Abstract
In earlier studies, we found profound alterations in specific signal transduction pathways such as mitogen-activated protein kinase signal pathway that mirrored neuronal cell death in Alzheimer disease (AD). To further delineate the mechanism(s) involved in such aberrant signaling, we subsequently showed that mGluR2 is increased in pyramidal neurons in the hippocampus of AD and often co-localizes with neurofibrillary pathology. Based on these data, we suggested that selective neuronal degeneration in AD may arise through the differential expression and activation of specific receptor populations, such as, mGluR2. In this study, to examine the mechanisticrelevance of the above-mentioned in vivo findings, we used cell culture models to show that the activation of mGluR2 leads to the activation of extracellular signal-related kinase (ERK) pathways. Importantly, attesting to the in vivo significance of our findings, this pro-survival signaling pathway is also found to be ectopically activated in AD. We also found that the activation of mGluR2 increases the phosphorylation of tau and that the specific activation of mGluR2 reduces oxidative stress mediated cytotoxicity in neuronal cells. Taken together our findings strongly suggest that mGluR2 may participate in mediating the survival of neurons in the face of selective neuronal dysfunction and degeneration in AD. Additionally, our findings lend support to the notion that tau phosphorylation is a neuroprotective antioxidant responsetocellular insults.
Keywords: ERK, mGluR2, Neuroprotection
1. Introduction
Metabotropic glutamate receptors (mGluRs) have been suggested as therapeutic targets for neurodegenerative diseases since their unique functions allow modulation of signals via G-protein dependent pathways and this may alter neuronal functions more precisely than targeting ionotropic GluRs (iGluRs) (Bruno et al., 2001). In this regard, it is notable that signal transduction by glutamate receptors is mediated by the activation of the MAP kinase pathways (Ferraguti et al., 1999) and that these signal transduction pathways are known to be altered in AD (Webber et al., 2005; Zhu et al., 2003). Additionally, agonists of mGluR lead to an increase in phosphorylated tau mediated through extracellular signal-related kinase (ERK)-mediated kinases and several lines of evidence suggest that the differential expression of glutamate receptors in select populations of neurons accounts for specific neuronal vulnerability (Bruno et al., 2001).
The activation of Group II mGluRs protects neurons against excitotoxic degeneration by the inhibition of glutamate release (Buisson and Choi, 1995; Buisson et al., 1996) such that potent and selective Group II mGluR agonists protect against excitotoxicity in vitro (Kingston et al., 1999) and global ischemia in vivo (Bond et al., 2000). Since mGluR2 appears to play a role in the pathogenesis of neuronal cell death and survival, it is not surprising that mGluR2 is specifically increased in hippocampal neurons in AD (Lee et al., 2004a) and that mGluR2 could play a key role in the pathogenesis of AD (Lee et al., 2004b). In contrast, the expression level of mGluR2 in the dentate gyrus granular neurons is unchanged.
Of interest, the aberrant expression of mGluR2 in the hippocampus is closely associated with phosphorylated tau containing neurofibrillary changes in the pyramidal neurons, which, while traditionally thoughtof as detrimental (Iqbal and Grundke-Iqbal, 2006), more recently, has been associated with neuronal survival (Lee et al., 2005; Morsch et al., 1999). In the latter, it is notable that the activation of mGluR2 by specific agonists activates the ERK pathway (Ferraguti et al., 1999; Otani et al., 1999), a pro-survival pathway, and that ERK phosphorylates tau at those sites known to be phosphorylated in AD (Reynolds et al., 2000). Based on this, we hypothesized that mGluR2 activation sequentially lead to the activation of the ERK pathway, tau phosphorylation and neurofibrillary pathology in neurons and ultimately neuroprotection. Given the importance of mGluR2 in cellular viability, such neurons are potentially protected from the disease process. To test this hypothesis, in this study, we investigated the effect of mGluR2 activation on cellular viability, ERK activation, and tau phosphorylation in neurons.
2. Results
2.1. The expression of mGluR2 and its effect on ERK in rat primary cortical neurons
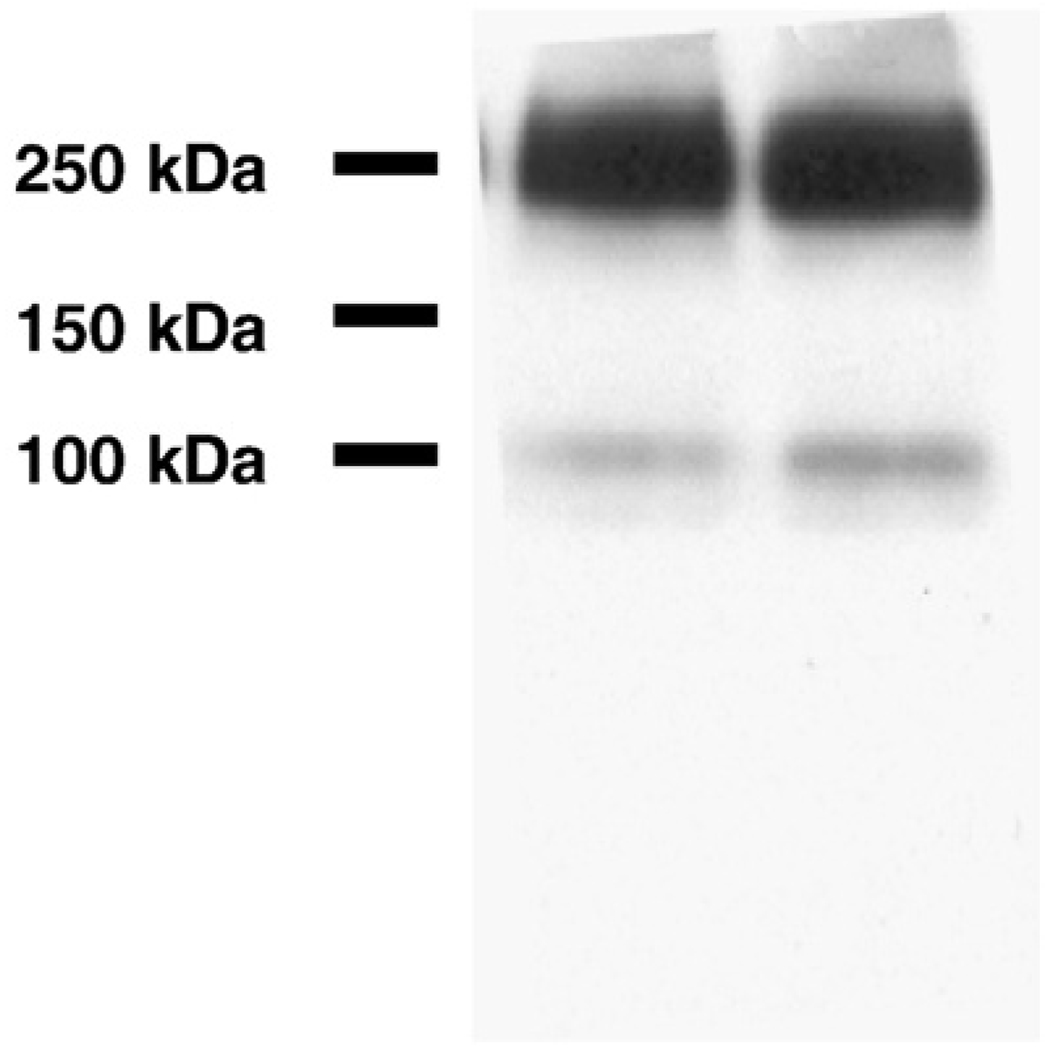
While the effect of mGluR2 on ERK pathway activation is known in CHO cells (Ferraguti et al., 1999; Phillips et al., 1998), the expression pattern of mGluR2 has not been examined in primary neurons or other neuronal cell culture models. Since primary neurons or neuronal cell lines such as SHSY5Y cells may provide a more physiologically relevant context to examine the effect of mGluR2 activation, we first determined the expression of mGluR2 protein in rat primary cortical neurons. The membrane fraction from DIV 7 to 10 neurons was separated by SDS-PAGE using a 7.5% acrylamide gel and detected by immunoblot analysis with an anti-mGluR2 antibody. Two bands were detected similar to immunoblot data from human brain samples (Lee et al., 2004a) (Fig. 1). The lower band corresponds to the calculated molecular weight of mGluR2 (~100 kDa) and the upper band is likely a receptor dimer and frequently appears in immunoblots of mGluRs (Reid and Romano, 2001; Romano et al., 1996). This data provides strong evidence that rat primary cortical neurons of DIV 7–10 express mGluR2 and, as such, can be used for our mGluR2 studies. Thus, DIV 7–10 neurons were used for the following experiments.
Fig. 1.
The expression of mGluR2 in rat primary cortical neurons (DIV 7–10). The membrane fraction rat primary cortical neurons were separatedon a 7.5% acrylamide gel and mGluR2 was detected by anti-mGluR2 rabbit polyclonal antibody. As expected, the monomer (~100 kDa) and dimer (~200 kDa) are observed and this staining pattern is the same as found for mGluR2 in human brain samples.
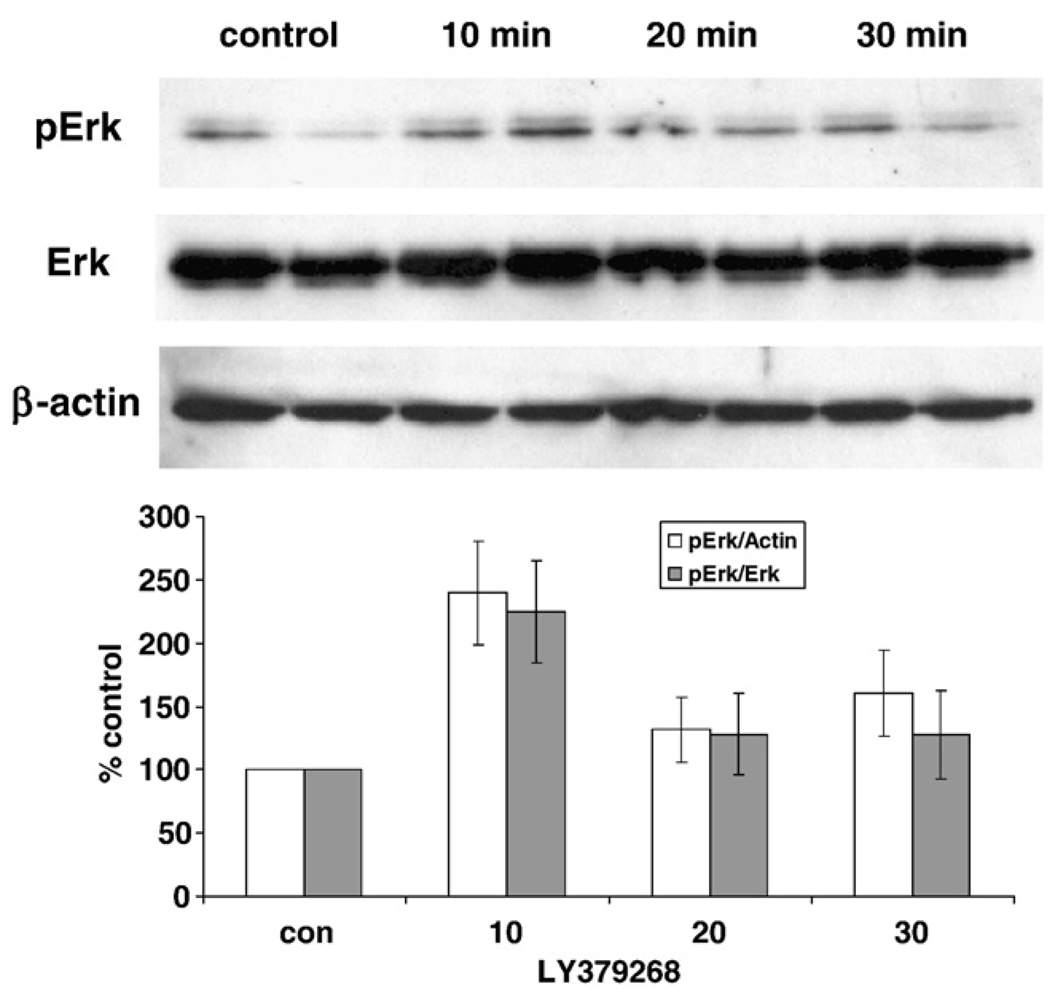
Cultures of rat cortical neurons were treated with LY379268 (10 µM) to test whether the activation of group II mGluR induces the ERK pathway. The activation of ERK can be measured using a phospho-specific antibody since the activation of ERK occurs through the phosphorylation of threonine and tyrosine (202 and 204 of human ERK or 183 and 185 of rat ERK). After treatment with LY379268, proteins were extracted at the indicated time point and analyzed by immunoblot with a phospho-ERK specific antibody. The immunoreactivity of phospho-ERK was significantly increased by LY379268 treatment after 10 min and returned to background levels at 20 and 30 min (Fig. 2). There was no significant change in the level of non-phosphorylated forms of ERK demonstrating the specific activation of ERK is through post-translational modification by phosphorylation rather than regulation of protein expression.
Fig. 2.
LY379268 induces the phosphorylation of ERK (Thr202/Tyr204). A primary cortical neuron culture (DIV 7–10) was treated with 10 µM LY379268 for indicated time and then the extracted proteins were subjected to immunoblot with phospho-ERK (Thr202/Tyr204) specific antibody. The phosphorylation level is increased after 10 min treatment and gradually decreased to basal level. The immunoblot with an antibody, which detects total ERK level, indicates that the total protein level of ERK is not changed by LY379268 treatment. The level of β-actin was used to normalize total ERK and phospho-ERK level. Data in the graph represent means ± SD values from triplicate samples from at least three separate experiments.
Since ERK is thought to contribute to tau phosphorylation (Kyoung Pyo et al., 2004; Reynolds et al., 2000), the major component of neurofibrillary tangles (NFT), we next examined whether the activation of group II mGluR induces tau phosphorylation in primary neurons.
2.2. The phosphorylation of tau by LY379268 in primary neurons
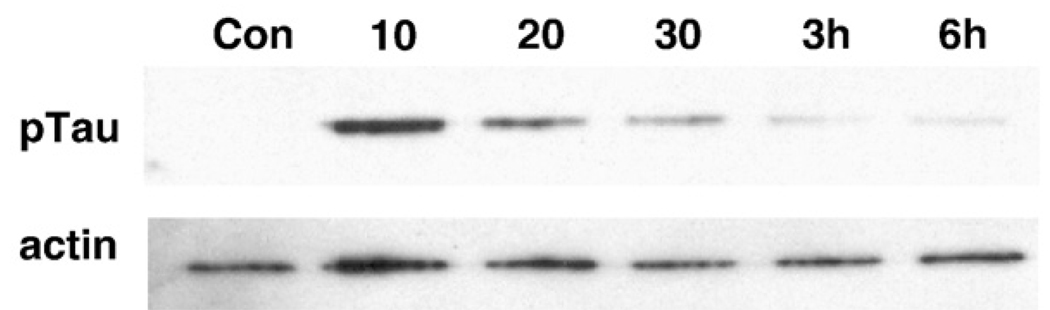
To determine whether group II mGluR activation by LY379268 contributes to tau phosphorylation, rat cortical neurons were treated with 10 µM LY379268 and the phosphorylation levels of tau protein were measured by immunoblot with the phospho-specific antibody, AT8. After 10 minutes treatment of LY379268, tau phosphorylation was dramatically increased and thereafter gradually decreased to background levels (Fig. 3). This finding supports the hypothesis that ERK-mediated tau phosphorylation is induced by group II mGluR since ERK is temporarily activated at the same time point as tau phosphorylation and the epitope for the AT8 antibody can be phosphorylated by ERK (Reynolds et al., 2000).
Fig. 3.
LY379268 induces the phosphorylation of tau in rat primary cortical neurons (DIV 7). The representative data of immunoblot with AT8 (phospho-tau) demonstrate the increase of tau phosphorylation by LY379268 treatment. After 10 min treatment with 10 µM of LY379268, the phosphorylation of tau is dramatically increased and gradually revertstobasal level over the next 6 h. The antibody for β-actin was used for internal protein loading control.
2.3. The establishment of stable cell culture overexpressing mGluR2
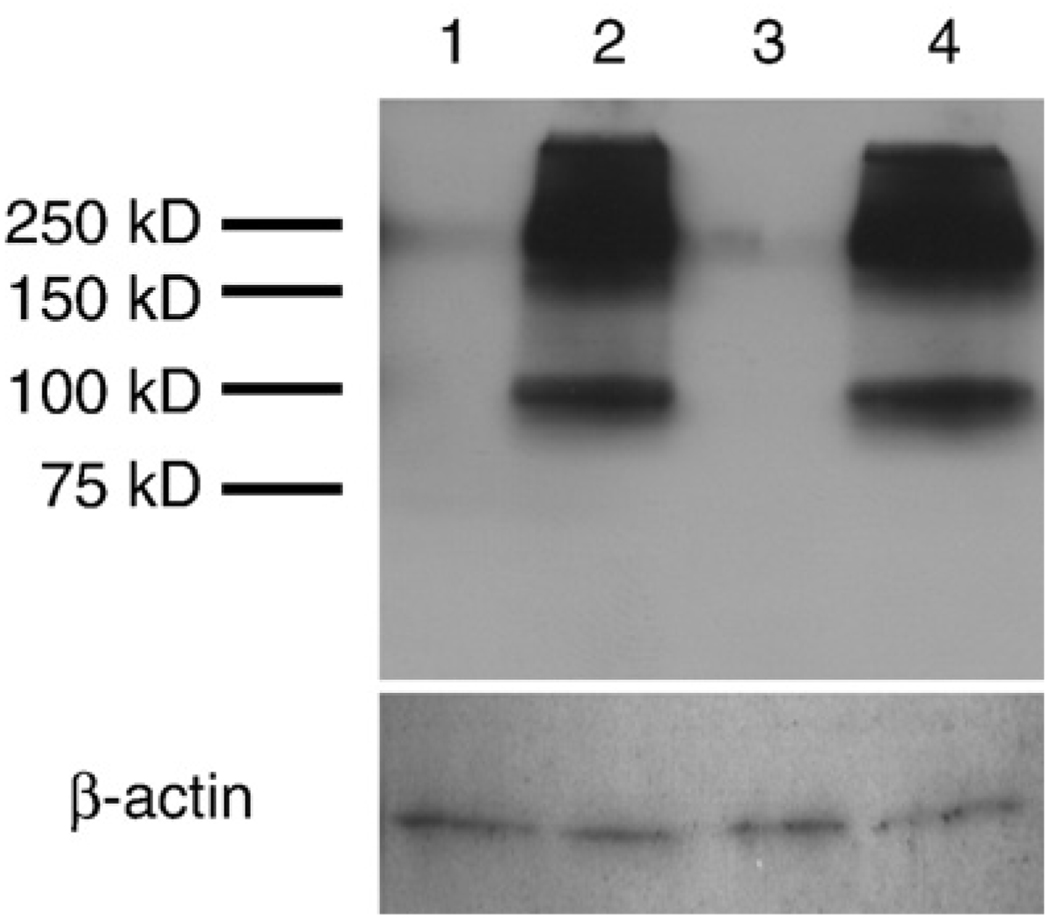
Previously, we hypothesized that the increase of mGluR2 in neurons of AD was a compensatory reaction to protect neurons from cytotoxic stress (Lee et al., 2004a). To test this hypothesis, we determined whether increased expression and activation of mGluR2 is protective. Specifically, mGluR2-overexpressing cell model was established using the human neuroblastoma cell line, SHSY5Y. mGluR2 cDNA was subcloned into a mammalian expression vector pCep4 which is an episomal expression vector and, as such, excludes artifacts created by non-specific insertion of the gene into genome. After subcloning, the purified plasmid, pCep4-mGluR2, was transfected into cells and then selected with hygromycin B. Additionally, a negative control cell line was also established by transfecting the empty vector. Selected cell populations were subjected to immunoblot to examine the level of mGluR2. In the control cell line (SHSY5Y-pCep4), mGluR2 was not detected by immunoblot analysis suggesting that SHSY5Y cells either do not express mGluR2 or express an amount that is below the detection limit of immunoblot (Fig. 4). On the other hand, prominent mGluR2 immunoreactive bands were observed in the mGluR2 transfected cells (SHSY5Y-mGluR2). The two bands, which represent monomer and dimer forms, match the results from human brains and rat cortical neurons (Fig. 1). Therefore, the SHSY5Y-mGluR2 cell line was used in the following studies as a tool to study the potential protective function of mGluR2 as well as to discriminate the function of mGluR2 from mGluR3. Since LY379268 binds and activates both mGluR2 and mGluR3, the combination of LY379268 and this cell line will delineate the specific effects of mGluR2 activation.
Fig. 4.
The expression of mGluR2 in stably transfected cell culture, SHSY5Y-mGluR2 and SHSY5Y-pCep4. mGluR2 is not expressed in vector transfected cells (1, 3) but is highly expressed in pCep4-mGluR2 transfected cells (2, 4). The two bands, which are monomer and dimer forms, match the results from human brains and rat primary neurons (Fig. 1).
2.4. The activation of ERK by LY379268 in mGluR2 overexpressing cell line
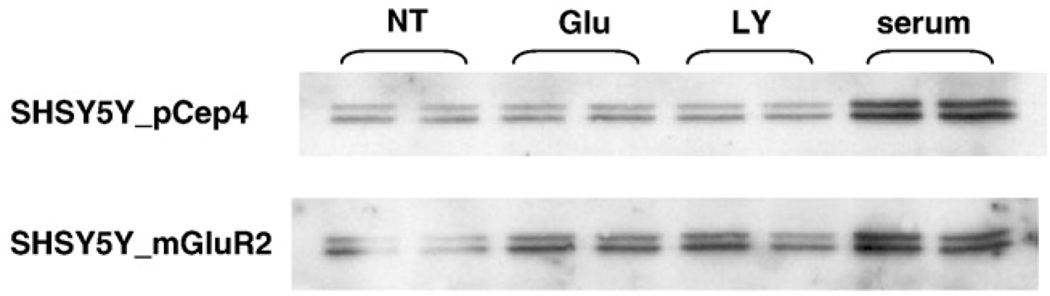
To examine whether overexpressed mGluR2 in SHSY5Y-mGluR2 activates ERK, protein levels were measured after agonist treatment. 10 µM of LY379268 increased the phosphorylation of ERK in SHSY5Y-mGluR2 cells but not in control cells, SHSY5Y-pCep4 (Fig. 5). Whereas, glutamate, (1 mM), another mGluR2 agonist, also induced phospho-ERK only in SHSY5Y-mGluR2. Treatment with 10% fetal bovine serum (FBS), a positive control of ERK activation without glutamate receptor activation, demonstrated equal ERK activation in both cell lines. These data demonstrate that while the general mechanism of phosphorylation of ERK is intact in both cells, LY379268-and glutamate-mediated phosphorylation of ERK in SHSY5Y is specific for cells engineered to express mGluR2.
Fig. 5.
LY379268 induces ERK phosphorylation in SHSY5Y-mGluR2 cells. Each cell culture was treated with LY379268, glutamate and FBS for 10 min and cell lysates was subjected to immunoblot with phospho-ERK (Thr202/Tyr204) specific antibody. The phosphorylation level is increased specifically in SHSY5Y-mGluR2 after 10 min treatment of LY379268 and glutamate but not in SHSY5Y-pCep4. FBS is used as a positive control and clearly shows that the phosphorylation of ERK by FBS is not different between the two cells. Thus, LY379268 and glutamate mediated ERK phosphorylation is mGluR2 specific. Glu; 1 mM glutamate, LY; 10 µM LY379268, serum; 10% FBS.
2.5. The effect of overexpression of mGluR2 or oxidative stress mediated cell death
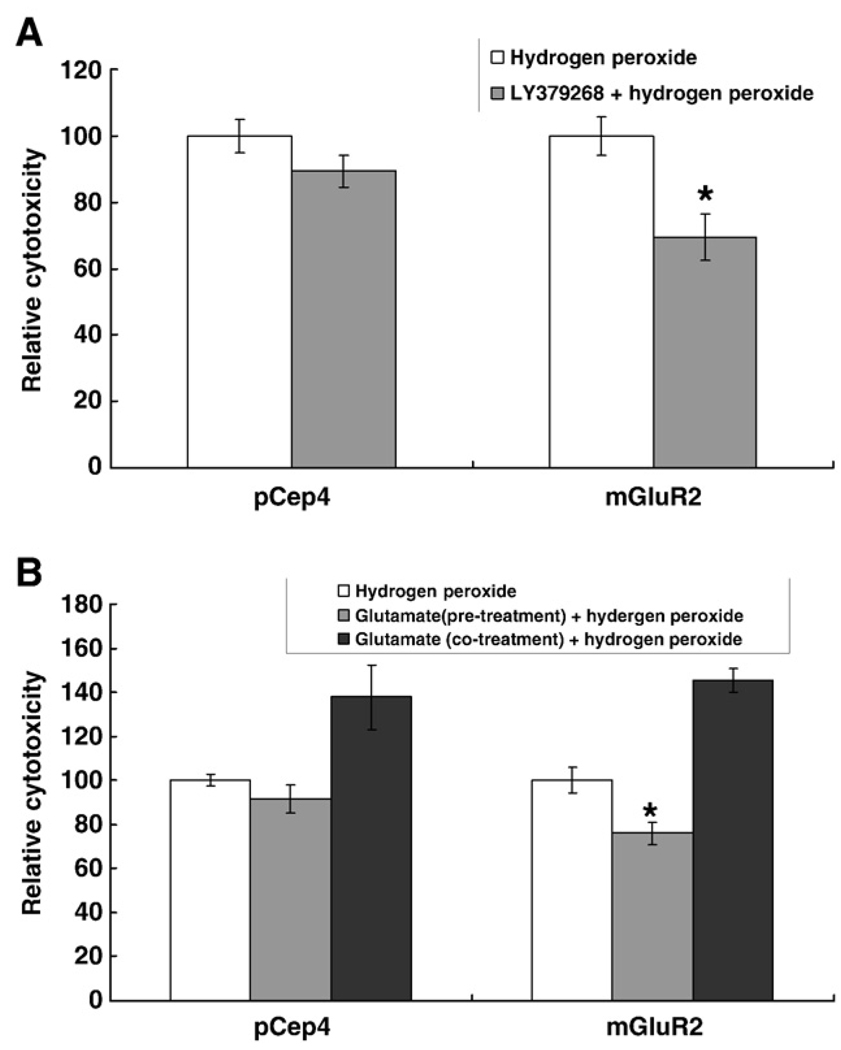
To address whether the overexpression of mGluR2 has a neuroprotective effect, 100 µM of hydrogen peroxide, as an oxidative stressor, was added after, or with, mGluR2 agonists and cytotoxicity was measured by an LDH assay. Basal toxicity of hydrogen peroxide without agonists was similar in both SHSY5Y-mGluR2 and SHSY5Y-pCep4 cell cultures (Fig. 6). Thus, the overexpression of mGluR2 itself does not affect cell viability or resistance to hydrogen peroxide in SHSY5Y cells. Next, the cell viability was measured after, or with, agonist treatment to examine the effect of mGluR2 activation. For LY379268, each cell culture was incubated with 10 µM LY379268 for 1 h and then 100 µM hydrogen peroxide was added into the medium and incubated for 24 h. Treatment with LY379268 significantly reduced cytotoxicity (~20%) in SHSY5Y-mGluR2 but had no significant effect in SHSY5Y-pCep4 (Fig. 6). To confirm the specificity of mGluR2 activation and exclude any possible non-mGluR2 specific effects of LY379268, glutamate was applied to each cell type and the cytotoxicity of hydrogen peroxide was measured again. The cytotoxicity of hydrogen peroxide was also reduced in SHSY5Y-mGluR2 by pretreatment of 1 mM glutamate for 1 h but the level of protection was less than in the LY379268-treated group (Fig. 6). The data convincingly shows that the activation of mGluR2 has a protective effect against hydrogen peroxide-mediated oxidative stress. Surprisingly, continuous incubation with glutamate after pretreatment potentiated the cytotoxic effect of hydrogen peroxide (Fig. 6) although this is unlikely related to mGluR2 activation since the level of potentiation is similar in both cells. Instead, the deleterious effect of long term incubation of glutamate may reflect excitotoxicity which is mediated by ionotropic glutamate receptors such as NMDA receptors. Consistent with this notion, it has been reported that the SHSY5Y cell line expresses NMDA receptors (Nair et al., 1996).
Fig. 6.
The activation of mGluR2 is protective against hydrogen peroxide mediated cytotoxicity. SHSY5Y-mGluR2 (mGluR2) and SHSY5Y-pCep4 (vector) were incubated with 10 µM LY379268 for 1 h and then 100 µM hydrogen peroxide was added into the medium and incubated for an additional 24 h (A). Cytotoxicity was measured with the LDH assay. Glutamate was similarly applied to the cells and the cytotoxicity of hydrogen peroxide was also measured (B). The cytotoxicity of hydrogen peroxide was reduced in SHSY5Y-mGluR2 by pretreatment with 10 µM LY379268 or 1 mM glutamate for 1 h. However, continuous glutamate treatment increased the susceptibility to hydrogen peroxide. Data in the graph represent means ± SD values from at least three separate experiments and each experiment contains five samples for each group. *p < 0.05, compared with hydrogen peroxide treated group.
3. Discussion
In this study, we found that the group II mGluR specific agonist, LY379268, induced ERK activation in rat primary cortical neurons. Moreover, mGluR2 stably transfected cells, which overexpress mGluR2 and do not express mGluR3, showed that ERK activation is specifically mediated by mGluR2 activation. These results demonstrate for the first time that group II mGluR induces ERK activation in primary neuronal cultures and neuronal cell lines in an mGluR2-specific manner. Activation of the ERK pathway may provide protection against apoptosis in some cell types. For example, in PC-12 cells, withdrawal of NGF leads to the inhibition of ERK and cell death, whereas the constitutive activation of ERK in these cells inhibits apoptosis (Xia et al., 1995). Additionally, blockade of ERK activation prevents protection against TNF-α-induced apoptosis that is mediated by FGF-2 in L929 cells (Gardner and Johnson, 1996). However, ERK can be harmful under certain conditions and ERK is activated in a number of pathophysio-logically-relevant conditions. For example, ERK activation specifically occurs in the rat hippocampus after bicucullin-induced seizures and ischemia (Baraban et al., 1993; Gass et al., 1993). Also, ERK activation plays a direct mechanistic role in 6-OHDA toxicity which may contribute to the dopaminergic neuronal cell death that occurs in Parkinson disease (Kulich and Chu, 2001). Finally, given that ERK plays a significant role in hippocampus-related learning and memory, it is perhaps not surprising that disease conditions, such as AD (Smith, 1998), which involve the hippocampus (Hirano and Iida, 2006) and affect learning and memory, also involve the ERK pathway (Perry et al., 1999; Zhu et al., 2001; Zhu et al., 2002). In this regard, the stimulation of sAβPP secretion by NGF is ERK activity-dependent (Desdouits-Magnen et al., 1998). Also very relevant to AD, ERK can phosphorylate tau protein in vitro in a PHF-tau manner (Reynolds et al., 2000) and phosphorylation of tau impairs its ability, at least in vitro (Cash et al., 2003) to assemble into microtubules (Smith, 1998).In the present study, we found that mGluR2 activation increased tau phosphorylation in primary neurons (Fig. 3). This is consistent with our findings in the AD brain showing co-localization of mGluR2 and hyperphosphorylated tau (Lee et al., 2004a). The dual functions of ERK in neuronal cells, i.e., neuroprotection and contribution to NFT formation, are difficult to reconcile using the established dogma that NFT formation is deleterious. In this vein, it is of interest to note that CA1 hippocampal neurons survive with NFT for about 20 years and that NFT may not be obligatory for death of CA1 hippocampal neurons in AD (Kril et al., 2002; Morsch et al., 1999). Therefore, mGluR2-mediated activation of ERK, as demonstrated here, and tau phosphorylation not only reflect the pathology found in AD but could reflect a compensatory response invoked by neurons to cytotoxic stressors (Lee et al., 2005; Li et al., 2007).
There are many reports of neuroprotection with mGluR, especially by group II mGluR agonists. For example, agonists for group II mGluR have been reported to protect against apoptotic and excitotoxic stimuli in vitro (Allen et al., 1999; Copani et al., 1995; Kingston et al., 1999; Matarredona et al., 2001). These in vitro studies have been supported by reports showing that group II mGluRs agonists are also neuroprotective in vivo (Bond et al., 2000; Chiamulera et al., 1992; Miyamoto et al., 1997). However, there are no studies on whether such protective effects are mediated by mGluR2 or mGluR3. However, in our studies, the cytotoxicity of hydrogen peroxide was significantly reduced by LY379268 in SHSY5Y-mGluR2 but not in SHSY5Y-pCep4. This clearly indicates that the activation of mGluR2 in isolation is sufficient to mediate protection in neuronal cells and, since this involves tau phosphorylation, strengthens the notion that tau phosphorylation is a protective antioxidant (Nunomura et al., 2001; Smith et al., 2002).
In summary, the activation of group II mGluR induces ERK activation in rat primary cortical neurons and the specific activation of mGluR2 also induces the ERK pathway. Importantly, mGluR2 activation is neuroprotective against oxidative stressors and further studies will help delineate the specific functions of mGluR2 in neurons. The therapeutic potential of targeting mGluR2 in AD and other neurodegenerative diseases is obvious.
4. Experimental procedures
4.1. Materials
LY379268, a specific agonist for group II mGluR (EC50mGluR2 = 2.69 nM and EC50mGluR3 = 4.58 nM) was provided by Eli Lilly and Co., Ltd, was dissolved in equimolar NaOH to facilitate solubility. For immunoblot analysis, anti-mGluR2 (Upstate, Lake Placid, NY), anti-phospho-ERK (Cell Signaling, Danvers, MA) and anti-phospho-tau (AT8, Pierce, Rockford, IL) antibodies were used as described previously (Lee et al., 2004a; Perry et al., 1999).
4.2. Cell culture
The SHSY5Y human neuroblastoma cell line was cultured in Opti-MEM I media (Invitrogen) containing 5% FBS. Hygromycin B (200 µg/ml) was added to maintain stably transfected cell cultures. Primary cortical neuron cultures were prepared from Sprague/Dawley rat brains at embryonic day 18. After dissection, the cerebral cortex was dissociated and plated in poly-D-lysine coated culture dishes and cultured in serum-free Neurobasal medium supplemented with B27 supplement, 0.5 mM glutamine, and 25 µM glutamate (Invitrogen, Carlsbad, CA). Half of the medium was replaced every 3–4 days with Neurobasal medium supplemented with B27 and 0.5 mM glutamine.
4.3. Plasmids and transfection
To subclone mGluR2 cDNA into the mammalian expression vector, mGluR2 cDNA was excised from pmGR2 (Tanabe et al., 1992) by the restriction enzymes, Hind III and Not I, and ligated into the mammalian expression vector pCep4 (Invitrogen, Carlsbad, CA). The purified pCep4-mGluR2 was transfected into SHSY5Y cells using Superfect agent (Qiagen, Valencia, CA) and selected by incubating with hygromycin B (200 µg/ml) for 4 weeks. After establishing stable cell cultures, the level of target protein was determined by immunoblot.
4.4. Immunoblot
To detect mGluR2 in primary neurons and stable cell cultures, membrane preparations and immunoblots were performed as described in the literature with minor modifications (Reid and Romano, 2001). Briefly, cells were washed twice with ice-cold phosphate-buffered saline and homogenized with buffer I (0.32 M sucrose, 2 mM EDTA, pH 7.5) plus protease inhibitors using a 22-gauge needle. The samples were then centrifuged at 1000 ×g for 10 min to remove nuclei and cell debris and the supernatant was further centrifuged at 30,000 ×g for 20 min. The pellet was homogenized in buffer II (2 mM HEPES/2 mM EDTA, pH 7.5, containing the protease inhibitor cocktail (Sigma, St. Louis, MO), again using a 22-gauge needle, and then kept on ice for 30 min. The membrane fraction was collected by centrifuging at 30,000 ×g for 20 min and then washed twice in TBS-containing protease inhibitor cocktail, and stored at −80 °C. Protein concentration was determined using the BCA method (Pierce, Rockford, IL). For electrophoresis, samples were dissolved in a sample buffer (pH 6.7) containing 100 mM dithiothreitol (DTT) and 10 µg of the proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) in a 7.5% polyacrylamide gel.
For phospho-ERK and tau protein detection, the lysis buffer (20 mM Tris-HCl, pH 7.5,150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1 mM PMSF) was directly added to cell culture dishes and keep on ice for 10 min. The samples were then centrifuged at 1000 ×g for 10 min to remove nuclei and cell debris. The protein concentration in the supernatant was measured by the BCA method and 10 µg of each protein was separated by SDS-PAGE on a 12% polyacrylamide gel.
4.5. Cytotoxicity test
The cytotoxicity of hydrogen peroxide was evaluated by the LDH assay (Roche, Indianapolis, IN). Briefly, cell media was collected after each treatment and the collected media mixed with LDH substrate in a 96-well plate. After incubation for 30 min at room temperature, the optical density was measured at 490 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The measured optical density was then converted after standardization with low (no treatment; 0% toxicity) and high controls (1% Triton X-100; 100% toxicity) by the following equation:
Acknowledgments
Work in the authors' laboratories is supported by the National Institutes of Health and the Alzheimer's Association.
Abbreviations
- AD
Alzheimer disease
- ERK
extracellular signal-related kinase
- MAPK
mitogen-activated protein kinase
- NFT
neurofibrillary tangle
- PHF
paired helical filaments
REFERENCES
- Allen JW, Ivanova SA, Fan L, Espey MG, Basile AS, Faden AI. Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury. J. Pharmacol. Exp. Ther. 1999;290:112–120. [PubMed] [Google Scholar]
- Baraban JM, Fiore RS, Sanghera JS, Paddon HB, Pelech SL. Identification of p42 mitogen-activated protein kinase as a tyrosine kinase substrate activated by maximal electroconvulsive shock in hippocampus. J. Neurochem. 1993;60:330–336. doi: 10.1111/j.1471-4159.1993.tb05855.x. [DOI] [PubMed] [Google Scholar]
- Bond A, Jones NM, Hicks CA, Whiffin GM, Ward MA, O'Neill MF, Kingston AE, Monn JA, Ornstein PL, Schoepp DD, Lodge D, O'Neill MJ. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: investigations into possible mechanism of action in vivo. J. Pharmacol. Exp. Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J. Cereb. Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Buisson A, Choi DW. The inhibitory mGluR agonist, S-4-carboxy-3-hydroxy-phenylglycine selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal death. Neuropharmacology. 1995;34:1081–1087. doi: 10.1016/0028-3908(95)00073-f. [DOI] [PubMed] [Google Scholar]
- Buisson A, Yu SP, Choi DW. DCG-IV selectively attenuates rapidly triggered NMDA-induced neurotoxicity in cortical neurons. Eur. J. Neurosci. 1996;8:138–143. doi: 10.1111/j.1460-9568.1996.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer's disease and aging is independent of tau filament formation. Am. J. Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Albertini P, Valerio E, Reggiani A. Activation of metabotropic receptors has a neuroprotective effect in a rodent model of focal ischaemia. Eur. J. Pharmacol. 1992;216:335–336. doi: 10.1016/0014-2999(92)90382-e. [DOI] [PubMed] [Google Scholar]
- Copani A, Bruno V, Battaglia G, Leanza G, Pellitteri R, Russo A, Stanzani S, Nicoletti F. Activation of metabotropic glutamate receptors protects cultured neurons against apoptosis induced by beta-amyloid peptide. Mol. Pharmacol. 1995;47:890–897. [PubMed] [Google Scholar]
- Desdouits-Magnen J, Desdouits F, Takeda S, Syu LJ, Saltiel AR, Buxbaum JD, Czernik AJ, Nairn AC, Greengard P. Regulation of secretion of Alzheimer amyloid precursor protein by the mitogen-activated protein kinase cascade. J. Neurochem. 1998;70:524–530. doi: 10.1046/j.1471-4159.1998.70020524.x. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C. Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur. J. Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- Gardner AM, Johnson GL. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J. Biol. Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- Gass P, Kiessling M, Bading H. Regionally selective stimulation of mitogen activated protein (MAP) kinase tyrosine phosphorylation after generalized seizures in the rat brain. Neurosci. Lett. 1993;162:39–42. doi: 10.1016/0304-3940(93)90554-x. [DOI] [PubMed] [Google Scholar]
- Hirano A, Iida M. Topographic study of Alzheimer's neurofibrillary changes: a personal perspective. J. Alzheimers Dis. 2006;9:53–60. doi: 10.3233/jad-2006-9s307. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J. Alzheimers Dis. 2006;9:219–242. doi: 10.3233/jad-2006-9s325. [DOI] [PubMed] [Google Scholar]
- Kingston AE, O'Neill MJ, Lam A, Bales KR, Monn JA, Schoepp DD. Neuroprotection by metabotropic glutamate receptor glutamate receptor agonists: LY354740, LY379268 and LY389795. Eur. J. Pharmacol. 1999;377:155–165. doi: 10.1016/s0014-2999(99)00397-0. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. (Berl) 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J. Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung Pyo H, Lovati E, Pasinetti GM, Ksiezak-Reding H. Phosphorylation of tau at THR212 and SER214 in human neuronal and glial cultures: the role of AKT. Neuroscience. 2004;127:649–658. doi: 10.1016/j.neuroscience.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Lee HG, Ogawa O, Zhu X, O'Neill MJ, Petersen RB, Castellani RJ, Ghanbari H, Perry G, Smith MA. Aberrant expression of metabotropic glutamate receptor 2 in the vulnerable neurons of Alzheimer's disease. Acta Neuropathol. (Berl) 2004a;107:365–371. doi: 10.1007/s00401-004-0820-8. [DOI] [PubMed] [Google Scholar]
- Lee HG, Zhu X, O'Neill MJ, Webber K, Casadesus G, Marlatt M, Raina AK, Perry G, Smith MA. The role of metabotropic glutamate receptors in Alzheimer's disease. Acta Neurobiol. Exp. (Wars) 2004b;64:89–98. doi: 10.55782/ane-2004-1494. [DOI] [PubMed] [Google Scholar]
- Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol. Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarredona ER, Santiago M, Venero JL, Cano J, Machado A. Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+induced neurotoxicity along with brain-derived neurotrophic factor induction. J. Neurochem. 2001;76:351–360. doi: 10.1046/j.1471-4159.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Ishida M, Shinozaki H. Anticonvulsive and neuroprotective actions of a potent agonist (DCG-IV) for group II metabotropic glutamate receptors against intraventricular kainate in the rat. Neuroscience. 1997;77:131–140. doi: 10.1016/s0306-4522(96)00442-3. [DOI] [PubMed] [Google Scholar]
- Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Nair VD, Niznik HB, Mishra RK. Interaction of NMDA and dopamine D2L receptors in human neuroblastoma SH-SY5Y cells. J. Neurochem. 1996;66:2390–2393. doi: 10.1046/j.1471-4159.1996.66062390.x. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J. Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris PL, Smith MA. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10:2411–2415. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- Phillips T, Barnes A, Scott S, Emson P, Rees S. Human metabotropic glutamate receptor 2 couples to the MAP kinase cascade in Chinese hamster ovary cells. Neuroreport. 1998;9:2335–2339. doi: 10.1097/00001756-199807130-00034. [DOI] [PubMed] [Google Scholar]
- Reid SN, Romano C. Developmental and sensory-dependent changes of group II metabotropic glutamate receptors. J. Comp. Neurol. 2001;429:270–276. doi: 10.1002/1096-9861(20000108)429:2<270::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J. Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Smith MA. Alzheimer disease. Int. Rev. Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-beta and tau serve antioxidant functions in the aging and Alzheimer brain. Free Radic. Biol. Med. 2002;33:1194–1199. doi: 10.1016/s0891-5849(02)01021-3. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992;8:169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Webber KM, Smith MA, Lee HG, Harris PL, Moreira P, Perry G, Zhu X. Mitogen- and stress-activated protein kinase 1: convergence of the ERK and p38 pathways in Alzheimer's disease. J. Neurosci. Res. 2005;79:554–560. doi: 10.1002/jnr.20380. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech. Ageing Dev. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- Zhu X, Sun Z, Lee HG, Siedlak SL, Perry G, Smith MA. Distribution, levels, and activation of MEK1 in Alzheimer's disease. J Neurochem. 2003;86:136–142. doi: 10.1046/j.1471-4159.2003.01820.x. [DOI] [PubMed] [Google Scholar]