Abstract
5-Lipoxygenase (5LO), by producing leukotrienes, is a proinflammatory enzyme, and there is evidence suggesting that it is up-regulated with aging and may be involved in Alzheimer’s disease (AD). In this paper, we studied the effect of 5LO-targeted gene disruption on the amyloid phenotype of a transgenic mouse model of AD, the Tg2576. Amyloid-β (Aβ) deposition in the brains of Tg2576 mice lacking 5LO was reduced by 64–80% compared with Tg2576 controls. This reduction was associated with a similar significant decrease in Aβ levels measured by sandwich ELISA. Absence of 5LO did not induce any significant change in amyloid-β precursor protein (APP) levels and processing, or Aβ catabolic pathways. Furthermore, in vitro studies showed that 5LO activation or 5LO metabolites increase, whereas 5LO inhibition decreases, Aβ formation, secondary to correspondent changes in γ-secretase activity. These data establish for the first time a novel functional role for 5LO in the pathogenesis of AD-like amyloidosis, thereby modulating γ-secretase activity. Our work suggests that pharmacological inhibition of 5LO could provide a novel therapeutic tool for AD.
Keywords: γ-secretase, Tg2576, Aβ, amyloid-β precursor protein, leukotrienes
Alzheimer’s disease (AD) is a neurodegenerative disorder that causes progressive loss of cognitive function and dementia. The pathological hallmarks in the AD brains are amyloid plaques in the extracellular parenchyma consisting mainly of Amyloid-β (Aβ) peptides (1), and intraneuronal tangles made of abnormally phosphorylated microtubule-associated tau protein (2).
Increasing evidence from human and mouse models suggests that inflammation is an important player in the pathogenesis of AD (3). Eicosanoids, including prostaglandins and leukotrienes, derive from arachidonic acid by the action of cyclooxygenase (COX-1 and COX-2), lipoxygenase (12/15 lipoxygenase (12/15LO), and 5-lipoxygenase (5LO) enzymes. They have important proinflammatory functions and a large array of other biological activities (4). The roles of cyclooxygenases (5-8) and 12/15LO (9, 10) in AD have been studied, but much less attention has been directed to the 5LO (11).
Previously, it has been shown that 5LO is present in the central nervous system (CNS) in neurons and other cell types in mice (12) and rats (13, 14). 5LO and leukotrienes in the CNS have neuromodulatory and neuroendocrine functions (14-16) and might play an important role in aging-associated neurodegenerative diseases (12, 13). 5LO and its metabolites have been also been reported to be associated with excitotoxic injury (17, 18) and cerebral ischemia (19). Taken together, these data would indirectly indicate that 5LO may have also a role in neurodegeneration, but only few studies have directly addressed this issue. It has been shown that 5LO promoter mutations cause a lower 5LO expression compared to wild-type (20). In a pilot study on the association of 5LO promoter polymorphism and the age of onset of AD, a trend toward higher frequency of wild-type 5LO promoter was found in early onset AD (21). Although because of the limited number of cases, no statistical significance was found between the two groups, this study implies that higher expression of 5LO in cases with wild-type 5LO promoter might be associated with earlier onset of AD (21).
In the present study, we investigated the effect of 5LO on the Aβ pathology of a transgenic mouse model of AD-like amyloidosis, the Tg2576 (22). To this end, we generated a double transgenic model crossing 5LO-deficient with Tg2576 mice. Genetic disruption of 5LO resulted in a significant reduction in Aβ deposits and Aβ42 levels. These changes were not associated with a significant alteration in amyloid-β precursor protein (APP) processing or Aβ catabolism. In vitro studies showed that 5LO activation and its metabolites increase, whereas 5LO inhibition decreases, Aβ formation by modulating the γ-secretase complex activity. Taken together, these data provide the first experimental evidence supporting a functional role of 5LO in the amyloid pathology of this mouse model of AD through a mechanism that involves the modulation of the γ-secretase activity.
MATERIALS AND METHODS
Mice and treatments
All animal procedures were approved by the Institutional Animal Care and Usage Committee, in accordance with the U.S. National Institute of Health guidelines. Tg2576 transgenic mice expressing hAPP with the Swedish mutation (K670N/M671L) (22) and 5-lipoxygenase-deficient (5LO−/−) mice (23) were both backcrossed onto C57BL/6SJL mice at least 5 times. Tg2576 mice were crossed to 5LO−/− to generate Tg2576 × 5LO−/− mice. These mice showed regular growth and looked outwardly healthy. All mice were genotyped by polymerase chain reaction (PCR) analysis using tail DNA, were kept in a pathogen-free environment on a 12-hour light-dark cycle, and had access to food and water ad libitum. All of the experiments presented in this paper were performed on female mice.
After sacrifice, animals were perfused intracardially with ice-cold 0.9% PBS containing 10 mM EDTA and 1% BHT. Brains were removed and dissected into two hemibrains by midsagittal dissection. The left hemibrain was used for biochemistry assays or total RNA extraction, and the right hemibrain was immersion-fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.6) overnight for immunohistochemistry studies.
Human subjects
Human hippocampus samples were obtained from patients with neuropathologically confirmed AD (3 females and 2 males) and normal control subjects (1 female and 1 male). Averages of age in AD and control groups (78.4±2.2 vs. 72.0±7.0 yr) were not significantly different. Additional human frontal cortex samples were obtained from patients with neuropathologically confirmed AD (3 females and 4 males) and normal control subjects (3 females and 1 male). Averages of age in AD and control groups (79.7±4.6 vs. 77.2±2.0 yr) were not significantly different. Postmortem diagnostic evaluation was performed in accordance with standard histopathological criteria and previously described procedures that have been used in earlier reports from our laboratory (24).
Immunohistochemistry
Immunostainings were performed as reported previously by our group (25-27). Serial 6-μm-thick coronal sections were mounted on 3-aminopropyl triethoxysilane (APES) -coated slides. Every eighth section from the habenular commissure to the posterior commissure (8–10 sections per animal) was examined using unbiased stereological principles. The sections were deparaffinized, hydrated, pretreated with formic acid (88%), and subsequently treated with 5% in H2O2 methanol. Sections were blocked in 2% fetal bovine serum before incubation with primary antibody overnight at 4°C. 4G8 (total Aβ) was diluted 1:10,000, whereas BA27 (Aβ40) and BC05 (Aβ42) (28) were used as supplied by the manufacturer (Wako Chemicals, Osaka, Japan). Subsequently, the sections were incubated with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) and then developed using the avidin-biotin complex method (Vector Laboratories) with 3,3′-diaminobenzidine (DAB) as a chromogen. Light microscopic images from the hippocampus, somatosensory cortex, and perihippocampal cortex were used to calculate the area occupied by Aβ-immunoreactivity using the software Image-Pro Plus for Windows version 5.0 (Media Cybernetics, Silver Spring, MD, USA). The threshold optical density that discriminated staining from background was determined and kept constant for all quantifications. The area occupied by Aβ-immunoreactivity was measured by the software and was divided by the total area of interest to calculate the percentage area occupied by Aβ-immunoreactivity.
Biochemical analyses
Sequential extractions of mouse brain samples in RIPA and then formic acid (FA) were performed as described previously (6). For measuring Aβ1–40 and Aβ1–42 levels, sensitive sandwich ELISA kits were used (IBL America, Minneapolis, MN, USA). FA samples were first neutralized in 1M Tris base at the ratio of 1:20. Aβ1–38 levels were detected by a sandwich ELISA using Ban50 as capture antibody (Wako Chemicals) and Ab14.1.1, a monoclonal antibody raised against Aβ38 (29), as detection antibody. Ab14.1.1 was a generous gift from Dr. Todd E. Golde (Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA). Analyses were always performed in duplicate and in a coded fashion.
Leukotriene B4 in brain, urine, and conditioned media was measured by an EIA kit (Assay Designs, Ann Arbor, MI, USA) after appropriate lipid extractions. Mouse TNF-α and 8-isoprostaglandin F2α (isoprostane F2α-III) were measured in cortex homogenates by ELISA (Pierce Biotechnology, Rockford, IL, USA) and EIA kits (Assay Designs), respectively.
γ-secretase activity in brain homogenates from 15-month-old Tg2576 × 5LO−/− and Tg2576 control mice was measured by using a kit from R&D Systems (Minneapolis, MN, USA) following the manufacturer’s instructions.
Western blot analyses
RIPA fractions of mouse and human brain homogenates were used for Western blot analyses. Samples were electrophoresed on 7.5% Tris-glycine polyacrylamide gels and transferred to nitrocellulose membranes. For detection of C-terminal fragments, samples were electrophoresed on 16.5% Tris/Tricine polyacrylamide gels. For detection of Aβ peptides in brain lysates, Tris/Tricine/8 M urea gel (12% T/3% C separation gel) was performed as described (30).
Antibodies and dilutions used for Western blot analysis were as follows: anti-Aβ N-terminal (82E1; 2.5 μg/ml; IBL America), anti-APP N-terminal raised against amino acids 66–81 for total APP (22C11; 1:1500; Chemicon International, Temecula, CA, USA), Anti-sAPPβswe (6A1; 2.5 μg/ml, IBL America), Anti-sAPPα (2B3; 2.5 μg/ml; IBL America), anti-GFAP (2.2B10; 1:2000; Invitrogen, Carlsbad, CA, USA), anti-APP C-terminal for CTFs (1:600; EMD Biosciences, La Jolla, CA, USA), anti-APP C-terminal for CTFs (369; 1:600; a generous gift from Dr. Sam Gandy, Thomas Jefferson University, Philadelphia, PA, USA), anti-BACE-1 (1:200; IBL America), anti-IDE N-terminal (1:1000; EMD Biosciences), antineprilysin (1:150; Santa Cruz Biotechnologies, Santa Cruz, CA, USA), anti-apoE (1:100; Santa Cruz Biotechnology), anti-5-lipoxygenase (clone 33; 1:500; BD Biosciences) and anti-β-actin (1:4000; Santa Cruz Biotechnology). HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology and Pierce Biotechnology.
Mouse embryonic fibroblast isolation
To isolate mouse embryonic fibroblasts (MEFs), pregnant female mice were sacrificed 13–14 days after observing vaginal plugs. Uterine horns were dissected out, briefly rinsed in 70% ethanol, and placed into a sterile petri dish containing PBS without bivalent cations. Each embryo was isolated and separated from its placenta and surrounding membranes. The head was separated and used for genotyping. Liver and kidneys were removed, and the remaining body tissue was placed in another sterile petri dish, washed thoroughly with PBS, and minced with crossed scalpels. Tissue was suspended in 1 ml trypsin-EDTA 0.15% and was repeatedly aspirated and expelled with a 1-ml pipette to break up all big pieces. The suspension was incubated at 37°C for 20–30 min and vortex mixed repeatedly. The resulting cell suspension was plated into a 10-cm dish with prewarmed MEF medium (DMEM with high glucose, 10% fetal bovine serum, L-glutamine (200 mM), and penicillin/streptomycin). The culture medium was replaced with fresh DMEM after 24 h. After 2 or 3 passages, MEFs were genotyped again to avoid possible errors in initial genotyping due to maternal tissue contamination. APP × 5LO+/+ and APP × 5LO−/− MEFs were always used for experiments. Leukotriene B4 and Aβ were measured in the media after 30 min and 3 h, respectively, after challenge with A23187. The kit from R&D Systems was used to measure the γ-secretase activity in MEFs, 3 h after the addition of A23187. As positive controls, we tested 2 different γ-secretase inhibitors (L-685,458 and DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester), which were able to suppress the signal to baseline at the concentration of 100 nM when incubated with MEFs. In contrast, GL189, a BACE-1 inhibitor, did not affect the γ-secretase activity, showing the specificity of the assay.
HEK293-C99 cells
HEK293-C99 cells stably transfected with human C-terminal fragment C99 of human APP containing Aβ sequence, were kindly provided by Dr. Robert W. Doms (Department of Microbiology, University of Pennsylvania, Philadelphia, PA, USA). Cells were maintained in DMEM (supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin) containing 200 μg/ml G418. For Aβ measurements, cells were challenged with 5LO metabolites for 8 h and with 5LO inhibitor for 24 h.
Real-time quantitative RT-PCR amplification
RNA isolation and reverse transcription were performed as described previously (12). The experiments were performed using TaqMan Gene Expression Assays consisting of a 20× mix of unlabeled primers and FAM™ (6-carboxyfluorescein) dye-labeled TaqMan MGB probe and performed using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) (12).
Data analysis
Data analyses were performed using SigmaStat for Windows version 3.00. Statistical comparisons between Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice were performed by unpaired Student’s t test or the Mann-Whitney rank sum test when a normal distribution could not be assumed. Comparisons between more than two groups were performed by one-way analysis of variance (ANOVA). Values in all figures represent mean ± SE.
RESULTS
5LO is up-regulated in old Tg2576 mice and in human AD brain
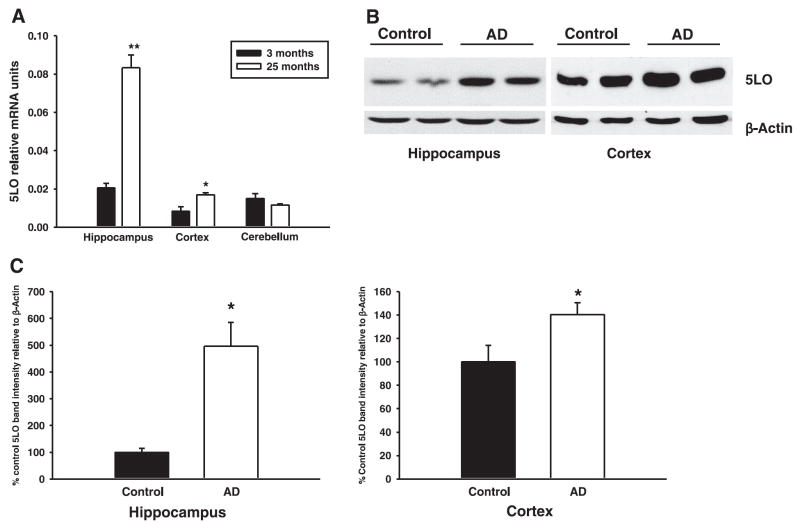
We sought to investigate the changes of 5LO expression levels in aging Tg2576 mice and in humans with AD. 5LO mRNA levels were measured by real-time quantitative RT-PCR amplification in the hippocampus, cortex, and cerebellum of 3- (young) and 24-month-old (old) Tg2576 mice. 5LO message levels were increased in cortex (2.0-fold increase) and especially in hippocampus (4.1-fold increase) of old vs. young Tg2576 mice (Fig. 1A). In contrast, cerebellar levels were not changed. Interestingly, at both ages, mRNA levels in the hippocampus were always higher than the levels in cerebellum and cortex in mice of the same age (Fig. 1A). Finally, 5LO protein levels in the hippocampus and frontal cortex of humans with AD, as determined by Western blot analysis, were significantly increased compared with controls (Fig. 1B, C).
Figure 1.
5LO is up-regulated in old Tg2576 mice and in AD brains. A) Relative mRNA levels of 5LO were measured in separate anatomical regions of the brains of 3- (young) and 24-month-old (old) Tg2576 mice by real-time quantitative RT-PCR amplification (n=3 mice per group). *P < 0.05; **P < 0.001 significant, old vs. young. B) Representative Western blot analysis of the hippocampal and cortical homogenates from control and AD brains probed with a specific antibody against 5LO. C) Densitometric analysis of 5LO immunoreactivity in control and AD brains. Values represent mean ± SE. *P < 0.05, significant vs. control.
Aβ deposition is decreased in the brains of Tg2576 mice deficient for 5LO
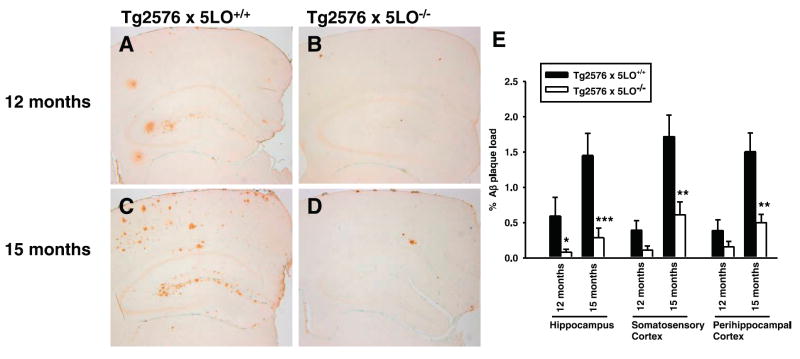
Brain sections of Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice were immunostained with 4G8, an anti-Aβ antibody reactive to amino acid residues 17–24 (Fig. 2A–D), and the immunoreactive area was calculated in three different brain regions: hippocampus, somatosensory, and perihippocampal cortex. At 12 months of age, we observed a significant reduction of Aβ immunoreactivity in the hippocampus of Tg2576 × 5LO−/− mice, when compared with Tg2576 × 5LO+/+ mice. Immunoreactivity in the somatosensory and perihippocampal cortex of the same animals was also decreased, but the difference did not achieve statistical significance (P=0.064 and 0.173, respectively) (Fig. 2A, B, E). In 15-month-old Tg2576 × 5LO−/− mice, Aβ immunoreactivity was significantly decreased in all three brain regions when compared with Tg2576 × 5LO+/+ (Fig. 2C–E). Similar to the younger animals, we found that 15-month-old mice had a more prominent reduction in the hippocampus (80.3%) compared to the somatosensory (64.4%) and perihippocampal cortex (66.8%).
Figure 2.
Genetic ablation of 5LO in Tg2576 mice markedly reduces Aβ deposition in different regions of the brain. A–D) Representative sections of brains of female Tg2576 × 5LO+/+ (A, C) and Tg2576 × 5LO−/− mice (B, D) at 12 and 15 months of age, immunostained with 4G8 antibody. E) Quantification of the area occupied by Aβ immunoreactivity in hippocampus, somatosensory cortex, and perihippocampal cortex in Tg2576 × 5LO−/− (open bars) at 12 (n=8) and 15 months of age (n=10) compared to age-matched Tg2576 × 5LO+/+ mice (solid bars, n=6 and 12, respectively). Values represent mean ± SE. *P < 0.05; **P < 0.01; ***P < 0.005, significant vs. control.
Genetic ablation of 5LO significantly reduces Aβ levels in the brains of Tg2576 mice
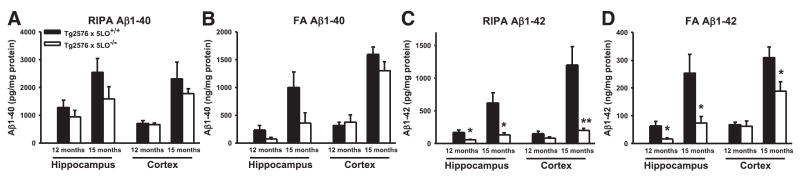
To assess the effect of genetic absence of 5LO on Aβ formation in vivo, we measured its levels in the hippocampus and cortex of Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice by sandwich ELISA. Compared with Tg2576 × 5LO+/+, RIPA-soluble (RIPA) and formic acid extractable (FA) Aβ1–40 levels were slightly but not significantly decreased in the hippocampus and cortex of 12- and 15-month-old Tg2576 × 5LO−/− mice (Fig. 3A, B). In contrast, RIPA and FA Aβ1–42 levels in the hippocampus of 5LO-deficient mice were significantly decreased at both 12 and 15 months (Fig. 3C, D). In the cortex of 5LO-deficient mice, RIPA and FA Aβ1–42 levels were significantly decreased at 15 months but not at 12 months (Fig. 3C, D).
Figure 3.
Aβ1–42, but not Aβ1–40, is significantly decreased in the brains of Tg2576 × 5LO−/− compared to Tg2576 × 5LO+/+ mice. RIPA-soluble (RIPA) and formic acid extractable (FA) Aβ1–40 (A, B) and Aβ1–42 (C, D) levels in the cortex and hippocampus from female Tg2576 × 5LO+/+ (solid bars) and Tg2576 × 5LO−/− mice (open bars) at 12 and 15 months of age were measured by sandwich ELISA. Values represent mean ± SE (n=5 per group for 12-month-olds; n=7–11 per group for 15-month-olds). *P < 0.05; **P < 0.01, significant vs. control.
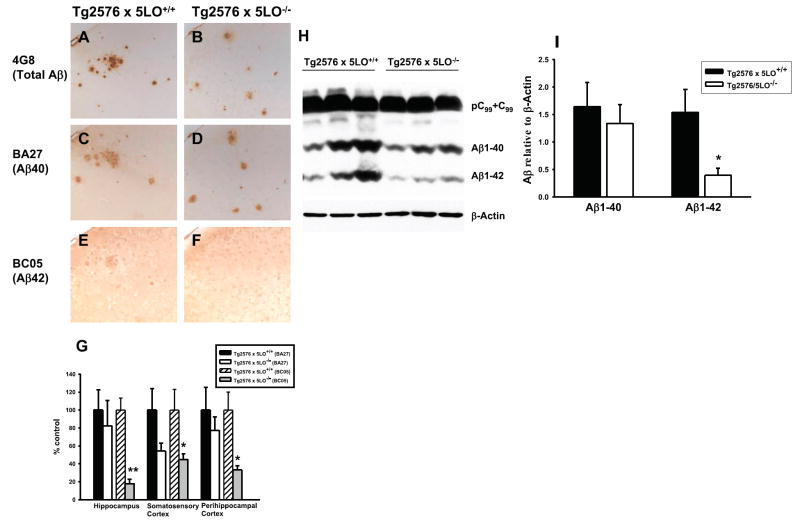
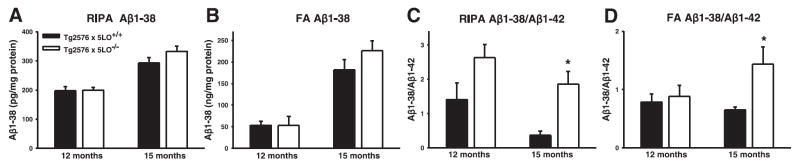
To further investigate the effect of genetic absence of 5LO on specific Aβ peptides, we immunostained brain sections from 15-month-old Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice with BA27 and BC05, two antibodies that specifically recognize Aβ40 and Aβ42, respectively (28). Similar to the data obtained by sandwich ELISA, we observed a slight nonsignificant decrease in Aβ40 and a significant reduction in Aβ42 immunoreactivity in Tg2576 × 5LO−/− mice (Fig. 4A–G). We also measured Aβ1–38 levels in the brains of these mice by sandwich ELISA. RIPA and FA Aβ1–38 levels in the cortex of 15-month-old Tg2576 × 5LO−/− mice were slightly increased, but these differences did not achieve statistical significance (P=0.144 and 0.210, respectively). No difference was observed at 12 months of age between the 2 groups (Fig. 5A, B). However, when we compared the ratio of Aβ1–38:Aβ1–42 between Tg2576 × 5LO+/+ and Tg2576 × 5LO−/−, we found a considerable increase of this ratio in the 5LO-deficient animals, which reached significance at 15 months of age (Fig. 5C, D). The ratio of Aβ1–40: Aβ1–42 was significantly increased in the RIPA extract of the cortex of Tg2576 × 5LO−/− mice at ages of 12 and 15 months (P=0.032 and P=0.004, respectively). However, no significant difference was found between the ratio of Aβ1–40:Aβ1–42 in the FA extract of the cortex between the two groups (data not shown).
Figure 4.
Immunohistochemistry and Western blot analyses confirm that Aβ42 is reduced in the brains of Tg2576 mice lacking 5LO. A–F) Representative sections of cortex from 15-month-old female Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice immunostained for total Aβ (4G8) (A, B), Aβ40 (BA27) (C, D) and Aβ42 (BC05) (E, F). G) Quantification of BA27 and BC05 immunoreactivities in hippocampus, somatosensory cortex, and perihippocampal cortex of Tg2576 mice deficient for 5LO compared to control (n=5–7 per group). Data are expressed as a percentage of control. H) Representative Western blots of cortex homogenates from 15-month-old Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice probed with 82E1 and anti-β-actin antibodies. I) Densitometric analysis of Aβ1–40 and Aβ1–42 immunoreactivities (n=8 per group). Values represent mean ± SE. *P < 0.05; **P < 0.001, significant vs. control.
Figure 5.
The ratio of Aβ1–38:Aβ1–42 is increased in Tg2576 × 5LO−/− compared to Tg2576 × 5LO+/+ mice. A, B) Aβ1–38 was measured in the RIPA (A) and FA (B) fractions of the cortex from female Tg2576 × 5LO+/+ (solid bars) and Tg2576 × 5LO−/− mice (open bars) at 12 (n=5 per group) and 15 months of age (n=6–8 per group) by sandwich ELISA. C, D) The ratio of Aβ1–38:Aβ1–42 in the same mice. Values represent mean ± SE. *P < 0.05, significant vs. control.
We also assayed Aβ levels in the cortex of Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice by Western blot. Densitometric analysis of immunoblots confirmed again the significant reduction of Aβ1–42 in Tg2576 mice deficient for 5LO (Fig. 4H, I). We attempted to measure Aβ1–38 also by immunoblotting, but the levels were below the sensitivity limit of the Western blot and could not be detected (data not shown).
Amyloid precursor protein metabolism, Aβ degrading enzymes, glial fibrillary acidic protein, and apoE in Tg2576 mice deficient for 5LO
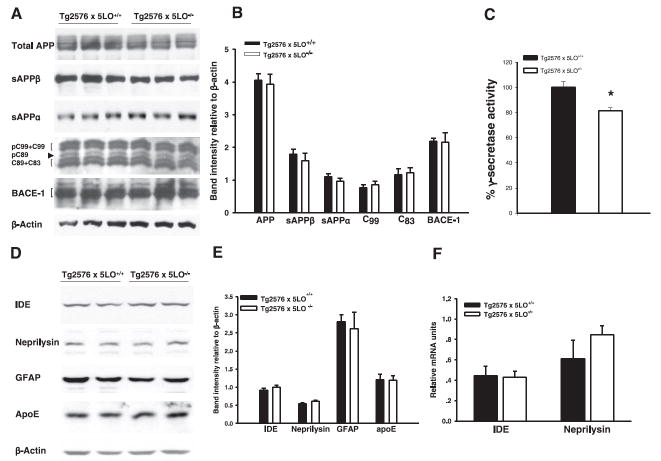
To start investigating the mechanisms responsible for the Aβ reduction in Tg2576 × 5LO−/− mice, we assessed the steady-state levels of APP and its cleavage products in the brains of these mice compared with Tg2576 × 5LO+/+ mice. Total APP was unaltered in Tg2576 mice deficient for 5LO. Similarly, β-secretase pathway represented by the β-site APP cleaving enzyme (BACE-1), secreted APPβ (sAPPβ), and C-terminal fragment-β (CTF-β; C99), did not differ between Tg2576 × 5LO−/− and Tg2576 × 5LO+/+ mice (Fig. 6A, B). 5LO deficiency did not alter α-secretase pathway either, as measured by the secreted APPα (sAPPα) and C-terminal fragment-α (CTF-α; C83) in the Tg2576 mice (Fig. 6A, B).
Figure 6.
APP metabolism mediated by α- and β-secretase is unaltered in Tg2576 mice deficient for 5LO. A) Representative Western blots of APP, its cleavage products (sAPPβ, sAPPα, and C-terminal fragments), and BACE-1 in the cortex of 15-month-old mice. B) Densitometric analyses of the immunoreactivities to the antibodies shown in panel A. C) γ-secretase activity was significantly decreased in brain homogenates from Tg2576 × 5LO−/− (n=3) compared to Tg2576 × 5LO+/+ (n=3). D) Representative Western blots of the cortex homogenates from the 15-month-old mice probed with specific antibodies against Aβ-degrading enzymes, IDE and neprilysin, and against GFAP and apoE. E) Densitometric analyses of the immunore-activities to the antibodies shown in panel C. F) Relative mRNA levels of IDE and neprilysin in the cortex of 15-month-old Tg2576 × 5LO+/+ (solid bars) and Tg2576 × 5LO−/− mice (open bars) as determined by real-time quantitative RT-PCR amplification (n=4 per group). Values represent mean ± SE. *P < 0.05, significant vs. control.
We measured γ-secretase activity in brain homogenates of these mice and found a significantly lower γ-secretase activity in 15-month-old Tg2576 × 5LO−/− compared to age-matched Tg2576 × 5LO+/+ mice (P=0.024) (Fig. 6C).
Next, we analyzed two of the major proteases involved in the catabolism of Aβ, i.e., insulin-degrading enzyme (IDE) and neprilysin (31). Protein levels of IDE and neprilysin in the cortex measured by Western blot were similar between 15-month-old Tg2576 × 5LO−/− and Tg2576 × 5LO+/+ mice (Fig. 6D, E). We quantified also the mRNA levels of these enzymes by real-time quantitative RT-PCR amplification and did not find any significant change between the two groups of animals (Fig. 6F). Searching for other possible mechanisms accountable for the Aβ pathology amelioration, we measured apoE and glial fibrillary acidic protein (GFAP) levels in these animals. As shown in Fig. 6D, E, we found no difference between Tg2576 × 5LO+/+ and Tg2576 × 5LO−/− mice for any of these proteins.
We also measured markers of inflammation and oxidative stress in the cortex of 5LO-deficient and control Tg2576 mice. TNF-α, a marker of inflammation, in Tg2576 × 5LO−/− (17.9±1.7 pg/mg protein) was not significantly different from Tg2576 × 5LO+/+ (19.1±1.9 pg/mg protein) (n=7 per group). Similarly, levels of the isoprostane F2α-III, a marker of oxidative stress, in Tg2576 × 5LO−/− (13.8±0.6 pg/mg tissue) and Tg2576 × 5LO+/+ mice (18.7±2.6 pg/mg tissue) were not significantly different (n=9 per group).
In vitro activation of 5LO increases Aβ levels and γ-secretase activity
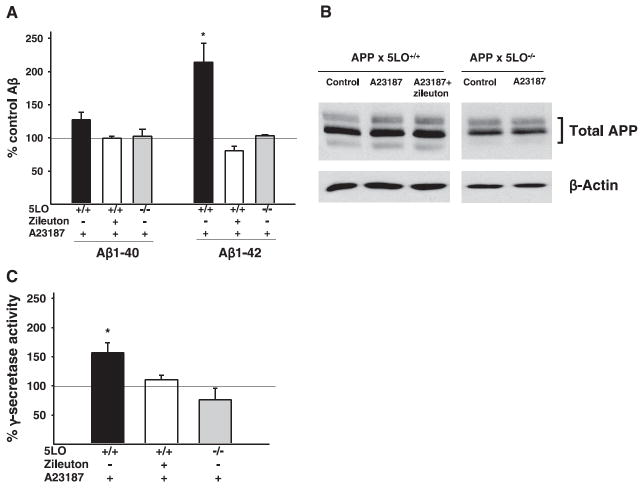
Next, we investigated the effect of 5LO activation on Aβ formation in MEFs with and without 5LO. The activation of 5LO was achieved by adding the Ca2+ ionophore A23187, which is known to be a potent activator of this enzyme (32). Enzyme activation was monitored by measuring leukotriene B4, a major 5LO metabolite in the conditioned media. As expected, A23187 induced a significant increase in leukotriene B4 in APP × 5LO+/+ but not in APP × 5LO−/− MEFs, and this effect was blocked in the presence of zileuton, a selective 5LO inhibitor (data not shown). Activation of 5LO was accompanied by a significant increase in Aβ levels (Fig. 7A). In contrast, when APP × 5LO−/− MEFs were challenged with A23187, no change in Aβ levels was observed, supporting the fact that 5LO is required for the observed effect of A23187 (Fig. 7A). Under our experimental conditions, A23187 did not induce any change in APP levels (Fig. 7B). Interestingly, γ-secretase activity was significantly increased after 5LO activation in APP × 5LO+/+ and the presence of zileuton was able to block this effect. No change in γ-secretase activity was observed in 5LO knockout MEFs (Fig. 7C).
Figure 7.
Activation of 5LO in MEFs by Ca2+ ionophore A23187 results in an increase in the γ-secretase activity and Aβ formation. A) 5LO enzyme was activated in APP × 5LO+/+ and APP × 5LO−/− MEFs by adding A23187 (100 nM) in the presence or absence of zileuton, and Aβ peptides secreted in the medium were measured. Data are expressed as percentage of control. Control for each genotype consisted of MEFs with the same genotype not treated with A23187 or zileuton. A23187 is present in all conditions except for control. Values represent mean ± SE of 3 experiments. B) Representative Western blots of total APP measured in cell lysates of APP ×5LO+/+ and APP × 5LO−/− MEFs. C) γ-secretase activity was measured in the harvested APP × 5LO+/+ and APP × 5LO−/− MEFs. *P < 0.05, significant vs. control.
5LO metabolites increase and 5LO inhibitors decrease Aβ secretion in HEK293-C99 cells
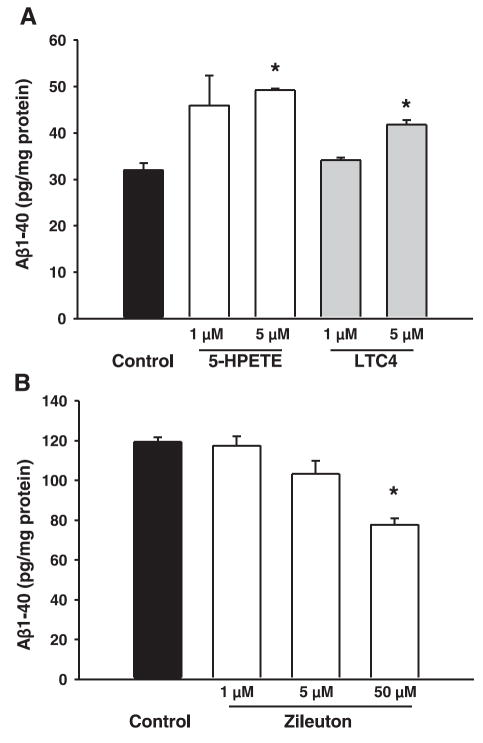
To further support the hypothesis that amyloidogenic effect of 5LO is mediated through γ-secretase, we used HEK293 cells stably expressing human APP C99, the direct substrate for γ-secretase. Incubation with two different 5LO metabolites, 5-hydroperoxyeicosatetraenoic acid (5-HPETE), and leukotriene C4 (LTC4) at the concentration of 5 μM induced significant increases in Aβ1–40 (Fig. 8A). On the other hand, when these cells were incubated with zileuton, a significant decrease in Aβ1–40 was observed (Fig. 8B). Aβ1–42 levels showed a small but nonsignificant increase after metabolite challenge (data not shown). GL189 (EMD Biosciences), a BACE-1 inhibitor, did not induce any change in Aβ secretion, confirming that γ-secretase is the main source of Aβ in these cells (data not shown).
Figure 8.
5LO metabolites increase and 5LO inhibitor decreases Aβ1–40 production in HEK293-C99 cells. A) HEK293-C99 cells were challenged with two different 5LO metabolites, 5-HPETE and LTC4. B) The same cells were treated with a 5LO inhibitor, zileuton (n=3 per condition). *P < 0.05, significant vs. control.
We also measured γ-secretase activity in these cells and found that it was significantly increased already after treatment with 1 μM 5-HPETE (P=0.020) and had a trend toward an increase with 1 μM LTC4 (P=0.13). By contrast, γ-secretase activity was significantly decreased after treatment with 5 μM zileuton (P=0.028) (data not shown).
DISCUSSION
The data presented in this study demonstrate that genetic disruption of 5LO results in a significant reduction of Aβ deposition and steady-state levels in the brain of the Tg2576 mouse model of AD and thereby provide the first evidence that 5LO might represent a novel target for modulating amyloidogenesis in AD.
Reports from our laboratory and another group have demonstrated that 5LO expression is strongly increased in aging rats and mice, especially in the hippocampus area, which is more susceptible to neurodegeneration (12, 33). In the present study, we showed that 5LO is up-regulated in old Tg2576 mice compared to young ones. Taking into account that aging is one of the strongest risk factors for AD development, these data provide an initial support for a possible link between 5LO and this disease. In line with these observations, we found that this enzyme is up-regulated in AD brains compared to controls.
In Tg2576 × 5LO−/− mice, total Aβ immunoreactivity to the 4G8 antibody was remarkably decreased compared with Tg2576 × 5LO+/+ controls. From our data, it appears that the rate of Aβ deposition was considerably slowed, because 15-month-old Tg2576 × 5LO−/− mice showed only a slight increase in amyloid plaque deposition compared to the 12-month-old group.
In our study, we observed that 5LO modulatory effect on Aβ manifests also some region dependency in the brain. Thus, both effects on Aβ deposition and Aβ reduction in 5LO-knockout mice were greater in the hippocampus than in the cortex. These data can be explained by previous findings in rodents, which show that 5LO expression in the hippocampus is higher than in the cortex, especially in older animals (12-14). Confirming these previous reports, in the current study we indeed observed that 5LO mRNA levels were higher in the hippocampus of young Tg2576 mice, and there was also a more robust age-associated increase in mRNA levels in this area compared to the cortex.
We performed extensive experiments with three different techniques to investigate the effect of the absence of 5LO on Aβ peptide levels. Thus, all of the data obtained with sandwich ELISA, immunohistochemistry with C-terminal-specific antibodies, and Western blot consistently pointed to the fact that the reducing effect on Aβ42 levels was always stronger than on Aβ40 levels in Tg2576 mice deficient for 5LO. The fact that significant lowering of Aβ42 alone resulted in a remarkable reduction of amyloid plaque deposition in the brain of these animals is not surprising after all, since the central role of Aβ42 in Aβ deposition has been demonstrated in several studies (34-36).
The significant reduction of Aβ42 and the increase in the ratio of Aβ1–38:Aβ1–42 in 5LO knockout mice would suggest the involvement of γ-secretase (37, 38). Furthermore, the absence of any significant change in sAPPα, sAPPβ, CTF-α, CTF-β, and BACE1 in the 5LO-deficient Tg2576 mice indicates that the involvement of α- and β-secretases is unlikely and is consistent with the hypothesis of an involvement of the γ-secretase. Thus, measurement of γ-secretase activity in brain homogenates of these animals confirmed that there was a significant reduction in the activity of this enzyme in the Tg2576 mice genetically deficient for 5LO, when compared with Tg2576 controls.
In line with the hypothesis of γ-secretase being the mechanism of Aβ reduction in 5LO knockout mice, the activation of 5LO enzyme in APP × 5LO+/+ MEFs led to an increase in γ-secretase activity. This effect could not be induced in APP × 5LO−/−, and could be reversed in APP × 5LO+/+ MEFs in the presence of zileuton, a selective 5LO inhibitor, indicating that 5LO is required for this effect on γ-secretase.
In another set of experiments, we used HEK293 cells that stably express C99 to further study the influence of 5LO on the γ-secretase complex. C99 is the precursor of Aβ and the immediate substrate for γ-secretase (1); therefore, these cells provide a good model to study this pathway. In these cells, 5LO metabolites induced an increase, and the 5LO inhibition induced a decrease, in Aβ formation and γ-secretase activity, further supporting the involvement of this secretase in the amyloidogenic effect of 5LO. However, differently from the in vivo studies, we did not find a stronger effect on Aβ1–42 compared with Aβ1–40. This dissimilarity can be explained by the rather complex structure of γ-secretase and the fact that different factors can considerably alter the response of this complex, depending on the in vivo vs. the in vitro system used. Different γ-secretase machinery in various species could also be another reason for these differences; in fact, data generated in MEFs obtained from Tg2576 mice, closely mimicked the findings in these mice.
We also sought to rule out other mechanisms that might be responsible for the reduction in Aβ levels in 5LO-deficient Tg2576 mice, such as Aβ-degrading enzymes, including neprilysin and IDE and also apoE alterations. In our study, no significant change was observed in either the protein or mRNA levels. We also assessed markers of inflammation and oxidative stress in Tg2576 × 5LO−/− and Tg2576 × 5LO+/+ and did not find any significant difference. This finding suggests that the antiamyloidogenic effect observed in these mice is not secondary to an anti-inflammatory or anti-oxidative effect resulting from the genetic absence of 5LO.
In conclusion, our studies unveil for the first time a novel functional role for 5LO enzyme in AD-like amyloidosis, since genetic absence of 5LO significantly reduces Aβ pathology in Tg2576 mice. We also provide several lines of evidence suggesting that this effect is mediated through the modulation of the γ-secretase pathway. Thus, 5LO could provide a novel and important target for the purpose of lowering Aβ pathology in AD.
Acknowledgments
This work was funded by the U.S. National Institutes of Health, grants AG22203, AG008051, and AG15408. The authors wish to thank Dr. V. M. Lee and Dr. J. Q. Trojanowski for their invaluable help and advice throughout this research. We thank Drs. T. E. Golde, S. Gandy, and R. W. Doms for providing antibodies and cells and Dr. K. Townsend for assistance in setting up the MEF experiments.
References
- 1.Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 4.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 5.Yermakova AV, O’Banion MK. Downregulation of neuronal cyclooxygenase-2 expression in end stage Alzheimer’s disease. Neurobiol Aging. 2001;22:823–836. doi: 10.1016/s0197-4580(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 6.Sung S, Yang H, Uryu K, Lee EB, Zhao L, Shineman D, Trojanowski JQ, Lee VM, Pratico D. Modulation of nuclear factor-kappa B activity by indomethacin influences A beta levels but not A beta precursor protein metabolism in a model of Alzheimer’s disease. Am J Pathol. 2004;165:2197–2206. doi: 10.1016/s0002-9440(10)63269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firuzi O, Pratico D. Coxibs and Alzheimer’s disease: should they stay or should they go? Ann Neurol. 2006;59:219–228. doi: 10.1002/ana.20774. [DOI] [PubMed] [Google Scholar]
- 9.Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 10.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mhatre M. The role of the 5-lipoxygenase pathway in Alzheimer’s disease. Drugs Future. 2006;31:83–89. [Google Scholar]
- 12.Chinnici CM, Yao Y, Pratico D. The 5-lipoxygenase enzymatic pathway in the mouse brain: Young versus old. Neurobiol Aging. 2006;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- 14.Lammers CH, Schweitzer P, Facchinetti P, Arrang JM, Madamba SG, Siggins GR, Piomelli D. Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J Neurochem. 1996;66:147–152. doi: 10.1046/j.1471-4159.1996.66010147.x. [DOI] [PubMed] [Google Scholar]
- 15.Morris AA, Rodger IW. Leukotrienes and the brain. Lancet. 1998;352:1487–1488. doi: 10.1016/S0140-6736(05)60322-4. [DOI] [PubMed] [Google Scholar]
- 16.Piomelli D. Eicosanoids in synaptic transmission. Crit Rev Neurobiol. 1994;8:65–83. [PubMed] [Google Scholar]
- 17.Manev H, Uz T, Qu T. Early upregulation of hippocampal 5-lipoxygenase following systemic administration of kainate to rats. Restor Neurol Neurosci. 1998;12:81–85. [PubMed] [Google Scholar]
- 18.Simmet T, Tippler B. Cysteinyl-leukotriene production during limbic seizures triggered by kainic acid. Brain Res. 1990;515:79–86. doi: 10.1016/0006-8993(90)90579-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Wei EQ, Fang SH, Chu LS, Wang ML, Zhang WP, Yu GL, Ye YL, Lin SC, Chen Z. Spatio-temporal properties of 5-lipoxygenase expression and activation in the brain after focal cerebral ischemia in rats. Life Sci. 2006;79:1645–1656. doi: 10.1016/j.lfs.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Drazen JM, Yandava CN, Dube L, Szczerback N, Hippen-steel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, Drajesk J. Pharmacogenetic association between ALOX5 promoter genotype and the response to antiasthma treatment. Nat Genet. 1999;22:168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- 21.Qu T, Manev R, Manev H. 5-Lipoxygenase (5-LOX) promoter polymorphism in patients with early-onset and late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:304–305. doi: 10.1176/jnp.13.2.304. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 23.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 24.Pratico D, Lee VMY, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 25.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 29.Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 30.Yagishita S, Morishima-Kawashima M, Tanimura Y, Ishiura S, Ihara Y. DAPT-induced intracellular accumulations of longer amyloid beta-proteins: further implications for the mechanism of intramembrane cleavage by gamma-secretase. Biochemistry. 2006;45:3952–3960. doi: 10.1021/bi0521846. [DOI] [PubMed] [Google Scholar]
- 31.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 32.Burkert E, Szellas D, Radmark O, Steinhilber D, Werz O. Cell type-dependent activation of 5-lipoxygenase by arachidonic acid. J Leukoc Biol. 2003;73:191–200. doi: 10.1189/jlb.0702354. [DOI] [PubMed] [Google Scholar]
- 33.Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurobiol Aging. 2000;21:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 34.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 35.Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 38.Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]