Abstract
Summary: We have developed a general scenario of prebiotic physicochemical evolution during the Earth's Hadean eon and reviewed the relevant literature. We suggest that prebiotic chemical evolution started in microspaces with membranous walls, where external temperature and osmotic gradients were coupled to free-energy gradients of potential chemical reactions. The key feature of this scenario is the onset of an emergent evolutionary transition within the microspaces that is described by the model of complex vectorial chemistry. This transition occurs at average macromolecular crowding of 20 to 30% of the cell volume, when the ranges of action of stabilizing colloidal forces (screened electrostatic forces, hydration, and excluded volume forces) become commensurate. Under these conditions, the macromolecules divide the interior of microspaces into dynamically crowded macromolecular regions and topologically complementary electrolyte pools. Small ions and ionic metabolites are transported vectorially between the electrolyte pools and through the (semiconducting) electrolyte pathways of the crowded macromolecular regions from their high electrochemical potential (where they are biochemically produced) to their lower electrochemical potential (where they are consumed). We suggest a sequence of tentative transitions between major evolutionary periods during the Hadean eon as follows: (i) the early water world, (ii) the appearance of land masses, (iii) the pre-RNA world, (iv) the onset of complex vectorial chemistry, and (v) the RNA world and evolution toward Darwinian thresholds. We stress the importance of high ionic strength of the Hadean ocean (short Debye's lengths) and screened electrostatic interactions that enabled the onset of the vectorial structure of the cytoplasm and the possibility of life's emergence.
Architecture is what ultimately distinguishes a living cell from a soup of the chemicals of which it is composed.
—Franklin Harold (40)
INTRODUCTION
The basic question of origin-of-life research, how living cells could have evolved from ions, molecules and polymers available on the Hadean Earth (see “Organic polymers and oligomers available on Hadean Earth” in the Appendix), was informally addressed by Darwin to explain why spontaneous emergence of life is not observed today. With some trepidation, Darwin suggested a prebiotic physicochemical evolution in “… a warm little pond with all sorts of ammonia and phosphoric salts, light, heat, electricity etc. present, that a protein compound was chemically formed, ready to undergo still more complex changes” (102). However, any such new life would not survive, according to Darwin's theory of natural selection, and hence, evolutionary theory throws no light on the issue of life's emergence. Since then, the origin-of-life question has been approached from the perspectives of other scientific disciplines, including organic chemistry, biochemistry, Earth and planetary sciences, molecular biology, computer modeling, and paleontology. Research into this question has grown rapidly in the last 20 years, as evidenced by a number of books on the subject (16, 20, 32, 35, 41, 45, 49, 72, 74, 102, 105, 107, 113, 129, 137).
Nevertheless, the question of the origin of life—how Hadean conditions and chemistries conspired to transform inanimate matter into evolving life—remains essentially unanswered. Rather, Darwin's speculation has been elaborated by other questions as scientific knowledge advanced. Was the emergence of life inevitable, or was it a unique convergence of factors hardly ever to be repeated anywhere and any time? What was it about the Hadean geophysical and geochemical conditions that caused life to emerge? Which came first, metabolism or replication? When did lipid membranes appear? Was the origin of life autotrophic or heterotrophic? What simpler chemical analogues might have fulfilled the roles of extant nucleic acids, proteins, and membranes? Which, if any, were the crucial transitional milestones in prebiotic evolution? How did the RNA world come about? Do we need new laws of physics and chemistry to deal with biological complexity? Was there only one last common ancestor or many? To deal with the questions above, many hypotheses have been put forward and diverse experimental approaches have been attempted, as discussed in the wide-ranging literature (3-5, 10-13, 15, 17, 18, 21, 25, 26, 29-31, 36, 37, 40, 42-44, 46-48, 50-53, 56-58, 61-63, 65-69, 71, 73, 75, 78-80, 82-86, 88, 93, 95, 98, 99, 103, 106, 115, 117, 124, 125, 128, 130-133, 135). These approaches to the origin-of-life research exemplify both “bottom-up” approaches (origins, syntheses, and complexifying of organic building blocks) and the “top-down” approaches (analyses and simplifications of extant bacterial cells); so far, these two types of approaches have not linked up to convey a coherent model of life's emergence. Currently, the most-recognized origin-of-life model is based on the “RNA world,” in which RNA-like informational polymers self-catalyze their own proliferation without the aid of proteinaceous enzymes (36, 43, 57a). However, how the RNA world arose, how membranes came to encapsulate the naked RNA protogenes, and how proteins assumed their catalytic function later are less certain; they remain a little mysterious from a physicochemical standpoint.
Here, we take a classical physical chemistry approach with emphasis on molecular confinement and crowding in “microspaces,” within which the precursors of nucleic acids, proteins, and other biomolecules coevolved in toto (15). By microspaces, we mean any spaces that have dimensions of about 0.1 to 1,000 μm and that are confined by semipermeable membranous walls about 0.005 to 0.1 μm thick. We propose that such microspaces could represent the basic physicochemical unit that generated, selected, and retained reacting molecules under the changing physicochemical gradients of the Hadean Earth. Within the microspaces, the reacting molecules and macromolecules could “recognize” each other through noncovalent physicochemical interactions, such as excluded volume interactions, hydration, and screened electrostatic interactions. When the ranges of action of these stabilizing, noncovalent interactions became commensurate, the “simple” prebiotic chemistry took a turn toward cytoplasmic vectorial chemistry (112). In this model, when biomacromolecules occupy on average 20 to 30% of cellular volume, the cytoplasm becomes structured into regions of crowded biomacromolecules and topologically complementary electrolytic pools and pathways; this dynamic architecture of a “living cytoplasm” is connected to external environments through the vectorial pathways of integral membrane proteins. The onset of cytoplasmic vectorial chemistry could thus represent a distinct (emergent) transition in prebiotic evolution. With this electrochemical model, which is described later in greater detail (see Fig. 1; also see “Model of complex vectorial biochemistry” in the Appendix), we emphasize the role of screened electrostatic interactions, which are known for a wide range of physicochemical and biochemical phenomena, as briefly summarized below.
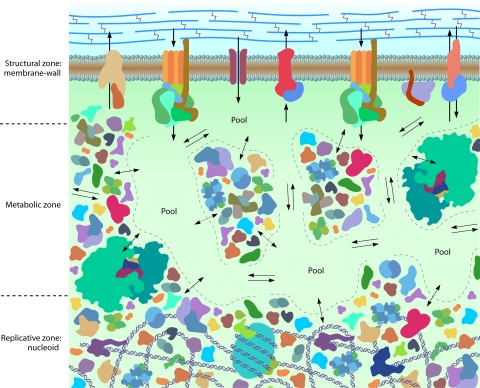
FIG. 1.
The model of complex vectorial biochemistry in a cell. The cell wall is schematically represented as thick dark lines cross-linked with thin, wiggly vertical lines (peptidoglycan); below is the plasma membrane populated with various proteins and their complexes that control the interaction of the cell with the outside environment. Potential cytoskeleton proteins on the cytoplasmic side of the membrane are not shown. The cell wall, membrane, and cytoskeleton proteins comprise a cell's structural zone. At the bottom of the picture is DNA, to which various proteins are attached and which is surrounded by other proteins; the DNA is partially wound up and supercoiled on histone-like proteins. DNA and associated proteins form the replicative zone. In between is the transient structure of the proteome, with electrolyte pools and crowded regions of proteins and their complexes, which form the metabolic zone. Ionic currents are depicted by arrows. Biochemical metabolite flows arising from enzymatic reactions are designated by single double-arrowed lines between the crowded biomacromolecular regions and the electrolyte pools. Ionic flows between the pools, designated by two arrows in opposite directions, are driven by bulk electrochemical potentials between the pools. The biomacromolecular crowded regions act in effect as three-dimensional membranes between the electrolyte pools, which are permeable to cations and conditionally to some anions. (Biomacromolecular structures are adapted with permission of David S. Goodsell and the RCSB PDB.)
THE UBIQUITOUS IONIC STRENGTH EFFECTS
It is well known from classical physical chemistry that the ionic strength of electrolyte solutions determines their bulk thermodynamic and transport properties, e.g., the activities of strong electrolytes (and hence also the activity of water), the solubility of ionic salts and minerals, and the dissociation of ligands from complex ions, as well as conductance and diffusion of electrolytes. The rates of reactions in which charged species are reactants or appear as products are also strongly influenced by ionic strength. These interionic effects were first explained by the concept of ionic atmosphere, described by the Poisson-Boltzmann equation of the Debye-Hückel theory (19, 92). Similarly, ionic strength plays an important role in phenomena that involve charged surfaces, such as the stability and repulsions between charged colloids (123), and in spontaneous self-assembly phenomena, e.g., formation of surfactant micelles, interfacial adsorption of ionic surfactants (94, 114), and formation of aqueous colloidal crystals (6, 24, 101). Ionic strength effects also play a role in film formation from “crowded” charged latexes (20% to 60% volume fraction) on drying (J. J. Spitzer, unpublished observations). Conformations of polyelectrolytes and their mutual repulsions and attractions also depend on the ionic strength of aqueous solutions (101). Many other physicochemical examples could be cited from the literature of natural and industrial colloids. In the case of charged colloidal surfaces, including polyelectrolytes, the ionic strength effects are further complicated by ion exchange between bulk ions and surface ions, sometimes described as ion-specific effects or ion exchange effects, e.g., in the behavior of colloidal clays (60).
Ionic strength and ion-specific effects are also well known in the biochemistry field because cells are composed of many charged molecular species. The membrane contains anionic phospholipids and embedded proteins with positive and negative charges, and in the cytoplasm, there are charged nucleic acids (DNA and RNA), water-soluble polypeptides and their quaternary complexes, phosphorylated and carboxylated substrates of metabolic and signaling pathways, and simple and complex ions. A well-known ionic strength effect is the lowering of the melting temperature of DNA on transfer from a high-ionic-strength solution to a low-ionic-strength solution (100); other examples are (to name a few) the intricate dependence of the association of RNA polymers on ionic strength and temperature (33), the arrest of RNA synthesis at low ionic strength (89), and the dependence of the catalytic conformation of riboswitches on ionic strength (96). Ionic strength also determines tertiary conformations of proteins and their associations into multienzyme complexes, as well as multistep self-constructions of larger assemblies, e.g., ATP-assisted self-construction of viruses in vitro (54). The kinetics of enzymatic reactions and the binding equilibria of substrates also typically depend on ionic strength; these electrolyte effects are often too complex to be described by classical equilibrium constants, which are found experimentally to depend on ionic strength and often on some specific ion, such as a magnesium or potassium ion. In these cases, the protein accommodates a more or less ion-specific binding site, which may allosterically control the enzyme's activity. In some instances, e.g., the binding of the lac repressor to DNA, it was found that the ionic strength dependence can be very pronounced in vitro but disappears in vivo (22, 91), possibly on account of high-level packing (crowding) of macromolecules in a living cell (27, 38, 134).
From a mechanistic perspective, the detection of ionic strength effects implies the underlying operation of screened electrostatic interactions. We have recently made this very point in connection with the mechanism of activation of osmoregulatory membrane transport proteins in Bacteria (87), which depends on the ionic content (and, in some cases, on specific ions) in the cytoplasm, on macromolecular cytoplasmic crowding, and on the concentration of anionic phospholipids in the membrane (7, 64, 87, 90, 97, 119). For at least two different types of membrane transport systems, it has been shown that the anionic membrane surface interacts electrostatically with protein domains, locking the systems in the inactive state. A high ionic strength screens the electrostatic interactions and allows the membrane protein to become activated (7, 87, 119). Further, since the bacterial cytoplasm contains strong electrolytes, and since it is crowded with many charged macromolecules, we have also outlined an electrochemical vectorial model of a bacterial cell (112), in which screened electrostatic forces play an important part. In this model, the cytoplasmic biomacromolecules occupy a large fraction (>20%) of the cell's volume, with the remaining volume being divided into many electrolyte pools that are connected by electrolyte pathways. The pathways are formed between charged macromolecules, and theoretically, they can have semiconducting properties; the charges also contribute to colloidal stability at close surface separations (108, 110, 111). Here, we refer to this combination of membrane vectorial biochemistry with vectorially structured cytoplasm as complex vectorial chemistry (Fig. 1). (We use the term “complex” here in its ordinary meaning of “composed of too many interacting parts to understand readily,” i.e., not in the sense of complexity theories of energy-dissipating, self-organizing systems, such as tornadoes or autocatalytic cycles and hypercycles [26, 45, 73].) This model of colloidally stabilized and crowded biomacromolecules is in accord with experimental observations of hindered diffusion (28, 118, 122), when biomacromolecular complexes persist longer at specific cellular locations (e.g., in response to environmental signals) and thereby trigger appropriate physiological responses. The concept of complex vectorial chemistry also fits well with the current view that a living bacterial cell is a robust, spatially and temporally self-constructing physicochemical device and not just a bag of nucleic acids with randomly diffusing enzymes (1, 40, 59, 131, 132). Within this context, screened electrostatic repulsions, in concert with other noncovalent forces, secure the colloidal stability of metabolic and replication “factories” enclosed by the membrane; hence, these forces must have also played a distinct role in the physical chemistry of life's emergence.
PHYSICOCHEMICAL APPROACH TO LIFE'S ORIGIN
Though research on the origin of life has been approached from various scientific disciplines, a classical physicochemical approach does not appear to have been given much emphasis. In adopting this general approach (57), we ask three closely related questions regarding prebiotic chemical evolution: where did it take place, by which physicochemical mechanisms did it take place, and what drove it? Physical chemistry, prokaryotic biology, and Hadean geophysics and geochemistry offer three interlocked general answers, summarized as follows: it took place within microspaces with membranous walls, it was brought about by noncovalent interactions of crowded reacting molecules, and it was driven by the physicochemical gradients of the Hadean Earth. Below, we discuss these three facets of prebiotic evolution without recourse to specific biochemical and geochemical reaction schemes.
The Necessity of Microspaces
The biological fact that a whole cell (and its “populations”), rather than “naked” nucleic acid, is the basic unit of animate matter (40, 131, 132) suggests to us that cells evolved from, or by the means of, analogous inanimate physicochemical units, which we give a general moniker of “microspaces.” In biology, there are many closed spaces that have dimensions of about 0.1 to 1,000 μm and that are enclosed by membranous walls with a thickness of about 0.005 to 0.1 μm. The walls give the microspaces their identity, a degree of mechanical stability, and the means of physicochemical communication with the outside, i.e., various degrees of permeability (23). Some examples of (organic and inorganic) microspaces and of their bioenergetic and self-organizational capacity have been suggested and experimentally explored before (3, 10, 11, 18, 31, 32, 48, 58, 61, 66, 79, 80, 86). Here, we generalize this concept and suggest that microspaces are the necessary locations for physicochemical molecular evolution toward “Hadean life.” In the words of Leslie Orgel, “Molecules that stay together evolve together” (83). The membranous walls could be purely inorganic or organic or an inorganic-organic composite; they likely evolved through all three compositional characteristics during the Hadean eon, providing both selective permeability and mechanical strength functions for the microspaces. The iron-sulfur-containing proteins in extant prokaryotic membranes could be the evolved vestiges of their physicochemical ancestors that originated in inorganic mineral environments (3, 48, 66, 98).
The necessity of microspaces is related to our other two points, the role of noncovalent interactions and the role of physicochemical gradients. First, microspaces provide the volume in which protobiomolecules can accumulate and crowd. As they do so, their thermal motions allow them to recognize each other through more and more frequent noncovalent interactions and, hence, to react in particular ways and to coevolve their reaction mechanisms (15). From a point of view of physical chemistry, it is hard to see how physicochemical evolution could progress toward biological evolution without microspaces that select (create, import, retain, or expel) protobiomolecules. Second, microspaces provide a convenient unit on which external physicochemical gradients can act. Again, from a point of view of physical chemistry, it is hard to see how physicochemical gradients could drive chemical evolution toward greater organization and complexity in unbounded solutions. In other words, a three-dimensional volume provides greater opportunities than a two-dimensional surface for biological complexity to arise and evolve (21). Consequently, “early” microspaces solve the concentration problem of chemical evolution (20). Furthermore, their evolution in toto does not require any subsequent “cellularization” mechanisms (encapsulation by a membrane and a cell wall) for chemically reacting metabolic primordial soups (81, 129) or for proliferating naked RNA autocatalysts (83) or for surface pyrite protobionts (125). Such later evolutionary encapsulations are unlikely from a practical physicochemical standpoint. Perhaps the occurrences of “racemic” d- or l-amino acids in extant bacterial peptidoglycans (120) attest to a prebiotic evolutionary stage, when homochirality was still evolving inside the crowded microspaces but was less important for the evolution of structural components of the cell, e.g., peptidoglycan of the cell wall. Indeed, if one of the functions of the cell wall is to maintain mechanical integrity by a cross-linked polymer, its strength will come predominantly from the cross-linked nature of the polymer (cross-link density) and less so from a particular chemistry of the cross-links.
Some “protolife” physicochemical functions and attributes of microspaces can be specified from the properties of extant Bacteria. (i) They divide macroscopic “aqueous liquid” into internal and external solutions. (ii) Their membranous wall is permeable to water and to at least some components of the internal and external solutions, depending on the chemical nature of the membrane. (iii) Chemical reactions can take place inside and on the outside of microspaces, and can be catalyzed by both the internal and external surfaces of their membranous walls. (iv) Reactions inside a microspace can give products that cannot escape through the membrane; the trapped products are subject to further reactions inside the microspaces. (v) Osmotic imbalances, driven by external conditions, can stretch or shrink the wall, allowing its chemical evolution; these imbalances can also break or collapse the microspaces and release new kinds of molecules into the external liquid and/or produce fresh microspaces.
When charged ions and polyelectrolytes become involved, microspaces develop surface potentials (charged moieties on the molecules forming on the inside and outside membrane wall surfaces), Donnan potentials (unequal concentrations of a salt across a membrane that is impermeable to polyelectrolyte ions), and membrane dynamic chemiosmotic potentials (reacting charged species moving across the membrane in one direction and falling back inside in the opposite direction). While the surface and Donnan potentials are well-known inanimate physicochemical concepts, the dynamic chemiosmotic potentials are quintessentially biochemical and characteristic of “living membranes.” Here, reacting charged molecules move vectorially through the membrane in definite directions. Though strictly manmade chemical microspaces (closed membrane wall systems and their populations), with simple vectorial reactions analogous to chemiosmosis, may exist, we are not aware of any that have been designed, e.g., with a view to synthesize a particular product for chemical industry. Interestingly, the electrodialysis of seawater to obtain drinkable water, driven by external electrical potentials through charged membranes, has some attributes of vectorial electrochemistry. For a further discussion, see “Electrostatic, electrochemical, and chemiosmotic potentials” in the Appendix.
We suggest that the existence and evolution of microspaces, confined by semipermeable membranous walls, must be a part of any scenario of the origin of life, and in principle, such microspaces can be constructed and investigated in the laboratory. Thus, in our view, the membranous walls that define microspaces were the first to exist. Their evolution could have proceeded in one of two ways: (i) via microspaces with dilute interiors that became crowded and concentrated or (ii) via microspaces with very crowded and concentrated interiors that became diluted. In both cases, the interior macromolecular crowding would converge to a fraction of about 20 to 30% of the cell volume, per the model of complex vectorial chemistry; replicative and metabolic reactions then coevolved inside the microspaces along with their membrane walls as single units (or, more likely, as a community of such units) toward cellular life. Possibly, the first communities of such physicochemical units were the gel-like membranous FeS bubbles produced at alkaline hydrothermal vents (66, 98). Later, with the appearance of land, organic microspaces could have appeared, e.g., they could have emerged and evolved by drying and rewetting cycles from dissolved and suspended “cosmological oligomers” derived from hydrogen cyanide and from polyaromatic hydrocarbons (12, 25, 68), as further described in “Organic polymers and oligomers available on Hadean Earth” in the Appendix.
Role of Physicochemical Gradients
From a physicochemical point of view, cellular life takes place away from chemical and physical equilibria when a cell grows, and it persists and evolves when a cell divides. In this sense, life can exist only under external physicochemical gradients, and therefore, inanimate Hadean matter could evolve into animate matter also only under the action of external physicochemical gradients. However, since bacterial life can survive in nonmetabolic and nonreplicative sporulated forms or in starvation modes with no metabolism or very slight metabolism without replication (14, 76), we assume that life originated in microspaces that could go from equilibrium or pseudoequilibrium states to evolving (chemically reacting) states under the action of external physicochemical gradients. In other words, the evolution of microspaces proceeded in a stop-and-go fashion; “partially evolved” microspaces could “survive” in unfavorable states, e.g., charged agglomerated macromolecules inside microspaces could redisperse under conditions of lower ionic strength, or dehydrated microspaces could rehydrate when the activity of water increased again. Conversely, e.g., under large osmotic stresses, microspaces could swell and burst (“perish”), releasing their new, partially evolved macromolecules into the external liquid for potential exploitation by microspaces that had survived.
Microspaces are subject to many external physicochemical gradients, e.g., temperature gradients, concentration gradients of salts and related osmotic and electrochemical gradients, and oceanic hydrodynamic and mechanical stress gradients. The physicochemical gradients and their flux responses are coupled in complex ways to free-energy gradients of possible chemical reactions. Temperature gradients cause changes in reaction rates by the Arrhenius effect on rate constants and by the accumulation of reactants by liquid/solid transitions (4) and by other mechanisms such as thermophoresis and microconvection (3). Concentration gradients give rise to diffusional flows; a cooling temperature gradient brings about the appearance of catalytic solids or gelled phases, through which new reaction mechanisms become possible. When ions are subject to temperature and osmotic gradients, “ionic currents” give rise to electrochemical potentials, and when the water activity outside of the microspaces changes, their membranous walls shrink or expand, changing their permeability. Clearly, microspaces offer a rich complexity of physicochemical gradients and fluxes that are coupled to free-energy gradients of possible reactions; simplified (but not too simplified) models and experiments can be designed to study such couplings.
We suggest that external temperature gradients were the initial drivers of physicochemical prebiotic evolution, and other physicochemical gradients, particularly the free-energy gradients of potential reactions, were strongly coupled to them. There were two kinds of temperature gradients on the Hadean Earth: (i) quasilinear gradients between the cold oceanic waters and the hot fluids of hydrothermal vents and (ii) diurnal cyclic gradients brought about by the Sun's radiation striking the surface of the rotating Earth.
From a theoretical viewpoint, physicochemical gradients and their couplings offer a number of possibilities, starting from Onsager's linear reciprocity relations for gradients and fluxes to the complexity theories of emergent phenomena motivated by Prigogine's dissipative structures (41). In general, we can view the response of an equilibrium system to perturbing gradients as follows: (small) linear gradients from equilibrium give Onsager's reciprocal relations and orderly steady states; larger departures from equilibrium lead to nonlinear responses, which may lead to chaotic behavior; and still larger departures may lead to the emergence of “energy-hungry,” self-organizing structures. Given the biological data on the precision of cell growth and division, on the various states of low metabolic activity or no metabolic activity (34), on the degree of homeostasis within the cell, and on the remarkable ability of a cell to resist injury and to self-repair (40), we doubt that life has much to do with the complexity of energy-dissipating structures at the edge of chaos (sensitivity to external initial conditions). Rather, the complexity seems to lie in the free-energy gradients of too many kinds of potential reactions and reaction mechanisms, which involve polymers (polyelectrolytes), ions, and molecules, and which are always coupled to external physical gradients through membranous walls. The biomacromolecular crowding leads to the emergent dynamic structuring of reaction pathways, as discussed later in the context of the concept of complex vectorial chemistry (112). Whether the self-organizing effects of dissipation of large amounts of metabolic energy (73, 74) cause the emergence of physical vectorial pathways remains to be determined.
Role of Noncovalent Interactions
We suggest that stabilizing noncovalent forces, i.e., molecular forces that prevent haphazard and uncontrolled agglomerations and precipitations within the microspaces, are essential for physicochemical evolution. Inside the microspaces, these forces allow the protobiomolecules to recognize each other and yet retain their individuality, in effect providing the physicochemical mechanism for molecular evolution. We suggest three such forces to be of particular importance.
(i) Hydrogen bonding and charge-dipole forces (hydration) impart stability by making any molecular species dissolved or nearly dissolved or by making hydrogen bonds between polar functionalities of macromolecules and water molecules. Since water molecules are strong dipoles, they also dissolve many inorganic and organic salts, providing a strong electrolyte solution in which charged protobiomacromolecules must retain their individuality and stability.
(ii) Screened electrostatic repulsions together with the hydration effects (cf. above) provide a degree of stability for charged macromolecules that are “bathed” by the electrolytes inside the microspaces. These forces arise from the nonuniform distribution of cations and anions around a charged surface or around a charged surface moiety; they represent a mechanism complementary to the hydration interactions to reduce the free energy of charged surfaces. In other words, a charged surface can reduce its electrostatic free energy by hydration interactions (Born hydration energy) and by redistributing free ions in the neighborhood (attracting oppositely charged ions and repelling ions of the same sign, thereby creating a diffuse ionic atmosphere). We discuss the role of these repulsions below.
(iii) Excluded volume repulsions arise from the nonbonding molecular orbitals of molecules and ions, and thus, they define their van der Waals shapes. At higher levels of crowding (in the biochemical sense), these repulsions become very frequent, and the shape and size of molecules then become important factors in their mutual recognition and reactivity, e.g., the familiar lock-and-key (or induced-fit) substrate-enzyme interactions. In fact, it is this shape of molecules that is subject to physicochemical evolution within the crowded microspaces.
These stabilizing interactions counteract self-assembling or “coagulating” or agglomerating (attractive) interactions arising from hydrophobic effects, hydrogen bonding and ion pairing within low-dielectric molecular environments, screened electrostatic attractions, and van der Waals attractions, including aromatic ring stacking interactions. The physicochemical mechanism of the evolution of protobiomolecules then can be seen in the balance between the repulsive and attractive noncovalent forces, as chemical reactions take place inside and outside of the microspaces and through their membranous walls. The chemical reactions are here conceived in the broadest sense and include all the protein/protein, protein/membrane, protein/nucleic acid, nucleic acid/membrane, and nucleic acid/nucleic acid associations (complexation) and their metabolic, replicative, and transport reactions. The repulsive screened electrostatic interactions are discussed below separately because of their importance in the electrochemical structure of the cytoplasm (112).
Screened electrostatic repulsions.
Screened electrostatic repulsions (interactions in general, including attractions) have been well documented in the physicochemical and biochemical literature over the last 100 years. They have been typically revealed by dependencies of various physicochemical phenomena on ionic strength. We have shown previously that the opposite case, i.e., the absence of ionic strength effects, does not necessarily imply the absence of screened electrostatic effects (108, 110, 111); the theory predicts that the dependence on ionic strength is generally reduced at electrostatic potentials greater than thermal energy, kT, when co-ions begin to be expelled away from a charged surface and when the interacting surfaces are very close. This reduction in ionic strength dependence is influenced by the kind of ions and charged moieties on the surface: the less they are able to be hydrated, the more the counterions become associated with the surface, which reduces the ionic strength dependence further (108, 110). Any particular case can be decided by chemical insight that defines analytical charge density used in the Poisson-Boltzmann equation. If there is no obvious chemical reason that the salts used to increase ionic strength react specifically with the charged moieties (leading to the elimination of coulombic charges or strong ion pairs within a low-permittivity molecular environment), then screened electrostatic interactions will persist at any ionic strength; only their range of action gets shorter with increasing ionic strength. In other words, one cannot always “screen away” these forces by high ionic strength (as has been sometimes assumed), and they remain relevant in the biochemical range of relatively high ionic strengths (87, 112), of about 0.1 to 2.5 molar.
The range of screened electrostatic interactions is defined by Debye's length, usually denoted as 1/κ, and defined as
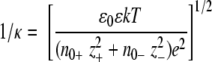 |
(1) |
The denominator expresses ionic concentrations (e is the electronic charge, z− and z+ are ionic valencies of anions and cations, and n0− and n0+ are their bulk concentrations); the numerator includes thermal energy (kT) multiplied by vacuum permittivity (ɛ0) and solvent permittivity (ɛ). For an electrolyte composed of singly charged cations and anions, Debye's length decreases inversely with the square root of their concentrations. For prokaryotes, the relevant ranges of background cytoplasmic electrolyte (mostly potassium salts of various phosphates and carboxylates, such as glutamate) are in the range of 0.1 molar to about 2.0 molar (for certain halophiles).
It is instructive to calculate the range of Debye's lengths at relatively high concentrations of electrolyte, as shown in Table 1. For this purpose, we used the measured dielectric constant of NaCl solutions (92). With increasing concentrations of NaCl (and other salts), the dielectric constant of solutions and, hence, Debye's length decrease (at 2.0 molar, Debye's length is reduced by about 15%, and at 0.1 molar, the small reduction of the dielectric constant has a negligible effect on Debye's length). The important point to notice is that in this range of physiological concentrations of strong electrolytes, the electrostatic interactions act over distances of about 10 to 2 Å, which correspond to the sizes of small molecules and ions and to the “protruding functionalities” of biomacromolecules (e.g., side chains of amino acids or phosphate moieties in a DNA helix). This length or size correspondence suggests that screened electrostatic forces participated in the evolution of the van der Waals surface topology of biochemical ions and polyelectrolytes within the increasingly crowded conditions of Hadean microspaces. Therefore, it is unlikely that life could have originated and evolved in pure water or in dilute electrolytes, as screened electrostatic forces are then too long-range. Under such conditions, close-range molecular encounters are infrequent, and hence, the chances of evolution of new reaction mechanisms and molecular shapes become less favorable.
TABLE 1.
The biological range of Debye's lengths in 1:1 aqueous electrolyte
| Molarity (mol/liter)a | 1/κb (Å) | Dielectric constant (ɛ) |
|---|---|---|
| 0.10 | 9.5 | 77.2 |
| 0.15 | 7.8 | 76.7 |
| 0.20 | 6.7 | 76.1 |
| 0.30 | 5.4 | 75.0 |
| 0.40 | 4.7 | 73.9 |
| 0.50 | 4.1 | 72.8 |
| 1.00 | 2.8 | 67.3 |
| 2.00 | 1.8 | 56.3 |
1:1 electrolyte.
Debye's length.
A number of locations for the origin of life on Hadean Earth, ranging from hot hydrothermal vents (67) to concentrated solutions of HCN in frozen ice, have been suggested (56). We notice that Debye's length is essentially independent of temperature, as shown in Table 2. Over the range of 100°C, between the freezing point and the boiling point of water (at about one atmosphere pressure), the theoretically required kelvin temperature T changes by about 37%, while the relevant theoretical quantity (ɛT)1/2 to which Debye's length is proportional, changes by only 7%. This effect comes from the experimental fact that the dielectric constant of water (ɛ) decreases with temperature at about the same rate as temperature itself increases (on the kelvin scale); this compensation effect is enhanced by the theoretical requirement of the square root of the product of temperature and dielectric constant, (ɛT)1/2. As a consequence, the temperature variations necessary to drive physicochemical evolution will drive only the rate of chemical reactions and hydration stabilizations (i.e., the “hydration evolution” of shapes of molecules and their reactivities), while the screened electrostatic interactions will provide a background stabilizing effect independent of temperature. The temperature dependence of dielectric constant of water is thus yet another unique property that makes water a suitable solvent for the evolution of biochemical ions and their reactions.
TABLE 2.
Temperature-independent Debye's length in 0.15 molar 1:1 salt in water
| T (°C) | T (K)a | ɛb | (ɛT)1/2 (K1/2)c | 1/κ (Å)d |
|---|---|---|---|---|
| 0 | 273 | 86.1 | 153 | 7.9 |
| 10 | 283 | 82.2 | 152 | 7.8 |
| 20 | 293 | 78.5 | 152 | 7.8 |
| 30 | 303 | 74.9 | 151 | 7.7 |
| 40 | 313 | 71.6 | 150 | 7.7 |
| 50 | 323 | 68.3 | 148 | 7.6 |
| 60 | 333 | 65.2 | 147 | 7.6 |
| 70 | 343 | 62.3 | 146 | 7.5 |
| 80 | 353 | 59.4 | 145 | 7.4 |
| 90 | 363 | 56.7 | 143 | 7.4 |
| 100 | 373 | 54.1 | 142 | 7.3 |
The percent change overall is 36%.
The percent change overall is 37%.
The percent change overall is 7%.
The percent change overall is 7%.
HADEAN EVOLUTION
The Salty Ocean
Our physicochemical approach stresses the importance of electrolytes in the geophysical and geochemical processes that gave rise to unicellular life. It is not unreasonable to suggest that extant bacterial life depends not only on water but also on relatively high levels of ions (potassium, magnesium, and other ions) within the cytoplasm. The importance of salts has not been much considered in origin-of-life research, though some interesting observations have been made already. For example, peptides were synthesized under the conditions of high salt content (85, 93), catalyzed by copper ions; also, the hydrolytic stability of RNA is reported to increase with a high salt content (4). The salts in the early oceans also provided UV protection to amino acids (13). It has been suggested, however, that life could not have started in salty oceans because the colloidal stability of fatty acid soap vesicles is poor in such solutions (18, 71): it is sensitive to pH and high ionic strength and to small amounts of divalent metal ions which precipitate insoluble salts and agglomerate such vesicles. However, template-directed synthesis of a genetic polymer can proceed in a high-ionic-strength buffered solution in model vesicles whose colloidal stability is enhanced by the incorporation of nonionic surfactants (65). This result points to the role of nonionic (or zwitterionic) surface-active molecules in the stability and permeability of early protocells (microspaces). In extant bacterial membranes, zwitterionic phospholipids act as diluents of anionic phospholipids to control the surface charge density, which in turn plays a role in the activation of osmosensing and osmoregulating membrane transporters (87).
Relatively little is known about the origin of large amounts of NaCl and other salts in early Hadean oceans. Cosmologically, sodium, potassium, and magnesium and their chlorides, cyanides, and isocyanides are observed in circumstellar envelopes of oxygen-rich and carbon-rich stars (135), from which they escape into the interstellar space; they could have been an integral part of the Earth accretion process from the solar nebula. It has been estimated that the salt content of early oceans was up to twice as high as it is today (47, 63, 75). The sodium and potassium salts are highly water soluble over large pH ranges, and their concentrations in the early ocean could have been established quickly by high temperatures of hydrothermal water/rock interactions and through volcanic activity. For simplicity, we therefore assume that physicochemical evolution started with microspaces that were formed in liquid water, which already had a relatively high ionic strength, possibly higher than that of the present-day ocean (from about 0.3 to 0.6 molar as a 1:1 electrolyte). This scenario suggests that either the initial individual microspaces were stabilized by zwitterionic and nonionic stabilization (less sensitive to divalent ions, high ionic strength, and pH) or the prebiotic evolution started in already agglomerated “inorganic bubbles” or “mats” and life arose in such “communal” physical form. The speed and success of physicochemical evolution in such communal settings are likely to have been greater than those of evolution of microspaces “going solo” in the Hadean oceans. For example, thermophoresis could have been one mechanism by which “protobiochemicals” could have become concentrated in such communal settings, as suggested for FeS porous minerals at alkaline hydrothermal vents (3). Such early communal physical form of prebiotic evolution is compatible with the much later “age of common ancestors” (52, 131, 132) and the mechanisms of horizontal gene transfer and Darwinian thresholds. We thus favor the notion that prebiotic physicochemical evolution took place in agglomerated microspaces rather than in single and independent microspaces.
Tentative Transitions
Just as major transitions appear in the evolution of biological species (107), the earlier physicochemical evolution might have also progressed through several major stages, when new environmental conditions were encountered. Below, we suggest a broad outline of stages of physicochemical evolution of microspaces, with the understanding that many inorganic, organometallic, and organic reactions were possible and took place inside and outside of the microspaces. Some prebiotic reactions are well documented in the literature (20, 29, 49, 74, 102, 105, 129, 137), but the combined effects of high ionic strength, macromolecular crowding in microspaces, and environmental physicochemical gradients have not been much investigated. In what follows, we use a list format (without literature citations) to shorten the description of this speculative scenario; some of the points can be expanded and elaborated as needed later with specific schemes of prebiotic (biochemical and geochemical) reactions.
Microspace evolution. (i) Water world.
The “water world” would mark the appearance of the first inorganic FeS-based microspaces at hydrothermal vents and the potential generation of organic compounds (formate, acetate, amino acids, and short peptides) by the carbon dioxide-hydrogen redox couple; there was accumulation of “cosmological” C-, H-, N-, and O-based compounds (brought in by asteroids, meteorites, comets, and interplanetary dust particles) and of their hydrolytic and UV catalyzed reaction products; reduction products arising from oxidation of elemental iron to Fe2+ and Fe3+.
(ii) Land appearance.
In tidal and pond-like settings, there was film formation from reduced organic compounds produced at hydrothermal vents and from C-, H-, N-, and O-based “cosmological” compounds; formation of organic microspaces that undergo drying and rewetting cycles; and microspaces subject to oscillatory temperature gradients driven by the Sun's electromagnetic irradiation of the rotating Earth.
(iii) Pre-RNA world.
In the “pre-RNA world,” there were increasing accumulations of organic building blocks; interactions and reconstitutions of microspaces of different chemistries; increasing concentrations and crowding of reacting molecules within microspaces; physicochemical coevolution of populations of microspaces driven by cyclic environmental gradients; coevolution of proto-membrane walls, protoenzymes, proto-nucleic acid, and proto-ATP-like molecules; chemical reactions catalyzed by proto-membrane walls (inside, outside, and within) and by the surfaces of minerals; transfer of RNA-like monomers and polymers to other microspaces when microspaces perish (analog of horizontal gene transfer); and reactions within microspaces strongly coupled to external physicochemical gradients.
(iv) The turn toward complex vectorial chemistry.
The crowding of protobiomacromolecules within microspaces reached “critical” volume fractions of 20 to 30%, which led to emergent structural segregations in time and space of protobiochemical electrolyte pools and electrolyte pathways; and the range of action of stabilizing noncovalent forces became commensurate (hydration, excluded volume, and screened electrostatic interactions).
(v) The RNA world: toward Darwinian thresholds.
In the “RNA world,” there were autocatalytic population explosions of pre-RNA and RNA autocatalysts within microspaces; a transition to self-sustaining energy production via ATP, partial decoupling from cyclic environmental gradients toward protohomeostasis; a differentiation of the lipid (alkane- and alkene-based) bilayer beneath the membranous walls; increasing decoupling of catalytic functions between proto-RNA and protoenzyme complexes; genetic code evolution toward cellular growth and protocell division; emergence of populations of common ancestors; horizontal genetic transfer and Darwinian thresholds; and “survival of the fittest.”
The first two stages were dictated by Earth's evolution of its atmosphere, oceans, and oceanic and continental lithospheres; the last two stages represent the partial decoupling of the evolution of microspaces from external geophysical and geochemical gradients, which eventually led to the life-dominated evolution of Earth by the end of the Hadean eon (95). The turn to complex vectorial chemistry could fall between stages iii and v; however, there might have been other major but as yet unknown physicochemical transitions that would enable the RNA world (within microspaces) and the evolution of genetic code toward cell division.
The above-described scheme of tentative physicochemical transitions can be also viewed from the “end” of the Hadean eon, during which the Earth was cooling from ∼100°C to ∼50°C and during which (for about 500,000,000 years) different kinds of microspaces would appear, and each kind would experiment with many reaction schemes. Since chemical reactions are faster by a factor of approximately 2 for each 10 kelvins, this hot Hadean physicochemical evolution could be equivalent to more than 2 billion years (depending on the cooling model) were it to take place at the lower temperature regimen at the end of the Hadean eon (somewhat similar to today's conditions). We cannot say whether during this long time a life was bound to emerge (16, 17, 20, 45, 72, 105) or whether it was a rather unusual sequence of nonequilibrium phenomena, driven by unique geophysical and geochemical gradients of the Hadean Earth (the unlikely “happy accident” scenario of Francis Crick [16]).
Below, we review and discuss in more detail transitions i and ii, with the emphasis on the role of electrolytes (high ionic strength) in the Hadean oceans. The emergent transition iv (complex vectorial chemistry) is discussed separately later, while the pre-RNA and RNA worlds are not elaborated further (15, 36, 43, 53, 57a).
Early water world.
The precise environmental conditions on the Hadean Earth are unknown, but various scenarios can be suggested based on our knowledge of planetary sciences and geochemistry. The details of how early oceans and atmosphere became established have remained contentious, e.g., the discussion on whether the early atmosphere was reducing or more neutral has been extensive and has been summarized in books and reviews (4, 41, 42, 53, 69, 84, 102, 129). Currently, geological arguments favor a relatively oxidized atmosphere (water vapor, carbon dioxide, and elemental nitrogen, though no free oxygen) (44, 128). This view is replacing the older view of a reducing and highly reactive atmosphere (water vapor, methane, and ammonia), which was simulated in the famous electrical discharge experiments of Stanley Miller (4, 69). The Earth was covered by a hot turbulent ocean that was subject to electromagnetic radiations and to continual bombardment by asteroids, comets, meteorites, and interplanetary dust particles. The Hadean ocean could periodically freeze, owing to the weak radiation of the young Sun; on the other hand, higher atmospheric concentrations of carbon dioxide (up to 1 MPa) and other greenhouse gases could have prevented the potential Hadean ice age. Under the less-favored assumption of a reducing atmosphere and a hot water world, hydrogen-rich molecules in “oil slicks” and in surface-active “aerosols” were suggested as possible initial locations for the emergence of life (50, 78, 115, 117, 130). It is possible that degradation products (hydrolytic products and UV radiation) of cosmological polymers derived from HCN and polyaromatic hydrocarbons began to accumulate in Hadean oceans (12, 25, 46, 68, 104); the HCN-derived compounds were likely to have been surface active, as evidenced by the foaming issues in the industrial production of HCN (2). Such organic materials might have accumulated on the ocean surface or sank to the bottom. On balance, we think that the interface of the liquid ocean (hot or frozen) and gaseous atmosphere was somewhat unfavorable to bring about and sustain viable microspaces.
In addition to the ocean/atmosphere interface, this early water world provided a relatively benign ocean/warm lithosphere interface at the bottom of the Hadean seas. Here, the oceanic lithosphere was cooled by hydrothermal convection of ocean water, responding to both magmatic heat and the geothermal gradient in the solid crust. Additional thermal energy to drive hydrothermal convection could have been provided by the exothermic serpentinization reaction, by which fractions of the circulating carbonic ocean water could have been reduced to hydrogen, methane and carboxylic acids (66, 67). At the same time, thermal convection in the mantle, driven by the rise of hot magma and the sinking of cooler lithosphere, produced widespread volcanic and hydrothermal activity at the bottom of the Hadean ocean (99). The volcanoes exhaled carbon dioxide and steam while submarine hydrothermal springs produced abundant hydrogen. The interface between hydrothermal springs and the ocean was relatively isolated from the irradiated and stormy ocean/atmosphere interface, and further, if the greenhouse gases were insufficient to prevent the freezing of the oceanic surfaces, then “lakes” were likely to exist under the sea ice.
It is to be expected that the ionic strength of the aqueous phases, from which the FeS-bearing bubbles are formed, would have a significant effect on their morphology, size, and possibly catalytic properties. A low-ionic-strength ocean may not “coagulate” the precipitating FeS fast enough, leading to breakage and colloidal dispersion into the ocean. At high ionic strength, however, the precipitation reactions are more likely to yield the morphology of coagulated membranous bubbles and pores. Thus, the ionic conditions (concentration of reactants) and the prevailing hydrodynamic conditions of the mixing of hot alkaline hydrothermal fluids with the cold acidic oceanic waters were critical to the appearance and catalytic potential of the bubble mats (98) that formed under the high-ionic-strength (coagulating) conditions of the ocean.
Land appearance.
As the interior of the young Earth cooled and less-dense granitic material differentiated from mafic magma and concentrated at subduction zones, persistent continental cratons (early islands and small continents) appeared to “float” in and above the submerged oceanic crust. The subduction of the oceanic crust under new continental cratons created new avenues for the formation of inorganic precipitates of various chemistries and morphologies through hydrothermal and volcanic activity. The cratons provided a new interface of solid rock and atmosphere, and a three-phase line boundary of gaseous atmosphere, liquid seas, and solid land. These new and evolving geophysical and geochemical conditions provided opportunities for the formation of new microspaces.
Once the rock/ocean/atmosphere line boundary became established, it underwent cyclical tidal movement up and down the shorelines in response to Earth's daily rotation in the presence of the Moon. This tidal movement was nearly twice as frequent as today's because of the still “young” age of the Earth-Moon system that is estimated to have come into existence ∼4.5 billion years ago. The best current estimate for the length of a Hadean day (∼3.9 billion years ago) is ∼14 h, with tides every 7 h (51). Because of the close proximity of the young Moon, the tides were also much higher than today. Assuming that oceans contained some kind of primordial soup of prebiotic organic molecules, probably derived from carbon dioxide reductions at hydrothermal vents and from molecules and polymers brought in via bombardments from outer space (see “Organic polymers and oligomers available on Hadean Earth” in the Appendix), the formation of gelled (or solid), salt-saturated organic films on the exposed mineral surfaces becomes inevitable.
The phenomenon of film formation (“tarring”) could have led to relatively thick films deposited on rocky (mineral) surfaces by the repeated drying of dissolved and suspended materials in the Hadean oceans, even if the concentrations of such materials were low. The buildup of such films on Hadean seashores could have provided new “concentrated” reaction media, in which new organic reactions could have been catalyzed by a high salt content, the mineral surfaces, and UV light. Such films were likely inhomogeneous, possibly providing new interior binding sites by the mechanism of “molecular imprints,” when “impurities” selectively leach out from the polymeric matrix (52). The films got thicker and eventually cracked and detached in pieces from the solid surfaces because of the increased stresses during the cycling of temperature and osmotic gradients. The atmosphere sides of such films were likely to be more hydrophobic than their mineral sides, which could have forced the films to acquire various “closed” configurations. The conjecture above appears to represent a plausible mechanism for the formation of robust organic microspaces with potentially concentrated or crowded interiors, which could have been accumulating on Hadean shorelines. However, the high ionic strength of the ocean could force such microspaces to agglomerate (or even to fuse), and thus, they were unlikely to survive as colloidally stabilized individual microspaces analogous to biochemists' lipid vesicles; nevertheless, the availability of zwitterionic or nonionic surfactant-like molecules would diminish the coagulating effects of the high-ionic-strength ocean.
The fate of the new microspaces could be multifarious, from perishing under large osmotic and temperature gradients (providing “food” for the formation of subsequent microspaces) to finding more hospitable circumstances. There is some evidence that such materials would actually sink, e.g., the density of HCN-derived cross-linked polymers has been estimated to be higher than that of seawater (46). They would accumulate at the bottom of the oceans and possibly interact with inorganic iron-sulfur minerals, forming organic-inorganic composites with new catalytic membranous capabilities. If they were lighter than seawater, they could survive at the ocean surface and be repeatedly subjected to deposition in the tidal areas and growing more in an agglomerated, communal fashion: they could have retained their original individuality or their membranous walls could have fused into still other microspaces. Thus, the densities of these materials could fractionate their heavier fractions to the sea bottom and their lighter fractions to the ocean and land surfaces. The evolution of these “lighter” microspaces would then take place on Earth's Hadean surfaces; it would be predominantly driven by oscillatory temperature gradients (Earth rotations) and by osmotic gradients (low-ionic-strength rains providing hypo-osmotic stresses and tidal flooding with seawater and evaporation providing hyperosmotic stresses).
As the continental cratons grew in number and with the continuing volcanic and hydrothermal activity, the FeS minerals were likely to be brought up from the sea bottom to form new tidal regions; the repeated deposition of oceanic organic films onto the new catalytic inorganic substrates could have led to yet another type of microspaces with unique inorganic/organic membranous walls, which would be now also subject to the cyclical temperature and osmotic gradients of the Hadean shorelines.
We thus envisage the continual chemical evolution of microspaces and their internal evolution of reaction mechanisms (to yield proto-nucleic acids and protoproteins) both in the initial hydrothermal surroundings of the Hadean oceanic lithosphere and later in the tidal areas of the emerging cratons. It is within such evolving microspaces that the cyclical temperature and chemiosmotic gradients provided the first impetus for the establishment of vectorial reaction pathways. For example, such temperature cycling could be important for the evolution of temperature-driven vectorial (template) syntheses of RNA type polymers (resembling the PCR) and for the establishment of the macromolecular “architecture” and ionic homeostasis in early microspaces. However, such protoreplicative and protometabolic vectorial reactions, spatially and temporally segregated within the microspaces, could coevolve only under the conditions of macromolecular crowding (27, 38, 112, 134); physicochemical evolution could then take a turn toward complex vectorial biochemistry and toward the possibility of life's emergence.
THE EMERGENCE OF COMPLEX VECTORIAL BIOCHEMISTRY
The model of complex vectorial biochemistry is a combination of current bioenergetic models of ion transport through lipid membranes and related transmembrane potentials (9, 39, 70, 77) with a semiquantitative electrochemical model of a dynamically structured (crowded) cytoplasm (112). Conceptually, this model is not new; in a way, it is a generalization of Mitchell's communication model (70) between the cytoplasmic citric acid cycle reactions (three-dimensional) and the electron transport and ionic translocation reactions (e.g., H+ and Na+) in cellular membranes (two-dimensional). We thus suggest that the cytoplasm has its own dynamic architecture, comprising a high-volume fraction of reacting macromolecules which divide the cytosol into the networks of real ion-conducting pathways and real electrolyte pools. This architecture extends throughout the entire volume of a cell and is connected through the membrane to the environment outside. This time-dependent structure of the cytoplasm has no (direct) relation to the time-invariant bioenergetic pathways of classical biochemistry: the cell's architecture predictably expands during the cell's growth with its own self-regulatory mechanisms, and it develops and expands again with each cell division. This cytoplasmic architecture, even though it is the product of gene expression, is to a degree autonomous, self-regulating, and inheritable with each cell division (10, 40, 131, 132), e.g., the autonomy of the cytoplasmic architecture, with its membrane (and walls), is exploited by viral nucleic acids, which can take over this architecture and modify it in order to replicate a different genome. We thus extend Mitchell's communication model (70) to include all cytoplasmic replicative and metabolic reactions and all membrane reactions, including membrane transport. The model of complex vectorial biochemistry thus reiterates the fact that a cell is the fundamental unit of biology and that it may have evolved from analogous physicochemical units, i.e., from various kinds of microspaces.
Commensuration of Stabilizing Noncovalent Interactions
The simplified model of complex vectorial chemistry is shown in Fig. 1 and is described in the legend and in “Model of complex vectorial biochemistry” in the Appendix. How can such cellular architecture remain stable in the colloidal sense and yet undergo a wide range of well-controlled biochemical reactions and transformations during a cell's growth and division? The model of complex vectorial chemistry suggests that such colloidal stability persists when the range of action of stabilizing noncovalent forces (excluded volume, screened electrostatic forces, and hydration forces) remains commensurate during growth and cell division. In some sense, this commensuration of stabilizing molecular forces reflects the biological principle of homeostasis (8), i.e., the necessity to maintain a constant environment (or architecture) inside the cell.
How can we estimate the range of action of hydration and screened electrostatic forces, and the distances between biomacromolecular surfaces for the emergence of such colloidally stable, homeostatic (self-regulating) vectorial architecture? Any detailed descriptions of such time-dependent cellular structures cannot be developed easily, as the cell contains many different macromolecules (and small molecules) with complex attractive interactions; their concentrations are strongly time dependent during growth and between cell divisions, as they respond to varying environmental conditions, e.g., to external water activity or the availability of food. However, we can make some average, rough order-of-magnitude estimates, using some basic knowledge and the Fermi method of educated guesses (“guesstimations”) (126).
The Fermi guesstimations.
We have already discussed some aspects of screened electrostatic forces described above; here, we elaborate the commensuration of these stabilizing interactions with hydration and excluded volume interactions in more detail.
The range of hydration interactions can be estimated from the size of a water molecule. We know that one water molecule must be the minimum needed, and we suspect that 10 water molecules may be enough. Hence, the average range of action of hydration forces (essentially hydrogen bonding) is about 3.2 water molecules (geometric means of 1 and 10, per Fermi's method), or 0.6 nm, assuming a water molecule is a sphere with radius of 0.1 nm (the length of the O-H bond). This Fermi estimate is in agreement with the radial distributions of water, where three water molecules' peaks can be detected at moderate temperatures (92); the third peak nearly disappears at high temperatures. We can thus expect that for polar functionalities, the range of hydration stabilization may vary in the 0.6- to 0.8-nm range in the lower temperature range of 0 to 50°C; in the range of 50 to 100°C, the hydration stabilization may act over only 0.4 to 0.6 nm.
The range of screened electrostatic interactions is given (for low potentials) by Debye's length, as shown in Table 1. We see that the required “saltiness” of the Hadean ocean must be in the range of 0.15 to 0.50 molar in order for hydration forces and screened electrostatic forces to stabilize molecular surfaces over a comparable range of distances. Somewhat lower or higher saltiness of the Hadean ocean would still keep the range of action of these stabilizing forces within the same order of magnitude. Extant bacteria generally have this order of magnitude of salt concentrations in the cytoplasm.
The range of excluded volume interactions, the sizes of macromolecules, and their crowding can be estimated, very roughly, by considering a simple model of crowding of monodisperse spheres of 5.0 nm in diameter in a simple cubic lattice. Assuming the densities to be 1.0 g/cm3 for both the spheres and the electrolyte solution, which then give a molecular mass of about 39,500 Da for the 5.0-nm-diameter spheres, roughly representing the molecular mass of the “average” protein in a cell. In a simple cubic lattice, each sphere has a cube available to it, the size of its side being 6.4 nm at a 25% volume fraction and 6.0 nm at a 30% volume fraction; thus, two neighboring spheres will have surface-to-surface distance of 1.4 (6.4 − 5.0) nm at a 25% volume fraction and 1.0 nm at a 30% volume fraction. At higher-volume fractions, the surface-to-surface distance is less than 1.0 nm, and the spheres would touch at a fraction with a cell volume just over 52%. Thus, this simple model would suggest that fractions of 30% and higher cell volume will make the average distances (surface-to-surface) commensurate with the ranges of hydration and screen electrostatic interactions. Extant bacteria have biomacromolecules packed at 20% to 25% volume fractions, which represent a range somewhat lower than but not that far from the prediction of such a simple model (and in accord with the suspected but unquantified requirement that biomacromolecules need some more room for diffusion, albeit hindered diffusion).
How to describe the structure of a bacterial cell?
Clearly, the above-described simplified estimates of the crowding of biomacromolecules within a bacterial cell are inadequate. However, as yet, no detailed views are available of the biomacromolecular crowding within biological cells (5, 40); the best impression, beautifully executed from known structures of individual biomacromolecules, could be drawn only with somewhat arbitrary spatial relations without considering intermolecular repulsions and attractions (38). The temporal and spatial evolution of such biomacromolecular crowding at the nanoscale level between cell divisions presently needs to be developed, perhaps by considering physiological data on cell growth and division. Currently, a “coarser”-level, time-dependent description of the morphological changes on cell division is being intensely studied at the mesoscale level (hundreds of nanometers); the large-scale, ATP-assisted assembly of cytoskeleton proteins that initiates DNA segregation and cell division of Escherichia coli, Bacillus subtilis, and Caulobacter crescentus has been recently reviewed (116). Another description of the cell architecture at the mesoscale could be based on three functional or physiological zones: the structural zones around the cell envelope (the cell wall, membrane, and cytoskeleton proteins), the replicative zones (nucleoid with nucleic acid/protein complexes and supercoiled nodes), and the metabolic zones in between (part of the proteome), as shown in Fig. 1. The physicochemical interrelationships of such “physiological” zones remain to be explored, e.g., would they be separated by actual “phase boundaries” with measurable interfacial tensions or merely differ by their viscoelastic properties? What would be the hydrodynamic flows within such an anisotropic environment, and how would such electrochemical and osmotic flows contribute to cell growth (136)? And what time scales do we need to consider in such mesoscale descriptions? Whatever the case may be, the nanoscale model of electrolyte pools and charged pathways would apply to all such higher-order functional zones as shown in Fig. 1 and further described in “Model of complex vectorial biochemistry” in the Appendix.
The nanoscale model of complex vectorial chemistry describes a dynamic architecture of bacterial cells; it suggests a structure of crowded biomacromolecular regions with complementary electrolyte pools, a structure in which the size and location of the electrolytes pools vary with time, as the crowded biomacromolecular regions become more numerous during the cell's growth. The pools contain various electrolytes, both simple ions, such as potassium and phosphate, and metabolic ions, such as ATP and other phosphate esters of metabolic intermediates, at different “local pool” concentrations. The pools are interconnected by electrolyte pathways, where the low-molecular-weight ionic traffic is driven by electrochemical potentials between the electrolyte pools. These electrolyte pathways can theoretically have semiconducting properties (111, 112) formed by the surfaces of charged biomacromolecules, which thus regulate the direction of ionic currents between the pools. Varying charge densities (or corresponding electrostatic potentials) at such biomacromolecular surfaces, brought about by phosphorylation and other reactions, then change the forces between biomacromolecules, allowing new macromolecular complexes to emerge or to disappear, thereby changing the distribution of electrolyte pools and their interconnectedness through electrolyte pathways.
This semiquantitative electrochemical picture of complex vectorial chemistry gives another reason why nature chose ions, to paraphrase the famous question of why “nature chose phosphates” (127); it is the ions, both the environmental or abiotic ions (potassium, sodium, magnesium, calcium, bicarbonate, dihydrogen and hydrogen phosphates, and sulfate) and the biochemical ions (the “genome-derived” phosphate esters and carboxylates of classical bioenergetic pathways and their respective polymers [RNA, DNA, and proteins]), which make up the workings of the electrolyte pools and pathways.
The necessity of the salty ocean.
When the ranges of the stabilizing noncovalent forces, i.e., the screened electrostatic, hydration, and excluded volume forces, become comparable in a confined place, only then can the cyclic metabolic and replicative reactions emerge and coevolve within the vectorially structured cytoplasm. According to our general model, such coevolution is driven by external physicochemical gradients coupled through the membranous walls to the free-energy gradients of potential reactions.
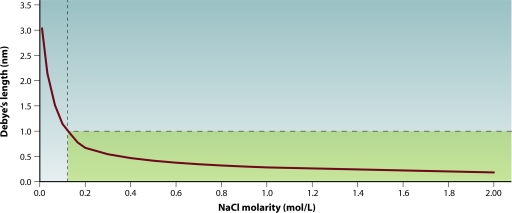
The graph of Debye's length for a 1:1 strong electrolyte, such as NaCl, is shown in Fig. 2. The rapid increase of Debye's lengths below ∼0.10 molar is noteworthy, as it indicates long-range screened electrostatic repulsions that would prevent close macromolecular contact needed for polyelectrolyte evolution (polypeptides, RNA, and DNA). The region in Fig. 2 bounded by the lines at approximately 0.10 molar and 1.0 nm signifies the commensurate ranges of hydration and Debye's lengths that are also commensurate with the surface-to-surface distances of crowded protobiomacromolecules at cell volume fractions higher than ∼25%. At fractions of <20% cell volume and/or a Debye's length larger than 1.5 nm, the hindered diffusional motions of biomacromolecules become more random and render unlikely the emergence of dynamic vectorial pathways and structures; physicochemical evolution toward life's emergence within the microspaces would not then take place.
FIG. 2.
Comparable hydration layers and Debye's length. The green zone between 0.10 molar and 1.0 nm signifies the ranges of hydration and Debye's lengths that are commensurate with the surface-to-surface distances of protobiomacromolecules inside a crowded cell.
One of the features of complex vectorial chemistry is the prediction of a switching condition for a pathway to become semiconducting, i.e., when the potential within a pathway becomes more negative, e.g., by a phosphorylation reaction, which prevents an anion from using that particular pathway (87, 112). The simplest switching condition is given by equation 2 as follows:
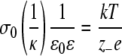 |
(2) |
Here, σ0 is the surface (negative) charge density that is related to Debye's length (1/κ) and the thermal energy (kT) and the charge of the anion (z−e); both of these quantities determine the switching state. Thus, the pathway becomes semiconducting either by increased surface charge or by the decrease of Debye's length.
For a given Debye's length, the critical surface charge density in equation 2, σ0, is also essentially independent of temperature, just like Debye's length, as again, the critical charge density is proportional to the square root of the product of the dielectric constant and temperature (Debye's κ is inversely proportional to the square root of the product of the dielectric constant and temperature, as in equation 1).
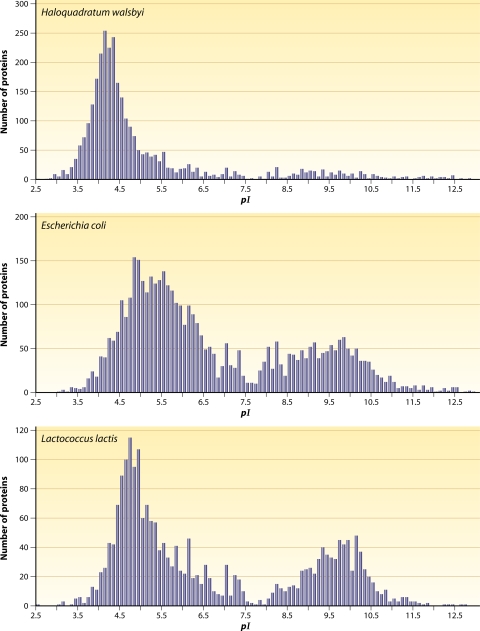
This general physicochemical result of the model of complex vectorial chemistry predicts that at high ionic strengths (higher Debye's κ), the cellular enzymes ought to have generally evolved with more-charged groups (higher σ0), e.g., more-anionic amino acids, on average, in order to maintain the same mechanism of lower-temperature switching conditions given by equation 2. It is noteworthy that there is good evidence that halophiles' cytoplasmic enzymes contain a high proportion of anionic amino acids and hydroxyl amino acids and less cationic and hydrophobic amino acids, possibly in order to maintain colloidal stability and functionality of their cellular architecture at very high ionic strengths (121). In Fig. 3, the in silico-generated proteomes of a halophilic bacterium, Haloquadratum walsbyi (a square-shaped halophile), are compared with the proteomes of Escherichia coli (a gram-negative bacterium) and Lactococcus lactis (a gram-positive bacterium). Both E. coli and L. lactis have an intracellular ionic strength that is low compared to that of H. walsbyi and typically live in moderate-salt environments, compared to the high-salt habitat of H. walsbyi. These pI distributions of proteins may indicate that the halophiles were to evolve first, as their proteome appears simpler. The halophiles can rely mainly on the hydrophobically driven formation of protein complexes, whereas the bimodal protein distributions for L. lactis and E. coli may indicate greater evolutionary possibilities in forming protein complexes, the formation of protein complexes being driven by both the hydrophobic effect and by the positive-negative charge attractions modulated by cytoplasmic pH and ionic strength.
FIG. 3.
Distribution of protein abundance as a function of the predicted isoelectric point (pI). pI values were calculated for all proteins specified by the genome of Haloquadratum walsbyi (a halophile, living in an environment of high ionic strength and predicted to have a high intracellular ionic strength), Escherichia coli (a gram-negative bacterium, growing optimally at low to moderate salt concentrations, below 0.5 M NaCl) and Lactococcus lactis (a gram-positive bacterium, growing optimally at low to moderate salt concentrations). Both E. coli and L. lactis have an intracellular ionic strength that is low compared to that of H. walsbyi. The distribution of proteins as a function of pI is bimodal with a minimum around pH 7.5, i.e., corresponding to the intracellular pH of the cytoplasm. In the vast majority of microorganisms, anionic proteins are far more abundant than cationic ones. As shown for H. walsbyi, the bias for anionic proteins is most extreme in halophiles, that is, when the high external salt concentration is (osmotically) balanced by a high concentration of ionic osmolytes in the cytoplasm.
CONCLUSIONS AND PERSPECTIVES
We have developed a general scenario of emergence of life on Hadean Earth, drawn from our current (albeit still evolving) knowledge of physical chemistry, biochemistry, microbiology, and Hadean geophysics and geochemistry. This scenario of life's emergence is a general physicochemical model, which is based on three premises.
The first premise is that prebiotic physicochemical evolution started in microspaces confined by membranous walls, the microspaces were inanimate precursors of the first unicellular organisms, and the precursors of extant biochemicals coevolved in toto inside the microspaces, which themselves were subject to prebiotic evolution. The evolution of microspaces thus leads directly to the emergence of unicellular organisms at the end of the Hadean eon.
The second premise is that prebiotic evolution was driven by external physicochemical gradients, particularly temperature and osmotic gradients, which were coupled to free-energy gradients of potential reactions, both within the microspaces and through their membranous walls. The organic reactants for such reactions could have originated on Hadean Earth (at hydrothermal vents by hydrogen reductions of carbon dioxide), and they could have been brought in by bombardments (meteorites and interplanetary dust particles) from outer space.
The third premise is that noncovalent stabilizing interactions were important to maintain colloidal stability of crowded macromolecular contents of the microspaces; in particular, hydration, excluded volume, and screened electrostatic interactions played an important part in shaping the topology of coevolving protobiomolecules.
From these premises, we draw some general conclusions. We stress the importance of a salty Hadean ocean as the necessary aqueous medium for providing short Debye's lengths, which allow close thermal interactions under high-crowding conditions of protobiomacromolecules within microspaces; further, these interactions provide a background colloidal stabilization that is independent of temperature. Hadean microspaces could have been stabilized by largely nonionic membranous walls, which could have enabled their independent evolution without being agglomerated by the high ionic strength of the Hadean oceans; more likely, Hadean microspaces were stabilized by a mixture of ionic and nonionic membranous walls, which were agglomerated by the high-ionic-strength contents of the Hadean oceans and hence evolved in communal physicochemical mats (possibly the precursors of extant biofilms).
We have briefly outlined a speculative sequence of tentative transitions between major evolutionary periods during the Hadean eon; these were (i) the early water world, (ii) the appearance of land masses, (iii) the pre-RNA world, (iv) the onset of complex vectorial chemistry, and (v) the RNA world and evolution toward Darwinian thresholds. We do not suggest any detailed biochemical or geochemical reaction schemata, which can still be stipulated to address more specific questions of life's emergence within a particular evolutionary period. The model of complex vectorial chemistry appears to represent a key turning point of the prebiotic evolution within microspaces. At this juncture, when the stabilizing noncovalent interactions became commensurate, the interior of the microspaces contained a 20 to 30% volume fraction of protobiomolecules; they were stabilized by commensurate ranges of hydration and screened electrostatic interactions, and they were subject to variable physicochemical gradients of the Hadean Earth. The high-level macromolecular crowding divided the cytosol into evolving networks of actual electrolyte pools and charged pathways. Under these conditions, cyclic environmental gradients drove the evolution of vectorial biochemical reactions within microspaces toward the RNA world and toward the emergence of Hadean life. We briefly touched on the issues related to the temporal and spatial descriptions of cell architecture at the nanoscale and mesoscale levels and on the possibility that halophiles were the first to evolve in the high-ionic-strength Hadean ocean.
One disadvantage of the (nanoscale) model of complex vectorial chemistry in crowded microspaces is its inherent temporal and spatial complexity that needs to be figured out in detail. How is it possible that so many different parts work so well together on size scales from nanometers to micrometers and on time scales from microseconds to seconds? How exactly are physicochemical gradients and their fluxes coupled to chemical reactions inside the microspaces? With new information-handling technologies now available, it seems possible to design relatively simple computer-controlled experiments to study artificial networks of electrochemical pools with conducting (both cations and anions) and semiconducting (only cations or anions) ionic pathways in microspaces under cyclic temperature and osmotic gradients. In this respect, some chemical engineering designs of artificial charged membranes already possess some aspects of vectorial electrochemistry and perhaps this and other chemical engineering knowledge could be exploited in studies of complex vectorial biochemistry. Hopefully, further elaborations of this model with specific reaction schemes for specific membranous microspaces will lead to such experiments, which could then also illuminate the emergent and evolutionary character of complex vectorial chemistry.
Acknowledgments
We thank Michael Russell for extensive comments and contributions to the manuscript, though not all views expressed here coincide with his. We thank Fabrizia Fusetti for the in silico analysis of the prokaryotic proteomes. The literature on the origin of life has grown enormously in the last 20 years, and we apologize to researchers whose publications are not referenced or are referenced inadequately. We thank the RCSB Protein Data Bank for permission to use the “Molecule of the Month” database of protein pictures of David Goodsell as templates for creating Fig. 1; we used artistic license to obtain protein and nucleic acid shapes at very coarse resolutions.
The research of B.P. is supported by grants from The Netherlands Science Foundation (NWO; CW “top subsidy”), EU (EDICT program), ESF (SYSMO-Kosmobac program), and The Netherlands Proteomics Centre.
Biography
 Jan Spitzer was educated at the Institute of Chemical Technology, Prague, Czech Republic (M.Sc. in Chemical Engineering), and at the University of London, London, United Kingdom, where he obtained his Ph.D. in Physical Chemistry in 1975 (Interionic Interactions, Solvation). He won the National Science and Engineering Research Council of Canada University Fellowship and taught at the University of Lethbridge, Alberta, Canada, and performed research on bitumen recovery from the oil sands in Alberta, Canada. Since 1985, he has worked in the synthetic latex industry (styrene-butadiene and acrylic polymer emulsions) in Canada and in the United States, developing new emulsion polymers and emulsion polymerization processes. His academic research interest has remained in the area of physical chemistry of solutions and colloids, where he has published over 40 original contributions, particularly on electrostatic interactions between ions, polyelectrolytes, and charged colloids, based on Poisson-Boltzmann models. His current interest is in colloidal stability of non-equilibrium-charged colloidal systems, such as high-volume fraction polymer colloids during their syntheses, and macromolecular crowding in biological cells during their growth or under osmotic stresses.
Jan Spitzer was educated at the Institute of Chemical Technology, Prague, Czech Republic (M.Sc. in Chemical Engineering), and at the University of London, London, United Kingdom, where he obtained his Ph.D. in Physical Chemistry in 1975 (Interionic Interactions, Solvation). He won the National Science and Engineering Research Council of Canada University Fellowship and taught at the University of Lethbridge, Alberta, Canada, and performed research on bitumen recovery from the oil sands in Alberta, Canada. Since 1985, he has worked in the synthetic latex industry (styrene-butadiene and acrylic polymer emulsions) in Canada and in the United States, developing new emulsion polymers and emulsion polymerization processes. His academic research interest has remained in the area of physical chemistry of solutions and colloids, where he has published over 40 original contributions, particularly on electrostatic interactions between ions, polyelectrolytes, and charged colloids, based on Poisson-Boltzmann models. His current interest is in colloidal stability of non-equilibrium-charged colloidal systems, such as high-volume fraction polymer colloids during their syntheses, and macromolecular crowding in biological cells during their growth or under osmotic stresses.
 Bert Poolman is a professor in biochemistry and program director of the Centre for Synthetic Biology. His research focuses on the elucidation of the mechanisms by which signals are transduced and small molecules are translocated across cellular membranes. Cells are reengineered for the production of correctly folded membrane proteins, and methods are developed to reconstitute complex molecular assemblies in synthetic membranes and to analyze their functional and structural properties. The in vitro mechanistic studies are used to strengthen in vivo physiological measurements and vice versa. Research topics of his group include (i) the mechanisms of translocation catalysis by ATP-binding cassette transporters; (ii) the molecular basis of cell volume regulation, including a comprehensive understanding of the role of lipid-protein interactions, (macro)molecular crowding, and other generic physicochemical factors in transmembrane signaling and transport; and (iii) membrane proteomics with a focus on nuclear and vacuolar membranes and the basis for molecule transport through the nuclear pore complex.
Bert Poolman is a professor in biochemistry and program director of the Centre for Synthetic Biology. His research focuses on the elucidation of the mechanisms by which signals are transduced and small molecules are translocated across cellular membranes. Cells are reengineered for the production of correctly folded membrane proteins, and methods are developed to reconstitute complex molecular assemblies in synthetic membranes and to analyze their functional and structural properties. The in vitro mechanistic studies are used to strengthen in vivo physiological measurements and vice versa. Research topics of his group include (i) the mechanisms of translocation catalysis by ATP-binding cassette transporters; (ii) the molecular basis of cell volume regulation, including a comprehensive understanding of the role of lipid-protein interactions, (macro)molecular crowding, and other generic physicochemical factors in transmembrane signaling and transport; and (iii) membrane proteomics with a focus on nuclear and vacuolar membranes and the basis for molecule transport through the nuclear pore complex.
APPENDIX
Organic polymers and oligomers available on Hadean Earth.
Geologists and paleontologists divide the history of Earth into eons (Phanerozoic, Proterozoic, Archean, and Hadean). The oldest eon is the Hadean, which approximately spans the period of 4,600 million to 3,800 million years ago, from the time of the Earth's formation (proto-Earth-asteroid impact creating the current Earth-Moon planetary system) to the first clues of life—a photosynthetically enriched 12C isotope in Isua rocks of western Greenland. The initial Hadean conditions were harsh (as viewed through our anthropocentric lenses), when high atmospheric pressures could allow water to exist at temperatures greater than 200°C and when there was extensive volcanic activity in the Earth's lithosphere. Our model of life emergence suggests that cosmological carbon-based chemicals (ions, molecules, oligomers, and polymers, including cross-linked polymers) became part of early semipermeable walls of the microspaces. Polymeric materials have been identified in interstellar space (in diffuse and molecular interstellar clouds) on planetary surfaces and in comets and meteorites; the infall of interplanetary dust particles (micrometeorites) is thought to bring large amounts of such polymers to the Earth's surface, a process still occurring today. Chemically, two kinds of carbon-based polymers have been identified: these are polyaromatic hydrocarbons and polymers resulting from polymerization or other reactions of hydrogen cyanide (HCN). The relation of these nitrogenous tars to adenine and to amino acids is quite plausible, as adenine is a pentamer of HCN and the oligomers of HCN can be hydrolyzed to amino acids. However, both of these kinds of polymers are intractable because they are highly cross-linked; physicochemical properties of laboratory analogs of such polymers, such as density, molecular structure, and molecular weight, have not yet been well established. They could have accumulated in the early oceans, where they also provided UV protection to other prebiotic molecules, such as amino acids and nucleotide precursors. Similar polymers were also observed in large yields in the prebiotic syntheses of amino acids and nucleosides; however, these tars have been regarded more as a nuisance rather than as raw material for the evolution of membranous walls of potential microspaces. Special kinds of such planetary materials are tholins, which are believed to be responsible for the orange haze of the surface of Titan (one of Saturn's satellites). Carbonaceous meteorites, such as the famous Murchinson meteorite that was recovered in Australia in 1969, also contain carbon-based polymers similar to biogeochemical kerogens (carbon-based polymers embedded in Earth's sedimentary rocks).
Electrostatic, electrochemical, and chemiosmotic potentials.
Electrostatic potential arises from a time-invariant (fixed, equilibrium, or instantaneous) distribution of charges, e.g., from various ions, either fixed on chemical surfaces, e.g., phosphate esters on DNA and carboxylates on acidic proteins, or free ions in solution, such as dihydrogen phosphate or sodium in water. The distribution of charges gives rise to a distribution of electrostatic potential that obeys Coulomb's law, one of the fundamental laws of nature that is valid also in the quantum mechanical world of chemically reacting molecules and atoms and even within the atomic nuclei. Within the electrostatic models of ions, charged chemical surfaces, and polyelectrolytes, Coulomb's law is expressed in a differential form as Poisson's equation, one the four equations of classical electromagnetism of James Clerk Maxwell. Together with the Boltzmann theorem, electrostatic models of distribution of free ions around charged systems (other ions, charged surfaces, and charged membranes) can be formulated and solved. From such solutions, the distribution of electrostatic potentials, e.g., the surface electrostatic potential of a charged molecular surfaces as well as mechanical forces that charged systems engender, can be computed. Since the energy of moving a charge in an electrostatic field is independent of the path, and since the addition of equal charges of the opposite sign give zero, electrostatic energies have the nature of thermodynamic free energy. Essentially all cytoplasmic bacterial biomacromolecules and metabolites, as well as the membrane bilayer, are ionic in nature; therefore, electrostatic interactions (direct charge interactions as well as screened electrostatic interactions), in addition to hydrophobic and hydration effects, contribute to the forces between charged molecular surfaces, i.e., to their associations and dissociations. The electrostatic potential is thus a surface property of an interface between a charged surface and a bulk solution.
Electrochemical potentials are thermodynamic potentials between two phases separated by an interface, such as an electrode immersed in an electrolyte solution (where electrochemical reductions or oxidation reactions take place) or two solutions of electrolytes separated by a membrane. The latter case is of particular importance for biological membranes. The simplest case is a situation in which the membrane is permeable only to cations and impermeable to anions, i.e., two solutions of an anionic polyelectrolyte at different concentrations (c2 and c1) separated by a membrane that is not permeable to the large polyelectrolyte anion. A simple equation for this case was derived in 1902 by Bernstein, as follows:
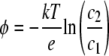 |
(3) |
Here, kT is the thermal energy, e is the electron charge, and c represents the concentrations of the polyelectrolyte in the respective compartments; this membrane potential is generally known as Donnan or Gibbs-Donnan potential. This is a limiting case of a diffusion potential, which arises from unequal permeabilities of cations and anions through the membrane, a situation that is described by Goldman's equation, in which the concentrations of charged ions on either side of the membrane are weighted by their permeabilities (Pi).
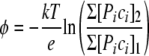 |
(4) |
An important point to realize is that these electrochemical potentials are established by movement of electroneutral combinations of ions across the membrane and express the properties of both the bulk concentrations of ions away from the membrane and the permeability properties of the membrane toward the respective ions. When the membrane is impermeable to all ions, then a chemical potential difference of only water is established across the membrane, which causes water to flow from where its thermodynamic activity (related to its concentration through its osmotic coefficient) is higher to a compartment where its activity is lower. Such osmotic flows can be stopped or even reversed by applying hydrostatic pressure to the compartment where water has lower activity levels. Hence, osmotic pressure differences do not give rise to electrochemical potentials.
Chemiosmotic membrane potentials are characteristic of living membranes; they arise from vectorial chemical reactions that involve the movement of ions and electrons within and across the membrane. They were discovered and conceptualized by biochemists in their analyses of energy-transducing membranes, such as the membranes of mitochondria in animal cells or chloroplast membranes of plant cells. Bacterial and archaeal membranes are also capable of energy transduction via various membrane-embedded electron transfer components and ATPases; the latter can sometimes operate reversibly and thus transport ions at the expense of ATP hydrolysis (hence, ATPases) or synthesize ATP at the expense of electrochemical ion gradients (hence, ATP synthetases). Chemiosmotic membrane potentials can be also established by various ion transport mechanisms across the biological bilayer membrane. There are no physicochemical analogs of chemiosmotic membrane potentials.
Model of complex vectorial biochemistry.
There are three main characteristics of the model of complex vectorial biochemistry, as shown in Fig. 1. First, the large proportion of different biomacromolecules in extant bacterial cytoplasm (20 to 30% by volume) leads to a situation in which the cytoplasm is structured into regions where there are pools of ionic, low-molecular-weight metabolic intermediates. Such pools have different bulk concentrations (and, hence, different electrochemical potentials) in respect to other pools. Ionic metabolites are thus driven from their higher-(electro)chemical-potential pools, into which they are biochemically produced, to lower-(electro)chemical-potential regions, where they are consumed, as a consequence of the Donnan disequilibrium.
Second, the pathways between the pools are lined with charged surfaces of protein complexes, protein/RNA complexes, and protein/DNA complexes with various surface charge distributions. The theory predicts that these metabolic and replicative vectorial pathways can have semiconducting properties. As the biomacromolecules are generally negatively charged, the cations can pass through all the pathways where the overall electrostatic potential is negative, while anions can pass only through pathways where the electrostatic potential is sufficiently low; further, singly and doubly charged anions are conditionally obliged to use different pathways depending on the electrostatic potential within the pathway; this is particularly applicable to the narrower pathways of the crowded biomacromolecular regions. Third, these pathways and pools are connected to membrane proteins (transporters, metabolic enzymes, and signal transduction components) and, through them, to the outside environment.
An important aspect of the model of complex vectorial chemistry is that large biomacromolecular complexes cannot diffuse freely. Collectively, they begin to create an emergent vectorial structure or architecture of the protocytoplasm. Such dynamic structures of crowded biomacromolecular regions persist on longer time scales than the fast-diffusing low-molecular-weight metabolites that participate in biochemical reactions. As a rough approximation, the ionic currents occur on a time scale of about microseconds, while the dynamic nanostructure, as depicted in Fig. 1, persists on a time scale of about milliseconds; it evolves during cell growth on a time scale of seconds, while the cell divisions take place on a time scale of hours. As the cell grows, the crowded regions of biomacromolecular complexes may “digest” some of their own proteins (diminish in size), synthesize other proteins (grow), and generate low-molecular-weight metabolites that are either used right away (transported vectorially within the crowded biomacromolecular regions) or released into nearby pools for vectorial transport to lower-electrochemical-potential pools.
The model has a certain aspect of a packet-switching network (as in an Internet communication network where each message is broken into “packets” that travel via different pathways to the same destination): anionic metabolites (“informational packets,” typically anionic phosphate esters into which the classical biochemical pathways are broken up) can reach their destination by different pathways, i.e., their delivery is not dependent on one particular (fixed) vectorial pathway. Hence, the crowded biomacromolecular regions in Fig. 1, which are topologically complementary to the electrolyte pools, do not need to contain enzymes that are associated with the classical (time-invariant) metabolic and signaling biochemical pathways.
REFERENCES
- 1.Alberts, B. 1998. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92291-293. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, T. J. October 1975. Reduction of foaming in ammonia recovery system in a HCN manufacturing process. U.S. patent 3,914,386.
- 3.Baaske, P., F. M. Weinert, S. Duhr, K. H. Lemke, M. J. Russell, and D. Braun. 2007. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. USA 1049346-9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bada, J. 2004. How life began on Earth: a status report. Earth Planet. Sci. Lett. 2261-15. [Google Scholar]
- 5.Betts, M. J., and R. B. Russell. 2007. The hard cell: from proteomics to a whole cell model. FEBS Lett. 5812870-2876. [DOI] [PubMed] [Google Scholar]
- 6.Bevan, M. A., J. A. Lewis, P. V. Braun, and P. Wiltzius. 2004. Structural evolution of colloidal crystals with increasing ionic strength. Langmuir 207045-7053. [DOI] [PubMed] [Google Scholar]
- 7.Biemans-Oldehinkel, H., N. A. Mahmood, and B. Poolman. 2006. A sensor for intracellular ionic strength. Proc. Natl. Acad. Sci. USA 10310624-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth, I. R. 1985. Regulation of cytoplasmic pH in bacteria. Microbial. Rev. 49359-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne, J. H., and S. G. Schultz. 1994. An introduction to membrane transport and bioelectricity. Raven Press, New York, NY.
- 10.Cavalier-Smith, T. 2001. Obcells as proto-organisms: membrane heredity, lithophosphorylation and the origin of genetic code, the first cells, and photosynthesis. J. Mol. Evol. 53555-595. [DOI] [PubMed] [Google Scholar]
- 11.Chen, I. A., R. W. Roberts, and J. W. Szostak. 2004. The emergence of competition between model protocells. Science 3051474-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chyba, C. F., and C. Sagan. 1992. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origin of life. Nature 355125-132. [DOI] [PubMed] [Google Scholar]
- 13.Cleaves, H. J., and S. L. Miller. 1998. Oceanic protection of prebiotic organic compounds from UV radiation. Proc. Natl. Acad. Sci. USA 957260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colwell, R. R., and D. J. Grimes. 2000. Nonculturable microorganisms in the environment. ASM Press, Washington, DC.
- 15.Copley, S. D., E. Smith, and H. J. Morowitz. 2007. The origin of RNA world: co-evolution of genes and metabolism. Bioorg. Chem. 35430-443. [DOI] [PubMed] [Google Scholar]
- 16.Crick, F. 1981. Life itself. Simon & Schuster, New York, NY.
- 17.Crick, F., and L. E. Orgel. 1973. Directed panspermia. Icarus 19314-346. [Google Scholar]
- 18.Deamer, D. W. 1997. The first living systems: a bioenergetic perspective. Microbiol. Mol. Biol. Rev. 61239-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debye, P., and E. Hückel. 1923. Zur Theorie der Elektrolyte. Phys. Z. 24185-206. [Google Scholar]
- 20.de Duve, C. 2005. Singularities—landmarks on the pathways to life. Cambridge University Press, Cambridge, United Kingdom.
- 21.de Duve, C., and S. L. Miller. 1991. Two-dimensional life? Proc. Natl. Acad. Sci. USA 8810014-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deHaseth, P. L., T. M. Lohman, and M. T. Record, Jr. 1977. Nonspecific interaction of lac repressor with DNA: an association reaction driven by counterion release. Biochemistry 164783-4790. [DOI] [PubMed] [Google Scholar]
- 23.Dmitriev, B., F. Toukach, and S. Ehlers. 2005. Towards a comprehensive view of the bacterial cell-wall. Trends Microbiol. 13569-574. [DOI] [PubMed] [Google Scholar]
- 24.Dogic, Z., and S. Fraden. 2001. Development of model colloidal liquid crystals and the kinetics of isotropic-smectic transition. Philos. Trans. R. Soc. Lond. A (London) 359997-1015. [Google Scholar]
- 25.Ehrenfreund, P., S. Rasmussen, J. Cleaves, and L. Chen. 2006. Experimentally tracing the key steps in the origin of life: the aromatic world. Astrobiology 6490-520. [DOI] [PubMed] [Google Scholar]
- 26.Eigen, M. 1971. Self-organization of matter and the evolution of biological macromolecules. Naturwissenschaften 58465-523. [DOI] [PubMed] [Google Scholar]
- 27.Ellis, R. J. 2001. Macromolecular crowding—obvious but underappreciated. Trends Biochem. Sci. 26597-604. [DOI] [PubMed] [Google Scholar]
- 28.Elowitz, M. B., M. G. Surette, P.-E. Wolf, J. B. Stock, and S. Leibler. 1999. Protein mobility in the cytoplasm of Escherichia coli. J. Bacteriol. 181197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eschenmoser, A. 2007. The search for the chemistry of life's origin. Tetrahedron 6312821-12844. [Google Scholar]
- 30.Ferris, J. P., P. C. Joshi, E. H. Edelson, and J. G. Lawless. 1978. HCN: a plausible source of purines, pyrimidines and amino acids on the primitive Earth. J. Mol. Evol. 11293-311. [DOI] [PubMed] [Google Scholar]
- 31.Folsome, C. E. 1976. Synthetic organic microstructures and the origin of cellular life. Naturwissenschaften 63303-306. [Google Scholar]
- 32.Fox, S. W. 1988. The emergence of life. Basic Books, New York, NY.
- 33.Franklin, R. M. 1967. Replication of bacteriophage ribonucleic acid: some physical properties of single stranded, double stranded, and branched viral ribonucleic acid. J. Virol. 164-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenkiel-Krispin, D., I. Ben-Avraham, J. Englander, E. Shimoni, S. G. Wolf, and A. Minsky. 2004. Nucleoid restructuring in stationary-state bacteria. Mol. Microbiol. 51395-405. [DOI] [PubMed] [Google Scholar]
- 35.Fry, I. 2000. The emergence of life on earth. Rutgers University Press, New Brunswick, NJ.
- 36.Gilbert, W. 1986. The RNA world. Nature 319618-622. [Google Scholar]
- 37.Glass, J. I., N. Assad-Garcia, N. Alperovich, S. Yooseph, M. R. Lewis, M. Maruf, C. A. Hutchinson III, H. O. Smith, and J. C. Venter. 2006. Essential genes of a minimal bacterium. Proc. Natl. Acad. Sci. USA 103425-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodsell, D. S. 1991. Inside a living cell. Trends Biochem. Sci. 16203-206. [DOI] [PubMed] [Google Scholar]
- 39.Harold, F. M. 1986. The vital force: a study of bioenergetics. W. H. Freeman and Co., New York, NY.
- 40.Harold, F. M. 2005. Molecules into cells: specifying spatial architecture. Microbiol. Mol. Biol. Rev. 69544-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazen, R. M. 2005. Genesis. Joseph Henry Press, Washington, DC.
- 42.Jortner, J. 2006. Conditions for the emergence of life on the early Earth: summary and reflections. Philos. Trans. R. Soc. Lond. B (London) 3611877-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joyce, G. F. 2002. The antiquity of RNA-based evolution. Nature 418214-221. [DOI] [PubMed] [Google Scholar]
- 44.Kasting, J. F., D. H. Eggler, and S. P. Raeburn. 1993. Mantle redox evolution and the oxidation state of the Archean atmosphere. J. Geol. 101245-257. [DOI] [PubMed] [Google Scholar]
- 45.Kauffman, S. A. 2000. Investigations. Oxford University Press, Oxford, United Kingdom.
- 46.Khare, B. N., C. Sagan, W. R. Thompson, E. T. Arakawa, C. Meisse, and P. S. Tuminello. 1994. Optical properties of poly-HCN and their astronomical applications. Can. J. Chem. 72678-693. [DOI] [PubMed] [Google Scholar]
- 47.Knauth, L. P. 2005. Temperature and salinity history of the precambrian ocean: implications for the course of microbial evolution. Paleogeogr. Paleoclimatol. Paleoecol. 21953-69. [Google Scholar]
- 48.Koonin, E. V., and W. Martin. On the origin genes and cells within inorganic compartments. Trends Biochem. Sci. 21647-654. [DOI] [PMC free article] [PubMed]
- 49.Lahav, N. 1999. Biogenesis: theories of life's origin. Oxford University Press, Oxford, United Kingdom.
- 50.Lasaga, A. C., and M. J. Dwyer. 1971. Primordial oil slick. Science 17453-55. [DOI] [PubMed] [Google Scholar]
- 51.Lathe, R. 2006. Early tides: response to Varga et al. Icarus 180277-280. [Google Scholar]
- 52.Lauterbur, P. C. 2008. The spontaneous development of biology from chemistry. Astrobiology 83-8. [DOI] [PubMed] [Google Scholar]
- 53.Lazcano, A., and S. L. Miller. 1996. The origin and early evolution of life: prebiotic chemistry, the pre-RNA world, and time. Cell 85793-798. [DOI] [PubMed] [Google Scholar]
- 54.Lee, C.-S., and P. Guo. 1995. In vitro assembly of infectious virions of double-stranded DNA phage φ29 from cloned gene products and synthetic nucleic acids. J. Virol. 695018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehn, J.-M. 2002. Toward self-organization and complex matter. Science 2952400-2403. [DOI] [PubMed] [Google Scholar]
- 56.Levy, M., S. L. Miller, K. Brinton, and J. L. Bada. 2000. Prebiotic synthesis of adenine and amino acids under Europe-like conditions. Icarus 145609-613. [DOI] [PubMed] [Google Scholar]
- 57.Lifson, S. 1997. On the crucial stages in the origin of animate matter. J. Mol. Evol. 441-8. [DOI] [PubMed] [Google Scholar]
- 57a.Lincoln, T. A., and G. F. Joyce. 2009. Self-sustained replication of an RNA enzyme. Science 3231229-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long, M. S., C. D. Jones, M. R. Helfrich, L. K. Mangeney-Slavin, and C. D. Keating. 2005. Dynamic microcompartmentation in synthetic cells. Proc. Natl. Acad. Sci. USA 1025920-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Losick, R., and L. Shapiro. 1999. Changing views on the nature of the bacterial cell: from biochemistry to cytology. J. Bacteriol. 1814143-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lubetkin, S. D., S. R. Middleton, and R. H. Ottewill. 1984. Some properties of clay-water dispersions. Philos. Trans. R. Soc. Lond. A (London) 311353-368. [Google Scholar]
- 61.Luisi, P. L., F. Ferri, and P. Stano. 2006. Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 931-13. [DOI] [PubMed] [Google Scholar]
- 62.Lupi, O., P. Dadalti, E. Cruz, and P. R. Sanberg. 2006. Are prions related to the emergence of early life? Med. Hypotheses 671027-1033. [DOI] [PubMed] [Google Scholar]
- 63.MacLeod, G., C. Mckeown, A. J. Hall, and M. J. Russell. 1994. Hydrothermal and oceanic pH conditions at 4 Gya relevant to the origin of life. Orig. Life Evol. Biosph. 2419-41. [DOI] [PubMed] [Google Scholar]
- 64.Mahmood, N. A., H. Biemans-Oldehinkel, J. Patzlaff, G. K. Schuurman-Wolters, and B. Poolman. 2006. Ion specificity and ionic strength dependence of the osmoregulatory ABC transporter OpuA. J. Biol. Chem. 28129830-29839. [DOI] [PubMed] [Google Scholar]
- 65.Mansy, S. S., J. P. Schrum, M. Krishnamurthy, S. Tobé, D. A. Treco, and J. W. Szostak. 2008. Template-directed synthesis of a genetic polymer in a model protocell. Nature 454122-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin, W., and M. J. Russell. 2007. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. Lond. B (London) 3621887-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin, W., J. Baross, D. Kelley, and M. J. Russell. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6805-814. [DOI] [PubMed] [Google Scholar]
- 68.Mathews, C. N., and R. D. Minard. 2006. Hydrogen cyanide polymers. Comets and the origin of life. Faraday Discuss. 133393-401. [DOI] [PubMed] [Google Scholar]
- 69.Miller, S. L., and H. J. Cleaves. 2006. Prebiotic chemistry on the primitive Earth, p. 3-56. In I. Ritgoutsos and G. Stephanopoulous (ed.), Systems biology. Oxford University Press, New York, NY.
- 70.Mitchell, P. 1979. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic, and chemiosmotic reaction systems. Eur. J. Biochem. 951-20. [DOI] [PubMed] [Google Scholar]
- 71.Monnard, P.-E., C. L. Apel, A. Kanavarioti, and D. W. Deamer. 2002. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2139-152. [DOI] [PubMed] [Google Scholar]
- 72.Monod, J. 1971. Chance and necessity. Knopf, New York, NY.
- 73.Morowitz, H., and E. Smith. 2007. Energy flow and the organization of life. Complexity 1351-59. [Google Scholar]
- 74.Morowitz, H. J. 1992. Beginnings of cellular life. Yale University Press, New Haven, CT.
- 75.Morse, J. W., and F. T. Mackenzie. 1998. Hadean ocean carbonate geochemistry. Aquat. Geochem. 4310-319. [Google Scholar]
- 76.Munn, C. B. 2004. Marine microbiology. BIOS Scientific Publishers, London, United Kingdom.
- 77.Nicholls, D. G., and S. J. Ferguson. 2002. Bioenergetics 3. Academic Press, London, United Kingdom.
- 78.Nilson, F. P. R. 2002. Possible impact of a primordial oil slick on atmospheric and chemical evolution. Orig. Life Evol. Biosph. 32247-253. [DOI] [PubMed] [Google Scholar]
- 79.Noireaux, V., and A. Libchaber. 2004. A vesicle bioreactor as a step toward an artifical cell assembly. Proc. Natl. Acad. Sci. USA 10117669-17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noireaux, V., R. Bar-Ziv, J. Godefroy, H. Salman, and A. Libchaber. 2005. Toward an artificial cell based on gene expression in vesicles. Phys. Biol. 2P1-P8. [DOI] [PubMed] [Google Scholar]
- 81.Oparin, A. I. 1957. Origin of life. Academic Press, New York, NY.
- 82.Orgel, L. E. 1998. The origin of life—a review of facts and speculations. Trends Biochem. Sci. 23491-495. [DOI] [PubMed] [Google Scholar]
- 83.Orgel, L. E. 2004. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 3999-123. [DOI] [PubMed] [Google Scholar]
- 84.Pascal, R., L. Boiteau, P. Forterre, M. Gargaud, A. Lazcano, P. López-García, D. Moreira, M.-C. Maurel, J. Peretó, D. Prieur, and J. Reisse. 2006. Prebiotic chemistry—biochemistry—emergence of life (4.4—2 Ga). Earth Moon Planets 98153-203. [Google Scholar]
- 85.Plankensteiner, K., H. Reiner, and B. M. Rode. 2005. Stereoselective differentiantion in the salt-induced reaction and its relevance for the origin of life. Peptides 26535-541. [DOI] [PubMed] [Google Scholar]
- 86.Pohorille, A., and D. Deamer. 2002. Artificial cells: prospects for biotechnology. Trends Biotechnol. 20123-128. [DOI] [PubMed] [Google Scholar]
- 87.Poolman, B., J. J. Spitzer, and J. M. Wood. 2004. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biophys. Biochim. Acta 166688-104. [DOI] [PubMed] [Google Scholar]
- 88.Pross, A. 2004. Causation and the origin of life: metabolism or replication first? Orig. Life Evol. Biosph. 34307-321. [DOI] [PubMed] [Google Scholar]
- 89.Qasba, P. K., and W. Zillig. 1969. Nature of RNA synthesized at low and high ionic strength by DNA-dependent RNA polymerase from Escherichia coli. Eur. J. Biochem. 7315-317. [DOI] [PubMed] [Google Scholar]
- 90.Racher, K. I., D. E. Culham, and J. M. Wood. 2001. Requirements for osmosensing and osmotic activation of transporter ProP from Escherichia coli. Biochemistry 407324-7333. [DOI] [PubMed] [Google Scholar]
- 91.Record, M. T., Jr., E. S. Courteney, S. Cayley, and H. J. Guttman. 1998. Biophysical compensation mechanisms buffering Escherichia coli protein-nucleic acid interactions against changing environments. Trends Biochem. Sci. 23190-194. [DOI] [PubMed] [Google Scholar]
- 92.Robinson, R. A., and R. H. Stokes. 1959. Electrolyte solutions. Butterworths, London, United Kingdom.
- 93.Rode, B. M. 1999. Peptides and the origin of life. Peptides 20773-786. [DOI] [PubMed] [Google Scholar]
- 94.Rosen, M. J. 1978. Surfactants and interfacial phenomena. John Wiley & Sons, New York, NY.
- 95.Rosing, M. T. 1999. C-13-depleted carbon microparticles in ∼3700-Ma sea-floor sedimentary rocks from west Greenland. Science 283674-676. [DOI] [PubMed] [Google Scholar]
- 96.Roth, A., A. Nahvi, M. Lee, I. Jona, and R. R. Breaker. 2008. Characteristics of the glmS ribozyme suggests only structural roles for divalent metal ions. RNA J. 12607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rübenhagen, R., S. Morbach, and R. Krämer. 2001. The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for the cytoplasmic K+. EMBO J. 205412-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Russell, M. J., R. M. Daniel, A. J. Hall, and J. Sherringham. 1994. A hydrothermally precipitated catalytic iron sulphide membrane as a first step towards life. J. Mol. Evol. 39231-243. [Google Scholar]
- 99.Russell, M. J., and N. T. Arndt. 2005. Geodynamic and metabolic cycles in the Hadean. Biogeosciences 297-111. [Google Scholar]
- 100.Schildkraut, C., and S. Lifson. 1965. Dependence of the melting temperature of DNA on salt concentration. Biopolymers 3195-208. [DOI] [PubMed] [Google Scholar]
- 101.Schmitz, K. S. 2002. Phase separation in macroion systems, p. 195-269. In S. K. Tripathy, J. Kumar, and H. S. Nalwa (ed.), Handbook of polyelectrolytes and their applications. American Scientific Publishers, Stevenson Ranch, CA.
- 102.Schopf, J. W. 2002. Life's origin. University of California Press, Berkeley.
- 103.Segré, D., and D. Lancet. 2000. Composing life, EMBO Rep. 1217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sephton, M. A. 2002. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 19292-311. [DOI] [PubMed] [Google Scholar]
- 105.Shapiro, R. 1986. Origins—a skeptic's guide to the creation of life on Earth. Simon & Schuster, Inc., New York, NY.
- 106.Shenhav, B., and D. Lancet. 2004. Prospects of a computational origin of life endeavor. Orig. Life Evol. Biosph. 34181-194. [DOI] [PubMed] [Google Scholar]
- 107.Smith, J. M., and E. Szathmary. 1995. The major transitions in evolution. Oxford University Press, New York, NY.
- 108.Spitzer, J. J. 1984. A re-interpretation of hydration forces. Nature 310396-397. [Google Scholar]
- 109.Reference deleted.
- 110.Spitzer, J. J. 2002. Dissociation of colloidal spheres according to LMO law. Langmuir 187906-7915. [Google Scholar]
- 111.Spitzer, J. J. 2003. Maxwellian double layer forces: from infinity to contact. Langmuir 197099-7111. [Google Scholar]
- 112.Spitzer, J. J., and B. Poolman. 2005. Electrochemical structure of the crowded cytoplasm. Trends Biochem. Sci. 30536-541. [DOI] [PubMed] [Google Scholar]
- 113.Sullivan, W. T., III, and J. A. Baross. 2007. Planets and life: the emerging science of astrobiology. Cambridge University Press, Cambridge, United Kingdom.
- 114.Tanford, C. 1980. The hydrophobic effect: formation of micelles and biological membranes. John Wiley & Sons, New York, NY.
- 115.Tervahatu, H., A. Tuck, and V. Vaida. 2005. Chemistry in prebiotic aerosols: a mechanism for the origin of life, p. 155-165. In J. Seckbach (ed.), Origins. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 116.Thanbichler, M., and L. Shapiro. 2008. Getting organized—how bacterial cells move proteins and DNA. Nat. Rev. Microbiol. 628-40. [DOI] [PubMed] [Google Scholar]
- 117.Trevors, J. T. 2003. Possible origin of a membrane in the subsurface of the Earth. Cell Biol. Int. 27451-457. [DOI] [PubMed] [Google Scholar]
- 118.van den Bogaart, G., N. Heermans, V. Krasnikov, and B. Poolman. 2007. Protein mobility and diffusive barriers in Escherichia coli: consequences of osmotic stress. Mol. Microbiol. 64858-871. [DOI] [PubMed] [Google Scholar]
- 119.van der Heide, T., M. C. A. Stuart, and B. Poolman. 2001. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 207022-7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18503-519. [DOI] [PubMed] [Google Scholar]
- 121.Ventosa, A., J. J. Nieto, and A. Oren. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verkman, A. S. 2002. Solute and macromolecular diffusion in cellular aqueous compartments. Trends Biochem. Sci. 2727-33. [DOI] [PubMed] [Google Scholar]
- 123.Verwey, E. J. W., and W. T. G. Overbeek. 1948. The theory of stability of lyophobic colloids. Elsevier, Amsterdam, The Netherlands.
- 124.von Kiedrowski, G., L.-H. Eckardt, K. Naumann, W. M. Pankau, M. Reimold, and M. Rein. 2003. Toward replicable, multifunctional, nanoscaffolded machines. A chemical manifesto. Pure Appl. Chem. 75609-619. [Google Scholar]
- 125.Wächterhäuser, G. 2006. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philos. Trans. R. Soc. Lond. B 3611787-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weinstein, L., and J. A. Adams. 2008. Guesstimation. Princeton University Press, Princeton, NJ.
- 127.Westheimer, F. H. 1987. Why nature chose phosphates? Science 2351173-1178. [DOI] [PubMed] [Google Scholar]
- 128.Wilde, S. A., J. W. Valley, W. H. Peck, and C. M. Graham. 2001. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 409175-178. [DOI] [PubMed] [Google Scholar]
- 129.Wills, C., and J. Bada. 2000. The spark of life: Darwin and primeval soup. Perseus Publishing, Cambridge, MA.
- 130.Woese, C. R. 1979. A proposal concerning the origin of life on the planet Earth. J. Mol. Evol. 1395-101. [DOI] [PubMed] [Google Scholar]
- 131.Woese, C. R. 2002. On the evolution of cells. Proc. Natl. Acad. Sci. USA 998742-8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woese, C. R. 2004. A new biology for a new century. Microbiol. Mol. Biol. Rev. 68173-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yockey, H. P. 2000. Origin of life on Earth and Shannon's theory of communication. Comput. Chem. 24105-123. [DOI] [PubMed] [Google Scholar]
- 134.Zimmerman, S. B., and A. P. Minton. 1993. Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 2227-65. [DOI] [PubMed] [Google Scholar]
- 135.Ziurys, L. M. 2006. The chemistry in circumstellar envelopes of evolved stars: following the origin of the elements to the origin of life. Proc. Natl. Acad. Sci. USA 10312274-12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zonia, L., and T. Munnik. 2007. Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci. 1290-97. [DOI] [PubMed] [Google Scholar]
- 137.Zubay, G. 1996. Origins of life on the Earth and in the cosmos. Wm. C. Brown Publishers, Dubuque, IA.