Abstract
Background
The mechanisms causing age-dependent changes in left ventricular (LV) structure and function are not completely understood. Matrix metalloproteinase (MMPs) and tissue inhibitors (TIMPs) constitute one important proteolytic pathway affecting LV remodeling. However, whether these determinants of ECM composition change as a function of age has not been examined in an aging population free of clinically significant cardiovascular disease.
Methods and Results
Subjects (n=77, age 20–90 years) with no evidence of cardiovascular disease underwent echocardiography and measurement of plasma MMP-2,-7,-8,-9 and TIMP-1,-2,-4 (ELISA). As subject age increased, volume/mass ratio decreased and mitral E/A ratio decreased. As subject age increased, MMP-2 increased (from 1188±99 to 1507±76 ng/mL), MMP-7 increased (from 1.2±0.1 to 3.1±0.6 ng/mL), MMP-9 decreased (from 29±7 to 8±2 ng/mL), TIMP-1,-2 and -4 increased (from 728±46 to 1093±73, from 34±5 to 53±6 and from 1.26±0.22 to 2.34±0.30 ng/mL, respectively), all p < 0.05. There were significant correlations between a decreased LV volume/mass and E/A ratio and increased MMP-7, and TIMP-1 and -4.
Conclusions
MMPs and TIMPs changed as a function of age in the absence of clinically significant cardiovascular disease. These age-dependent alterations in MMP & TIMP profiles favor extracellular matrix accumulation and were associated with concentric remodeling and decreased LV diastolic function. Because of these age-dependent changes in this proteolytic system, the superimposition of a disease processes such as myocardial infarction or hypertensive heart disease in the older subject may result in different myocardial ECM remodeling then that seen in a younger subject.
Keywords: Extracellular Matrix, Matrix Metalloproteinase, Age
Introduction
Left ventricular (LV) structural remodeling, such as changes in LV mass, volume, and geometry, are important predictors of functional and clinical outcomes (1–4). Advancing age, independent of any concurrent cardiovascular disease, can itself be associated with significant LV structural remodeling (5–8). These age-dependent changes in LV structure may play an important role in the functional limitations which occur in advancing age (5,7). However, the pathophysiologic mechanisms by which advancing age cause structural remodeling are not completely understood. This study will focus on one potential pathophysiologic mechanism of LV remodeling, changes in extracellular matrix (ECM) regulatory proteases. The matrix metalloproteinases (MMPs), and their tissue inhibitors, the TIMPs, constitute one important proteolytic pathway which can affect remodeling of the left ventricle by altering cardiomyocyte and/or ECM structure and composition (9–13). MMPs degrade fibrillar proteins, activate biologically important molecules, and modulate ECM remodeling (9,10,12–19). TIMPs inhibit active MMPs as well as modify other growth regulatory effects (9,10,12,15,20). Myocardial MMP and TIMPs levels are reflected in and can be determined by using plasma sampling (21–23). Therefore, the purpose of this study was to test the hypothesis that MMP and TIMP plasma profiles change as a function of age and are associated with age-dependent structural and functional LV remodeling in patients with advancing age but without coexistent, clinically significant, cardiovascular disease.
Methods
Subjects
Seventy-seven subjects were prospectively enrolled in this cross-sectional study which examined the effect of age on MMP/ & TIMP profiles. Informed consent was obtained from all subjects. The criteria for enrollment included men and women greater than 18 years of age without evidence of clinically significant cardiovascular disease. Potential subjects were carefully screened ruling out subjects with co-morbid conditions that would alter plasma MMP and TIMP profiles. All subjects underwent a complete medical history, comprehensive physical exam, electrocardiogram (ECG), and echocardiogram. Subjects were excluded from enrollment if they had evidence of coronary heart disease: previous history or ECG evidence of myocardial infarction, previous or planned coronary revascularization (surgery or percutaneous coronary intervention), LV ejection fraction < 50%, echocardiographic evidence of a regional wall motion abnormality, left heart catheterization showing coronary artery disease, or an abnormal exercise tolerance test. Subjects were also excluded if they had evidence of hypertensive heart disease: left ventricular hypertrophy determined echocardiographically as LV wall thickness of > 1.2 cm and/or LV mass index ≥ 125g/m2, or current evidence of arterial hypertension in which blood pressure was not pharmacologically treated to meet the Joint National Committee VII criteria for therapeutic control (i.e., < 140/90 mmHg) (24). Subjects were excluded if they had other cardiovascular disease states such as amyloidosis, sarcoidosis, genetic hypertrophic obstructive cardiomyopathy, valvular heart disease, ventricular or atrial arrhythmias. Finally, subjects were excluded if they had non-cardiovascular processes that alter MMP & TIMP levels: history of active malignancy in the past 3 years, ongoing or active rheumatological disease requiring significant anti-inflammatory agents, steroids, or immunosuppression, significant hepatic or renal dysfunction, HIV, or significant history of substance abuse.
The research protocol used in this study was reviewed and approved by the institutional review board at the Medical University of South Carolina.
Echocardiography
All echocardiograms were performed by one experienced sonographer (CM) using a Sonos 5500 system with an S-4 MHz transducer. Measurements were made using American Society of Echocardiography criteria (25,26). Two-dimensional echocardiographic studies of the left ventricle included the following views: parasternal long and short axis, apical 4- and 2- chamber, 2D derived M-mode images from the parasternal short axis. Doppler-echocardiographic mitral valve inflow velocities were recorded from an apical view with a cursor at the tips of the mitral valve leaflets. Tissue Doppler velocities were recordings from the lateral mitral annulus. Left ventricular end diastolic and end systolic volumes were calculated using the method of discs (26). Ejection fraction was calculated using the standard formula. Left ventricular mass was calculated using the formula of Reichek and Devereux (27). Doppler measurements of mitral inflow E wave velocity, A wave velocity, and E wave deceleration time were made. Tissue Doppler measurement of mitral E′ was made. Pulmonary capillary wedge pressure (PCWP) was calculated using the formula: 2 + 1.3(E/E′) (28). An average of 3 beats was used for every measurement. Images were coded and read in a blinded fashion.
MMP and TIMP Profiles
The MMP & TIMP species selected for this study were chosen because they have been previously identified in animal and clinical studies to be associated with myocardial matrix remodeling (18,21–23,29–32). MMPs can be categorized into functional/structural classes which include the interstitial collagenases (MMP-1,-8,-13), the gelatinases (MMP-2,-9), the stromelysins/matrilysins (MMP-3,-7), and membrane-type MMPs. Representative MMPs from most of these MMP classes were measured. Previous studies have shown that myocardial MMP-1 levels are reduced in patients with LV remodeling (29,33). In preliminary studies, we examined plasma MMP-1 in a subset of the study cohort. MMP-1 levels in these subjects were below acceptable detectable limits for this assay. These preliminary studies also revealed a low incidence of detectable plasma levels of the interstitial collagenase MMP-13. Therefore, these MMP species (MMP-1 and MMP-13) were not examined in the current study. However, the neutrophil associated collagenase MMP-8 was within acceptable detection limits and was measured in the current study. MMP-2 and MMP-9 were studied from the gelatinase class. MMP-7, matrilysin, a unique MMP type, (shown to be increased in models of myocardial infarction) was selected for the current study (33,34). The membrane-type MMPs (MT-MMPs) are a transmembrane bound class of MMPs, therefore plasma measurement would likely be problematic and were not pursued in the current study. TIMP-1 and TIMP-2 are the most extensively studied TIMPs, have been shown to change in a robust fashion in the myocardial tissue in animal models and clinical examples of cardiac disease, and therefore were selected for the current study (9,10,12,15,20). In addition, a recently developed and validated method provided the means to measure the cardiovascular specific TIMP-4 (50,51). TIMP-4 is expressed only in the heart and aorta, thus provided cardiovascular specificity to the plasma measurements made in the current study. All patients fasted overnight prior to each study, but took their morning medications as prescribed. All studies were performed between 9 AM and 12 PM.
All MMP & TIMP measurements were made using a high sensitivity two-site enzyme-linked immunosorbent assay (ELISA; Amersham Pharmacia Biotech, Buckinghamshire, UK) from a 5 cc blood sample. Briefly, blood was collected after the subject had remained supine for 20 minutes. Samples were immediately centrifuged and the plasma layer removed. The separated plasma was divided into 3 equal aliquots and frozen at -80°C. Plasma and the respective MMP standards were added to precoated wells containing the antibody to the MMP or TIMP of interest and washed. The resultant reaction was read at wavelength of 450 nm (Labsystems Multiskan MCC/340, Helsinki, Finland). The MMP-2 assay (Amersham, RPN 2617) detects the proform of MMP-2 and that complexed with TIMP-2. The MMP-9 assay (Amersham, RPN 2614) detects the proform of the enzyme that is complexed with TIMP-1. The MMP-8 assay system (Amersham, RPN 2619) detects the proform and active form. The MMP-7 assay (R&D Systems; DMP700) detects the proform and active form. The TIMP-1 assay (Amersham, RPN 2611) detects both free TIMP-1 and that complexed with MMPs. The TIMP-2 assay (Amersham, RPN 2618) detects both free TIMP-2 and that complexed with active MMPs. All samples were analyzed in duplicate and averaged. The intra-assay coefficient of variation for these measurements was less than 6%.
A semiquantitative immunoblot assay to assess plasma TIMP-4 levels was initially developed (50, 51). Then, an ELISA method was developed and validated in normal subjects and patients following a myocardial infarction (51). This ELISA, with a high sensitivity (0.005 ng/mL) and high specificity (no cross reactivity with other TIMPs or proteases), was utilized in the current study (R&D Systems, MN). This assay measured both free and bound TIMP-4 with high linearity (r2= 0.95) over a wide range of TIMP-4 standards (0.078-5.0 ng/mL). This ELISA was also cross-calibrated and validated utilizing the semiquantitative immunoblot assay described by this laboratory previously (51).
Statistical Analysis
The distribution of measurements derived from echocardiograms and plasma measurements of MMPs and TIMPs was tested for normality based on tests of skewness and kurtosis. Data which were not normally distributed were log transformed. Analysis of non normal parameters using both log transformed and untransformed values yielded similar results. Therefore, all data are presented in an untransformed manner. The subject sample was divided for analysis into quintiles, five sub-groups each with an equal number of participants. Differences between individual quintiles were determined using a one-way analysis of variance (ANOVA) followed by pair-wise comparison using Tukey’s method. For all continuous variables, a simple linear regression and a parametric correlation analysis (Pearson’s coefficient, r) were used to examine the relationship between age (measured as a continuous variable) and MMP & TIMP plasma levels and LV structure and function. The association between age (measured in quintiles) and plasma MMP & TIMP values and LV structure and function were evaluated using a non-parametric rank-order correlation analysis (Spearman’s coefficient, rho ρ), the non-parametric analog of Pearson’s correlation coefficient. Univariate analyses with an analysis of variance (ANOVA) were used to examine the effects of age on MMPs, TIMPs, indices of LV structure and function. Multivariate analyses with a two-way ANOVA were used to examine the effects of age, gender, medications or age/gender interaction (on MMPs, TIMPs, indices of LV structure and function) after controlling for the effects of the other covariates. A p value of < 0.05 was considered significant. All values are presented as the mean and standard error of the mean (SEM). All statistical procedures were performed utilizing STATA statistical software (StataCorp, Rel 8.0, College Station, TX).
Results
Demographics, LV Structure and Function (Table 1)
Table 1.
| Table 1 A. Age Dependent changes with Left Ventricular Structure and Function | |||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Demographics | |||||
| Number | 15 | 16 | 16 | 15 | 15 |
| Male/Female | 2/13 | 8/8 | 6/10 | 5/10 | 6/9 |
| Age range(years) | 22–29 | 31–48 | 49–56 | 57–64 | 68–90 |
| Systolic BP(mmHg) | 117±2 | 116±3 | 128±3* | 128±4* | 127±4* |
| Diastolic BP(mmHg) | 69±2 | 73±3 | 77±2 | 75±2 | 73±3 |
| LV Geometry | |||||
| Mass (grams) | 125±4 | 137±8 | 149±8 | 148±10 | 147±8 |
| End Diastolic Volume(mL) | 102±6 | 103±4 | 98±4 | 95±5 | 91±5 |
| Vol/Mass ratio(mL/gram) | 0.82±0.03 | 0.78±0.04 | 0.67±0.03* | 0.66±0.02* | 0.64±0.04* |
| IVSd (cm) | 0.80±0.02 | 0.87±0.03 | 0.94±0.04* | 0.93±0.04* | 0.97±0.04* |
| LVPWd (cm) | 0.81±0.02 | 0.84±0.03 | 0.95±0.03* | 0.95±0.03* | 0.94±0.04* |
| Mean WT (cm) | 0.80±0.02 | 0.86±0.03 | 0.95±0.03* | 0.94±0.03* | 0.95±0.04* |
| Relative wall thickness | 0.34±0.02 | 0.36±0.01 | 0.42±0.02* | 0.42±0.01* | 0.42±0.02* |
| LV Function | |||||
| Ejection Fraction(%) | 65±2 | 66±1 | 65±1 | 67±1 | 65±1 |
| Ees(mmHg/mL) | 2.5±0.2 | 2.6±0.2 | 2.9±0.2 | 3.2±0.3 | 3.1±0.2 |
| Mitral E wave(cm/sec) | 87±4 | 73±4* | 64±3* | 60±3* | 59±4* |
| Mitral A wave(cm/sec) | 54±3 | 54±3 | 70±5* | 68±2* | 73±4* |
| Mitral E/A ratio | 1.7±0.1 | 1.4±0.1 | 1.0±0.1* | 0.9±0.1* | 0.8±0.1* |
| E′(cm/sec) | 16.5±2.5 | 12.5±1.3 | 10.5±0.6* | 9.9±0.5* | 8.8±0.6* |
| PCWP(mmHg) | 9.9±0.8 | 8.6±0.5 | 10.3±0.6 | 10.1±0.5 | 10.8±0.6 |
| PCWP/EDV(mmHg/mL) | 0.08±0.02 | 0.09±0.01 | 0.11±0.01 | 0.11±0.04 | 0.12±0.01* |
| IVRT(msec) | 77±4 | 78±4 | 77±4 | 81±5 | 88±4 |
| Ea (mmHg/mL) | 1.4±0.08 | 1.4±0.08 | 1.5±0.09 | 1.5±0.07 | 1.6±0.11 |
| Left atrial dimension(cm) | 3.1±0.1 | 3.2±0.1 | 3.4±0.1 | 3.3±0.5 | 3.6±0.2 |
| Table 1 B. Age Dependent changes with Left Ventricular Structure and Function (No hypertensive patients) | |||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Demographics | |||||
| Number | 15 | 16 | 10 | 10 | 12 |
| Male/Female | 2/13 | 8/8 | 4/6 | 3/7 | 3/9 |
| Age range(years) | 22–29 | 31–48 | 49–56 | 57–64 | 68–90 |
| Systolic BP(mmHg) | 117±2 | 116±3 | 130±4* | 127±5* | 126±4* |
| Diastolic BP(mmHg) | 69±2 | 73±3 | 77±3 | 75±3 | 71±2 |
| LV Geometry | |||||
| Mass (grams) | 125±4 | 137±8 | 146±9 | 147±10 | 146±8 |
| End Diastolic Volume(mL) | 102±6 | 103±4 | 99±4 | 96±5 | 92±5 |
| Vol/Mass ratio(mL/gram) | 0.82±0.03 | 0.78±0.04 | 0.65±0.03* | 0.67±0.03* | 0.65±0.04* |
| IVSd (cm) | 0.80±0.02 | 0.87±0.03 | 0.94±0.04* | 0.93±0.04* | 0.97±0.04* |
| LVPWd (cm) | 0.81±0.02 | 0.84±0.03 | 0.95±0.03* | 0.95±0.03* | 0.94±0.04* |
| Mean WT (cm) | 0.80±0.02 | 0.86±0.03 | 0.95±0.03* | 0.94±0.03* | 0.95±0.04* |
| Relative wall thickness | 0.34±0.02 | 0.36±0.01 | 0.42±0.02* | 0.42±0.01* | 0.42±0.02* |
| LV Function | |||||
| Ejection Fraction(%) | 65±2 | 66±1 | 65±1 | 66±1 | 66±1 |
| Ees(mmHg/mL) | 2.5±0.2 | 2.6±0.2 | 2.9±0.2 | 3.2±0.3 | 3.1±0.2 |
| Mitral E wave(cm/sec) | 87±4 | 73±4* | 63±3* | 59±3* | 60±4* |
| Mitral A wave(cm/sec) | 54±3 | 54±3 | 67±5* | 67±2* | 71±3* |
| Mitral E/A ratio | 1.7±0.1 | 1.4±0.1 | 1.0±0.1* | 0.9±0.1* | 0.8±0.1* |
| E′(cm/sec) | 16.5±2.5 | 12.5±1.3 | 10.5±0.6* | 9.9±0.5* | 8.8±0.6* |
| PCWP(mmHg) | 9.9±0.8 | 8.6±0.5 | 9.8±0.5 | 10.0±0.4 | 11.0±0.5 |
| PCWP/EDV(mmHg/mL) | 0.08±0.02 | 0.09±0.01 | 0.11±0.01 | 0.11±0.04 | 0.12±0.01* |
| IVRT(msec) | 77±4 | 78±4 | 77±4 | 81±5 | 88±4 |
| Ea (mmHg/mL) | 1.4±0.08 | 1.4±0.08 | 1.5±0.09 | 1.5±0.07 | 1.6±0.11 |
| Left atrial dimension(cm) | 3.1±0.1 | 3.2±0.1 | 3.4±0.1 | 3.3±0.5 | 3.6±0.2 |
Abbreviations: BP = Blood Pressure, LV = Left Ventricular, Ees = end systolic elastance, Mitral E wave = peak early diastolic filling, Mitral A wave = peak late diastolic filling, E′ = peak early diastolic velocity of the lateral mitral annulus, PCWP = pulmonary capillary wedge pressure, EDV = end diastolic volume, IVRT = isovolumic relaxation time, Ea = effective arterial elastance,
= p < 0.05 vs. Quintile #1, Data are mean ± SEM.
The study participants ranged in age from 20 to 90 years, and included 50 females and 27 males. There was a small, statistically significant increase in systolic blood pressure as a function of increasing age. All resting blood pressure values fell below the definition for hypertension established by The Joint National Committee VII (<140/90 mmHg) (24). However, ambulatory and exercise blood pressures were not measured. There was a significant decrease in the LV volume to mass ratio and an increase in the relative wall thickness as a function of age; these changes were indicative of concentric LV remodeling. The mitral E/A ratio fell significantly as a function of age, indicative of impaired ventricular filling. Furthermore, as age increased from 20 to 90 years, Tissue Doppler E′ decreased (from 16.5±2.5 to 9.6±0.04 cm/sec), mitral E wave deceleration time increased (from 194±9 to 239±17 msec), both p < 0.05. In addition to these changes in indices of active relaxation, there was a trend toward changes in indices of passive stiffness. As age increased from 20 to 90 years, the ratio between calculated pulmonary capillary wedge pressure and end diastolic volume (an index of instantaneous end diastolic operative chamber stiffness) increased (from 0.08±0.01 to 0.12±0.01 mmHg/mL), p < 0.05.
Effects of age on MMP and TIMP plasma profiles (Tables 2 & 3)
Table 2.
| Table 2 A. Age Dependent changes in plasma MMP and TIMP profiles | |||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Gelatinases | |||||
| MMP-2 | 1188±99 | 1218±88 | 1287±63 | 1400±78 | 1507±76* |
| MMP-9 | 29±7 | 13±4 | 11±2 | 11±9 | 8±2* |
| Matrilysin | |||||
| MMP-7 | 1.2±0.09 | 1.4±0.15 | 2.3±0.37* | 2.3±0.18* | 3.1±0.6* |
| Neutrophil Collagenase | |||||
| MMP-8 | 2.3±0.4 | 3.2±0.9 | 2.1±0.2 | 4.2±1.8 | 1.9±0.3 |
| Tissue Inhibitor of MMPs | |||||
| TIMP-1 | 728±46 | 910±40 | 924±58 | 1006±76* | 1093±73* |
| TIMP-2 | 34±5 | 50±14 | 33±5 | 44±7 | 53±6* |
| TIMP-4 | 1.26±0.22 | 1.35±0.13 | 1.68±0.15 | 1.77±0.16 | 2.34±0.30* |
| MMP/TIMP Ratios | |||||
| MMP-2/TIMP-1 | 1.73±0.18 | 1.39±0.13 | 1.45±0.09 | 1.50±0.13 | 1.46±0.12 |
| MMP-2/TIMP-4 | 1199±207 | 992±112 | 905±58 | 849±109 | 744±69* |
| MMP-9/TIMP-1 | 0.041±0.009 | 0.015±0.004 | 0.014±0.003 | 0.012±0.007 | 0.008±0.002* |
| MMP-9/TIMP-4 | 23.6±4.2 | 10.0±2.6 | 8.4±2.0 | 8.0±4.0 | 5.0±1.7* |
| Table 2 B. Age Dependent changes in plasma MMP and TIMP profiles (No hypertensive patients) | |||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Gelatinases | |||||
| MMP-2 | 1188±99 | 1218±88 | 1284±61 | 1366±65 | 1510±80* |
| MMP-9 | 29±7 | 13±4 | 9±2 | 8±9 | 7±2* |
| Matrilysin | |||||
| MMP-7 | 1.2±0.09 | 1.4±0.15 | 2.0±0.17* | 2.3±0.20* | 2.8±0.6* |
| Neutrophil Collagenase | |||||
| MMP-8 | 2.3±0.4 | 3.2±0.9 | 2.1±0.2 | 3.2±1.0 | 1.8±0.3 |
| Tissue Inhibitor of MMPs | |||||
| TIMP-1 | 728±46 | 910±40 | 940±63 | 1058±86* | 1097±61* |
| TIMP-2 | 34±5 | 50±14 | 33±5 | 44±7 | 53±6* |
| TIMP-4 | 1.26±0.22 | 1.35±0.13 | 1.76±0.15 | 1.84±0.16 | 2.30±0.30* |
| MMP/TIMP Ratios | |||||
| MMP-2/TIMP-1 | 1.73±0.18 | 1.39±0.13 | 1.45±0.09 | 1.40±0.13 | 1.51±0.12 |
| MMP-2/TIMP-4 | 1199±207 | 992±112 | 905±58 | 845±109 | 725±51* |
| MMP-9/TIMP-1 | 0.041±0.009 | 0.015±0.004 | 0.011±0.003 | 0.010±0.007 | 0.007±0.001* |
| MMP-9/TIMP-4 | 23.6±4.2 | 10.0±2.6 | 6.7±1.6 | 4.8±1.0 | 3.7±0.8* |
Abbreviations: MMP = Matrix Metalloproteinase, TIMP = Tissue Inhibitor of MMP, all MMP/TIMP values are in ng/ml units,
= p < 0.05 vs. Quintile #1, Data are mean ± SEM.
Table 3.
Correlation between age, volume/mass (mL/gram), and Mitral E/A ratio with plasma MMP & TIMP levels
| Age (quintile) |
Age (continuous) |
LV volume/mass (mL/gram) |
Mitral E/A (ratio) |
|
|---|---|---|---|---|
| MMP-2 | 0.35 (<0.01) | 0.14 (<0.01) | −0.10 (0.34) | −0.30 (<0.01) |
| MMP-7 | 0.58 (<0.01) | 0.30 (<0.01) | −0.38 (<0.01) | −0.53 (<0.01) |
| MMP-8 | −0.07 (0.55) | 0.001 (0.9) | 0.03 (0.83) | 0.08 (0.51) |
| MMP-9 | −0.34 (<0.01) | 0.04 (0.07) | 0.19 (0.10) | 0.24 (0.04) |
| TIMP-1 | 0.43 (<0.01) | 0.23 (<0.01) | −0.32 (<0.01) | −0.42 (<0.01) |
| TIMP-2 | 0.32 (<0.01) | 0.22 (<0.01) | −0.03 (0.78) | −0.22 (0.06) |
| TIMP-4 | 0.49 (<0.01) | 0.32 (<0.01) | −0.21 (0.07) | −0.44 (<0.01) |
Abbreviations: LV = Left Ventricular, MMP = Matrix Metalloproteinase, TIMP = Tissue Inhibitor of MMP, all MMP/TIMP values are in ng/ml units. Data were analyzed using a Spearman’s correlation coefficient (ρ) except for “age (continuous)” which was analyzed using a Pearson’s correlation coefficient (r), p value for each correlation coefficient is in parenthesis.
MMP-2 increased and MMP-9 decreased as a function of age. There was no significant change in MMP-8 as a function of age. MMP-7 increased significantly as a function of increasing age. TIMP-1,-2 and -4 all increased significantly as a function of increasing age. The relationship between MMP and TIMP plasma profiles and age was further examined with age expressed in quintiles using a non-parametric correlation analysis (Spearman’s coefficient, ρ) and with age expressed as a continuous variable using a parametric correlation analysis (Pearson’s coefficient, r) (Table 3). There were significant positive correlations between MMP-2, MMP-7, TIMP-1, -2, and -4 and age as well as a significant negative correlation between MMP-9 and age.
The overall proteolytic potential, as reflected by the MMP/TIMP ratios, is an important determinant of ECM degradative capacity. Accordingly, selected MMP/TIMP ratios were examined as a function of age (Table 2). These data suggest that the MMP/TIMP ratios, and therefore net MMP activity, were lower in the older subjects compared to younger subjects suggesting a decreased ECM degradative capacity. In particular, the MMP-9/TIMP-1, MMP-9/TIMP-4 and MMP-2/TIMP-4 ratios all decreased as subject age increased.
Data presented in Table 1A and Table 2A were redone after excluding the patients receiving antihypertensive medications and are presented as Table 1B and 2B. In each case, there are minor numerical changes but no changes to the statistical analysis, results, or conclusions.
Correlation between MMP & TIMP plasma profiles and LV structure and function (Table 3)
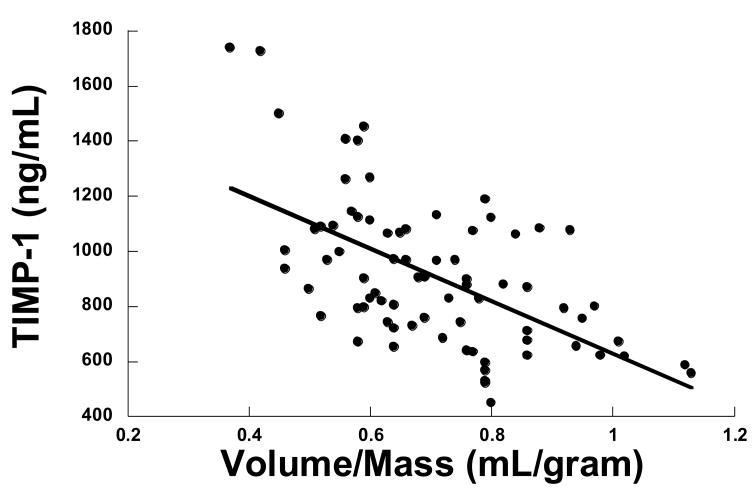
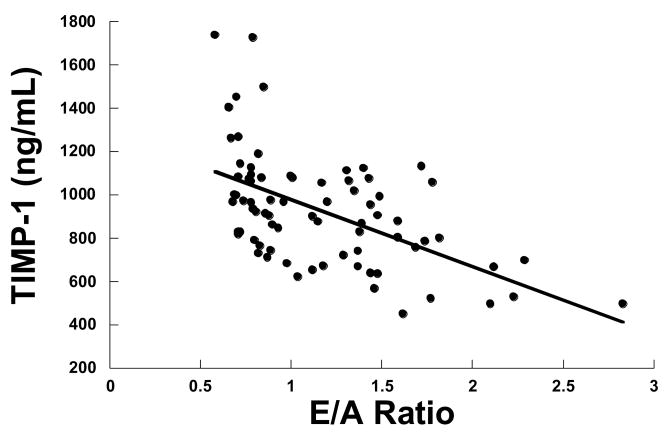
There were significant correlations between the volume to mass ratio and selective MMP & TIMP plasma levels (Table 3, Figure 1). Specifically, there was a significant negative correlation between MMP-7 and LV volume/mass ratio, such that as MMP-7 increased the volume/mass ratio decreased. There was a negative correlation between TIMP-1,-4 and volume/mass ratio, such that as TIMP-1,-4 increased the volume/mass ratio decreased. These findings suggested that concentric LV remodeling was associated with increased MMP-7, TIMP-1 and TIMP-4. In addition, there were significant correlations between plasma MMP & TIMP profiles and the mitral E/A ratio (Table 3, Figure 2). Specifically, there was a significant negative correlation between MMP-7 and mitral E/A ratio, such that as MMP-7 increased the mitral E/A ratio decreased. There was a negative correlation between TIMP-1,-4 and mitral E/A ratio, such that as TIMP-1,4 increased the mitral E/A ratio decreased. These findings suggested that slowing of active relaxation was associated with increased MMP-7 and TIMP-1.-4. While these univariate analyses suggest a relationship between MMP & TIMP plasma levels and volume/mass and E/A ratios, there was also a significant relationship between increasing age and these indices of LV structure and function. Therefore, multivariate analyses were performed in which MMP and TIMPs were added as covariates in a model examining E/A and volume/mass versus age, gender and medications. In this multivariate analysis, the effect of age predominated in predicting changes in the E/A ratio, less then 50% of the variance in the E/A ratio could be explained based on the MMP and TIMP changes alone, suggesting that other age-dependent determinants played a significant role in the changes in LV structure and function which occurred. Similar results were found for volume/mass ratio.
Figure 1.
Relationship between TIMP-1 and the left ventricular (LV) volume to mass ratio. As TIMP-1 increased the volume/mass ratio decreased (y = − 434x + 1244, p = 0.02, r = 0.3)
Figure 2.
Relationship between TIMP-1 and the mitral E/A ratio. As TIMP-1 increased the E/A ratio decreased (y = −196x +1161, p = 0.001, r = 0.4)
Relationships between plasma MMP/TIMP, medications, gender, and age/gender interaction (Tables 4 & 5)
Table 4.
Gender dependent changes in plasma MMP and TIMP profiles
| Men | Women | All | p-value | |
|---|---|---|---|---|
| Age (yrs) | 52 ± 3 | 48 ± 2 | 49 ± 2 | 0.361 |
| MMP-2 | 1352 ± 58 | 1300 ± 49 | 1319 ± 37 | 0.512 |
| MMP-7 | 2.3 ± 0.4 | 1.9 ± 0.2 | 2.0 ± 0.2 | 0.814 |
| MMP-8 | 4.8 ± 2.2 | 3.3 ± 0.9 | 3.8 ± 1.0 | 0.128 |
| MMP-9 | 16.5 ± 6.3 | 19.4 ± 3.8 | 18.3 ± 3.3 | 0.948 |
| TIMP-1 | 1058 ± 54 | 867 ± 37 | 934 ± 29 | 0.001 |
| TIMP-2 | 43 ± 6 | 43 ± 5 | 43 ± 4 | 0.895 |
| TIMP-4 | 1.8 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.903 |
Abbreviations: MMP = Matrix Metalloproteinase, TIMP = Tissue Inhibitor of MMP, all MMP/TIMP values are in ng/ml units, Data are mean ± SEM. The differences in the mean values for men vs women were compared using a Student’s T test.
Table 5.
Multivariate Analyses Examining the Effects of Age on MMPs and TIMPs
| Age | Gender | Medications | Age/Gender Interaction | |
|---|---|---|---|---|
| MMP-2 | 0.04 | 0.56 | 0.39 | 0.44 |
| MMP-7 | 0.0001 | 0.51 | 0.38 | 0.006 |
| MMP-8 | 0.24 | 0.30 | 0.44 | 0.39 |
| MMP-9 | 0.02 | 0.38 | 0.59 | 0.67 |
| TIMP-1 | 0.0009 | 0.012 | 0.02 | 0.05 |
| TIMP-2 | 0.28 | 0.77 | 0.61 | 0.33 |
| TIMP-4 | 0.003 | 0.98 | 0.43 | 0.52 |
| Vol/Mass (mL/gm) | 0.0001 | 0.19 | 0.45 | 0.03 |
| RWT | 0.0007 | 0.36 | 0.85 | 0.16 |
| Mitral E/A | 0.0001 | 0.18 | 0.57 | 0.46 |
| E′ (cm/sec) | 0.0004 | 0.14 | 0.44 | 0.78 |
Abbreviations: MMP = Matrix Metalloproteinase, TIMP = Tissue Inhibitor of MMP, all MMP/TIMP values are in ng/ml units, Vol= left ventricular volume, RWT = relative wallthickness, Mitral E/A ratio = peak early diastolic filling (Mitral E) vs peak late diastolic filling (Mitral A) velocity ratio, E′ = peak early diastolic velocity of the lateral mitral annulus,
No subjects were excluded from the current study because of their medication profiles. By study design, in each subject, medications were unchanged for a minimum of 90 days prior to study enrollment. Fifteen (19%) of the study population were taking anti-hypertensive medications as directed by their primary care physicians including diuretics, β-Blockers, Angiotensin Receptor Blockers, and Angiotensin Converting Enzyme Inhibitors. There were nine (12%) subjects taking statins, and six (8%) taking anti-inflammatories (COX-2 inhibitors). There were no significant relationships between medication profiles and LV geometry/function or MMP & TIMP plasma profiles. When the subjects taking medications were analyzed separately, they had normal values for plasma MMP and TIMP and normal LV structure and function. In addition, when the subjects taking medications were excluded from analysis, the results of the study did not differ significantly. Finally, a multivariate analysis was constructed which specifically examined the effect of medications on MMPs, TIMPs, and LV structure/function (Table 5). In this multivariate analysis, MMPs and TIMPs, indices of LV structure and function were examined with covariates of age, gender, medications and age/gender interactions. The data listed in Table 5 present the probability (p value) that an individual covariate (age, gender, medication or age/gender interaction) had an independent effect on MMPs, TIMPs, indices of LV structure or function. These analyses indicate that the most important determinant of MMPs, TIMPs, indices of LV structure or function was age. Therefore, these findings demonstrate that medications did not have an independent effect on MMPs, TIMPs, indices of LV structure or function.
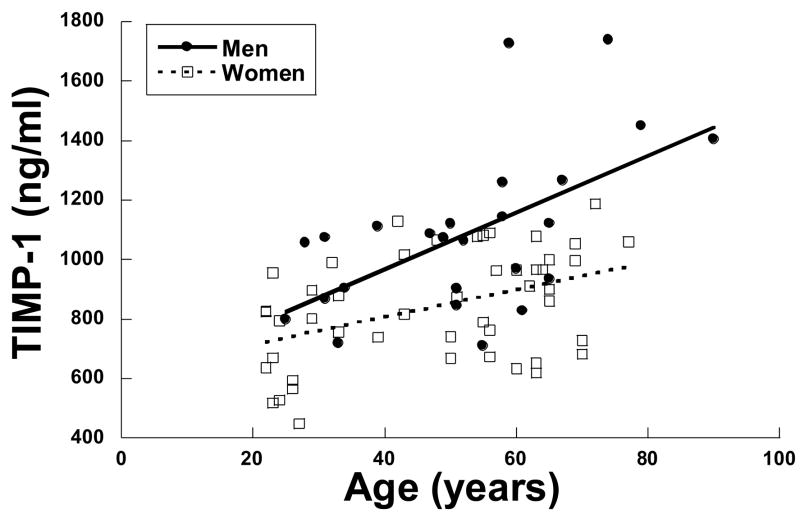
The effects of gender on MMP & TIMP profiles are presented in Tables 4 and 5 and Figure 3. For the group as a whole, there were no differences between men and women in MMP-2, MMP-7, MMP-8, and MMP-9. In addition, there were no differences in TIMP-2 or TIMP-4. In all of these measurements, women tended to have a somewhat lower value than men, however, this difference did not reach statistical significance. On the other hand, there were significantly lower values of TIMP-1 in women than men. This difference was present in the univariate analysis shown in Table 4 and in the multivariate analysis shown in Table 5. Based on this difference, TIMP-1 was examined in women and men as a function of age as a continuous variable shown in Figure 3. It was clear that TIMP-1 increased with age in both men and women, however, this increase was significantly more robust in men than women. These data must be interpreted with caution however because of the small sample size.
Figure 3.
Relationship between TIMP-1 and age for men vs. women examined using an analysis of covariates. TIMP-1 increased in both men and woman as a function of increasing age. However, the slope of the TIMP-1 vs. age relationship in men (y= 9.5x + 587, r= 0.6) was significantly steeper then that for women (y= 4.6x + 622, r= 0.4), p < 0.05.
Relationships between plasma MMPs, TIMPs and systolic BP (Table 6)
Table 6.
Correlation between Systolic Blood Pressure and Plasma MMP and TIMP levels
| p | r | r2 | |
|---|---|---|---|
| MMP-2 | 0.35 | 0.11 | 0.01 |
| MMP-7 | 0.04 | 0.24 | 0.06 |
| MMP-8 | 0.23 | 0.14 | 0.02 |
| MMP-9 | 0.09 | 0.20 | 0.04 |
| TIMP-1 | 0.001 | 0.40 | 0.16 |
| TIMP-2 | 0.05 | 0.23 | 0.05 |
| TIMP-4 | 0.20 | 0.15 | 0.02 |
Abbreviations: MMP = Matrix Metalloproteinase, TIMP = Tissue Inhibitor of MMP. Data were analyzed using a Pearson’s coefficient with (p-value), (r), (r2) listed for each variable tested.
The relationship between systolic BP and MMP and TIMP values was examined using a simple linear regression and a parametric correlation analysis (Pearson’s coefficient, r). There was a weak but statistically significant relationship between TIMP-1 and systolic BP such that higher systolic BP values were associated with higher TIMP-1 values. There were no significant relationships between the other MMP and TIMP species and systolic blood pressure.
Discussion
Data presented in the current study support two unique conclusions. First, both MMPs and TIMPs changed as a function of age in the absence of clinically significant cardiovascular disease. Second, changes in these proteolytic determinants of ECM composition were associated with age-dependent alterations in LV structure and function. In particular, MMP & TIMP profiles which favor extracellular matrix accumulation were associated with concentric remodeling and decreased LV diastolic function. Because of these age-dependent changes in this proteolytic system, the superimposition of a disease processes such as myocardial infarction or hypertensive heart disease in the older subject may result in different myocardial ECM remodeling then that seen in a younger subject. It should be noted however, that the current study used a cross-sectional design. This design imposed significant limitations. While generating correlative hypotheses relating age and MMP & TIMP profiles, this study did not establish a true mechanistic link between age, changes in MMPs, TIMP, and LV structure/function. Such mechanistic links can only be defined using longitudinal ageing studies of sufficient size and duration.
MMPs
The MMPs comprise a family of zinc-dependent enzymes which proteolytically degrade ECM structural proteins and process biologically active molecules including other MMPs. While pleotropic in their substrates and actions, changes in myocardial MMPs have predictable effects on ECM composition.
MMP-2 is a gelatinase which degrades the basement membrane proteins collagen IV, fibronectin, and laminin (14,15,32,35). It also degrades fibrillar collagen peptides (collagen fibrils that had been partially but incompletely degraded) (32,35). MMP-2 degrades newly synthesized collagen fibers (prior to lysyl oxidase induced cross-linking).
MMP-9, also a gelatinase, has a very low catalytic capacity for structural proteins (32,35). While MMP-9 processes similar structural proteins to that of MMP-2, this occurs at a lower biochemical/catalytic efficiency. However, MMP-9 is involved in the proteolytic activation of other important biologically active proteins/peptides such as TGF-β, and other “pro-fibrotic” proteins and pathways (12,18,32,35,36). Increased MMP-2 would be anticipated that the lead to increased ECM degradation, decreased MMP-9 would be expected to lead to decreased synthesis of ECM proteins. However, the overall proteolytic potential of MMPs is reflected by the MMP/TIMP ratios, therefore the “net MMP activity” is the important determinant of ECM degradative capacity. The data presented in this study suggest that the MMP/TIMP ratios, and therefore net MMP activity, were lower in the older subjects compared to younger subjects suggesting a decreased ECM degradative capacity.
MMP-7 is a matrilysin and has a low catalytic capacity for ECM structural proteins. But, MMP-7 has the widest portfolio of biologically active proteins/peptide substrates of any MMP. These substrates include matrikines such as osteopontin, thrombospondin, and TGF-β, all pro-fibrotic in nature (32–35,38). Increased MMP-7 would be expected to contribute to changes in ECM composition by activating profibrotic proteins/peptides which increase fibrillar collagen. In the current study MMP-7 was increased as a function of age, and in fact, increased before changes in other MMP species.
TIMPs
In addition to changes in MMPs, the current study demonstrated age-dependent changes in TIMPs. MMP activity is regulated at several levels that not only includes transcriptional regulation, but also includes post-translational modification such as TIMP binding (10,20,32,39). The TIMPs bind to active MMPs in a 1:1 stoichiometric relationship, inhibit MMP enzymatic activity and thereby form an important control point with respect to net ECM proteolytic activity (9,10,18,20,39). The current study showed that plasma levels of TIMP-1,-2 and -4 increased with age. As a result, the stoichiometric balance between MMPs and TIMPs was altered in favor of reduced ECM proteolytic activity which would therefore facilitate ECM accumulation. In addition to binding to active MMP sites, TIMPs possess other biological functions (15,20,39). For example, TIMPs can influence myocardial growth regulation (15). Increased TIMPs would be expected to promote cardiomyocyte and LV hypertrophy. These structural changes in turn can lead to changes in diastolic function.
Therefore, changes in relative MMP and TIMP levels will not only directly alter ECM turnover and structure, but would likely alter other determinants of ECM homeostasis. For example, on the one hand, MMPs are able to degrade the ECM structural proteins (52); on the other hand, they are capable of activating ECM signaling molecules such as matrix bound TGF-β, a potent profibrogen, contributing to collagen synthesis by myocardial fibroblasts (53). In addition, in addition to inhibiting active MMPs, TIMPs, especially TIMP-1, may also regulate proliferation of and collagen production by fibroblasts (54). These data emphasize the importance of profiling the entire cassette of TIMPs in the context of myocardial remodeling
While MMPs and TIMPs were the specific focus of the current study, it is also clear that they are not the only determinants of LV structure and function that change with increasing age. These determinants include changes in cardiac muscle cell processes such as calcium homeostasis, energetics, myofilament and extra myofilament proteins, changes in the level of neurohumoral activation, and changes in the vasculature (7,40). It is likely that each of these determinants, both individually and in concert, play a critical role in age-dependent structural and functional remodeling. This conclusion is supported, at least indirectly, by the data presented in the current study. For example, using a univariate analysis, it was shown that increased MMP-7 and TIMP-1,-4 correlated significantly with decreased E/A ratio. However, when age was added in a multivariate analysis, the effect of age predominated in predicting changes in the E/A ratio, less then 50% of the variance in the E/A ratio could be explained based on the MMP and TIMP changes alone, suggesting that other age-dependent determinants played a significant role in the changes in LV structure and function which occurred. Similar results were found for volume / mass ratio. However, these analyses must be interpreted with caution because these multivariate analyses contained a number of covariates which varied in a collinear fashion with age (or at least created redundancy and overlap). Therefore, these analyses neither prove nor disprove a causal mechanistic link between MMPs, TIMPs and LV structure/function. None-the-less, it is clear that MMPs and TIMPs change significantly with increasing age. Whether there is a true mechanistic link between age, changes in MMPs, TIMPs and LV structure/function must await future longitudinal ageing studies of sufficient size and duration.
Previously Published Studies
To our knowledge, only three previous studies have examined the effects of age on MMPs & TIMPs (41–43). However, the current study is the first to study a well defined population with no evidence of clinically significant CV disease, to examine a broad range of MMP & TIMP species, and to relate changes in MMPs & TIMPs to LV structure and function. There is significant discordance between current and previous studies. For example, Tayebjee et al. found no changes in MMP-2, MMP-9 and decreased TIMP-1 and TIMP-2 with increasing age (41). In contrast, in the Framingham study, Sundstrom et al. found an increase in TIMP-1 with increasing age (42). Some of this variability may be based on the methods used to make the plasma measurements. For example, some studies used ELISA methods while other studies used zymography and reverse-zymography to measure MMP & TIMP plasma levels. Zymography is an elecrophoretic separation technique in which a substrate is incorporated into the electrophoretic gel (44). While this method has been successfully employed in tissue extracts, there are some limitations to this approach, particularly for plasma samples. First, a number of binding proteins exist within the plasma, such as alpha 2-macroglobulin, which covalently bind MMPs and thereby make their extraction from plasma samples difficult (45). Second, plasma protein binding of MMPs and TIMPs will yield multiple bands on zymography and reverse-zymography which make quantification and accurate identification of specific MMP and TIMP types problematic (46). Third, the zymographic approach does not readily yield an absolute MMP or TIMP value, but rather yields relative comparative values (47). In the current study, a validated and calibrated high sensitivity ELISA provided a quantitative means to determine total concentration of a specific MMP or TIMP. As a consequence, direct comparisons between current and previous studies regarding MMP and TIMP levels must be done with caution.
In addition to technical differences, it is likely that some of the variability in MMP & TIMP values between studies were based on differences in the patient populations themselves. The patients examined in the current study had no evidence of clinically significant CV diseases by history, physical exam, ECG, echocardiogram, or chest x-ray. However, they did not undergo exercise tolerance testing, left heart catheterization, or other cardiac diagnostic testing. Patient examination was directed by the patients own physician and not the investigators. None-the-less, exclusion criteria prevented inclusion of clinically significant coronary heart disease, hypertensive heart disease, valvular heart disease, congenital heart disease, heart failure, or general cardiomyopathy. If coronary artery disease was present it is unlikely to contribute to changes in MMP and TIMP levels since these patients had no evidence of active myocardial ischemia or myocardial infarction. The data from the current study provide age-dependent baseline parameters which will allow future studies to define the effects of cardiovascular disease in an aging population.
Plasma MMP & TIMP Profiles
The current study utilized plasma levels of MMPs and TIMPs as surrogate markers to reflect changes in myocardial levels of these enzymes and peptides. However, there are several limitations to this approach that must be acknowledged. First, MMP activation and TIMP binding is a compartmentalized process that occurs within the myocardial interstitium (9,10,16). Thus, plasma levels do not necessarily reflect the net ECM proteolytic activity that occurs within the myocardium. Virtually the only way to directly and serially assess MMP and TIMP levels within the myocardium is to perform multiple LV biopsies. This approach is obviously problematic. Fortunately, data from previous clinical and experimental studies suggest that the differences in plasma MMP and TIMP levels observed in the current study are likely to reflect differences at the myocardial level (21–23). Thus, while plasma levels of MMPs and TIMPs are an indirect measure of the local myocardial ECM proteolytic processes, there are likely to reflect the relative protein abundance within the myocardium. A second limitation to plasma sampling of MMPs and TIMPs is that it is possible that the myocardium is not the only source of MMPs and TIMPs in the subjects examined in this study. Therefore, measurements of plasma MMP and TIMP levels represent the summation of MMPs and TIMPs released from both cardiac as well as non-cardiac sources. While, the specific exclusion criteria utilized in the current study helped to eliminate significant changes in the major non-cardiac sources of MMPs and TIMPs, not all potential non-cardiovascular sources could be excluded. For example, we did not calculate, estimate, or measure creatinine clearance. It should be recognized that creatinine clearance decreases with age and that impaired creatinine clearance may influence any surrogate plasma marker of ECM remodeling.. In addition, ageing may affect non cardiovascular organs as it does cardiovascular organs. These age-related changes may contribute to plasma levels of MMPs & TIMPs. Therefore, a new ELISA was developed and verified to quantify plasma measurements of cardiovascular specific TIMP-4. This new assay provides cardiovascular specificity to plasma measurements. TIMP-4 is only expressed in the heart and aorta; therefore, plasma measurements reflect changes limited to the cardiovascular system. These data support the conclusions that plasma profiles of MMPs and TIMPs change as function of age in the absence of clinically significant cardiovascular disease. Never the less, it must be recognized that subjects may have changes in other non-cardiac tissues, such as the kidneys and the vasculature that may contribute to MMP and TIMP release into the plasma. It is clearly recognized however, that the findings of the current study demonstrating age-dependent differences in plasma MMP and TIMP levels are associative findings. Whether these changes are mechanistically related to age associated myocardial remodeling and ECM accumulation warrant further study.
Acknowledgments
Support This study was supported by the Research Service of the Department of Veterans Affairs (MR Zile, FG Spinale), the National Heart, Lung, and Blood Institute Grants PO1-HL-048788 (MR Zile, FG Spinale), RO1-HL-059165 (FG Spinale), and MO1-RR-01070-251 (MR Zile, FG Spinale).
Footnotes
Conflict of Interest Disclosures None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elhendy A, Modesto KM, Mahoney DW, Khandheria BK, Seward JB, Pellikka PA. Prediction of mortality in patients with left ventricular hypertrophy by clinical, exercise stress, and echocardiographic data. J Am Coll Cardiol. 2003;41:129–135. doi: 10.1016/s0735-1097(02)02667-0. [DOI] [PubMed] [Google Scholar]
- 2.McMurray J, Pfeffer MA. New therapeutic options in congestive heart failure: Part I. Circulation. 2002;105:2099–2106. doi: 10.1161/01.cir.0000014763.63528.9d. [DOI] [PubMed] [Google Scholar]
- 3.Gaballa MA, Goldman S. Ventricular remodeling in heart failure. J Card Fail. 2002;8:S476–S485. doi: 10.1054/jcaf.2002.129270. [DOI] [PubMed] [Google Scholar]
- 4.Udelson JE, Konstam MA. Relation between left ventricular remodeling and clinical outcomes in heart failure patients with left ventricular systolic dysfunction. J Card Fail. 2002;8:S465–S471. doi: 10.1054/jcaf.2002.129289. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 6.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90:1231–1236. doi: 10.1016/s0002-9149(02)02840-0. [DOI] [PubMed] [Google Scholar]
- 7.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 8.Ganau A, Saba PS, Roman MJ, de Simone G, Realdi G, Devereux RB. Ageing induces left ventricular concentric remodeling in normotensive subjects. J Hypertens. 1995;13:1818–1822. [PubMed] [Google Scholar]
- 9.Chapman RE, Spinale FG. Extracellular protease activation and unraveling of the myocardial interstitium: critical steps toward clinical applications. Am J Physiol Heart Circ Physiol. 2004;286:H1–H10. doi: 10.1152/ajpheart.00609.2003. [DOI] [PubMed] [Google Scholar]
- 10.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 11.Singh RB, Dandekar SP, Elimban V, Gupta SK, Dhalla NS. Role of proteases in the pathophysiology of cardiac disease. Mol Cell Biochem. 2004;263:241–256. doi: 10.1023/B:MCBI.0000041865.63445.40. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- 13.Janicki JS, Brower GL, Gardner JD, Chancey AL, Stewart JA., Jr The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev. 2004;9:33–42. doi: 10.1023/B:HREV.0000011392.03037.7e. [DOI] [PubMed] [Google Scholar]
- 14.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 15.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 16.Spinale FG. Bioactive peptide signaling within the myocardial interstitium and the matrix metalloproteinases. Circ Res. 2002;91:1082–1084. doi: 10.1161/01.res.0000047874.80576.5a. [DOI] [PubMed] [Google Scholar]
- 17.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunasinghe SK, Ikonomidis J, Spinale FG. Contributory role of matrix metalloproteinases in cardiovascular remodeling. Curr Drug Targets Cardiovasc Haematol Disord. 2001;1:75–91. doi: 10.2174/1568006013337953. [DOI] [PubMed] [Google Scholar]
- 19.Dollery CM, McEwan JR, Henney AM. Matrix Metalloproteinases and Cardiovascular Disease. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 20.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 21.Bradham WS, Gunasinghe H, Holder JR, Multani M, Killip D, Anderson M, Meyer D, Spencer WH, 3rd, Torre-Amione G, Spinale FG. Release of matrix metalloproteinases following alcohol septal ablation in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2002;40:2165–2173. doi: 10.1016/s0735-1097(02)02595-0. [DOI] [PubMed] [Google Scholar]
- 22.Wilson EM, Gunasinghe HR, Coker ML, Sprunger P, Lee-Jackson D, Bozkurt B, Deswal A, Mann DL, Spinale FG. Plasma matrix metalloproteinase and inhibitor profiles in patients with heart failure. J Card Fail. 2002;8:390–398. doi: 10.1054/jcaf.2002.129659. [DOI] [PubMed] [Google Scholar]
- 23.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, Crawford FA, Spinale FG. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001;71:1518–1523. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ the National High Blood pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 25.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 26.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 29.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ., Jr A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 30.Peterson JT, Hallak H, Johnson L, Li H, O’Brien PM, Skliskovic DR, Bocan TMA, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 31.Mann DL, Spinale FG. Activation of matrix metalloproteinases in the failing human heart: breaking the tie that binds. Circulation. 1998;98:1699–1702. doi: 10.1161/01.cir.98.17.1699. [DOI] [PubMed] [Google Scholar]
- 32.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 33.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, Jr, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 34.Wielockx B, Libert C, Wilson C. Matrilysin (matrix metalloproteinase-7): a new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev. 2004;15:111–115. doi: 10.1016/j.cytogfr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 37.Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001;122:1739–1756. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 38.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- 39.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 40.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 41.Tayebjee MH, Lip GY, Blann AD, Macfadyen RJ. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res. 2005;115:205–210. doi: 10.1016/j.thromres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PWF, Vasa RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PWF, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 44.Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 45.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 46.Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 47.Barrett AJ. Alpha 2-macroglobulin. Methods Enzymol. 1981;80(Pt C):737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- 48.Stroud RE, DesChamps AM, Lowry AS, Harden AE, Mukherjee R, Lindsey ML, Ramamoorthy S, Zile MR, Spencer WH, Spinale FG. Plasma monitoring of the myocardial specific tissue inhibitor of metalloproteinase-4 after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Journal of Cardiac Failure. 2005;11:124–130. doi: 10.1016/j.cardfail.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) Journal of Biological Chemistry. 1997;33:20479–20483. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- 50.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–80. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 51.Stroud RE, Deschamps AM, Lowry AS, Hardin AE, Mukherjee R, Lindsey ML, Ramamoorthy S, Zile MR, Spencer WH, Spinale FG. Plasma monitoring of the myocardial specific tissue inihibitor of metalloproteinase-4 after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. J Card Fail. 2005 Mar;11(2):124–30. doi: 10.1016/j.cardfail.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004 Nov 16;110(20):3221–8. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 53.Mookerjee I, Unemori EN, DU XJ, Tregear GW, Samuel CS. Relaxin modulates fibroblast function, collagen production, and matrix metalloproteinase-2 expression by cardiac fibroblasts. Ann N Y Acad Sci. 2005 May;1041:190–3. doi: 10.1196/annals.1282.028. [DOI] [PubMed] [Google Scholar]
- 54.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288(2):H461–8. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]