Abstract
A Chinese hamster ovary (CHO) cell line, producing recombinant secreted human placental alkaline phosphatase (SEAP) was investigated under three different culture conditions (suspension cells, cells attached to Cytodex 3 and Cytopore 1 microcarriers) in a biphasic culture mode using a temperature shift to mild hypothermic conditions (33 °C) in a fed-batch bioreactor. The cell viability in both the suspension and the Cytodex 3 cultures was maintained for significantly longer periods under hypothermic conditions than in the single-temperature cultures, leading to higher integrated viable cell densities. For all culture conditions, the specific productivity of SEAP increased after the temperature reduction; the specific productivities of the microcarrier cultures increased approximately threefold while the specific productivity of the suspension culture increased nearly eightfold. The glucose and glutamine consumption rates and lactate and ammonia production rates were significantly lowered after the temperature reduction, as were the yields of lactate from glucose. However, the yield of ammonia from glutamine increased in response to the temperature shift.
Keywords: rCHO cells, Bioreactor, Hypothermia, Microcarrier cultures, SEAP
Introduction
With the increase in demand for therapeutic proteins and the shortage of reactor capacity for mammalian cell cultures, there have been numerous efforts to increase the production of recombinant therapeutic proteins. Strategies to improve productivity include developing and screening better cell lines, engineering cellular metabolism (Seth et al. 2006), applying environmental stresses such as hyperosmolarity (Chua et al. 1994), and optimization of process conditions (Trummer et al. 2006a, b). Mild hypothermia has been reported as a simple way to improve the production of recombinant therapeutic proteins in mammalian cell cultures (Furukawa and Ohsuye 1998). The benefits of mild hypothermia include improved cell viability, reduced cell lysis from dead cells, reduced nutrient consumption rates, and often, improved productivity (Yoon et al. 2003; Fox et al. 2004; Bollati-Fogolin et al. 2005; Trummer et al. 2006a). Despite the potential usefulness of mild hypothermia, there are significant limitations including G0/G1 arrest (Moore et al. 1997) and suppression of the cell growth, leading to low volumetric productivity. Biphasic culture, in which cells are cultivated at 37 °C until they reach the maximum viable cell density, followed by a reduction in temperature to prolong cell longevity, presents a possible strategy to alleviate the disadvantages of hypothermia. Biphasic cultures have demonstrated a higher volumetric productivity and final product titer in several studies (Bollati-Fogolin et al. 2005; Rodriguez et al. 2005; Trummer et al. 2006b).
Microcarrier culture presents another opportunity to increase culture and volumetric productivity. Microcarriers permit high-cell density cultures, by providing high cell surface/volume ratios; moreover, microcarrier culture simplifies supernatant clarification facilitating downstream processing. Microcarriers are particularly effective for perfusion cultures. Currently, microcarrier cultures are used extensively for viral vaccine production (Junker et al. 1992; Mendonça et al. 1993; Berry et al. 1999; Percheson et al. 1999; Doi et al. 2001; Frazzati-Gallina et al. 2001; Sugawara et al. 2002; Wu and Huang 2002; Choi et al. 2003; Kallel et al. 2003), primarily using VERO cells, although other cells lines such as MRC-5 are also used. Microcarriers have also been investigated for production of recombinant therapeutics using either Chinese hamster ovary (CHO) (Kong et al. 1998; Kong et al. 1999a, b; Xiao et al. 1999; Hu et al. 2000; Wang et al. 2002; Landauer et al. 2003) or baby hamster kidney (BHK) (Schmid et al. 1992; Kallel et al. 2003) cells. Recently, Tharmalingam and coworkers found that a biphasic temperature shift increased the productivity for interferon-β produced in CHO cells grown on macroporous microcarriers as well, as decreasing the aggregation (Tharmalingam et al. 2008). Moreover, they found that in comparison with suspension cultures at 37 °C, both microcarrier culture and temperature reduction increased productivity in a synergistic manner. However, it is well established that these effects are often cell-line specific.
We have previously explored the effects of microcarrier cultures on growth and productivity for fed-batch cultures of CHO cells producing recombinant proteins at a single temperature (Nam et al. 2007). In this paper, we investigated the effects of a mild temperature reduction on growth, metabolic activity, and productivity of secreted human placental alkaline phosphatase (SEAP) for CHO cell cultures in suspension and attached to both solid and macroporous microcarriers. The results of our studies stand in sharp contrast to those of Tharmalingam et al. as we found that microcarrier cultures exhibited lower productivities than suspension cultures and that the beneficial effects of temperature reduction were substantially reduced in microcarrier cultures as compared to suspension cultures.
Materials and methods
Cell line and culture conditions
CHO cell line TR2-255, producing secreted human placental alkaline phosphatase (SEAP), was derived from CHO-K1 in a similar manner as the C142 cell line described previously (Ermonval et al. 1997). Briefly, 3 × 106 CHO-K1 cells in a suspension containing a mixture of 1 μg pSV2Neo (coding for neomycin resistance), 20 μg pBC12RSVSEAP (coding for SEAP), and 20 μg pKCKd (coding for the murine MHC class I molecule) were electroporated at 280 V and 960 μF (average time 11 μs). Adherent cells were allowed to recover for 48 h and then plated in selective medium under clonal limiting dilution conditions (104 cells per well in 96-wellmicroplates). TR2-255 expressed SEAP, but not the murine MHC class I molecule. The TR2-255 cell line was adapted to the protein-free medium, HyQ SFM4CHO-Utility (Hyclone, Logan, UT). HyQ SFM4CHO-Utility was used for all culture conditions. Cells were grown under three different culture conditions, suspension culture and cultures attached to two different microcarriers, Cytodex 3 and Cytopore 1. All cultures were performed in a fed-batch culture mode in a Biostat B bioreactor (Sartorius BBI systems, Bethlehem, PA). Cytodex 3 (GE Healthcare, NJ) is a non-porous, surface-type microcarrier which consists of a surface layer of denatured collagen covalently bound to a matrix of cross-linked dextran. Cytopore 1 (GE Healthcare, NJ) is a macroporous microcarrier composed of cross-linked cotton cellulose with positively charged N, N-diethylaminoethyl (DEAE) groups on the surface.
Inoculation and bioreactor operating conditions
Fed-batch cultures were performed in a 2 L Biostat B bioreactor with a 1 L working volume. Exponentially growing cells were inoculated at ~2 × 105 cells/mL into the bioreactor. A marine impeller was used for stirring. The stirring speed was set at 70 rpm for suspension cultures. For microcarrier cultures, cells were intermittently (1 min out of every 30 min) stirred at 30 rpm for ~12 h after inoculation followed by continuous stirring at 30 rpm for another ~12 h to facilitate the cells’ attachment to the microcarriers. Subsequently, the stirring rate was increased to 100 rpm for Cytodex 3 cultures and 70 rpm for Cytopore 1 cultures for the duration of the experiment. The temperature was controlled at 37 °C until the end of the exponential growth phase, then changed to 33 °C during the stationary phase, and maintained at 33 °C until the end of the culture.
The dissolved oxygen (DO) level was set to 50% of air saturation and controlled by a PID controller. Oxygen was provided via surface aeration where oxygen-enriched air was supplied to the headspace of the reactor. The pH was maintained at 7.2 ± 0.05 using PID control with intermittent supply of CO2 and 1 M NaOH. Concentrated glucose (100 g/L) and glutamine (10 g/L) were added intermittently to maintain the concentration of glucose between 2.0 and 2.5 g/L and the concentration of glutamine between 0.2 and 0.25 g/L.
Microcarriers were prepared according to the manufacturer’s protocol. Microcarriers were swollen and hydrated in PBS (Ca2+ and Mg2+ free, pH 7.4) overnight. After hydration, the PBS was removed and replaced with fresh PBS (50 mL/g microcarriers) and the microcarriers were sterilized by autoclaving at 121 °C for 20 min. Prior to use, the PBS was removed aseptically, and the sterilized microcarriers were pre-conditioned with the protein-free medium (HyQ SFM4CHO-Utility) overnight at 4 °C.
The glass bioreactor culture vessel was siliconized before each microcarrier culture using Sigmacote (Sigma, St. Louis, MO) according to the manufacturer’s protocol to prevent the absorptive losses of microcarriers.
Determination of cell concentration and cell viability
Duplicate 2 mL samples were taken from the bioreactor twice daily to determine cell density and viability. After removing an aliquot for cell enumeration, the remaining sample was centrifuged for 2 min at 16,000 g, and the supernatant was stored at −20 °C for later analysis. For Cytodex 3 cultures, cells were trypsinized before cell counting. Briefly, 1 mL of each sample was collected and allowed to settle by gravity. The supernatant was aspirated and the microcarriers were washed with 1.5 mL of Ca2+ and Mg2+-free PBS. When the microcarriers had settled, the PBS was replaced by 1 mL of 0.05% (w/v) HyQ-trypsin-EDTA solution (Hyclone, Rogan, Utah) and incubated at 37 °C for 15 min with mild vortexing every 5 min.
For suspension and Cytodex 3 cultures, cell densities and viabilities were estimated by hemacytometer counts (Hausser Scientific, PA) after diluting 1:1 with 0.4% trypan blue solution (ICN Biomedicals, OH). As it was not possible to quantitatively remove all the cells from the microporous Cytopore 1 microcarriers by trypsinization, the total cell number was determined by counting the nuclei after crystal violet treatment. One milliliter of suspended Cytopore 1 microcarriers with cells attached was placed in a 1.5 mL microcentrifuge tube. The microcarriers were allowed to settle by gravity and the supernatant was aspirated and PBS was added. Cytopore 1 microcarriers were allowed to settle again, and the PBS was aspirated. A solution of 0.1 M citric acid and 0.1% crystal violet was added to a final volume of 1 mL. The solution was incubated at 37 °C overnight to remove the nuclei from the microcarrier beads. After vortexing, the nuclei were then counted on a hemacytometer.
All cultures were terminated when the viability dropped below 60% except for the Cytopore 1 culture for which the viability could not be determined; it was arbitrarily terminated at ~203 h.
Assay for SEAP activity
For SEAP activity measurement, 50 μL of the supernatant from each sample described above was incubated at 65 °C for 10 min to remove any endogenous alkaline phosphatase activity. The SEAP activity was determined by measuring the hydrolysis of p-nitrophenyl phosphate in 0.1 M Tris, 0.1 M NaCl, 50 mM MgCl2 (pH 9.5) at 405 nm using a Genesis 6 spectrophotometer (Thermo Spectronic, Rochester, NY). The ΔA405/min was converted to SEAP units per milliliter using a standard curve prepared with human placental alkaline phosphatase (Sigma).
Measurement of metabolic activities
Glucose, glutamine and lactate concentrations were measured using an YSI 2700 biochemical analyzer (Yellow Springs Instruments, Yellow Springs, OH). Ammonia concentrations were measured using the pHoenix ammonia gas-sensing electrode (pHoenix Electrode Co., Houston, Texas). One milliliter of sample was diluted with water to a final volume of 10 mL. About 100 μL of 10 M NaOH was added into the sample just before the measurement to convert the ammonium ion in the sample to ammonia by changing the pH. The ammonia electrode was connected to an Accumet pH meter (Fisher Scientific, NJ). The electrode was immersed in the stirred sample, covered with Parafilm to prevent ammonia release. The voltage signal (mV) was recorded and was converted to ammonia concentration (moles/L) using a standard curve made by serial dilution of 0.1 M NH4Cl (10−1–10−6 M).
Calculation of specific growth, productivity, and metabolic rates
The specific growth rate (μ) was calculated by plotting the logarithm of the viable cell density versus culture time during the exponential growth phase, based on the equations according to Pirt (1985);
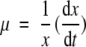 |
1 |
where x is viable cell density (cells/mL).
The volumetric productivity was calculated by dividing the accumulated SEAP activity by the corresponding culture time.
The specific production rate (qSEAP) was calculated as the slope of a plot of SEAP activity versus the time integral of viable cells 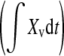 during the culture period (Buntemeyer et al. 1991);
during the culture period (Buntemeyer et al. 1991);
 |
2 |
where SEAP(t) and SEAP(t0) are SEAP activity at time t and t0, respectively and 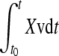 is the integral of Xv (viable cell number) between t and t0.
is the integral of Xv (viable cell number) between t and t0.
The specific consumption or production rates of glucose (qGlc), glutamine (qGln), lactate (qLac) and ammonia (qAmm) were based on the data collected during the growth phase and evaluated from a plot of the substrate and product concentrations against the time integral of growth curve (Renard et al. 1988). Yield (lactate/glucose) was calculated by dividing the specific production rate (lactate) by the specific consumption rate (glucose). Yield (ammonia/glutamine) was calculated by dividing the specific production rate (ammonia) by the specific consumption rate (glutamine).
Results
Growth characteristics of SEAP-producing CHO cells under different culture conditions
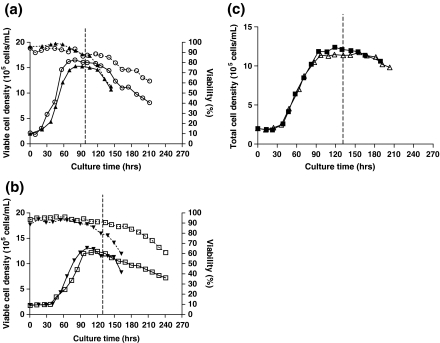
The cell line TR2-255, producing recombinant SEAP, was investigated in a biphasic culture mode using mild hypothermia for three different culture conditions, suspension, attached to Cytodex 3 microcarriers, and attached to Cytopore 1 microcarriers. Initially, CHO cells were grown at 37 °C. Once the cultures reached their maximum viable cell density and entered into the stationary phase, the culture temperature was reduced to 33 °C, without changing any other operating conditions.
In this study, the culture temperature was reduced to 33 °C at ~95 h for suspension culture and at ~133 h for microcarrier cultures. In the biphasic culture mode, the viabilities of the suspension and Cytodex 3 cultures were significantly extended compared to the respective single-temperature cultures (Fig. 1; Table 1). As observed previously (Nam et al. 2007), the suspension culture reached a higher maximum viable cell density and showed a slightly higher specific growth rate than the other cultures (Table 1). The Cytodex 3 culture remained viable longer than the suspension culture; however, the maximum viable cell density in the Cytodex 3 culture was lower than in the suspension culture, yielding a lower final integrated viable cell density (Table 1). For the Cytopore 1 culture, the culture was arbitrarily terminated at 203 h. As viable cell density measurements could not be performed, the integrated viable cell density was approximated from the total cell density. The Cytopore 1 culture showed the lowest maximum cell density among the cultures (Table 1).
Fig. 1.
Comparison of growth and viability between single-temperature and biphasic cultures. a Suspension cultures. b Cytodex 3 cultures. c Cytopore 1 cultures. Solid symbols [suspension (▲), Cytodex 3 (▼), Cytopore 1(■)] represent single-temperature cultures. Open symbols [suspension (○), Cytodex 3 (□), Cytopore 1 (△)] represent biphasic cultures. Solid lines indicate viable cell density; dashed lines indicate percent viability. Vertical dashed line indicates the temperature reduction from 37 °C to 33 °C
Table 1.
Summary of growth and production characteristics of SEAP-producing CHO cells in response to temperature reduction
| Culture conditions | Specific growth rate (h−1) | Max. viable cell conc. (106 cells/mL) | qSEAP (10−6U/cells/day) | Max. SEAP activity (U/mL) | Max. volumetric productivity (U/mL/day) | Max. IVCD (106cells/mL × day) | Culture Timee (h) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singleb | Single | Bib | Single | Bi | Single | Bi | Single | Bi | ||||
| Beforec | Afterd | |||||||||||
| Suspension | 0.032 | 1.65 | 0.045 | 0.20 | 0.68 | 0.034 | 0.077 | 6.2 | 10 | 135 | 212 | |
| 0.013 | 0.105 | |||||||||||
| Microcarrier (Cytodex 3) | 0.029 −9%a |
1.25 −24% |
0.027 | 0.12 | 0.27 −60% |
0.017 | 0.027 −65% |
5.4 | 8.0 −21% |
164 | 241 +14% |
|
| 0.017 +31% |
0.042 −60% |
|||||||||||
| Microcarrier (Cytopore 1)f | 0.025 −22% |
1.14 −31% |
0.017 | 0.11 | 0.15 −78% |
0.013 | 0.018 −77% |
6.7 | 7.2 −29% |
184 | 203 n.a. |
|
| 0.010 −23% |
0.030 −71% |
|||||||||||
aPercent change compared to suspension culture
bSingle: single-temperature cultures; Bi: biphasic cultures
cSpecific productivity before low temperature shift
dSpecific productivity after low temperature shift
eCulture time is the time when culture viability decreases to ~60%; for Cytopore 1 culture, the time is termination time
fData based on the total cell density
Productivities of SEAP-producing CHO cells under different culture conditions
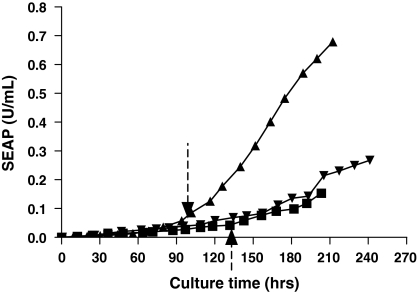
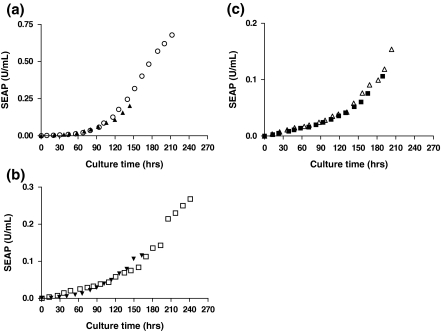
As in the single-temperature cultures, both Cytodex 3 and Cytopore 1 microcarrier cultures showed much lower maximum accumulated SEAP activity than the suspension culture (Fig. 2). The suspension culture showed the highest specific productivity; followed by the Cytodex 3 culture and the Cytopore 1 culture (Table 1). In all biphasic cultures, maximum accumulated SEAP activities, volumetric and specific SEAP productivities were improved compared to the respective single-temperature cultures although the effect was much smaller for Cytopore 1 cultures (Fig. 3; Table 1). Overall, all biphasic cultures outperformed the single-temperature cultures for SEAP production. The improved SEAP production in the biphasic cultures was due to the combined effects of extended viability and increased specific productivity due to the low temperature shift (Table 1). Biphasic microcarrier cultures underperformed the suspension cultures as in the single-temperature cultures. This was due to both lower maximum viable cell densities and lower specific productivities (Table 1).
Fig. 2.
Comparison of the productivities of SEAP-producing CHO cells under three different culture conditions in biphasic culture mode. Accumulated activity of SEAP in different culture conditions. [suspension (▲), Cytodex 3 (▼), Cytopore 1(■)]. Vertical dashed arrows at 95 and 133 h indicate the time of temperature reduction from 37 °C to 33 °C for suspension and microcarrier cultures, respectively
Fig. 3.
Comparison of the accumulated productivities of SEAP-producing CHO cells between single-temperature and biphasic culture mode in different culture conditions. a Suspension cultures. b Cytodex 3 cultures. c Cytopore 1 cultures. Solid symbols [suspension (▲), Cytodex 3 (▼), Cytopore 1(■)] represent single-temperature cultures. Open symbols [suspension (○), Cytodex 3 (□), Cytopore 1 (△)] represent biphasic cultures
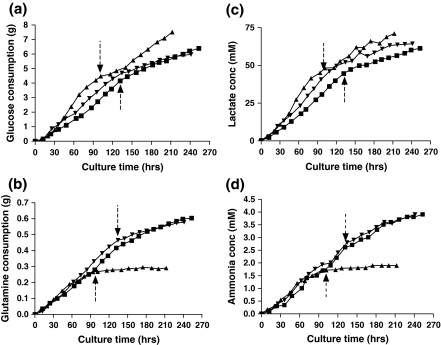
Metabolic activities of SEAP-producing CHO cells under different culture conditions
As described in “Materials and methods”, the cultures were intermittently fed glucose and glutamine to delay the onset of apoptosis. Figure 4 shows changes in metabolic activities of CHO cells in response to the temperature shift under the different culture conditions. As in the single-temperature cultures, the glucose and glutamine consumption rates of the Cytodex 3 culture were higher than the other cultures before the temperature reduction (Table 2). After the temperature shift, the glucose consumption rates were significantly lowered in all three culture conditions (Table 2), with the suspension culture having a slightly higher consumption rate than the microcarrier cultures. All of the single-temperature cultures showed a reduction in glucose consumption when the cells entered stationary phase (data not shown); however, the responses of the biphasic cultures to the temperature shift cannot be explained simply by the transition from exponential to stationary phase. When the glucose consumption rates of the biphasic cultures (after the temperature shift) were compared with the consumption rates in the stationary phase of corresponding single-temperature experiments, the suspension cultures showed a moderate increase, the Cytodex 3 cultures a moderate decrease, and the Cytopore 1 cultures were largely unchanged (data not shown).
Fig. 4.
Comparison of the metabolic activities of SEAP-producing CHO cells under three different culture conditions. a Glucose consumption. b Glutamine consumption. c Lactate production. d Ammonia production. Vertical dashed arrows at 95 and 133 h indicate the time of temperature reduction from 37 °C to 33 °C for suspension and microcarrier cultures, respectively. [suspension (▲), Cytodex 3 (▼), Cytopore 1(■)]
Table 2.
Summary of metabolic activities of SEAP-producing CHO cells in response to temperature reduction
| Culture conditions | qGlC (μmol/106 cells/h) | qLac (μmol/106 cells/h) | YLac/Glc (mol/mol) | qGln (nmol/106 cells/h) | qAmm (nmol/106 cells/h) | YAmm/Gln (mol/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beforea | Afterb | Before | After | Singlec | Before | After | Before | After | Single | |||
| Before | After | Before | After | |||||||||
| Suspension | 0.28 | 0.12 | 0.51 | 0.17 | 1.7 | 18 | 1.2 | 17 | 1.4 | 1.1 | ||
| 1.82 | 1.42 | 0.95 | 1.2 | |||||||||
| Microcarrier (Cytodex 3) | 0.32 +14%d |
0.07 −42% |
0.67 +31% |
0.13 −24% |
1.9 | 35 +89% |
7.8 +550% |
29 +66% |
11 +655% |
1.0 | ||
| 2.09 +15% |
1.82 +28% |
0.83 −13% |
1.4 +16% |
|||||||||
| Microcarrier (Cytopore 1) | 0.24 −14% |
0.10 −17% |
0.47 −8% |
0.12 −29% |
1.9 | 29 +57% |
11 +870% |
27 +54% |
13 +804% |
0.9 | ||
| 1.96 +8% |
1.20 −16% |
0.93 −2% |
1.1 −7% |
|||||||||
aBefore low temperature shift
bAfter low temperature shift
cSingle-temperature cultures
dPercent change compared to suspension culture
All of the cultures showed a substantial decrease in glutamine consumption rate upon temperature reduction; however, the relative consumption rates changed dramatically between the different cultures. In contrast to the changes in glucose consumption, the specific glutamine consumption rates, which were somewhat higher in the microcarrier cultures before the shift, were dramatically higher in the microcarrier cultures, with the Cytopore 1 culture exhibiting the highest specific consumption rate. When compared with the stationary phase of the single-temperature experiments (all of which showed a decrease in glutamine consumption rate upon entering stationary phase), the suspension cultures showed a dramatic decrease in glutamine consumption rate, the Cytodex 3 cultures, a slight decrease in glutamine consumption rate, and the Cytopore 1 cultures, a slight increase in glutamine consumption rate (data not shown).
The lactate and ammonia production rates showed similar trends as the glucose and glutamine consumption rates. After the temperature reduction, the lactate and ammonia production rates were also significantly decreased in all cultures compared to the rates before the temperature shift (Table 2). A similar decrease was also seen in the single-temperature cultures when they entered stationary phase (data not shown). Before the temperature shift, the yields of lactate from glucose in the suspension culture were lower than in the other cultures. After the temperature shift, the Cytopore 1 culture showed a lower yield of lactate from glucose than the suspension and Cytodex 3 cultures (Table 2); however, in all cases the yields of lactate from glucose were lower in the biphasic cultures (after the temperature shift) than in the stationary phase of the corresponding single-temperature cultures (data not shown). The yields of ammonia from glutamine in the suspension and Cytopore 1 cultures showed similar values while Cytodex 3 culture showed a slightly lower value before the temperature shift. After the temperature shift, the Cytodex 3 culture showed a slightly higher value than the other cultures (Table 2). There was no clear trend when comparing the yields of ammonia in the biphasic cultures with the corresponding yields in the stationary phases of the single-temperatures; some increased and some decreased. In general, the yields of lactate from glucose decreased after the temperature switch, while the yields of ammonia from glutamine increased.
Discussion
The growth, production and metabolic activities of SEAP-producing CHO cells under three different culture conditions (suspension, Cytodex 3, Cytopore 1) were investigated under biphasic hypothermic conditions. The suspension and the Cytodex 3 culture remained viable for a significantly longer period in the biphasic cultures than in the single-temperature cultures, leading to higher integrated cell densities. Moore and coworkers reported that low temperature culture leads to growth arrest in the G0/G1 phase of the cell cycle and delays the onset of apoptosis (Moore et al. 1997). Slikker and co-workers reported that hypothermia enhanced expression of the anti-apoptosis protein, bcl-2, and protected against oxidative stress-induced cell death in CHO cells by increasing the activity of anti-oxidant enzyme, glutathione peroxidase (GSH-Px) (Slikker et al. 2001). Some or all of these mechanisms may play a role in the extended viability of the cell lines in this study.
Mild hypothermia has been reported to increase the specific productivity of a number of recombinant proteins (Furukawa and Ohsuye 1998; Kaufmann et al. 1999; Hendrick et al. 2001; Ducommun et al. 2002; Yoon et al. 2003; Fox et al. 2004). In our studies, the specific productivity of SEAP at 33 °C was higher than the productivity before the temperature shift or the productivity of the single-temperature culture, regardless of culture condition (i.e., suspension or microcarrier). However, the specific productivity of the microcarrier cultures increased approximately threefold while the specific productivity of the suspension culture increased nearly eightfold. This is a significant difference from the results of Tharmalingam and coworkers, who found a twofold increase in productivity of interferon-β upon temperature reduction in suspension culture and a 2.3-fold increase in productivity in microcarrier culture (Tharmalingam et al. 2008). Some of the differences are most likely due to the different protein products (SEAP vs. interferon-β), while other differences may be due to the timing of the temperature shift. In our studies, the temperature shift was applied as the cells entered stationary phase, while Tharmalingam et al. applied their temperature shift at 2 days after inoculation, while the cells were still in the exponential growth phase. In addition, our studies were performed in fed-batch culture while their studies were performed in batch culture, potentially causing differences in nutrient availability. Moreover, the feeding strategy allowed for the accumulation of ammonia which was particularly significant in the microcarrier cultures. This ammonia accumulation may have had a detrimental effect on the protein production. In a similar study performed with CHO cells producing tissue plasminogen activator (t-PA), we found that the temperature shift led to a complete cessation of t-PA productivity when performed in late exponential phase, further reinforcing the effects of both protein product and culture conditions on the utility of temperature reduction (data not shown).
As other investigators have reported (Reuveny et al. 1986; Borth et al. 1992; Weidemann et al. 1994; Furukawa and Ohsuye 1998), glucose and glutamine consumption rates and lactate and ammonia production rates decreased after the temperature switch, in all culture conditions. Before the temperature shift, the Cytodex 3 microcarrier culture exhibited higher nutrient consumption than the suspension culture, as in the single-temperature cultures. However, those trends were different after applying the mild hypothermia, suggesting that the metabolic responses of CHO cells to mild hypothermia were culture-condition dependent. All cultures showed a decrease in the yield of lactate from glucose with temperature reduction. However, there was an increased yield of ammonia from glutamine.
Conclusion
While a biphasic temperature shift increased specific productivity in all three culture conditions, suspension and attached to both solid and macroporous microcarriers, the effects were much more pronounced in the suspension cultures. Similarly, the biphasic temperature shift had the most beneficial effect on the integrated viable cell density (IVCD) for suspension cultures, leading to the greatest total enhancement in productivity in suspension cultures. Other investigators have reported different results, reinforcing the view that the beneficial effects of temperature reduction are cell-line and protein-product specific. Moreover, we have recently observed that temperature reduction can significantly alter the glycan profiles for glycoproteins produced from mammalian cell cultures (Nam et al. 2008), clearly indicating that more research is necessary to understand the fundamental physiological changes that occur during temperature reduction, particularly in the protein processing and post-translational pathways, to most effectively employ temperature reduction to improve overall productivity.
Acknowledgments
This work was supported in part by the National Science Foundation (BES-0075336). Cytodex 3 and Cytopore 1 microcarriers were generously provided by GE Healthcare.
References
- Berry JM, Barnabé N, Coombs KM, Butler M (1999) Production of reovirus type-1 and type-3 from Vero cells grown on solid and macroporous microcarriers. Biotechnol Bioeng 62:12–19. doi:10.1002/(SICI)1097-0290(19990105)62:1<12::AID-BIT2>3.0.CO;2-G [DOI] [PubMed]
- Bollati-Fogolin M, Forno G, Nimtz M, Conradt HS, Etcheverrigaray M, Kratje R (2005) Temperature reduction in cultures of hGM-CSF-expressing CHO cells: effect on productivity and product quality. Biotechnol Prog 21:17–21. doi:10.1021/bp049825t [DOI] [PubMed]
- Borth N, Heider R, Assadian A, Katinger H (1992) Growth and production kinetics of human x mouse and mouse hybridoma cells at reduced temperature and serum content. J Biotechnol 25:319–331. doi:10.1016/0168-1656(92)90164-5 [DOI] [PubMed]
- Buntemeyer H, Lutkemeyer D, Lehmann J (1991) Optimization of serum-free fermentation process for antibody production. Cytotechnology 5:57–67. doi:10.1007/BF00365534 [DOI] [PubMed]
- Choi Y, Ahn CJ, Seong KM, Jung MY, Ahn BY (2003) Inactivated Hantaan virus vaccine derived from suspension culture of Vero cells. Vaccine 21:1867–1873. doi:10.1016/S0264-410X(03)00005-7 [DOI] [PubMed]
- Chua FK, Yap MG, Oh SK (1994) Hyper-stimulation of monoclonal antibody production by high osmolarity stress in eRDF medium. J Biotechnol 37:265–275. doi:10.1016/0168-1656(94)90133-3 [DOI] [PubMed]
- Doi Y, Abe S, Yamamoto H, Horie H, Ohyama H, Satoh K, Tano Y, Ota Y, Miyazawa M, Wakabayashi K, Hashizume S (2001) Progress with inactivated poliovirus vaccines derived from the Sabin strains. Dev Biol 105:163–169 [PubMed]
- Ducommun P, Ruffieux PA, Kadouri A, von Stockar U, Marison IW (2002) Monitoring of temperature effects on animal cell metabolism in a packed bed process. Biotechnol Bioeng 77:838–842. doi:10.1002/bit.10185 [DOI] [PubMed]
- Ermonval M, Cacan R, Gorgas K, Haas IG, Verbert A, Buttin G (1997) Differential fate of glycoproteins carrying a monoglucosylated form of truncated N-glycan in a new CHO line, MadIA214214, selected for a thermosensitive secretory defect. J Cell Sci 110(Pt 3):323–336 [DOI] [PubMed]
- Fox SR, Patel UA, Yap MG, Wang DI (2004) Maximizing interferon-gamma production by Chinese hamster ovary cells through temperature shift optimization: experimental and modeling. Biotechnol Bioeng 85:177–184. doi:10.1002/bit.10861 [DOI] [PubMed]
- Frazzati-Gallina NM, Paoli RL, Mourão-Fuches RM, Jorge SA, Pereira CA (2001) Higher production of rabies virus in serum-free medium cell cultures on microcarriers. J Biotechnol 92:67–72. doi:10.1016/S0168-1656(01)00362-5 [DOI] [PubMed]
- Furukawa K, Ohsuye K (1998) Effect of culture temperature on a recombinant CHO cell line producing a C-terminal á-amidating enzyme. Cytotechnology 26:153–164. doi:10.1023/A:1007934216507 [DOI] [PMC free article] [PubMed]
- Hendrick V, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36:71–83. doi:10.1023/A:1014088919546 [DOI] [PMC free article] [PubMed]
- Hu XW, Xiao CZ, Huang ZC, Guo ZX, Zhang ZG, Li ZH (2000) Pilot production of u-PA with porous microcarrier cell culture. Cytotechnology 33:13–19. doi:10.1023/A:1008127310890 [DOI] [PMC free article] [PubMed]
- Junker BH, Wu F, Wang S, Waterbury J, Hunt G, Hennessey J, Aunins J, Lewis J, Silberklang M, Buckland BC (1992) Evaluation of a microcarrier process for large-scale cultivation of attenuated hepatitis A. Cytotechnology 9:173–187. doi:10.1007/BF02521745 [DOI] [PubMed]
- Kallel H, Rourou S, Majoul S, Loukil H (2003) A novel process for the production of a veterinary rabies vaccine in BHK-21 cells grown on microcarriers in a 20-l bioreactor. Appl Microbiol Biotechnol 61:441–446 [DOI] [PubMed]
- Kaufmann H, Mazur X, Fussenegger M, Bailey JE (1999) Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng 63:573–582. doi:10.1002/(SICI)1097-0290(19990605)63:5<573::AID-BIT7>3.0.CO;2-Y [DOI] [PubMed]
- Kong D, Gentz R, Zhang JL (1998) Long-term stable production of monocyte-colony inhibition factor (M-CIF) from CHO microcarrier perfusion cultures. Cytotechnology 26:131–138. doi:10.1023/A:1007997412002 [DOI] [PMC free article] [PubMed]
- Kong D, Cardak S, Chen M, Gentz R, Zhang J (1999a) High cell density and productivity culture of Chinese hamster ovary cells in a fluidized bed bioreactor. Cytotechnology 29:215–220. doi:10.1023/A:1008064217040 [DOI] [PMC free article] [PubMed]
- Kong D, Chen M, Gentz R, Zhang J (1999b) Cell growth and protein formation on various microcarriers. Cytotechnology 29:149–156 [DOI] [PMC free article] [PubMed]
- Landauer K, Wiederkum S, Dürrschmid M, Klug H, Simic G, Blüml G, Doblhoff-Dier O (2003) Influence of carboxymethyl dextran and ferric citrate on the adhesion of CHO cells on microcarriers. Biotechnol Prog 19:21–29. doi:10.1021/bp025568l [DOI] [PubMed]
- Mendonça RZ, Ioshimoto LM, Mendonça RM, De-Franco M, Valentini EJ, Beçak W, Raw I and Pereira CA (1993) Preparation of human rabies vaccine in VERO cell culture using a microcarrier system. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al.] 26: 1305–1317 [PubMed]
- Moore A, Mercer J, Dutina G, Donahue CJ, Bauer KD, Mather JP, Etcheverry T, Ryll T (1997) Effects of temperature shift on cell cycle, apoptosis and nucleotide pools in CHO cell batch cultures. Cytotechnology 23:47–54. doi:10.1023/A:1007919921991 [DOI] [PMC free article] [PubMed]
- Nam JH, Ermonval M, Sharfstein ST (2007) Cell attachment to microcarriers affects growth, metabolic activity, and culture productivity in bioreactor culture. Biotechnol Prog 23:652–660. doi:10.1021/bp070007l [DOI] [PubMed]
- Nam JH, Zhang F, Ermonval M, Linhardt RJ, Sharfstein ST (2008) The effects of culture conditions on the glycosylation of secreted human placental alkaline phosphatase produced in Chinese hamster ovary cells. Biotechnol Bioeng 100:1178–1192. doi:10.1002/bit.21853 [DOI] [PMC free article] [PubMed]
- Percheson PB, Trépanier P, Dugré R, Mabrouk T (1999) A phase I, randomized controlled clinical trial to study the reactogenicity and immunogenicity of a new split influenza vaccine derived from a non-tumorigenic cell line. Dev Biol Stand 98:127–132 discussion 133–4 [PubMed]
- Pirt SJ (1985) Parameters of growth and analysis of growth data. Principles of microbe and cell cultivation. Blackwell Scientific Publications, Oxford, pp 4–14
- Renard JM, Spagnoli R, Mazier C, Salles MF, Mandine E (1988) Evidence that monoclonal antibody production kinetics is related to the integral of the viable cells curve in batch systems. Biotechnol Lett 10:91–96. doi:10.1007/BF01024632 [DOI]
- Reuveny S, Velez D, Macmillan JD, Miller L (1986) Factors affecting cell growth and monoclonal antibody production in stirred reactors. J Immunol Methods 86:53–59. doi:10.1016/0022-1759(86)90264-4 [DOI] [PubMed]
- Rodriguez J, Spearman M, Huzel N, Butler M (2005) Enhanced production of monomeric interferon-beta by CHO cells through the control of culture conditions. Biotechnol Prog 21:22–30. doi:10.1021/bp049807b [DOI] [PubMed]
- Schmid G, Zilg H, Johannsen R (1992) Repeated batch cultivation of rBHK cells on Cytodex 3 microcarriers: antithrombin III, amino acid, and fatty acid metabolic quotients. Appl Microbiol Biotechnol 38:328–333. doi:10.1007/BF00170081 [DOI] [PubMed]
- Seth G, Hossler P, Yee JC, Hu W-S (2006) Engineering cells for cell culture bioprocessing? Physiological fundamentals. Adv Biochem Eng Biotechnol 101:119–164. doi:10.1007/10_017 [DOI] [PubMed]
- Slikker W 3rd, Desai VG, Duhart H, Feuers R, Imam SZ (2001) Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in Chinese hamster ovary cells. Free Radic Biol Med 31:405–411. doi:10.1016/S0891-5849(01)00593-7 [DOI] [PubMed]
- Sugawara K, Nishiyama K, Ishikawa Y, Abe M, Sonoda K, Komatsu K, Horikawa Y, Takeda K, Honda T, Kuzuhara S, Kino Y, Mizokami H, Mizuno K, Oka T, Honda K (2002) Development of Vero cell-derived inactivated Japanese encephalitis vaccine. Biologicals J Int Assoc Biol Stand 30:303–314 [DOI] [PubMed]
- Tharmalingam T, Sunley K, Butler M (2008) High yields of monomeric recombinant beta-interferon from macroporous microcarrier cultures under hypothermic conditions. Biotechnol Prog 24:832–838. doi:10.1002/btpr.8 [DOI] [PubMed]
- Trummer E, Fauland K, Seidinger S, Schriebl K, Lattenmayer C, Kunert R, Vorauer-Uhl K, Weik R, Borth N, Katinger H, Muller D (2006a) Process parameter shifting: part I. Effect of DOT, pH, and temperature on the performance of Epo-Fc expressing CHO cells cultivated in controlled batch bioreactors. Biotechnol Bioeng 94:1033–1044. doi:10.1002/bit.21013 [DOI] [PubMed]
- Trummer E, Fauland K, Seidinger S, Schriebl K, Lattenmayer C, Kunert R, Vorauer-Uhl K, Weik R, Borth N, Katinger H, Muller D (2006b) Process parameter shifting: part II. Biphasic cultivation—a tool for enhancing the volumetric productivity of batch processes using Epo-Fc expressing CHO cells. Biotechnol Bioeng 94:1045–1052. doi:10.1002/bit.20958 [DOI] [PubMed]
- Wang MD, Yang M, Huzel N, Butler M (2002) Erythropoietin production from CHO cells grown by continuous culture in a fluidized-bed bioreactor. Biotechnol Bioeng 77:194–203. doi:10.1002/bit.10144 [DOI] [PubMed]
- Weidemann R, Ludwig A, Kretzmer G (1994) Low temperature cultivation—a step towards process optimisation. Cytotechnology 15:111–116. doi:10.1007/BF00762385 [DOI] [PubMed]
- Wu SC, Huang GY (2002) Stationary and microcarrier cell culture processes for propagating Japanese encephalitis virus. Biotechnol Prog 18:124–128. doi:10.1021/bp010120q [DOI] [PubMed]
- Xiao CZ, Huang ZC, Li WQ, Hu XW, Qu WL, Gao LH, Liu GY (1999) High density and scale-up cultivation of recombinant CHO cell line and hybridomas with porous microcarrier Cytopore. Cytotechnology 30:143–147. doi:10.1023/A:1008038609967 [DOI] [PMC free article] [PubMed]
- Yoon SK, Song JY, Lee GM (2003) Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Bioeng 82:289–298. doi:10.1002/bit.10566 [DOI] [PubMed]