients to the particular group of viruses, together with clinical and epidemiological data, led us to assume the existence of a natural anti-HPV barrier. To elucidate the function of this barrier and the pathogenesis of EV, we had to first identify the genetic background underlying EV.
In Search of the Cellular Gene(s) Controlling Beta-HPV Infection
Although an X-linked recessive (3) or autosomal dominant mode of inheritance has been reported for EV, the disease is usually considered to be an autosomal recessive disorder (128, 174). The familial occurrence of the disease led to the search for a putative EV gene involved in the control of infection with HPV in infected families.
Genome-wide approach: discovery of EVER genes.
Studies with the homozygosity mapping strategy of three consanguineous families led to the identification of a major predisposition locus located in 17q25.3 (175). A second locus was subsequently identified on chromosome 2p24 in a French family (177). Genetic analysis of the 17q25.3 region led to the identification of two adjacent genes (EVER1 and EVER2), the mutation of which segregates with the disease (176). Homozygous mutations in either gene were shown to confer susceptibility to EV. Codons spanning the full length of the predicted ORFs encode a putative EVER1 protein (805 amino acids) and an EVER2 protein (726 amino acids) that share 28.6% amino acid homology. Both proteins are predicted to be integral membrane proteins, with 10 and 8 transmembrane domains, respectively. The EVER genes are thought to be transcribed in numerous tissues including the skin.
Six analogs of EVER genes have been identified in humans and mice and have been shown to constitute a novel gene family, designated TMC, for “transmembrane channel-like,” including EVER1 (TMC6) and EVER2 (TMC8). Homologues of TMC genes have been identified in invertebrates (Drosophila melanogaster, Anopheles gambiae, and Caenorhabditis elegans) and nonmammalian vertebrates (Fugu fish) (101). The function of TMC proteins is largely unknown, although it has been postulated that TMC1 may serve as an ion channel, or transporter, and it might be involved in the modulation of signal transduction (109). The discovery of EVER genes has opened a new avenue in the search for the molecular background of the anti-HPV barrier.
In Search of EVER Protein Functions
Cellular partners of EVER proteins.
To elucidate the physiological role of the EVER proteins, we performed a yeast two-hybrid screening assay to find their cellular partners. Indeed, protein partners of known function could provide clues as to EVER function and the cellular signaling pathway(s) affected by EVER mutations in patients suffering from EV. To this end, we screened a human keratinocyte cDNA library using a GAL4-based yeast two-hybrid system. To avoid problems with hydrophobic transmembrane domains (10 and 8 transmembrane domains for EVER1 and EVER2, respectively), which are susceptible to preventing the GAL4 fusion proteins from reaching the yeast nucleus, three truncated segments lacking the transmembrane domains and spanning the N-terminal or C-terminal luminal loops and the highly conserved TMC domain were designed.
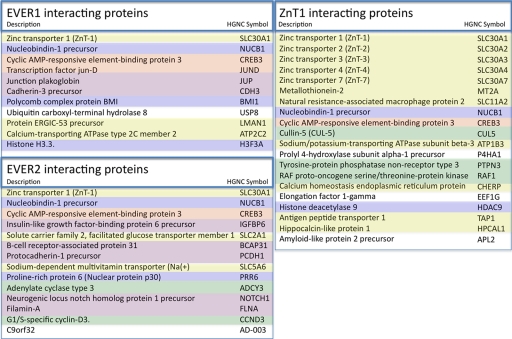
We found that both EVER1 and EVER2 interacted with zinc transporter 1 (ZnT-1), a membrane protein responsible for zinc efflux and resistance to zinc toxicity (158). This discovery prompted us to extend the yeast two-hybrid screen and search for cellular partners of ZnT-1 as well. In the end, this approach allowed us to map an integrated protein interaction network, illustrated in Fig. 4. Eleven, 14, and 20 interacting partners were found for EVER1, EVER2, and ZnT-1, respectively (Fig. 4). The topology of this network is highly suggestive of the implication of the EVER proteins and ZnT-1 in a heteromeric complex anchored in the endoplasmic reticulum (ER) membrane. Such heteromeric complexes formed by different cation diffusion facilitator members could be a common phenomenon for this ubiquitous family of metal ion transporters, and the recruitment of other cellular partners could participate in their functional fine-tuning.
FIG. 4.
Cellular proteins interacting with EVER1, EVER2, and ZnT-1. HGNC, HUGO Gene Nomenclature Committee.
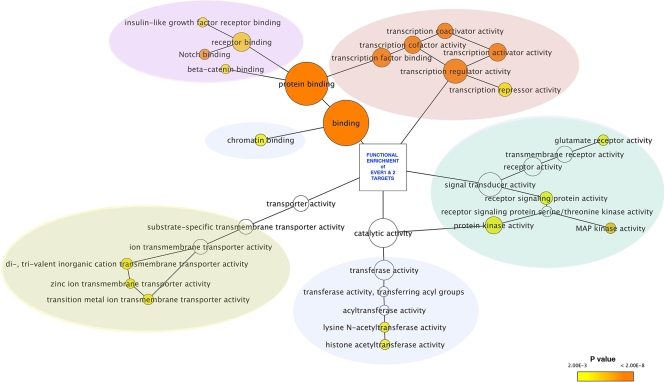
Interestingly, the EVER/ZnT-1 local interactome has been merged into the Human Protein Reference Database, which contains the total known human protein-protein interaction data set, in order to identify the nearest neighbors of the EVER1, EVER2, and ZnT-1 protein partners (data not shown). The resulting network is composed of 320 target proteins linked by 1,078 interactions. High-confidence sets of protein interactions were identified. They were selected for their significant enrichment in functional clusters. This selection, highlighted according to Gene Ontology (GO) term attributes, provides a list of targets for future studies of human cells (Fig. 5). This analysis is based on the cooccurrence of proteins in the same functional cluster and provides insight into the cellular signaling pathways that are susceptible to being regulated by the EVER/ZnT-1 complex. It must be stressed that the EVER/ZnT-1 interactome is connected to transcription regulator activity, signal transduction, chromatin modeling, and some specific epidermal signaling pathways such as NOTCH.
FIG. 5.
Cytoscape software subgraph illustration of the EVER1 and EVER2 interactome data sets filtered for the overrepresentation of GO attributes (GO terms) using the Bingo plug-in (194). The node color is proportional to the P value for GO term enrichment. Clusters overlaid by color are related to the functional categories listed in Fig. 4.
EVER proteins as constituents of the zinc transporter.
Since ZnT-1, a membrane protein responsible for zinc efflux and resistance to zinc toxicity (158), has been found to be a shared partner for both EVER1 and EVER2, we hypothesized that EVER proteins might be involved in the regulation of cellular zinc homeostasis. First, we confirmed the findings from the yeast two-hybrid screen by glutathione S-transferase pull-down and immunoprecipitation experiments (116). In keratinocytes, EVER and ZnT-1 were found to colocalize in the ER. We subsequently demonstrated that the EVER/ZnT-1 complex does not influence the total cellular zinc content but does modify the intracellular zinc distribution. Indeed, EVER2 was found to inhibit the influx of free zinc into nucleoli, where the active synthesis of rRNA takes place. In transiently and stably transfected keratinocytes, EVER and ZnT-1 downregulated the zinc-inducible transcription factor (metal-responsive transcription factor 1 [MTF-1]). Conversely, the level of luciferase activity driven by a promoter specific for MTF-1 was significantly higher in keratinocytes with a mutated EVER2 than in wild-type keratinocytes (116). These data indicate that EVER and ZnT-1 are negative regulators of transcription factors induced by zinc and that they regulate the zinc balance in keratinocytes.
CELLULAR ZINC AND THE PAPILLOMAVIRUS LIFE CYCLE
Zinc Homeostasis
Metals such as iron, calcium, zinc, manganese, and copper are all essential “trace elements,” even though they play distinct and important roles in cell biochemistry. Within the cell, these metals often bind to enzymes or regulatory proteins, serving as cofactors that modify their activities. Among these metals, zinc perhaps plays an exceptional role as an integral constituent of zinc fingers. Zinc fingers are distinct cysteine-based protein domains that bind at least one Zn2+ ion, and in the case of eukaryotic proteins, the most commonly found domains are zinc fingers with a canonical Cys2His2 (CCHH) motif (113). Apart from zinc fingers, zinc also binds to multiple other cellular proteins as well as to amino acids, lipids, DNA, or organic anions. In this context, one can discriminate two pools of cellular zinc: (i) tightly bound to cellular proteins and (ii) loosely bound or unbound, so-called “free” zinc. Comprehension of the mutual relationships between these two zinc compartments is crucial to a full understanding of cellular zinc biochemistry. It is certain that a great majority of the total cellular zinc, probably >99.99%, is tightly bound to different molecules and is excluded from the free-zinc pool. It is still a matter of debate whether Zn2+ is present in the cytoplasm in a truly unbound form or whether it is rather kept loosely bound and is transported by tentative “metallochaperones” (48, 156, 209). Regardless of these considerations, the pool of free cellular zinc is undoubtedly extremely small, in the low-nanomolar range. Whereas the importance of zinc as a structural constituent of multiple proteins is relatively well studied, the physiological significance of the free Zn2+ ions is still being revealed.
Zinc is known to modify signal transduction, but its excess might be toxic for the cell and can induce apoptosis (74, 133, 193). The exact mechanism by which zinc serves as a modulator of the cellular signaling network is certainly not fully understood, but one probable explanation could be the influence of Zn2+ on cellular phosphatases. Zinc ions bind to the thiolate groups of cysteine residues of the phosphatases and suppress their enzymatic activity (15, 74). What needs to be stressed is that already within the range of known in vivo concentrations, Zn2+ inhibits phosphatases and favors the phosphorylated state of a few proteins (232). By this mechanism, zinc is supposed to modulate the strength and duration of a signal that might eventually lead to the phosphorylation and activation of some transcription factors, for instance, AP-1 (102). The latest investigations go even one step further, suggesting that zinc ions can serve as a classical second messenger, analogically to what has been shown for Ca2+. Yamasaki and colleagues have recently demonstrated that upon the triggering of the cell surface receptor, free zinc ions are released from the ER and thereafter are spread throughout the cell, a phenomenon that has been designated a “zinc wave” (232). It might be suggested that in the steady state, a given pool of zinc ions is stored within the ER in order to be ready for release into the cytoplasm as a second messenger.
In the context of its biological activities, it is not surprising that both the distribution of free zinc and its concentration are tightly controlled. A detailed description of the cellular system maintaining cellular zinc homeostasis is beyond the scope of this paper and has recently been comprehensively reviewed (48, 122, 192). In short, at least three distinct elements of this rather complex system could be defined: (i) factors that serve as cellular zinc sensors detecting fluctuations of unbound Zn2+ in the cell, (ii) molecules that directly buffer Zn2+ ions by binding excess zinc and excluding it from the pool of free zinc, and (iii) zinc transporters. The best-characterized zinc sensor is MTF-1 (158, 173). In response to Zn2+, MTF-1 is translocated from the cytoplasm to the nucleus, where it can induce the expression of the proteins that represent the second and third categories listed above (8, 84, 114, 195). These proteins in turn decrease the level of free zinc ions in the cell, completing the feedback loop. A family of metallothioneins is an example of the proteins that directly buffer free zinc. These are metal-binding proteins, which protect the cell against metal-induced toxicity. It is tempting to speculate that metallothioneins might also contribute to zinc-dependent signaling by controlling the natural fluctuations of free-zinc concentrations. The third element of the zinc-managing system is a group of zinc transporters. These are transmembrane proteins that are classified into two families: (i) SLC39, also known as Zrt, i.e., Irt-like proteins (ZIP), and (ii) SLC30, also known as cation diffusion facilitator (CDF) or zinc transporters (ZnT) (48, 122). The ZIP proteins transport zinc in the direction of the cytoplasm, both from the extracellular space and from the organellar lumen, depending on the subcellular localization of a given protein. Conversely, ZnT family members transport Zn2+ out of the cytoplasm, either into the cellular organelles or to the outside of the cell, with the only known exception of a bidirectional transporter being ZnT-5b (216). Altogether, ZIP and ZnT working in opposition ensure the appropriate zinc balance, and this is likely to regulate zinc-mediated signaling. Not surprisingly, the disruption of any element of this controlling system might have a profound influence on the cell metabolism as well as on the function of the whole organism, and indeed, a homozygous ZnT-1 knockout is embryonically lethal (2).
Free Zinc Ions and the Papillomaviruses
Above, we have discussed an important role of zinc homeostasis in cell physiology, but it needs to be emphasized that zinc constitutes an element that is also needed by the virus. Although the mechanism of interplay between cellular zinc and the virus is almost completely unknown, at least three types of host-virus interactions on the ground of zinc could be considered: (i) cellular zinc as a cofactor of viral proteins, (ii) Zn2+ as a constituent of the intracellular signaling network, and (iii) the virus as a “zinc predator” or “zinc scavenger” that might affect the biology of the host cell, exploiting its zinc stock. Indeed, multiple viral proteins comprise a motif that resembles the classical zinc fingers found in eukaryotic proteins, and these zinc-binding domains are usually crucial for the structure and function of viral proteins. On the other hand, by a participation in signal transduction, Zn2+ might activate the cellular transcription factors that are relevant for the virus. It seems that all these possibilities are true in the case of HPVs.
Zn2+ as a cofactor of HPV proteins.
At least three HPV proteins contain the sequences that are potential zinc-binding sites. Among them, only E6 and E7 comprise the Cys-X-X-Cys motif (CXXC) that resembles classical zinc fingers (14), whereas the E4 protein binds zinc by histidine rather than by cysteine residues (183). The E6 protein has four, and E7 has two, CXXC motifs separated by a 29-amino-acid spacer, which seems to be important for protein stability (223). It has been demonstrated that under reducing conditions, i.e., under the conditions typical of the intracellular environment, the E6 and E7 proteins encoded by high-risk HPV bind zinc ions by the thiolate groups of these zinc finger-like motifs (14, 72, 97, 98, 121, 135, 182, 185). These viral zinc fingers are highly conserved, and cysteine substitution destabilizes the protein (97) and interferes with its biological activity (29, 125). For instance, such a substitution prevents E6 nuclear localization and abolishes its transforming activity (97). The binding of zinc to the CXXC motifs changes the E7 conformation (98) and thus enables dimer formation, even though Zn2+ itself does not seem to participate directly in the interaction between the monomers (135, 149). In terms of biological significance, these zinc-binding motifs of the E7 protein are crucial for cell immortalization and malignant transformation (29, 125, 135) as well as for the stable maintenance of the episomal HPV genome (124).
Zn2+ as a tentative regulator of viral gene expression.
As part of the cellular signaling network, Zn2+ can change the activity of the cellular transcription factors that are exploited by the virus. In the case of papillomaviruses, such factors, which on the one hand are inducible by zinc and on the other hand play a pivotal role in the HPV life cycle, are exemplified by the transcription factor AP-1. AP-1 consists of a heterogeneous group of proteins bound as a homo- or heterodimer. The main constituents of AP-1 belong to the Jun or Fos family, and the composition of the AP-1 dimer determines its function (83). AP-1 activity can be stimulated by Zn2+ ions, and it seems that both Jun and Fos might contribute to this phenomenon. Zinc triggers signaling pathways such as phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase/JNK (15, 50, 102, 193), presumably as a consequence of the inhibition of the relevant phosphatases (102). This inhibition might be direct, due to the binding of zinc to the phosphatase thiolate groups, or indirect, mediated by radical oxygen species released by zinc (193). This mechanism probably incorporates the Zn2+ stimulation of JNK, a kinase involved in Jun phosphorylation, and the consequent activation of AP-1 (50, 102). Since AP-1 is a central transcription factor in the HPV life cycle, it is tempting to speculate that through the activation of this transcription factor, Zn2+ might have a profound influence on viral genome expression. The transcription of HPV genes is driven by the viral E2 protein, which cooperates with AP-1 (30, 129, 215). Furthermore, there are multiple AP-1-binding sites within the LCR of the HPV genome (Fig. 1) (111, 162, 203) that enhance E6/E7 expression (discussed below) (111, 136, 147, 162). Moreover, AP-1 contributes significantly to HPV-mediated carcinogenesis (119), and the composition of AP-1 clearly changes during the course of the transformation process (170, 197).
Accessibility of free zinc—a limiting factor for papillomaviruses?
As we have argued above, papillomaviruses might need zinc at different stages of their life cycle, and obviously, they have to rely entirely on the cellular resources of this element. Even though the total cellular zinc content is relatively high, ∼108 atoms per cell, the pool of labile, easily accessible zinc remains much lower (∼103 atoms per cell or even much less). Due to the scarcity of free Zn2+, especially in the context of the high number of viral particles per cell, one might wonder whether the cellular pool of free zinc is sufficient for HPV, whether an increase in its concentration would be beneficial for HPV, and, possibly, how Zn2+ is delivered to HPV proteins. It remains to be determined whether cellular factors indeed “deliver” this element to the viral proteins, serving as metallochaperones (48, 156), or, conversely, whether Zn2+ is acquired directly by HPV proteins from the pool of free ions. Recent studies suggest that most cellular metalloproteins acquire the respective metal directly from the cellular pool, without the mediation of metallochaperones, and that protein folding in a particular subcellular compartment ensures the binding of an appropriate metal (209). Nevertheless, we have recently observed that the HPV E6 protein binds to metallothioneins (115; our unpublished data). It is still obscure whether E6 could directly acquire Zn2+ from this source or whether, rather, it releases Zn2+ from metallothioneins, increasing the pool of free zinc.
A distinct issue is the relationships between Zn2+ as a signaling molecule and the HPV life cycle. It would be particularly interesting to elucidate whether physiological oscillations of the amounts of free zinc affect HPV gene expression and, if so, whether AP-1 in involved. Diminishing the free-zinc level also suppresses AP-1 activity when it is triggered by a natural cell surface receptor (81), supporting the notion that intracellular Zn2+ indeed modulates AP-1 activity. However, it remains to be clarified whether a reduction of the free-zinc pool (or the prevention of its increase in infected cells) could in turn provoke a protective effect toward HPV through the inhibition of AP-1 activity.
Zinc Balance and the Immune System
It has been known for decades that breaking zinc homeostasis affects immune system function. This notion comes predominantly from studies of zinc deficiency caused by nutritional insufficiency or by inherited defects in zinc transporters. An extreme example of inherited zinc deficiency is acrodermatitis enteropathica, a rare autosomal recessive disease caused by a mutation in the ZIP4 gene, which encodes a zinc transporter belonging to the SLC39 family (221). ZIP4 is involved in zinc absorption from diet, in the intestine, and in the maintenance of the zinc balance at the whole-organism level (123). Zinc malabsorption in acrodermatitis enteropathica leads to the development of skin lesions and is notably also associated with immune defects (6). Regardless of the exact causative factor, a major reprogramming of the immune system takes place in the course of disorders associated with zinc deficiency (for a review, see reference 58). Importantly, such clinical states are accompanied by an increased frequency of viral, bacterial, and fungal infections, suggesting that an appropriate global zinc balance is essential for an effective response to microbial pathogens.
Zinc imbalance at the whole-organism level.
Zn2+ exerts a multidirectional effect on distinct leukocyte subsets, and both an excess and a deficit of zinc impair T- and B-lymphocyte function as well as that of NK cells and monocytes (181). Interestingly, the basal concentration of cellular free Zn2+ and the sensitivity to an excess of zinc are not identical among the leukocyte subsets. T cells are much more zinc sensitive than are, for instance, monocytes (225). Also, in vivo data confirm that zinc deficiency affects mainly lymphopoiesis, whereas myelopoiesis remains relatively unaltered (58). In zinc-deficient mice, an important thymus involution, low lymphoid tissue weight, and a reduction in lymphocyte numbers have been observed (55, 104). However, this could be a consequence of an improper function of thymulin, a zinc-dependent hormone crucial for T-cell differentiation, and not necessarily a direct effect of a zinc deficiency on T cells (34, 167). In addition to the deviations in the total numbers of lymphocytes, relationships between distinct subsets of T cells are changed upon zinc deficiency. It has been postulated that a low level of zinc favors Th2 rather than Th1 differentiation and might thus disfavor cell-mediated immunity (167, 181).
Zinc imbalance at the single-cell level.
A zinc-induced blast transformation of human lymphocytes was reported more than 30 years ago (17, 105), but the exact mechanism of this effect still remains unclear. It was postulated that an excess of zinc induces the expression of interleukin-2 (IL-2) and a high-affinity IL-2 receptor in T-cells and that this is thought to confer a mitogenic activity of zinc (205, 222). However, apart from IL-2, zinc also stimulates the expression of other cytokines such as IL-1β, IL-6, tumor necrosis factor (TNF), and gamma interferon (43, 222), and at least some of the reported Zn2+ effects on T cells are indirect, being mediated, e.g., by monocyte activation (186). In addition, it has to be kept in mind that observations made upon exposure of the cells to high concentrations of zinc might have an impact on plasma membrane fluidity (206, 219) or the function of ion exchangers (148, 160), which does not give a deep insight into the physiological role of intracellular Zn2+ in T-cell biology.
Few data on the physiological role of Zn2+ ions in lymphocytes come from the studies of T-cell receptor (TCR)-triggered signaling. It has been found that Zn2+ is indispensable for the association of Lck with CD4 and CD8 coreceptors (88, 120), even though it has never been demonstrated that alterations in levels of free intracellular zinc regulate this interaction. The binding of the Lck kinase to the TCR complex is crucial for the transmission of the signal from this complex upon the recognition of the peptide presented in the major histocompatibility complex (MHC) context (99). Zn2+ is involved in the heterodimerization of CD4 (as well as CD8) with Lck by bridging two couples of cysteine residues from each of the respective molecules (102). Interestingly, whereas in mature effector T cells, Lck is constantly bound to the coreceptor molecule, in naive T-cells, Lck is found predominantly in the unbound form and is recruited upon cell activation (11). Moreover, it seems that T-cell activation triggers rearrangements in cellular zinc homeostasis, and coordinated alterations in the expression of zinc transporters and metallothioneins in the course of T-cell activation and differentiation have been observed (M. Lazarczyk, unpublished data). One can speculate that the activation of naive T cells would be associated with an increase in the concentration of free cellular zinc, but the biological consequences of such a phenomenon remain obscure. Whether TCR-mediated signaling could be modulated by alterations in intracellular free-zinc concentrations remains to be elucidated. Interestingly, in dendritic cells (DC), physiological fluctuations in free zinc have recently been observed, and the impacts of this on DC activation have been studied. It has been demonstrated that immature DC display a relatively high level of free zinc ions. In the course of DC maturation, a rapid reduction in the level of free cellular zinc takes place, and this is essential for maturation. The suppression of the free-zinc level was shown to be required for the prevention of the internalization of MHC class II molecules from the cell surface (106). An important role of the physiological fluctuations of intracellular zinc in mast cell activation has also recently been demonstrated (232).
Apparently, the significance of zinc ions in the virus life cycle might be not limited to a direct effect of Zn2+ within the host cell but could involve the zinc-dependent regulation of an immune antiviral response. It is noteworthy that in a great majority of infected individuals, the immune system is able to eradicate papillomavirus infections within months, pointing to viral clearance as a major controlling mechanism. The maturation of locally residing antigen-presenting cells and the subsequent activation of cytotoxic T cells are crucial for viral clearance (208), and a dysfunction of this process could lead to a persistence of papillomavirus infection. Whether an improper zinc balance in cytotoxic T cells and/or DC could indeed contribute to the ineffective clearance of HPV infection observed in some patients remains an exciting question.
PROPOSED MECHANISM OF EVER-MEDIATED RESISTANCE TO HPV INFECTION AND CARCINOGENESIS
EVER proteins constitute a member of the zinc-transporting complex, and we hypothesized that by maintaining the cellular zinc balance, they could control HPV infections (116). The zinc imbalance imposed by an EVER deficiency would have to disrupt the anti-HPV barrier, with all the attendant clinical consequences. Knowing the possible importance of zinc, both for the HPV life cycle in the host cell and for immune cells and the clearance of already established infections, the EVER-based barrier might theoretically have two arms: one directly limiting virus replication in the infected cells and the other ensuring an adequate antiviral response. Fragmentary data available suggest that in EV patients, both of these elements could be disrupted. Notably, in EV-derived keratinocytes, an increased replication rate has been reported (116), indicating local EVER-deficiency-based defects. However, the lack of an efficient antiviral response observed in the majority of EV cases, despite the highly productive life cycle and evidence that the immune system has been exposed to HPV antigens, rather points to an immune defect as a result of EVER mutation.
EVER Proteins in Keratinocytes
Normal keratinocytes.
In human keratinocytes, we have previously found that EVER proteins interact with ZnT-1 and form a complex within the ER (116). It has been postulated that the EVER/ZnT-1 complex participates in the regulation of cellular zinc fluxes and allows the maintenance of the zinc balance in keratinocytes (116). The physiological fluctuations of free zinc in the cell might serve as a factor that modulates and integrates the global signaling network. In this context, one might expect that the constituents of the EVER complex contribute to this modulation, and by controlling the cellular zinc balance, this complex could modify the activity of a broad range of transcription factors and not only those like MTF-1 that are classical zinc sensors. Indeed, EVERs and ZnT-1 were found to suppress the activity of the AP-1 and Elk-1 transcription factors in human keratinocytes (116). In the case of AP-1, this effect seems to be related to an influence on the transactivation domain of Jun. Jun is an important constituent of the AP-1 dimer, and the function of its transactivation domain is regulated by distinct kinases. Phosphorylation by JNK leads to the activation of the transcription factor, whereas phosphorylation by GSK-3 suppresses Jun activity. It has been found that the EVER/ZnT-1 complex inhibits the c-Jun transactivation domain, possibly in a GSK-3-independent manner, and this effect could be mediated by a modulation of JNK activity (116).
Although it has not yet been formally proven, this influence of EVER on AP-1 activity is likely to be indirect, constituting a consequence of EVER-mediated changes in the cellular zinc balance. Zinc ions have previously been shown to induce JNK and to enhance the activity of AP-1 in this mechanism (50, 103). EVER proteins are thought to decrease the level of free zinc ions in keratinocytes, and one can expect that they could indirectly alter the phosphorylation pattern of cellular proteins. The inhibition of JNK by the EVER complex constitutes a tentative mechanism of EVER influence on AP-1 function in keratinocytes. Interestingly, a similar influence on AP-1 transcriptional activity was observed when a zinc-specific chelator (TPEN) was added to keratinocyte cultures (our unpublished data), whereas an excess of zinc exerts a reverse effect, further supporting the idea that the cellular zinc balance could control AP-1 activity in keratinocytes. The downregulation of AP-1 by EVER and ZnT-1 also takes place when AP-1 is activated by other physiological triggers, for instance, growth factors such as epidermal growth factor (EGF) or TGF-alpha (116). One can hypothesize that under such conditions, the EVER complex would inhibit the release of endogenous free zinc from the intracellular stores. Whether the activation of the EGF receptor in keratinocytes, analogically to what has been demonstrated for mast cells (232), can initiate a “zinc wave” remains to be determined. If so, the EVER complex might interfere with the spread of such a zinc wave and could modulate phosphatase activity and the cellular response to different cytokines. As the level of AP-1 activity is significantly higher in EVER2−/− keratinocytes than in cells with the wild-type EVER2 gene (116), we propose that one of the physiological roles of the EVER complex in keratinocytes might be to serve as a constitutive inhibitor of AP-1. In addition, one might expect that by touching zinc homeostasis, the EVER complex would broadly modify the phosphorylation pattern, with the final outcome not being limited to the modulation of only one or a very few transcription factors. Indeed, recent observations favor this possibility since EVER and ZnT-1 have also been found to downregulate the expression of the Elk-1 and Fos transcription factors (116).
EVER deficiency in keratinocytes and papillomaviruses.
It has to be kept in mind that even though beta-HPVs are ubiquitous in the general population, the viral load is very low compared to that for EV patients, and the HPV genome is usually detected only by very sensitive methods such as nested PCR (75, 76, 165), suggesting that the infection can be entirely controlled in healthy individuals. The fact that AP-1 activity is elevated in EVER-deficient keratinocytes could have a profound effect on virus biology. This suggests that EVER might act as a constitutive AP-1 inhibitor, serving as an internal anti-HPV barrier. The increased level of activity of AP-1 in EVER-deficient cells would be involved in the enhancement of viral gene expression, and it might also promote replication and the persistence of the viral genome in keratinocytes. Possibly, an uncoordinated overexpression of viral oncogenes would confer malignant transformation on infected cells.
However, although the contribution of HPV5 or HPV8 to carcinogenesis in EV patients is doubtless, it has been proposed that mutations in EVER genes might also contribute to oncogenesis in an HPV-independent manner (115, 116). Indeed, a constitutively active AP-1 mutant is known to exert a transforming activity, demonstrating that the uncontrolled activation of AP-1 itself can be involved in malignant transformation. The high growth rate of HPV-uninfected EVER2−/− keratinocytes derived from EV patients compared to that of cells with a wild-type EVER2 gene further supports this hypothesis (116). Nevertheless, to definitively confirm these preliminary observations, the effect of EVER knockdown on established cell lines with a homogenous genetic background should also be assessed. Moreover, the effect on the cell growth rate of the reconstitution of EVER2 expression in EVER2−/− keratinocytes, together with further analysis of proliferation and apoptosis state, should also be performed.
EVER gene polymorphism and papillomaviruses.
Besides nonsense mutations found in EV patients, it is possible that polymorphisms in EVER genes could also be important for papillomavirus replication and the development of NMSC in the general population.
It has been proposed that cutaneous HPV might contribute to the pathogenesis of NMSC in humans (165), but their exact role in these diseases is still controversial (153). Although a primary role of UV irradiation in basal cell carcinomas and squamous cell carcinomas is generally recognized, HPVs might serve as cocarcinogens, promoting the development of already-initiated malignancies. Such polymorphism, even though not leading to a massive EV-like skin eruption, would have to facilitate viral oncogene expression and/or the persistence of viral infections, increasing a “dosage of the oncoprotein” or “exposition span” to this particular cocarcinogen, respectively.
Indeed, a very common polymorphism in the EVER2 gene has recently been described, and the correlation between seropositivity for beta-HPVs and the risk of squamous cell carcinoma has been studied (159). This is a single nucleotide polymorphism in the transmembrane region in codon 306 within exon 8, which results in a change from isoleucine to asparagine (I306N). The replacement of a nonpolar amino acid by a polar one is thought to change the conformation of the EVER2 protein. Currently, it is difficult to dissect the influence of this polymorphism on the keratinocyte from its effect on immune system function. Whether there is a similar correlation between squamous cell carcinoma incidence and any polymorphism in the EVER1 gene also remains to be established. Nevertheless, preliminary observations have already opened a new avenue for the role of the EVER complex in the pathogenesis of NMSC in the general population.
EVER Proteins in Immune Cells
The recent discovery of the molecular mechanism of EVER protein action in keratinocytes has provided a first substantial clue in an understanding of the pathogenesis of EV, and more globally, it has given a mechanistic insight into the function of the natural anti-HPV barrier in keratinocytes. Despite that, we are still far from a comprehension of the whole complexity of the disease and the host-virus interplay on the grounds of zinc homeostasis. It must be stressed that the clinical effect of EVER deficiency is not necessarily limited to keratinocytes. EVER proteins are supposed to be ubiquitously expressed (152), and it is tempting to speculate that the lack of EVER in other cellular compartments contributes to EV pathogenesis. Indeed, whereas in a great majority of HPV-infected individuals, the immune system is able to eradicate the virus within months, pointing to viral clearance as a pivotal controlling mechanism (208), EV patients consistently display multiple immune deviations, and despite exposition to viral antigens, they are unable to clear HPV infections (for reviews, see references 132 and 152).
It remains unclear to what extent EV, seen as an immunodeficiency, would result from the dysfunction of innate or adaptive immunity. It has been reported that blast transformation of T cells (168) and nonspecific cell-mediated immunity (68, 172) are largely diminished in the majority of EV cases. Moreover, an anergy to dinitrochlorobenzene, a skin contact sensitizer, has also constantly been observed (69). On the other hand, distinct clinical states associated with immunodeficiency, either iatrogenic (127) or idiopathic (10), are sometimes accompanied by typical EV-like eruptions in the skin. In addition, an EV case with impaired Fas function and an Ala91Val variation in perforin has recently been described, and this was associated with a variant of autoimmune lymphoproliferative syndrome (234).
Although the presence of EVER proteins in lymphocytes has never been demonstrated, it has been suggested that the basal EVER mRNA expression level could be even higher in lymphocytes than in the epidermis (101). More importantly, preliminary experiments with immortalized human lymphocytes derived from EVER-deficient patients, or their healthy relatives, seem to confirm the assumption of a role of the EVER complex in the maintenance of zinc homeostasis in lymphocytes. Indeed, the lack of one of the EVER proteins leads to a disruption of the zinc balance in these cell lines. However, even though these results demonstrate that the EVER complex participates in the maintenance of the cellular zinc balance in leukocytes, more precise analyses of the role of the EVER proteins in the regulation of physiological zinc fluxes in primary lymphocytes are needed, and appropriate studies are currently being performed in our laboratories.
In this context, a burning question is what might be the consequences of the EVER-deficiency-induced zinc imbalance for the function of lymphocytes. It is likely that the effect of an EVER deficiency, like in keratinocytes, does not selectively affect the transcription factor MTF-1 but also changes cell signaling through other pathways, e.g., by modifying the transcriptional activity of AP-1. This in turn could change the expression pattern of multiple genes, including those that regulate lymphocyte differentiation and maturation, as well as cytokine expression. Indeed, we have found that EVER-deficient lymphocytes from EV patients of different unrelated families produce significantly more TNF than cells obtained from their healthy relatives (M. Lazarczyk and M. Favre, unpublished data). These results are in accordance with data from previous reports, which demonstrated that TNF transcription can be activated in response to an increased concentration of free zinc (232) and that the level is increased in the skin lesions of EV patients (131). Whether these alterations in cytokine expression levels truly contribute to the pathogenesis of EV is a distinct issue and remains uncertain, but at least, our data demonstrate that the effect of a disruption of the EVER complex is not restricted to keratinocytes.
Apart from the tentative effect of EVER deficiency in “professional” immune cells, other cells can also contribute to the final outcome of an anti-HPV immune response and might be touched by the zinc imbalance imposed by a lack of EVER. It is well established that keratinocytes participate in local inflammatory reactions in the skin through the secretion of, or the response to, various cytokines (type I interferons, TNF, IL-1, IL-6, IL-10, IL-12, and IL-15, etc.), growth factors (TGF and granulocyte-macrophage colony-stimulating factor), and chemokines (SDF1 and IL-8). Our preliminary results showed a decreased level of production of IL-6 and an increased level of secretion of IL-8 by EVER2−/− keratinocytes compared to those of cells with wild-type EVER2. IL-6 is a proinflammatory cytokine secreted by T cells, macrophages, and keratinocytes to stimulate the immune response to pathogens, leading to inflammation. IL-8 is a proinflammatory CXC chemokine that mediates the activation and migration of neutrophils into tissue from peripheral blood. The altered pattern of secretion observed in EVER2−/− keratinocytes may be involved in the lifelong persistence of lesions.
GENITAL HPV AND THE EVER-BASED BARRIER
Although the first step toward an understanding of the nature of the natural anti-HPV barrier has been reached, this still did not allow a few important questions to be answered. Why does this barrier not confer resistance to the related group of genital HPVs? Is this barrier really as selective as one can judge from the epidemiological data from EV patients? Or it is rather that genital HPVs have developed a mechanism that allows them either to omit or to break the barrier? If the latter were true, one of the genital HPV-derived proteins would have to inhibit or disrupt the EVER complex, whereas either beta-HPVs should be devoid of this protein or their counterpart of such a protein should be unable to affect the EVER complex.
Beta-HPVs, like gamma- and mu-HPVs, lack the E5 ORF, which is present in the genome of genital HPV. Moreover, some beta-HPVs are detected together with HPV3 or related genotypes (146), and such a codetection with E5-containing HPVs suggests that beta-HPVs are defective for the E5 protein and that they benefit from E5 delivered by the coinfecting HPV (152). In this context, the E5 protein emerged as a candidate factor that might confer resistance to the EVER-based barrier.
The HPV16 E5 Protein Inhibits EVER and ZnT-1 Activity
The fact that the EVER/ZnT-1 complex is involved in the control of the life cycle of beta-HPV but not that of alpha-HPV prompted us to search for a possible interaction between viral early proteins specific for cutaneous and mucosal HPVs and EVER1, EVER2, or ZnT-1. No interaction was detected with the E6 or E7 oncoprotein (116). In contrast, we found that the E5 protein of HPV16 binds to EVER1, EVER2, and ZnT-1. More importantly, HPV16 E5 prevented the ZnT-1/EVER-mediated inhibition of MTF-1 transcriptional activity (115, 116). On the other hand, no effect of HPV16 E5 was observed in EVER2−/− keratinocytes originating from a Polish EV patient, which further suggests that the intact EVER/ZnT-1 complex is an important target for the HPV16 E5 protein in human keratinocytes.
The HPV E5 ORF, which is localized just downstream of the E2 ORF, has recently been classified into four different groups: alpha, beta, gamma, and delta (22). Interestingly, the different forms of the E5 proteins appeared to correlate with different clinical manifestations, in particular with oncogenic potential (190). The most extensively studied is the E5-alpha protein, which is encoded by high-risk alpha-HPV, whereas low-risk genital HPVs encode two distinct forms: E5-gamma and E5-delta (62). In this context, it is tempting to speculate that different forms of HPV E5 could differ in their capacities to bind and to inhibit the EVER proteins. One might suppose that E5-alpha could disturb the function of the EVER complex to a higher extent than E5-beta, E5-gamma, or E5-delta. If so, the remnant activity of the EVER complex upon the partial inhibition by low-risk HPV-derived E5 proteins might still be sufficient to protect the cell from malignant transformation. This intriguing issue needs urgent elucidation.
In the following paragraphs, we focus on the E5-alpha protein encoded by HPV16.
Basic Biological Properties of the HPV16 E5 Protein
HPV16 E5 encodes an 83-amino-acid hydrophobic membrane protein. This protein is distributed mainly in the Golgi apparatus and the ER. In contrast to the bovine papillomavirus type 1 E5 protein, HPV16 E5 is unable to form transformed foci on cell monolayers and is thus considered to have only moderate transforming activity. This notion is further supported by the inability of HPV16 E5 to immortalize primary human keratinocytes (200). Because of its weak transforming activity, and the fact that the E5 ORF is often deleted after the viral genome has integrated into the host DNA, it is admitted that this viral protein plays a role during the early stages of HPV infection and transformation of infected keratinocytes.
Among the known functions of HPV16 E5 is the stimulation of EGF-dependent proliferation and the inhibition of endosome acidification, which leads to the activation of signaling pathways mediated by the EGF receptors (EGFRs). It is known that HPV16 E5 is able to increase the number of EGFRs at the cell surface due to an abnormal recycling of EGFR. This process is thought to be mediated by the interaction of the HPV16 E5 protein with the 16-kDa pore-forming subunit of the vacuolar H+-ATPase proton pump located in the ER (32, 184). Recently, karyopherin beta 3 (KNb3) has also been identified as a cellular target for HPV16 E5 (108). Karyopherin is localized mainly in the cytoplasm near the nuclear envelope, and it has been suggested that a KNb3/HPV16 E5 complex plays an important role in vesicle trafficking. A direct association of HPV16 E5 with EGFR or erbB4 has also been observed in HPV16-infected lesions (212). This may influence the EGF signal transduction that leads to the synthesis of transcription factors such as cJun, Jun B, or Fos needed for viral genome expression. However, an EGFR-independent mechanism has been proposed to function in the E5-activated signaling cascade, involving mitogen-activated protein kinase p38 and extracellular signal-regulated kinase 1/2 pathways in human keratinocytes.
HPV16 E5 can also activate the synthesis of Jun transcription factors via both the protein kinase C signaling pathway and the ras-dependent pathway. It thus appears that the HPV16 E5 protein stimulates E6 and E7 gene expression and regulates E7-mediated cell transformation by the activation of cellular genes. It has been reported that HPV16 E5 downregulates the expression of the p21 tumor suppressor gene in immortalized keratinocytes, and this could promote cell proliferation and contribute to cell immortalization in the course of primary infection of genital keratinocytes (213). In addition, the inhibition of the p21 regulation protein by HPV16 E5 may facilitate the activation of Cdk4-cyclin D complexes that play an important role in the phosphorylation of pRb and favor the passage from the G1 phase to the S phase needed for viral DNA replication.
Finally, the E5 proteins of HPV16 and HPV31 were found to target B-cell-associated protein 31 (Bap31) (179). This interaction is believed to promote the proliferative capacity of keratinocytes in differentiated cells and prevent apoptosis.
HPV16 E5 contribution to the evasion of immune surveillance.
The HPV16 E5 downregulation of the expression of surface HLA class I genes of the major histocompatibility complex has been reported (7). This inhibition is linked to the specific interaction of the E5 protein with the heavy chain of HLA class I and involves the first hydrophobic domain of HPV16 E5. Thus, it is likely that in infected keratinocytes, E5 interferes with the presentation of viral antigens to the immune cells. As it has been demonstrated that elevated levels of free zinc change the vesicular circulation and turnover of MHC proteins in DCs (106), it is tempting to speculate that E5 could exert a similar effect in activated keratinocytes by the upregulation of the free zinc in the cell (116).
Interestingly, it has also been shown that HPV16 E5 inhibits Fas-induced apoptosis by decreasing the cell surface expression of the Fas receptor and downregulating the cleavage of procaspase-8 and procaspase-3 (95). Interference with antigen presentation mediated by the E5 protein, and impairing death receptor signaling, may allow the virus to establish a persistent infection by avoiding the clearance of infected keratinocytes by NK cells or cytotoxic T lymphocytes. Whether E5 plays a role in establishing a latent infection in stem cells remains to be determined.
MODEL OF AN EVER-BASED BARRIER PROTECTING AGAINST HPV
An extraordinarily high sensitivity to infections by cutaneous beta-HPVs in otherwise-healthy individuals carrying a mutation in one of the EVER genes suggests that in humans, a natural EVER-based barrier exists, which protects the host from papillomaviruses. Obviously, this observation does not indicate whether the barrier truly confers resistance to infection or, rather, that it ensures a rapid clearance of the virus. Nevertheless, judging by the clinical consequences of EVER deficiency, the EVER proteins apparently constitute an indispensable element of this barrier, which is crucial for the functional integrity of the EVER/ZnT-1 complex (116, 176). Herein, we present a model of this natural anti-HPV barrier.
We propose that a cellular zinc imbalance constitutes an important, perhaps even a crucial, step in the HPV life cycle. Putting the EVER/ZnT-1 complex in a central place might be partially supported on the one hand by the observations of the highly limited EV-HPV replication rate in the general population (i.e., in cells with wild-type EVER genes and maintained zinc homeostasis) compared to that in EV patients (36) and on the other hand by the function of the virally derived EVER inhibitor HPV E5 in the productive stages of the HPV life cycle. Moreover, it seems that breaking the barrier that imposes a cellular zinc imbalance is an important element of the pathogenesis of both alpha- and beta-papillomaviruses, and the main difference between these two groups would be a mechanism employed to achieve the same goal: EVER mutation (beta-HPV) versus E5-mediated EVER/ZnT-1 complex suppression (alpha-HPV).
At the cellular level, the EVER-based barrier would consist of two major elements: (i) the immune system, which is important for the control of already-established HPV infections and is usually capable of eliminating the virus, and (ii) keratinocytes, a natural cellular environment for the papillomavirus life cycle where the direct control of replication and viral genome expression by host factors could take place. The proper function of both of these elements would depend on the activity of the EVER/ZnT-1 complex.
EVER/ZnT-1 Complex and Immune Control of HPV-Infected Cells
In immune cells, the proper zinc balance controlled by the EVER/ZnT-1 complex would be important for lymphocyte differentiation and/or activation, for instance, for the generation of HPV-responsive cytotoxic T cells. It can be assumed that mutations in either of the EVER genes disrupts the EVER complex and that this is responsible for compromising virus clearance by the immune system, finally leading to a severe phenotype associated with the persistence of established papillomavirus infections and the malignant transformation of some of the lesions.
EVER/ZnT-1 Complex and Control of Viral Expression
In keratinocytes, the EVER/ZnT-1 complex would be responsible for maintaining a low level of free zinc, tonically inhibiting the activity of the AP-1 transcription factors needed for viral genome expression (Fig. 6). Dysfunction of the complex in keratinocytes can be caused by (i) virus-related factors or (ii) intrinsic host-related factors.
FIG. 6.
Model proposed for the limitation of expression of HPV in keratinocytes. (A) In normal keratinocytes, the activation of AP-1 transcription factor family synthesis by Zn2+ is thwarted by the EVER1/EVER2/ZnT-1 complex. Infection of keratinocytes by HPV16 allows the synthesis of the HPV16 E5 protein, which alleviates the inhibition of the EVER/ZnT-1 complex and facilitates the expression of the transcription factors necessary for the expression and replication of the viral genome. (B) The lack of the E5 protein encoded by the genome of beta-HPV does not allow the vegetative replication of these viruses and protects the general population. (C) In epidermodysplasia verruciformis, a mutation in the EVER1 or EVER2 gene would block the formation of the EVER/ZnT-1 complex and would allow the expression of AP-1 transcription factors and viral replication.
The HPV E5 protein is currently the only known viral factor that can inhibit the EVER/ZnT-1 complex (Fig. 6A). Although a role of the E5 protein in differentiating keratinocytes has been reported (179), in HPV16-induced lesions, the viral protein is expressed mainly in the basal and parabasal layers of the epidermis (27). This indicates that the E5 protein may favor E6 and E7 expression in the first layers of the epidermis, a step essential for the passage of cells from the G1 to the S phase (Fig. 2). The lack of an E5 ORF in certain HPVs (22) may account for their asymptomatic natures and the highly limited replication of beta-HPVs and most gamma-HPVs in healthy subjects (Fig. 6B). However, it cannot be excluded that in certain HPV genotypes, other viral proteins apart from E5 might display activity toward the EVER complex. This notion could be supported by the fact that a few E5-lacking gamma-HPVs are found in productive lesions, such as HPV4 and HPV60 in palmar warts and plantar cysts, respectively. Whether these viruses inhibit EVER function or are indicative of the existence of an additional cellular barrier protecting against HPV remains to be elucidated.
In terms of intrinsic factors, the dysfunction of the EVER/ZnT-1 complex can result from mutations in either of the EVER genes, as observed in EV patients. This would upregulate the free-zinc levels in keratinocytes and support virus replication, finally leading to a severe phenotype associated with a malignant conversion of some of the lesions (Fig. 6C).
Nevertheless, the surprising disruption of the cellular zinc-transporting complex that is achieved by two completely unrelated “strategies” sculptured during a long coevolution of HPV and the human species underlines the general importance of the cellular zinc (im)balance in the papillomavirus life cycle.
Commensalic or Symbiotic Nature of Beta-HPV?
The human skin harbors a surprising multiplicity of HPVs, most of them belonging to the beta and gamma genera (4). These viruses have been evolving for hundreds of thousands of years in humans, and they are extremely well adapted to their host. Beta-HPVs and most gamma-HPVs are found only in trace amounts in the skin, hardly ever causing symptomatic infections. Although beta-HPVs could be considered to be defective viruses (152), it remains to be determined whether these viruses are commensal or whether they may establish symbioses with the human being and play a beneficial role under certain conditions.
The very low viral load in subjects from the general population on the one hand and the scarcity of EV patients on the other hand may lead us to consider what the reservoir of beta-HPV in the human population is. Admittedly, the general acquisition of beta- and gamma-HPVs in early infancy has been demonstrated by Antonsson and colleagues (5). This clearly points to the fact that individuals from the general population can constitute a source of infection in spite of a very low viral load, at least when the physical contact is very close. It is noteworthy that in the cases reported by Antonsson, a majority of the genotypes found in mothers and fathers from the same family were different from each other (5). On the other hand, it is not entirely certain whether this mechanism might be sufficient to explain multiple reinfections in the adult throughout their whole life. However, another possible reservoir of beta-HPV has been proposed, which is psoriasis. Indeed, patients suffering from psoriasis display a high prevalence of antibodies raised against HPV5 and HPV36, with both these viruses being beta-HPVs (53). On top of that, beta-HPV DNA has been detected in nearly all psoriasis patients tested, demonstrating that psoriatic individuals have an alleviated restriction toward HPV. As psoriasis is a common dermatosis affecting 2 to 5% of the human population, regardless of a contribution of beta-HPV to the pathogenesis of this disease (130), it can be assumed that psoriatic patients constitute a reservoir for HPV (53). In addition, beta-HPV DNA sequences and HPV5 antibodies were detected in patients with bullous disease and autoimmune connective tissue disorders with cutaneous involvement as well as in burned subjects (52). In all these clinical states, the epidermal repair processes are augmented, with increased keratinocyte proliferation, which might constitute a benefit for HPV and explain an alleviated restriction toward these viruses. This in turn would ensure the lifelong maintenance of HPV infections in the general population.
The nature of the cohabitation of HPV and humans is an open, and not a trivial or weightless, issue. The ubiquity of beta-HPVs and their astonishing genomic diversity, together with the asymptomatic course of infection, obviously led to considerations of their commensalic nature (4). Nevertheless, it can be speculated that beta-HPV could actively participate in the hyperproliferation of keratinocytes during wound healing and, under certain conditions, implement symbiosis with human beings. The involvement of beta-HPV in the regulation of keratinocyte proliferation is poorly understood, but early proteins of beta-HPV have been shown to inhibit TGF-beta-triggered signaling and are expected to stimulate the proliferation of keratinocytes (137). Furthermore, the HPV8 E2 protein was suggested to promote the transition of keratinocytes into the transiently amplifying compartment (150). Therefore, through the stimulation of keratinocyte proliferation and their migration, beta-HPVs might promote epithelial repair processes, constituting an obvious benefit for the human when skin repair is needed. Under such conditions, locally in the epidermis, the expression of members of the EVER/ZnT-1 complex might be transiently decreased (for instance, in response to local inflammation), and the barrier against beta-HPV could be temporarily alleviated. Therefore, the relationships between the EVER complex, beta-HPV, and epidermal healing constitute an exciting question, and in this context, the elucidation of the mechanism controlling EVER gene expression seems to be an even more burning issue.
Acknowledgments
We thank Katherine Kean for critical reading of the manuscript.
This work was supported by grants from the Contrats de la Ligue Nationale contre le Cancer (grants R05/75-129 and RS07/75-75), the Association pour la Recherche sur le Cancer (grants 3731XA0531F and 4867), and the Agence Nationale de la Recherche (EPI-HPV-3D). M.L. was supported by postdoctoral fellowships from the Association pour la Recherche sur le Cancer and the Foundation for Polish Science (FNP).
Biography
Maciej Lazarczyk, M.D., Ph.D., was born in 1977 in Warsaw, Poland. His residency in dermatology and venerology was initiated in the clinic of S. Jablonska and S. Majewski, Warsaw, Poland. He received his Ph.D. in the Center of Biostructure Research in 2004 in Warsaw. In 2005, he joined Michel Favre's group at the Pasteur Institute, Paris, France. He was working on the host-related mechanisms protecting against HPV infections. Currently, he is at the Department of Immunology and Infectious Diseases in Toulouse, France, continuing the studies on the EVER protein function in collaboration with the Genetics, Papillomavirus and Human Cancer Unit.
Patricia Cassonnet was born in l'Haÿ les Roses, France, in 1960. She received her technician certificate in biophysics in 1980 and entered the papillomavirus diagnostic laboratory at the Pasteur Institute in 1985. She joined the Genetics, Papillomavirus and Human Cancer Unit in 2004 to work on papillomavirus disease pathogenesis and biological properties of viral early proteins.
Christian Pons was born in Figeac, France, in 1972. He joined the Pasteur Institute in 1998 and worked as laboratory technician in the Genetics, Papillomavirus and Human Cancer Unit. He is involved in the characterization of EVER genes for papillomavirus infection.
Yves Jacob was born in Paris, France, in 1957. He received his medical doctorate in 1983 from the University of Medicine Lariboisière-Saint Louis (Paris VII) followed by a Ph.D. degree in molecular biology from Paris VII University in 1992. He worked at different research organizations in Paris before joining the Pasteur Institute in 1994. His current research interests include HPV disease pathogenesis and interactomics.
Michel Favre was born in Tarascon/Ariège, France, in 1949. After completing a Master's program in Biochemistry in 1971 at Paul Sabatier University in Toulouse and Paris VII University, he spent 8 years at the Institut Gustave Roussy, Villejuif, France. He joined the Pasteur Institute in 1979. His main research interests were biochemical and biological properties of human papillomaviruses and characterization of human genes involved in resistance to papillomavirus infection. He has been the head of the Genetics, Papillomavirus and Human Cancer Unit since 2004.
REFERENCES
- 1.Akgul, B., M. Curten, H. Haigis, I. Rogosz, and H. Pfister. 2006. Interferon regulatory factor 5.2 acts as a transcription repressor of epidermodysplasia verruciformis-associated human papillomaviruses. Arch. Virol. 1512461-2473. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, G. K., H. Wang, S. K. Dey, and R. D. Palmiter. 2004. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis 4074-81. [DOI] [PubMed] [Google Scholar]
- 3.Androphy, E. J., I. Dvoretzky, and D. R. Lowy. 1985. X-linked inheritance of epidermodysplasia verruciformis. Genetic and virologic studies of a kindred. Arch. Dermatol. 121864-868. [PubMed] [Google Scholar]
- 4.Antonsson, A., O. Forslund, H. Ekberg, G. Sterner, and B. G. Hansson. 2000. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 7411636-11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonsson, A., S. Karanfilovska, P. G. Lindqvist, and B. G. Hansson. 2003. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 412509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anttila, P. H., E. von Willebrand, and O. Simell. 1986. Abnormal immune responses during hypozincaemia in acrodermatitis enteropathica. Acta Paediatr. Scand. 75988-992. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafi, G. H., M. Haghshenas, B. Marchetti, and M. S. Campo. 2006. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 1192105-2112. [DOI] [PubMed] [Google Scholar]
- 8.Auf der Maur, A., T. Belser, Y. Wang, C. Gunes, P. Lichtlen, O. Georgiev, and W. Schaffner. 2000. Characterization of the mouse gene for the heavy metal-responsive transcription factor MTF-1. Cell Stress Chaperones 5196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ault, K. A. 2006. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect. Dis. Obstet. Gynecol. 2006(Suppl.)40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzimonti, B., M. Mondini, M. De Andrea, D. Gioia, U. Dianzani, R. Mesturini, G. Leigheb, R. Tiberio, S. Landolfo, and M. Gariglio. 2005. CD8+ T-cell lymphocytopenia and lack of EVER mutations in a patient with clinically and virologically typical epidermodysplasia verruciformis. Arch. Dermatol. 1411323-1325. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann, M. F., A. Gallimore, S. Linkert, V. Cerundolo, A. Lanzavecchia, M. Kopf, and A. Viola. 1999. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J. Exp. Med. 1891521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balabanian, K., B. Lagane, J. L. Pablos, L. Laurent, T. Planchenault, O. Verola, C. Lebbe, D. Kerob, A. Dupuy, O. Hermine, J. F. Nicolas, V. Latger-Cannard, D. Bensoussan, P. Bordigoni, F. Baleux, F. Le Deist, J. L. Virelizier, F. Arenzana-Seisdedos, and F. Bachelerie. 2005. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 1052449-2457. [DOI] [PubMed] [Google Scholar]
- 13.Balabanian, K., A. Levoye, L. Klemm, B. Lagane, O. Hermine, J. Harriague, F. Baleux, F. Arenzana-Seisdedos, and F. Bachelerie. 2008. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J. Clin. Investig. 1181074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbosa, M. S., D. R. Lowy, and J. T. Schiller. 1989. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J. Virol. 631404-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barthel, A., E. A. Ostrakhovitch, P. L. Walter, A. Kampkotter, and L. O. Klotz. 2007. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: mechanisms and consequences. Arch. Biochem. Biophys. 463175-182. [DOI] [PubMed] [Google Scholar]
- 16.Beaudenon, S., F. Praetorius, D. Kremsdorf, M. Lutzner, N. Worsaae, G. Pehau-Arnaudet, and G. Orth. 1987. A new type of human papillomavirus associated with oral focal epithelial hyperplasia. J. Investig. Dermatol. 88130-135. [DOI] [PubMed] [Google Scholar]
- 17.Berger, N. A., and A. M. Skinner. 1974. Characterization of lymphocyte transformation induced by zinc ions. J. Cell Biol. 6145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard, H. U. 2005. The clinical importance of the nomenclature, evolution and taxonomy of human papillomaviruses. J. Clin. Virol. 32(Suppl. 1)S1-S6. [DOI] [PubMed] [Google Scholar]
- 19.Bernard, H. U., and D. Apt. 1994. Transcriptional control and cell type specificity of HPV gene expression. Arch. Dermatol. 130210-215. [PubMed] [Google Scholar]
- 20.Bouwes Bavinck, J. N., E. I. Plasmeijer, and M. C. Feltkamp. 2008. Beta-papillomavirus infection and skin cancer. J. Investig. Dermatol. 1281355-1358. [DOI] [PubMed] [Google Scholar]
- 21.Braakhuis, B. J., P. J. Snijders, and C. R. Leemans. 2007. Human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 3571157; author reply 1157-1158. [PubMed] [Google Scholar]
- 22.Bravo, I. G., and A. Alonso. 2004. Mucosal human papillomaviruses encode four different E5 proteins whose chemistry and phylogeny correlate with malignant or benign growth. J. Virol. 7813613-13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo, I. G., and A. Alonso. 2007. Phylogeny and evolution of papillomaviruses based on the E1 and E2 proteins. Virus Genes 34249-262. [DOI] [PubMed] [Google Scholar]
- 24.Burk, R. D., M. Terai, P. E. Gravitt, L. A. Brinton, R. J. Kurman, W. A. Barnes, M. D. Greenberg, O. C. Hadjimichael, L. Fu, L. McGowan, R. Mortel, P. E. Schwartz, and A. Hildesheim. 2003. Distribution of human papillomavirus types 16 and 18 variants in squamous cell carcinomas and adenocarcinomas of the cervix. Cancer Res. 637215-7220. [PubMed] [Google Scholar]
- 25.Caldeira, S., I. Zehbe, R. Accardi, I. Malanchi, W. Dong, M. Giarre, E. M. de Villiers, R. Filotico, P. Boukamp, and M. Tommasino. 2003. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J. Virol. 772195-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellsague, X., M. Diaz, S. de Sanjose, N. Munoz, R. Herrero, S. Franceschi, R. W. Peeling, R. Ashley, J. S. Smith, P. J. Snijders, C. J. Meijer, and F. X. Bosch. 2006. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J. Natl. Cancer Inst. 98303-315. [DOI] [PubMed] [Google Scholar]
- 27.Chang, J. L., Y. P. Tsao, D. W. Liu, S. J. Huang, W. H. Lee, and S. L. Chen. 2001. The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J. Biomed. Sci. 8206-213. [DOI] [PubMed] [Google Scholar]
- 28.Cheng, S., D. C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 92335-2349. [DOI] [PubMed] [Google Scholar]
- 29.Chesters, P. M., K. H. Vousden, C. Edmonds, and D. J. McCance. 1990. Analysis of human papillomavirus type 16 open reading frame E7 immortalizing function in rat embryo fibroblast cells. J. Gen. Virol. 71449-453. [DOI] [PubMed] [Google Scholar]
- 30.Chong, T., D. Apt, B. Gloss, M. Isa, and H. U. Bernard. 1991. The enhancer of human papillomavirus type 16: binding sites for the ubiquitous transcription factors oct-1, NFA, TEF-2, NF1, and AP-1 participate in epithelial cell-specific transcription. J. Virol. 655933-5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clifford, G. M., J. S. Smith, M. Plummer, N. Munoz, and S. Franceschi. 2003. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 8863-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad, M., V. J. Bubb, and R. Schlegel. 1993. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 676170-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuberos, V., J. Perez, C. J. Lopez, F. Castro, L. V. Gonzalez, L. A. Correa, G. Sanclemente, A. Gaviria, M. Muller, and G. I. Sanchez. 2006. Molecular and serological evidence of the epidemiological association of HPV 13 with focal epithelial hyperplasia: a case-control study. J. Clin. Virol. 3721-26. [DOI] [PubMed] [Google Scholar]
- 34.Dardenne, M., W. Savino, S. Berrih, and J. F. Bach. 1985. A zinc-dependent epitope on the molecule of thymulin, a thymic hormone. Proc. Natl. Acad. Sci. USA 827035-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Koning, M. N., L. Struijk, J. N. Bavinck, B. Kleter, J. ter Schegget, W. G. Quint, and M. C. Feltkamp. 2007. Betapapillomaviruses frequently persist in the skin of healthy individuals. J. Gen. Virol. 881489-1495. [DOI] [PubMed] [Google Scholar]
- 36.Dell'oste, V., B. Azzimonti, M. De Andrea, M. Mondini, E. Zavattaro, G. Leigheb, S. J. Weissenborn, H. Pfister, K. M. Michael, T. Waterboer, M. Pawlita, A. Amantea, S. Landolfo, and M. Gariglio. 2009. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J. Investig. Dermatol. 1291026-1034. [DOI] [PubMed] [Google Scholar]
- 37.de Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 32417-27. [DOI] [PubMed] [Google Scholar]
- 38.DiMaio, D., and J. B. Liao. 2006. Human papillomaviruses and cervical cancer. Adv. Virus Res. 66125-159. [DOI] [PubMed] [Google Scholar]
- 39.Doorbar, J. 2006. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. (London) 110525-541. [DOI] [PubMed] [Google Scholar]
- 40.Doorbar, J. 2005. The papillomavirus life cycle. J. Clin. Virol. 32(Suppl. 1)S7-S15. [DOI] [PubMed] [Google Scholar]
- 41.Doorbar, J. 2007. Papillomavirus life cycle organization and biomarker selection. Dis. Markers 23297-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doorbar, J., S. Ely, J. Sterling, C. McLean, and L. Crawford. 1991. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352824-827. [DOI] [PubMed] [Google Scholar]
- 43.Driessen, C., K. Hirv, L. Rink, and H. Kirchner. 1994. Induction of cytokines by zinc ions in human peripheral blood mononuclear cells and separated monocytes. Lymphok. Cytok. Res. 1315-20. [PubMed] [Google Scholar]
- 44.D'Souza, G., A. R. Kreimer, R. Viscidi, M. Pawlita, C. Fakhry, W. M. Koch, W. H. Westra, and M. L. Gillison. 2007. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 3561944-1956. [DOI] [PubMed] [Google Scholar]
- 45.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 9710002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunne, E. F., E. R. Unger, M. Sternberg, G. McQuillan, D. C. Swan, S. S. Patel, and L. E. Markowitz. 2007. Prevalence of HPV infection among females in the United States. JAMA 297813-819. [DOI] [PubMed] [Google Scholar]
- 47.Egawa, K. 2003. Do human papillomaviruses target epidermal stem cells? Dermatology 207251-254. [DOI] [PubMed] [Google Scholar]
- 48.Eide, D. J. 2006. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763711-722. [DOI] [PubMed] [Google Scholar]
- 49.Elgui de Oliveira, D. 2007. DNA viruses in human cancer: an integrated overview on fundamental mechanisms of viral carcinogenesis. Cancer Lett. 247182-196. [DOI] [PubMed] [Google Scholar]
- 50.Eom, S. J., E. Y. Kim, J. E. Lee, H. J. Kang, J. Shim, S. U. Kim, B. J. Gwag, and E. J. Choi. 2001. Zn(2+) induces stimulation of the c-Jun N-terminal kinase signaling pathway through phosphoinositide 3-kinase. Mol. Pharmacol. 59981-986. [DOI] [PubMed] [Google Scholar]
- 51.Favre, M., F. Breitburd, O. Croissant, and G. Orth. 1977. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J. Virol. 211205-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favre, M., S. Majewski, B. Noszczyk, F. Maienfisch, A. Pura, G. Orth, and S. Jablonska. 2000. Antibodies to human papillomavirus type 5 are generated in epidermal repair processes. J. Investig. Dermatol. 114403-407. [DOI] [PubMed] [Google Scholar]
- 53.Favre, M., G. Orth, S. Majewski, S. Baloul, A. Pura, and S. Jablonska. 1998. Psoriasis: a possible reservoir for human papillomavirus type 5, the virus associated with skin carcinomas of epidermodysplasia verruciformis. J. Investig. Dermatol. 110311-317. [DOI] [PubMed] [Google Scholar]
- 54.Favre, M., N. Ramoz, and G. Orth. 1997. Human papillomaviruses: general features. Clin. Dermatol. 15181-198. [DOI] [PubMed] [Google Scholar]
- 55.Fernandes, G., M. Nair, K. Onoe, T. Tanaka, R. Floyd, and R. A. Good. 1979. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc. Natl. Acad. Sci. USA 76457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forslund, O., A. Antonsson, P. Nordin, B. Stenquist, and B. G. Hansson. 1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 802437-2443. [DOI] [PubMed] [Google Scholar]
- 57.Forslund, O., T. Iftner, K. Andersson, B. Lindelof, E. Hradil, P. Nordin, B. Stenquist, R. Kirnbauer, J. Dillner, and E. M. de Villiers. 2007. Cutaneous human papillomaviruses found in sun-exposed skin: beta-papillomavirus species 2 predominates in squamous cell carcinoma. J. Infect. Dis. 196876-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraker, P. J., and L. E. King. 2004. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 24277-298. [DOI] [PubMed] [Google Scholar]
- 59.Frattini, M. G., and L. A. Laimins. 1994. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc. Natl. Acad. Sci. USA 9112398-12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frattini, M. G., and L. A. Laimins. 1994. The role of the E1 and E2 proteins in the replication of human papillomavirus type 31b. Virology 204799-804. [DOI] [PubMed] [Google Scholar]
- 61.Frisch, M. 2002. On the etiology of anal squamous carcinoma. Dan. Med. Bull. 49194-209. [PubMed] [Google Scholar]
- 62.Garcia-Vallve, S., A. Alonso, and I. G. Bravo. 2005. Papillomaviruses: different genes have different histories. Trends Microbiol. 13514-521. [DOI] [PubMed] [Google Scholar]
- 63.Gaspar, H. B., C. Harwood, I. Leigh, and A. J. Thrasher. 2004. Severe cutaneous papillomavirus disease after haematopoietic stem-cell transplantation in patients with severe combined immunodeficiency. Br. J. Haematol. 127232-233. [DOI] [PubMed] [Google Scholar]
- 64.Gewirtzman, A., B. Bartlett, and S. Tyring. 2008. Epidermodysplasia verruciformis and human papilloma virus. Curr. Opin. Infect. Dis. 21141-146. [DOI] [PubMed] [Google Scholar]
- 65.Giannoudis, A., and C. S. Herrington. 2001. Human papillomavirus variants and squamous neoplasia of the cervix. J. Pathol. 193295-302. [DOI] [PubMed] [Google Scholar]
- 66.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 751565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giuliano, A. R., B. Lu, C. M. Nielson, R. Flores, M. R. Papenfuss, J. H. Lee, M. Abrahamsen, and R. B. Harris. 2008. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J. Infect. Dis. 198:827-835. [DOI] [PubMed] [Google Scholar]
- 68.Glinski, W., S. Jablonska, A. Langner, S. Obalek, M. Haftek, and M. Proniewska. 1976. Cell-mediated immunity in epidermodysplasia verruciformis. Dermatologica 153218-227. [DOI] [PubMed] [Google Scholar]
- 69.Glinski, W., S. Obalek, S. Jablonska, and G. Orth. 1981. T cell defect in patients with epidermodysplasia verruciformis due to human papillomavirus type 3 and 5. Dermatologica 162141-147. [DOI] [PubMed] [Google Scholar]
- 70.Goldschmidt, M. H., J. S. Kennedy, D. R. Kennedy, H. Yuan, D. E. Holt, M. L. Casal, A. M. Traas, E. A. Mauldin, P. F. Moore, P. S. Henthorn, B. J. Hartnett, K. I. Weinberg, R. Schlegel, and P. J. Felsburg. 2006. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J. Virol. 806621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottschling, M., A. Stamatakis, I. Nindl, E. Stockfleth, A. Alonso, and I. G. Bravo. 2007. Multiple evolutionary mechanisms drive papillomavirus diversification. Mol. Biol. Evol. 241242-1258. [DOI] [PubMed] [Google Scholar]
- 72.Grossman, S. R., and L. A. Laimins. 1989. E6 protein of human papillomavirus type 18 binds zinc. Oncogene 41089-1093. [PubMed] [Google Scholar]
- 73.Gulino, A. V., D. Moratto, S. Sozzani, P. Cavadini, K. Otero, L. Tassone, L. Imberti, S. Pirovano, L. D. Notarangelo, R. Soresina, E. Mazzolari, D. L. Nelson, L. D. Notarangelo, and R. Badolato. 2004. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 104444-452. [DOI] [PubMed] [Google Scholar]
- 74.Haase, H., and W. Maret. 2003. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 291289-298. [DOI] [PubMed] [Google Scholar]
- 75.Harwood, C. A., T. Surentheran, J. M. McGregor, P. J. Spink, I. M. Leigh, J. Breuer, and C. M. Proby. 2000. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J. Med. Virol. 61289-297. [DOI] [PubMed] [Google Scholar]
- 76.Harwood, C. A., T. Surentheran, P. Sasieni, C. M. Proby, C. Bordea, I. M. Leigh, F. Wojnarowska, J. Breuer, and J. M. McGregor. 2004. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br. J. Dermatol. 150949-957. [DOI] [PubMed] [Google Scholar]
- 77.Hazard, K., K. Andersson, J. Dillner, and O. Forslund. 2007. Human papillomavirus subtypes are not uncommon. Virology 3626-9. [DOI] [PubMed] [Google Scholar]
- 78.Hebert, A. A., and M. D. Lopez. 1997. Oral lesions in pediatric patients. Adv. Dermatol. 12169-193; discussion 194. [PubMed] [Google Scholar]
- 79.Hebner, C. M., and L. A. Laimins. 2006. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 1683-97. [DOI] [PubMed] [Google Scholar]
- 80.Heck, D. V., C. L. Yee, P. M. Howley, and K. Munger. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 894442-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herbein, G., A. Varin, and T. Fulop. 2006. NF-kappaB, AP-1, zinc-deficiency and aging. Biogerontology 7409-419. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez, P. A., R. J. Gorlin, J. N. Lukens, S. Taniuchi, J. Bohinjec, F. Francois, M. E. Klotman, and G. A. Diaz. 2003. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 3470-74. [DOI] [PubMed] [Google Scholar]
- 83.Hess, J., P. Angel, and M. Schorpp-Kistner. 2004. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 1175965-5973. [DOI] [PubMed] [Google Scholar]
- 84.Heuchel, R., F. Radtke, O. Georgiev, G. Stark, M. Aguet, and W. Schaffner. 1994. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 132870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hildesheim, A., R. Herrero, P. E. Castle, S. Wacholder, M. C. Bratti, M. E. Sherman, A. T. Lorincz, R. D. Burk, J. Morales, A. C. Rodriguez, K. Helgesen, M. Alfaro, M. Hutchinson, I. Balmaceda, M. Greenberg, and M. Schiffman. 2001. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br. J. Cancer 841219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horn, S., H. Pfister, and P. G. Fuchs. 1993. Constitutive transcriptional activator of epidermodysplasia verruciformis-associated human papillomavirus 8. Virology 196674-681. [DOI] [PubMed] [Google Scholar]
- 87.Huh, K., X. Zhou, H. Hayakawa, J. Y. Cho, T. A. Libermann, J. Jin, J. W. Harper, and K. Munger. 2007. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 819737-9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huse, M., M. J. Eck, and S. C. Harrison. 1998. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J. Biol. Chem. 27318729-18733. [DOI] [PubMed] [Google Scholar]
- 89.Iversen, T., and S. Tretli. 1998. Intraepithelial and invasive squamous cell neoplasia of the vulva: trends in incidence, recurrence, and survival rate in Norway. Obstet. Gynecol. 91969-972. [DOI] [PubMed] [Google Scholar]
- 90.Jablonska, S., J. Dabrowski, and K. Jakubowicz. 1972. Epidermodysplasia verruciformis as a model in studies on the role of papovaviruses in oncogenesis. Cancer Res. 32583-589. [PubMed] [Google Scholar]
- 91.Jackson, S., L. Ghali, C. Harwood, and A. Storey. 2002. Reduced apoptotic levels in squamous but not basal cell carcinomas correlates with detection of cutaneous human papillomavirus. Br. J. Cancer 87319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson, S., C. Harwood, M. Thomas, L. Banks, and A. Storey. 2000. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 143065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jamieson, D. J., P. Paramsothy, S. Cu-Uvin, A. Duerr, and the HIV Epidemiology Research Study Group. 2006. Vulvar, vaginal, and perianal intraepithelial neoplasia in women with or at risk for human immunodeficiency virus. Obstet. Gynecol. 1071023-1028. [DOI] [PubMed] [Google Scholar]
- 94.Joyce, J. G., J. S. Tung, C. T. Przysiecki, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 2745810-5822. [DOI] [PubMed] [Google Scholar]
- 95.Kabsch, K., and A. Alonso. 2002. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J. Virol. 7612162-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalantari, M., L. L. Villa, I. E. Calleja-Macias, and H. U. Bernard. 2008. Human papillomavirus-16 and -18 in penile carcinomas: DNA methylation, chromosomal recombination and genomic variation. Int. J. Cancer 1231832-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanda, T., S. Watanabe, S. Zanma, H. Sato, A. Furuno, and K. Yoshiike. 1991. Human papillomavirus type 16 E6 proteins with glycine substitution for cysteine in the metal-binding motif. Virology 185536-543. [DOI] [PubMed] [Google Scholar]
- 98.Kang, J. H., S. W. Jin, H. S. Yoon, W. D. Yoo, H. S. Kim, K. S. Hahm, and S. N. Park. 1997. Analysis of the conformational change of recombinant human papilloma virus type 18 E7 protein induced by metal binding. Virus Res. 49147-154. [DOI] [PubMed] [Google Scholar]
- 99.Kappes, D. J. 2007. CD4 and CD8: hogging all the Lck. Immunity 27691-693. [DOI] [PubMed] [Google Scholar]
- 100.Kawai, T., U. Choi, N. L. Whiting-Theobald, G. F. Linton, S. Brenner, J. M. Sechler, P. M. Murphy, and H. L. Malech. 2005. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp. Hematol. 33460-468. [DOI] [PubMed] [Google Scholar]
- 101.Keresztes, G., H. Mutai, and S. Heller. 2003. TMC and EVER genes belong to a larger novel family, the TMC gene family encoding transmembrane proteins. BMC Genomics 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim, P. W., Z. Y. Sun, S. C. Blacklow, G. Wagner, and M. J. Eck. 2003. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science 3011725-1728. [DOI] [PubMed] [Google Scholar]
- 103.Kim, Y. M., W. Reed, W. Wu, P. A. Bromberg, L. M. Graves, and J. M. Samet. 2006. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290L1028-L1035. [DOI] [PubMed] [Google Scholar]
- 104.King, L. E., F. Osati-Ashtiani, and P. J. Fraker. 2002. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J. Nutr. 132974-979. [DOI] [PubMed] [Google Scholar]
- 105.Kirchner, H., and H. Ruhl. 1970. Stimulation of human peripheral lymphocytes by Zn2+ in vitro. Exp. Cell Res. 61229-230. [DOI] [PubMed] [Google Scholar]
- 106.Kitamura, H., H. Morikawa, H. Kamon, M. Iguchi, S. Hojyo, T. Fukada, S. Yamashita, T. Kaisho, S. Akira, M. Murakami, and T. Hirano. 2006. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 7971-977. [DOI] [PubMed] [Google Scholar]
- 107.Koshiol, J., L. Lindsay, J. M. Pimenta, C. Poole, D. Jenkins, and J. S. Smith. 2008. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am. J. Epidemiol. 168123-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krawczyk, E., J. A. Hanover, R. Schlegel, and F. A. Suprynowicz. 2008. Karyopherin beta3: a new cellular target for the HPV-16 E5 oncoprotein. Biochem. Biophys. Res. Commun. 371684-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurima, K., Y. Yang, K. Sorber, and A. J. Griffith. 2003. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics 82300-308. [DOI] [PubMed] [Google Scholar]
- 110.Kutler, D. I., V. B. Wreesmann, A. Goberdhan, L. Ben-Porat, J. Satagopan, I. Ngai, A. G. Huvos, P. Giampietro, O. Levran, K. Pujara, R. Diotti, D. Carlson, L. A. Huryn, A. D. Auerbach, and B. Singh. 2003. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J. Natl. Cancer Inst. 951718-1721. [DOI] [PubMed] [Google Scholar]
- 111.Kyo, S., D. J. Klumpp, M. Inoue, T. Kanaya, and L. A. Laimins. 1997. Expression of AP1 during cellular differentiation determines human papillomavirus E6/E7 expression in stratified epithelial cells. J. Gen. Virol. 78401-411. [DOI] [PubMed] [Google Scholar]
- 112.Laffort, C., F. Le Deist, M. Favre, S. Caillat-Zucman, I. Radford-Weiss, M. Debre, S. Fraitag, S. Blanche, M. Cavazzana-Calvo, G. de Saint Basile, J. P. de Villartay, S. Giliani, G. Orth, J. L. Casanova, C. Bodemer, and A. Fischer. 2004. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common gammac cytokine receptor subunit or JAK-3 deficiency. Lancet 3632051-2054. [DOI] [PubMed] [Google Scholar]
- 113.Laity, J. H., B. M. Lee, and P. E. Wright. 2001. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol. 1139-46. [DOI] [PubMed] [Google Scholar]
- 114.Langmade, S. J., R. Ravindra, P. J. Daniels, and G. K. Andrews. 2000. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 27534803-34809. [DOI] [PubMed] [Google Scholar]
- 115.Lazarczyk, M., and M. Favre. 2008. Role of Zn2+ ions in host-virus interactions. J. Virol. 8211486-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lazarczyk, M., C. Pons, J. A. Mendoza, P. Cassonnet, Y. Jacob, and M. Favre. 2008. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 20535-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Deist, F., C. Laffort, G. Orth, C. Bodemer, and A. Fischer. 2005. Severe combined immunodeficiency: susceptibility to HPV? Med. Sci. (Paris) 21125-127. (In French.) [DOI] [PubMed] [Google Scholar]
- 118.Leverrier, S., D. Bergamaschi, L. Ghali, A. Ola, G. Warnes, B. Akgul, K. Blight, R. Garcia-Escudero, A. Penna, A. Eddaoudi, and A. Storey. 2007. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis 12549-560. [DOI] [PubMed] [Google Scholar]
- 119.Li, J. J., J. S. Rhim, R. Schlegel, K. H. Vousden, and N. H. Colburn. 1998. Expression of dominant negative Jun inhibits elevated AP-1 and NF-kappaB transactivation and suppresses anchorage independent growth of HPV immortalized human keratinocytes. Oncogene 162711-2721. [DOI] [PubMed] [Google Scholar]
- 120.Lin, R. S., C. Rodriguez, A. Veillette, and H. F. Lodish. 1998. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 27332878-32882. [DOI] [PubMed] [Google Scholar]
- 121.Lipari, F., G. A. McGibbon, E. Wardrop, and M. G. Cordingley. 2001. Purification and biophysical characterization of a minimal functional domain and of an N-terminal Zn2+-binding fragment from the human papillomavirus type 16 E6 protein. Biochemistry 401196-1204. [DOI] [PubMed] [Google Scholar]
- 122.Liuzzi, J. P., J. A. Bobo, L. A. Lichten, D. A. Samuelson, and R. J. Cousins. 2004. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA 10114355-14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liuzzi, J. P., and R. J. Cousins. 2004. Mammalian zinc transporters. Annu. Rev. Nutr. 24151-172. [DOI] [PubMed] [Google Scholar]
- 124.Longworth, M. S., and L. A. Laimins. 2004. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J. Virol. 783533-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Longworth, M. S., and L. A. Laimins. 2004. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu, X. M., S. Monnier-Benoit, L. Z. Mo, S. Y. Xu, J. L. Pretet, Z. Liu, D. A. Vuitton, and C. Mougin. 2008. Human papillomavirus in esophageal squamous cell carcinoma of the high-risk Kazakh ethnic group in Xinjiang, China. Eur. J. Surg. Oncol. 34765-770. [DOI] [PubMed] [Google Scholar]
- 127.Lutzner, M., O. Croissant, M. F. Ducasse, H. Kreis, J. Crosnier, and G. Orth. 1980. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J. Investig. Dermatol. 75353-356. [DOI] [PubMed] [Google Scholar]
- 128.Lutzner, M. A. 1978. Epidermodysplasia verruciformis. An autosomal recessive disease characterized by viral warts and skin cancer. A model for viral oncogenesis. Bull. Cancer 65169-182. [PubMed] [Google Scholar]
- 129.Mack, D. H., and L. A. Laimins. 1991. A keratinocyte-specific transcription factor, KRF-1, interacts with AP-1 to activate expression of human papillomavirus type 18 in squamous epithelial cells. Proc. Natl. Acad. Sci. USA 889102-9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Majewski, S., M. Favre, G. Orth, and S. Jablonska. 1998. Is human papillomavirus type 5 the putative autoantigen involved in psoriasis? J. Investig. Dermatol. 111541-542. [DOI] [PubMed] [Google Scholar]
- 131.Majewski, S., N. Hunzelmann, R. Nischt, B. Eckes, L. Rudnicka, G. Orth, T. Krieg, and S. Jablonska. 1991. TGF beta-1 and TNF alpha expression in the epidermis of patients with epidermodysplasia verruciformis. J. Investig. Dermatol. 97862-867. [DOI] [PubMed] [Google Scholar]
- 132.Majewski, S., and S. Jablonska. 1997. Human papillomavirus-associated tumors of the skin and mucosa. J. Am. Acad. Dermatol. 36659-685. [DOI] [PubMed] [Google Scholar]
- 133.Mann, J. J., and P. J. Fraker. 2005. Zinc pyrithione induces apoptosis and increases expression of Bim. Apoptosis 10369-379. [DOI] [PubMed] [Google Scholar]
- 134.May, M., K. Grassmann, H. Pfister, and P. G. Fuchs. 1994. Transcriptional silencer of the human papillomavirus type 8 late promoter interacts alternatively with the viral trans activator E2 or with a cellular factor. J. Virol. 683612-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McIntyre, M. C., M. G. Frattini, S. R. Grossman, and L. A. Laimins. 1993. Human papillomavirus type 18 E7 protein requires intact Cys-X-X-Cys motifs for zinc binding, dimerization, and transformation but not for Rb binding. J. Virol. 673142-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Medina-Martinez, O., V. Vallejo, M. C. Guido, and A. Garcia-Carranca. 1997. Ha-ras oncogene-induced transcription of human papillomavirus type 18 E6 and E7 oncogenes. Mol. Carcinog. 1983-90. [PubMed] [Google Scholar]
- 137.Mendoza, J. A., Y. Jacob, P. Cassonnet, and M. Favre. 2006. Human papillomavirus type 5 E6 oncoprotein represses the transforming growth factor β signaling pathway by binding to SMAD3. J. Virol. 8012420-12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moerman, M., V. G. Danielides, C. S. Nousia, F. Van Wanzeele, R. Forsyth, and H. Vermeersch. 2001. Recurrent focal epithelial hyperplasia due to HPV13 in an HIV-positive patient. Dermatology 203339-341. [DOI] [PubMed] [Google Scholar]
- 139.Mohr, I. J., R. Clark, S. Sun, E. J. Androphy, P. MacPherson, and M. R. Botchan. 1990. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science 2501694-1699. [DOI] [PubMed] [Google Scholar]
- 140.Molano, M., A. Van den Brule, M. Plummer, E. Weiderpass, H. Posso, A. Arslan, C. J. Meijer, N. Munoz, and S. Franceschi. 2003. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am. J. Epidemiol. 158486-494. [DOI] [PubMed] [Google Scholar]
- 141.Munger, K., and P. M. Howley. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89213-228. [DOI] [PubMed] [Google Scholar]
- 142.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348518-527. [DOI] [PubMed] [Google Scholar]
- 143.Munoz, N., X. Castellsague, A. B. de Gonzalez, and L. Gissmann. 2006. HPV in the etiology of human cancer. Vaccine 24(Suppl. 3)1-10. [DOI] [PubMed] [Google Scholar]
- 144.Narisawa-Saito, M., and T. Kiyono. 2007. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 981505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Notarangelo, L., J. L. Casanova, A. Fischer, J. Puck, F. Rosen, R. Seger, and R. Geha. 2004. Primary immunodeficiency diseases: an update. J. Allergy Clin. Immunol. 114677-687. [DOI] [PubMed] [Google Scholar]
- 146.Obalek, S., M. Favre, J. Szymanczyk, J. Misiewicz, S. Jablonska, and G. Orth. 1992. Human papillomavirus (HPV) types specific of epidermodysplasia verruciformis detected in warts induced by HPV3 or HPV3-related types in immunosuppressed patients. J. Investig. Dermatol. 98936-941. [DOI] [PubMed] [Google Scholar]
- 147.Offord, E. A., and P. Beard. 1990. A member of the activator protein 1 family found in keratinocytes but not in fibroblasts required for transcription from a human papillomavirus type 18 promoter. J. Virol. 644792-4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohana, E., D. Segal, R. Palty, D. Ton-That, A. Moran, S. L. Sensi, J. H. Weiss, M. Hershfinkel, and I. Sekler. 2004. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J. Biol. Chem. 2794278-4284. [DOI] [PubMed] [Google Scholar]
- 149.Ohlenschlager, O., T. Seiboth, H. Zengerling, L. Briese, A. Marchanka, R. Ramachandran, M. Baum, M. Korbas, W. Meyer-Klaucke, M. Durst, and M. Gorlach. 2006. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene 255953-5959. [DOI] [PubMed] [Google Scholar]
- 150.Oldak, M., H. Smola, M. Aumailley, F. Rivero, H. Pfister, and S. Smola-Hess. 2004. The human papillomavirus type 8 E2 protein suppresses β4-integrin expression in primary human keratinocytes. J. Virol. 7810738-10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Orth, G. 1987. Epidermodysplasia verruciformis, p. 199-243. In N. P. Salzman and P. M. Howley (ed.), the papovaviridae: the papillomaviruses. Plenum Press, New York, NY.
- 152.Orth, G. 2006. Genetics of epidermodysplasia verruciformis: insights into host defense against papillomaviruses. Semin. Immunol. 18362-374. [DOI] [PubMed] [Google Scholar]
- 153.Orth, G. 2005. Human papillomaviruses associated with epidermodysplasia verruciformis in non-melanoma skin cancers: guilty or innocent? J. Investig. Dermatol. 125xii-xiii. [DOI] [PubMed] [Google Scholar]
- 154.Orth, G. 1999. Papillomaviruses—human (papovaviridae): general features, p. 1105-1114. In A. Granoff and R. G. Webster (ed.), Encyclopedia of Virology, 2nd ed. Academic Press, London, United Kingdom.
- 155.Orth, G., M. Favre, F. Breitburd, O. Croissant, S. Jablonska, and S. Obalek. 1980. Epidermodysplasia verruciformis: a model for the role of papillomaviruses in human cancer. Cold Spring Harb. Conf. Cell Prolif. 7259-282. [Google Scholar]
- 156.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2922488-2492. [DOI] [PubMed] [Google Scholar]
- 157.Palefsky, J. M. 2007. HPV infection in men. Dis. Markers 23261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palmiter, R. D., and S. D. Findley. 1995. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 14639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patel, A. S., M. R. Karagas, M. Pawlita, T. Waterboer, and H. H. Nelson. 2008. Cutaneous human papillomavirus infection, the EVER2 gene and incidence of squamous cell carcinoma: a case-control study. Int. J. Cancer 1222377-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patrick, J., J. Michael, M. N. Golden, B. E. Golden, and P. J. Hilton. 1978. Effect of zinc on leucocyte sodium transport in vitro. Clin. Sci. Mol. Med. 54585-587. [DOI] [PubMed] [Google Scholar]
- 161.Peitsaro, P., B. Johansson, and S. Syrjanen. 2002. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J. Clin. Microbiol. 40886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peto, M., I. Tolle-Ersu, H. G. Kreysch, and G. Klock. 1995. Epidermal growth factor induction of human papillomavirus type 16 E6/E7 MRNA in tumor cells involves two AP-1 binding sites in the viral enhancer. J. Gen. Virol. 761945-1958. [DOI] [PubMed] [Google Scholar]
- 163.Pett, M., and N. Coleman. 2007. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J. Pathol. 212356-367. [DOI] [PubMed] [Google Scholar]
- 164.Pfefferle, R., G. P. Marcuzzi, B. Akgul, H. U. Kasper, F. Schulze, I. Haase, C. Wickenhauser, and H. Pfister. 2008. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J. Investig. Dermatol. 1282310-2315. [DOI] [PubMed] [Google Scholar]
- 165.Pfister, H., P. G. Fuchs, S. Majewski, S. Jablonska, I. Pniewska, and M. Malejczyk. 2003. High prevalence of epidermodysplasia verruciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent population. Arch. Dermatol. Res. 295273-279. [DOI] [PubMed] [Google Scholar]
- 166.Praetorius, F. 1997. HPV-associated diseases of oral mucosa. Clin. Dermatol. 15399-413. [DOI] [PubMed] [Google Scholar]
- 167.Prasad, A. S. 1998. Zinc and immunity. Mol. Cell. Biochem. 18863-69. [PubMed] [Google Scholar]
- 168.Prawer, S. E., F. Pass, J. C. Vance, L. J. Greenberg, E. J. Yunis, and A. S. Zelickson. 1977. Depressed immune function in epidermodysplasia verruciformis. Arch. Dermatol. 113495-499. [PubMed] [Google Scholar]
- 169.Premoli-De-Percoco, G., J. P. Cisternas, J. L. Ramirez, and I. Galindo. 1993. Focal epithelial hyperplasia: human-papillomavirus-induced disease with a genetic predisposition in a Venezuelan family. Hum. Genet. 91386-388. [DOI] [PubMed] [Google Scholar]
- 170.Prusty, B. K., and B. C. Das. 2005. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 113951-960. [DOI] [PubMed] [Google Scholar]
- 171.Purdie, K. J., T. Surentheran, J. C. Sterling, L. Bell, J. M. McGregor, C. M. Proby, C. A. Harwood, and J. Breuer. 2005. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. J. Investig. Dermatol. 12598-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pyrhonen, S., S. Jablonska, S. Obalek, and E. Kuismanen. 1980. Immune reactions in epidermodysplasia verruciformis. Br. J. Dermatol. 102247-254. [DOI] [PubMed] [Google Scholar]
- 173.Radtke, F., R. Heuchel, O. Georgiev, M. Hergersberg, M. Gariglio, Z. Dembic, and W. Schaffner. 1993. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 121355-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rajagopalan, K., J. Bahru, D. S. Loo, C. H. Tay, K. N. Chin, and K. K. Tan. 1972. Familial epidermodysplasia verruciformis of Lewandowsky and Lutz. Arch. Dermatol. 10573-78. [PubMed] [Google Scholar]
- 175.Ramoz, N., L. A. Rueda, B. Bouadjar, M. Favre, and G. Orth. 1999. A susceptibility locus for epidermodysplasia verruciformis, an abnormal predisposition to infection with the oncogenic human papillomavirus type 5, maps to chromosome 17qter in a region containing a psoriasis locus. J. Investig. Dermatol. 112259-263. [DOI] [PubMed] [Google Scholar]
- 176.Ramoz, N., L. A. Rueda, B. Bouadjar, L. S. Montoya, G. Orth, and M. Favre. 2002. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 32579-581. [DOI] [PubMed] [Google Scholar]
- 177.Ramoz, N., A. Taieb, L. A. Rueda, L. S. Montoya, B. Bouadjar, M. Favre, and G. Orth. 2000. Evidence for a nonallelic heterogeneity of epidermodysplasia verruciformis with two susceptibility loci mapped to chromosome regions 2p21-p24 and 17q25. J. Investig. Dermatol. 1141148-1153. [DOI] [PubMed] [Google Scholar]
- 178.Reeves, W. C., S. S. Ruparelia, K. I. Swanson, C. S. Derkay, A. Marcus, and E. R. Unger. 2003. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 129976-982. [DOI] [PubMed] [Google Scholar]
- 179.Regan, J. A., and L. A. Laimins. 2008. Bap31 is a novel target of the human papillomavirus E5 protein. J. Virol. 8210042-10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reh, H., and H. Pfister. 1990. Human papillomavirus type 8 contains cis-active positive and negative transcriptional control sequences. J. Gen. Virol. 712457-2462. [DOI] [PubMed] [Google Scholar]
- 181.Rink, L., and P. Gabriel. 2001. Extracellular and immunological actions of zinc. Biometals 14367-383. [DOI] [PubMed] [Google Scholar]
- 182.Ristriani, T., Y. Nomine, M. Masson, E. Weiss, and G. Trave. 2001. Specific recognition of four-way DNA junctions by the C-terminal zinc-binding domain of HPV oncoprotein E6. J. Mol. Biol. 305729-739. [DOI] [PubMed] [Google Scholar]
- 183.Roberts, S., I. Ashmole, T. M. Sheehan, A. H. Davies, and P. H. Gallimore. 1994. Human papillomavirus type 1 E4 protein is a zinc-binding protein. Virology 202865-874. [DOI] [PubMed] [Google Scholar]
- 184.Rodriguez, M. I., M. E. Finbow, and A. Alonso. 2000. Binding of human papillomavirus 16 E5 to the 16 kDa subunit c (proteolipid) of the vacuolar H+-ATPase can be dissociated from the E5-mediated epidermal growth factor receptor overactivation. Oncogene 193727-3732. [DOI] [PubMed] [Google Scholar]
- 185.Roth, E. J., B. Kurz, L. Liang, C. L. Hansen, C. T. Dameron, D. R. Winge, and D. Smotkin. 1992. Metal thiolate coordination in the E7 proteins of human papilloma virus 16 and cottontail rabbit papilloma virus as expressed in Escherichia coli. J. Biol. Chem. 26716390-16395. [PubMed] [Google Scholar]
- 186.Ruhl, H., and H. Kirchner. 1978. Monocyte-dependent stimulation of human T cells by zinc. Clin. Exp. Immunol. 32484-488. [PMC free article] [PubMed] [Google Scholar]
- 187.Sastre-Garau, X., M. Favre, J. Couturier, and G. Orth. 2000. Distinct patterns of alteration of myc genes associated with integration of human papillomavirus type 16 or type 45 DNA in two genital tumours. J. Gen. Virol. 811983-1993. [DOI] [PubMed] [Google Scholar]
- 188.Schaper, I. D., G. P. Marcuzzi, S. J. Weissenborn, H. U. Kasper, V. Dries, N. Smyth, P. Fuchs, and H. Pfister. 2005. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 651394-1400. [DOI] [PubMed] [Google Scholar]
- 189.Schiffman, M., P. E. Castle, J. Jeronimo, A. C. Rodriguez, and S. Wacholder. 2007. Human papillomavirus and cervical cancer. Lancet 370890-907. [DOI] [PubMed] [Google Scholar]
- 190.Schiffman, M., R. Herrero, R. Desalle, A. Hildesheim, S. Wacholder, A. C. Rodriguez, M. C. Bratti, M. E. Sherman, J. Morales, D. Guillen, M. Alfaro, M. Hutchinson, T. C. Wright, D. Solomon, Z. Chen, J. Schussler, P. E. Castle, and R. D. Burk. 2005. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 33776-84. [DOI] [PubMed] [Google Scholar]
- 191.Schneider-Maunoury, S., O. Croissant, and G. Orth. 1987. Integration of human papillomavirus type 16 DNA sequences: a possible early event in the progression of genital tumors. J. Virol. 613295-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sekler, I., S. L. Sensi, M. Hershfinkel, and W. F. Silverman. 2007. Mechanism and regulation of cellular zinc transport. Mol. Med. 13337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Seo, S. R., S. A. Chong, S. I. Lee, J. Y. Sung, Y. S. Ahn, K. C. Chung, and J. T. Seo. 2001. Zn2+-induced ERK activation mediated by reactive oxygen species causes cell death in differentiated PC12 cells. J. Neurochem. 78600-610. [DOI] [PubMed] [Google Scholar]
- 194.Shannon, P., A. Markiel, O. Ozier, N. S. Baliga, J. T. Wang, D. Ramage, N. Amin, B. Schwikowski, and T. Ideker. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 132498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smirnova, I. V., D. C. Bittel, R. Ravindra, H. Jiang, and G. K. Andrews. 2000. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J. Biol. Chem. 2759377-9384. [DOI] [PubMed] [Google Scholar]
- 196.Smith, J. S., L. Lindsay, B. Hoots, J. Keys, S. Franceschi, R. Winer, and G. M. Clifford. 2007. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer 121621-632. [DOI] [PubMed] [Google Scholar]
- 197.Soto, U., C. Denk, P. Finzer, K. J. Hutter, H. zur Hausen, and F. Rosl. 2000. Genetic complementation to non-tumorigenicity in cervical-carcinoma cells correlates with alterations in AP-1 composition. Int. J. Cancer 86811-817. [DOI] [PubMed] [Google Scholar]
- 198.Srodon, M., M. H. Stoler, G. B. Baber, and R. J. Kurman. 2006. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). Am. J. Surg. Pathol. 301513-1518. [DOI] [PubMed] [Google Scholar]
- 199.Stoppler, M. C., S. W. Straight, G. Tsao, R. Schlegel, and D. J. McCance. 1996. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology 223251-254. [DOI] [PubMed] [Google Scholar]
- 200.Straight, S. W., B. Herman, and D. J. McCance. 1995. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 693185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Straight, S. W., P. M. Hinkle, R. J. Jewers, and D. J. McCance. 1993. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 674521-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Struijk, L., J. N. Bouwes Bavinck, P. Wanningen, E. van der Meijden, R. G. Westendorp, J. Ter Schegget, and M. C. Feltkamp. 2003. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 1211531-1535. [DOI] [PubMed] [Google Scholar]
- 203.Stunkel, W., and H. U. Bernard. 1999. The chromatin structure of the long control region of human papillomavirus type 16 represses viral oncoprotein expression. J. Virol. 731918-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sutton, B. C., R. A. Allen, W. E. Moore, and S. T. Dunn. 2008. Distribution of human papillomavirus genotypes in invasive squamous carcinoma of the vulva. Mod. Pathol. 21345-354. [DOI] [PubMed] [Google Scholar]
- 205.Tanaka, Y., S. Shiozawa, I. Morimoto, and T. Fujita. 1989. Zinc inhibits pokeweed mitogen-induced development of immunoglobulin-secreting cells through augmentation of both CD4 and CD8 cells. Int. J. Immunopharmacol. 11673-679. [DOI] [PubMed] [Google Scholar]
- 206.Teresa, G., A. Anna, P. Maria, J. Zofia, and B. Gerard. 2002. Carp erythrocyte lipids as a potential target for the toxic action of zinc ions. Toxicol. Lett. 13257-64. [DOI] [PubMed] [Google Scholar]
- 207.Thierry, F., and P. M. Howley. 1991. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 390-100. [PubMed] [Google Scholar]
- 208.Tindle, R. W. 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 259-65. [DOI] [PubMed] [Google Scholar]
- 209.Tottey, S., K. J. Waldron, S. J. Firbank, B. Reale, C. Bessant, K. Sato, T. R. Cheek, J. Gray, M. J. Banfield, C. Dennison, and N. J. Robinson. 2008. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 4551138-1142. [DOI] [PubMed] [Google Scholar]
- 210.Trottier, H., and E. L. Franco. 2006. The epidemiology of genital human papillomavirus infection. Vaccine 24(Suppl. 1)S1-S15. [DOI] [PubMed] [Google Scholar]
- 211.Trottier, H., and E. L. Franco. 2006. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am. J. Manag. Care 12S462-S472. [PubMed] [Google Scholar]
- 212.Tsai, T. C., and S. L. Chen. 2003. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch. Virol. 1481445-1453. [DOI] [PubMed] [Google Scholar]
- 213.Tsao, Y. P., L. Y. Li, T. C. Tsai, and S. L. Chen. 1996. Human papillomavirus type 11 and 16 E5 represses p21(WafI/SdiI/CipI) gene expression in fibroblasts and keratinocytes. J. Virol. 707535-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Underbrink, M. P., H. L. Howie, K. M. Bedard, J. I. Koop, and D. A. Galloway. 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J. Virol. 8210408-10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ushikai, M., M. J. Lace, Y. Yamakawa, M. Kono, J. Anson, T. Ishiji, S. Parkkinen, N. Wicker, M. E. Valentine, I. Davidson, et al. 1994. trans activation by the full-length E2 proteins of human papillomavirus type 16 and bovine papillomavirus type 1 in vitro and in vivo: cooperation with activation domains of cellular transcription factors. J. Virol. 686655-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Valentine, R. A., K. A. Jackson, G. R. Christie, J. C. Mathers, P. M. Taylor, and D. Ford. 2007. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 28214389-14393. [DOI] [PubMed] [Google Scholar]
- 217.van de Nieuwenhof, H. P., I. A. van der Avoort, and J. A. de Hullu. 2008. Review of squamous premalignant vulvar lesions. Crit. Rev. Oncol. Hematol. 68131-156. [DOI] [PubMed] [Google Scholar]
- 218.van Zeeburg, H. J., P. J. Snijders, T. Wu, E. Gluckman, J. Soulier, J. Surralles, M. Castella, J. E. van der Wal, J. Wennerberg, J. Califano, E. Velleuer, R. Dietrich, W. Ebell, E. Bloemena, H. Joenje, C. R. Leemans, and R. H. Brakenhoff. 2008. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J. Natl. Cancer Inst. 1001649-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Verstraeten, S. V., M. P. Zago, G. G. MacKenzie, C. L. Keen, and P. I. Oteiza. 2004. Influence of zinc deficiency on cell-membrane fluidity in Jurkat, 3T3 and IMR-32 cells. Biochem. J. 378579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.von Knebel Doeberitz, M. 2002. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur. J. Cancer 382229-2242. [DOI] [PubMed] [Google Scholar]
- 221.Wang, K., B. Zhou, Y. M. Kuo, J. Zemansky, and J. Gitschier. 2002. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 7166-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Warner, G. L., and D. A. Lawrence. 1988. The effect of metals on IL-2-related lymphocyte proliferation. Int. J. Immunopharmacol. 10629-637. [DOI] [PubMed] [Google Scholar]
- 223.Watanabe, S., H. Sato, A. Furuno, and K. Yoshiike. 1992. Changing the spacing between metal-binding motifs decreases stability and transforming activity of the human papillomavirus type 18 E7 oncoprotein. Virology 190872-875. [DOI] [PubMed] [Google Scholar]
- 224.Weissenborn, S. J., I. Nindl, K. Purdie, C. Harwood, C. Proby, J. Breuer, S. Majewski, H. Pfister, and U. Wieland. 2005. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J. Investig. Dermatol. 12593-97. [DOI] [PubMed] [Google Scholar]
- 225.Wellinghausen, N., H. Kirchner, and L. Rink. 1997. The immunobiology of zinc. Immunol. Today 18519-521. [DOI] [PubMed] [Google Scholar]
- 226.Wetzler, M., M. Talpaz, E. S. Kleinerman, A. King, Y. O. Huh, J. U. Gutterman, and R. Kurzrock. 1990. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am. J. Med. 89663-672. [DOI] [PubMed] [Google Scholar]
- 227.Wilson, V. G., M. West, K. Woytek, and D. Rangasamy. 2002. Papillomavirus E1 proteins: form, function, and features. Virus Genes 24275-290. [DOI] [PubMed] [Google Scholar]
- 228.Winer, R. L., Q. Feng, J. P. Hughes, S. O'Reilly, N. B. Kiviat, and L. A. Koutsky. 2008. Risk of female human papillomavirus acquisition associated with first male sex partner. J. Infect. Dis. 197279-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Winer, R. L., S. K. Lee, J. P. Hughes, D. E. Adam, N. B. Kiviat, and L. A. Koutsky. 2003. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am. J. Epidemiol. 157218-226. [DOI] [PubMed] [Google Scholar]
- 230.Woodman, C. B., S. Collins, H. Winter, A. Bailey, J. Ellis, P. Prior, M. Yates, T. P. Rollason, and L. S. Young. 2001. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 3571831-1836. [DOI] [PubMed] [Google Scholar]
- 231.Yamamoto, Y., J. M. Huibregtse, and P. M. Howley. 1997. The human E6-AP gene (UBE3A) encodes three potential protein isoforms generated by differential splicing. Genomics 41263-266. [DOI] [PubMed] [Google Scholar]
- 232.Yamasaki, S., K. Sakata-Sogawa, A. Hasegawa, T. Suzuki, K. Kabu, E. Sato, T. Kurosaki, S. Yamashita, M. Tokunaga, K. Nishida, and T. Hirano. 2007. Zinc is a novel intracellular second messenger. J. Cell Biol. 177637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.You, J., J. L. Croyle, A. Nishimura, K. Ozato, and P. M. Howley. 2004. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117349-360. [DOI] [PubMed] [Google Scholar]
- 234.Zavattaro, E., B. Azzimonti, M. Mondini, M. De Andrea, C. Borgogna, V. Dell'Oste, M. Ferretti, S. Nicola, G. Cappellano, A. Carando, G. Leigheb, S. Landolfo, U. Dianzani, and M. Gariglio. 2008. Identification of defective Fas function and variation of the perforin gene in an epidermodysplasia verruciformis patient lacking EVER1 and EVER2 mutations. J. Investig. Dermatol. 128732-735. [DOI] [PubMed] [Google Scholar]
- 235.Zheng, Z. M., and C. C. Baker. 2006. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 112286-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2342-350. [DOI] [PubMed] [Google Scholar]
- 237.Zur Hausen, H. 2009. Papillomaviruses in the causation of human cancers—a brief historical account. Virology 384260-265. [DOI] [PubMed] [Google Scholar]