Abstract
In vitro cultures of cardiomyocytes have proven to be a useful tool for toxicological, pharmacological, and developmental studies, as well as for the study of the cellular and molecular mechanisms responsible for proper myocyte function. One deficient area of research is that of myocyte proliferation. Cardiomyocyte proliferation dramatically diminishes soon after birth and has a very limited occurrence within the adult heart, thus limiting the use of adult cells for proliferation studies. An improved understanding of the requirements for myocyte proliferation will allow for the development of better approaches to repair damaged heart tissue. Here, we provide a protocol for the reliable isolation of embryonic mouse myocytes. These myocytes behave similarly to those in vivo, including their ability to proliferate, providing an ideal system for the study of cardiomyocyte proliferation.
Keywords: Embryo, Cardiomyocyte culture, Myocardium, Mouse
Introduction
Cardiomyocytes have been studied in vitro for more than 40 years. In vitro cultures are useful for the study of myocytes because they allow for elimination of hemodynamic influences and control over the factors surrounding cardiac muscle cells. Due to the fragility of myocytes, isolation of these cells requires careful washes with enzymatic solutions and diligent adherence to purification steps. Previous protocols for myocyte isolation have been successful with adult and neonatal hearts; however, they have been less so with embryonic hearts.
In vitro studies with cardiomyocytes have been instrumental in advancing our knowledge into the causes of cardiac diseases, including myocyte cell death, but have been limited for understanding the dynamics of myocyte cell proliferation. One important key to the heart’s survival after cardiac infarction is the ability to induce myocyte proliferation as part of the repair process. Myocytes proliferate rapidly during development, but rely predominantly on cell growth for heart enlargement after birth (Li et al. 1997a, b). Recent studies of myocyte proliferation have focused on zebrafish, myocytes induced from stem cells, knockout mice, and myocyte cultures from neonatal rats (Pasumarthi et al. 1996; Poss et al. 2002; Beltrami et al. 2003; Ahuja et al. 2007; Qi et al. 2007; van Laake et al. 2008). Adult and neonatal rat myocytes are the predominant source of myocytes for cardiac muscle studies. However, the direct, in vitro study of mouse embryonic myocyte proliferation would provide further insight into the requirements for myocyte proliferation and a valuable tool for myocyte maturation studies. Due to the difficulty of isolating embryonic myocytes, very few myocyte studies focus on embryonic myocytes. Here we provide an enhanced protocol for the consistent and efficient collection and cultivation of embryonic myocytes. These in vitro cultures are comparable to in vivo myocytes due to their pervasiveness for proliferation, as well as for the production of growth factors necessary for proper heart development and regeneration.
Materials
Solutions
Isolation media
Two hundred and fifty milligram Fetuin (Sigma, St. Louis, MO, #F-2379), 20 mg Ascorbic Acid (Sigma #A2218), 10 g Bovine Serum (Gemini Bio Products, West Sacramento, CA., #100-106), 10 mL pen/strep (Invitrogen, Carlsbad, CA, #600-5140) was added to 1L liquid Ham’s media (Thermo Fisher, Waltham, MA, #SH30026.02). 1.176 g NaHCO3 (Mallinckrodt Baker, Phillipsburg, NJ, #3506) was added to adjust the pH to 7.4. The solution was filter-sterilized through a 0.22 μm pore filter.
Culture media
Two hundred and fifty microliter Non-essential Amino Acids (MediaTech #25-025-l) and 250 μL Pen/Strep was added to 250 mL DMEM (MediaTech, Manassas, VA, #15-017-CM) with 25 mL Fetal Bovine Serum. The solution’s pH was adjusted to 7.4 and filter-sterilized.
Enzyme buffer
The following reagents were dissolved in 1 L 1× PBS: 8.0 g NaCl (VWR, West Chester, PA, #BDH0286), 2.0 g Dextrose (Sigma #DP434), 200 mg KCl (Fisher #P217), 5.75 mg NaH2PO. 4 H2O (Thermo Fisher #S374), and 1.0 g NaHCO3. pH was adjusted to 7.4 and filter sterilized.
Enzyme solution
Hundred milliliter enzyme buffer was combined with 1 mL 2.5 mg/mL Pancreatin (Invitrogen 02-0036DG). The solution’s pH was adjusted to 7.4 and filter sterilized. This solution was made fresh for each use.
Tyrodes Buffer (Sigma #T2145) and 1× PBS solution (MediaTech #55-0310PC) were prepared according to manufacturers’ directions. pH was adjusted to 7.4 and filter sterilized.
Supplies
Cell culture plates (10 cm) were purchased from Sarstedt (Nümbrecht, Germany, #831802). Tissue culture dishes (35 mm) and conical tubes (50 mL) were purchased from Corning (Lowell, MA, #430165, #430829). Anti-α-sarcomeric actin primary antibody was purchased from Sigma (#A2547). Anti-phosphorylated histone H3 primary antibody was purchased from Millipore (Billerica, MA, #06-570). Vascular endothelial growth factor (VEGF) and transforming growth factor-beta two (TGF-β2) ELISA kits were purchased from R&D biosystems (Minneapolis, MN, #MMV00, #DB250). Murine laminin was purchased from Sigma (#L2020). Donkey anti-mouse and goat anti-rabbit secondary antibodies conjugated to alexa-flourescence were purchased from Invitrogen (#A21203, #A11034) and used at the indicated concentration.
Procedures
Preparation of embryonic cardiomyocyte culture
At least 48 h prior to myocyte cell collection, culture dishes were coated with laminin and incubated at 37 °C until the day of collection. For a 12 well plate, 500 μL 1× PBS and 6 μL laminin (6 μg) were added per well. On the morning of cell collection the laminin solution was removed and the plate was returned to 37 °C until needed. To isolate sufficient numbers of myocytes, a minimum of six timed-pregnant Swiss Webster mice were anesthetized in a CO2 chamber. E12.0 embryos (average ten embryos per litter) were removed and rinsed in Tyrodes salts buffer. Embryonic hearts were microdissected away from the embryo and placed in a 35 mm diameter dish containing culture medium prewarmed to 37 °C. The hearts were maintained at 37 °C in culture media until completion of collection of all hearts. Once all hearts were collected, they were combined and rinsed five times with culture media.
After rinsing, the hearts were placed in 10 mL of enzyme solution in a 50 mL conical tube (Tube A). The tube was slowly rotated for 10 min in a 37 °C incubator. Myocyte cells were not removed during this first incubation period and therefore the solution was discarded without removing the hearts. Next, fresh 10 mL of enzyme solution was added to Tube A (containing the heart samples) and incubated at 37 °C with slow rotation for 10 min. After this incubation, the solution (excluding the heart samples) was transferred to a new 50 mL tube (Tube B), and 10 mL culture medium was added to Tube B in order to stop the enzymatic reaction. Tube B was spun for 10 min at 1000 rpm. After centrifugation, the supernatant was removed from Tube B. The cells were resuspended in 1 mL culture media and stored in a third 50 mL tube (Tube C) at 37 °C. Concurrently, fresh 10 mL of enzyme solution was added to Tube A and rotated for 10 min at 37 °C.
These incubation and centrifugation steps were repeated six to eight times until the heart cells were completely removed and the heart structures were reduced to a single matrix conglomerate. All cell collections were combined into Tube C at the end of each centrifugation. After the enzymatic incubations were complete, Tube C was spun for 10 min at 1000 rpm. The supernatant was removed and cells were resuspended with 10 mL isolation medium. The resuspended cells were then placed in a 10 cm culture dish and incubated at 37 °C for 2–3 h.
During this 2–3 h period the non-myocyte cell types (predominantly fibroblast cells) adhered to the culture dish. After incubation, the isolation medium, containing predominantly myocyte cells, was removed and spun for 10 min at 1000 rpm. The cells were then resuspended in culture medium and counted using a trypan blue exclusion assay and a hemocytometer. Two and a half to three million cells were cultured per well in a 12 well plate with 1 mL culture medium. Due to the presence of red blood cells, cells which will not survive, and residual fibroblast cells, this number does not represent the final myocyte cell count. However, it will yield 50–60% confluency of adherent cells. Due to cell proliferation 100% confluency will be reached within 24–48 h.
After 24 h incubation, the culture medium containing red blood cells and dead cell material was removed. The cells were rinsed and then given fresh culture medium. Primary embryonic myocyte cultures were given 1-2 mL of culture media per well. Half of the media was replaced every 48 h. If desired, confluent myocyte cultures can be divided 1:2 after 1 week of culture by use of the above enzymatic steps.
Immunoflourescent labeling assay and cell counts
Cells used for immunofluorescent staining were cultured on four well chamber slides (Thermo Fisher, #177437). Anti-α-sarcomeric actinin (diluted 1:100) and anti-phosphorylated histone H3 (diluted 1:100) antibodies labeled myocytes and proliferating cells in embryonic myocyte culture. Donkey anti-mouse alexaflor-594 (diluted 1:1000) and goat anti-rabbit alexaflor-488 (diluted 1:1000) were used as secondary antibodies. All immunostainings were performed as previously reported by Rodgers et al. (2006). Cells were fixed with four percent paraformaldehyde for 10 min and permeabilized for 5 min with 0.5% (v/v) Triton-X-100 (Fisher #BP151) in 10 mM Pipes (Sigma #P1851) solution. Slides were blocked with 3% (w/v) BSA (Sigma #A3311) and 0.05% (v/v) Tween 20 for 2 h at room temperature and incubated overnight at 4 °C with the primary antibodies. After 8 h of washing with 0.2% (w/v) BSA and 0.05% (v/v) Tween 20 the slides were incubated overnight with the secondary antibodies. Following another 8 h of washes with the above washing solution, the slides were mounted with aquapolymount mounting medium (Polyscience Inc, Warrington, PA, #H3569). A single wash with bis-benzymide (1:500, Invitrogen #18606) was included during the second day of washes. The percentages of myocyte cells (actinin positive cells) were calculated based on the total number of nuclei within the culture.
Proliferation analysis
Myocyte proliferation was determined by labeling with an anti-phosphorylated histone H3 antibody. The cultures were co-stained with anti-α-sarcomeric actin per the above procedure. The percentage of proliferating myocyte cells was calculated by comparing the total number of phosphorylated histone H3 labeled myocyte cells to the total number of myocyte cells.
rtPCR
Total cell and tissue RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA, #74104) according to the manufacturer’s instructions. cDNA was synthesized from 2 μg RNA using a Transcriptor First Strand cDNA kit (Roche, Indianapolis, IN, #04379012001) with an anchored oligo-dT primer. Reverse transcription PCR products were generated using 1 μg cDNA, 1 U Taq polymerase (Promega, Madison, WI, #M3008), 0.25 μL of 100 mmol/L dNTP mix (Invitrogen #10297-018), 2.5 μL 10× Taq buffer (Promega #M190G), 1.5 μL of 25 mM MgCl2 (Promega #A351H), 2 μL of 10 μM forward and reverse primers, and 17.5 μL dH2O. Amplicon sizes and primers were as follows: 343 bp α-actinin 2 (forward: 5′-GAA ATA GTC GAT GGC AAT GTG A-3′, reverse: 5′-CAA TGT CTT CAG CAT CCA ACA T-3′), 389 bp cardiac Troponin I (forward: 5′-GTT CTC TGC CTC TGG AGA TCA T, reverse: 5′-GTT CTT GGT GAC TTT TGC TTC C-3′), and 335 bp GAPDH (forward: 5′-GAT GAC ACT AAG AAG GTG GTG A-3′, reverse: 5′-AAT TGT GAG GGA GAT GCT CAG T-3′). The thermocycling conditions were 94 °C for 2 min; then 26 cycles of 94 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a 5 min 72 °C final extension. PCR products were separated on 1% tris-borate-EDTA (TBE) agarose gels, stained with ethidium bromide, and visualized using a Gel Logic 100 Image system (Kodak).
ELISA analysis
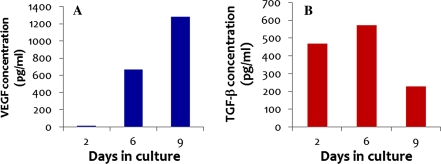
Detection of VEGF and TGF-β2 was by ELISA analysis. Culture media for this test were collected 2, 6, and 9 days after isolation of cells and used immediately without dilution. The ELISA analysis was carried out per manufacturer’s directions (R&D Systems). The activation step was included for accurate detection of TGF-β2.
Results and discussion
Although our protocol is similar to other myocyte isolation procedures, we have made several key improvements which allow for the enhanced and consistent isolation of primary embryonic myocytes. Of most significance, we found that embryonic myocytes are highly susceptible to cellular damage from enzymatic solutions in standard isolation protocols. Trypsin is frequently used for the digestive step during isolating of myocyte cells (Wang and Kang 1999; Fu et al. 2005; Nickson et al. 2007; Sreejit et al. 2008). Many enzymatic steps also involve lengthy digestive time periods (Burt 1982; Wang and Kang 1999; Song et al. 2000; Nickson et al. 2007). While determining the modifications necessary for a reliable embryonic isolation, we identified that cell viability is greatly reduced by the use of trypsin or extensive incubation periods with pancreatin. Either one of these methods, or the combination of both, will lead to few or no viable cells. Previous researchers keep the harvested myocyte cells on ice until all digestive reactions are completed (Burt 1982; Nuss and Marban 1994; Fu et al. 2005; Sreejit et al. 2008). We determined that cell viability can be doubled by incubating the primary myocytes in complete culture medium at 37 °C during the procedures (Tube C). Past studies have used serum levels varying from 5% to 20% of either FBS or Horse serum (Burt 1982; Wang and Kang 1999; Nickson et al. 2007; Sreejit et al. 2008). We found that embryonic myocyte cultures grow well 10% FBS. More importantly, the addition of nonessential amino acids was essential for long term viability of cells. Nonessential amino acids are not often reported as required additive for the culture of cardiomyocyte cells (Burt 1982; Sreejit et al. 2008). Though Sreejit et al. (2008) and Song et al. (2000) report using 1% gelatin and Ahuja et al. (2004) reported using fibronectin as a pre-coat for myocyte culture dishes, such pre-coat steps are not often reported (Burt 1982; Nuss and Marban 1994; Fu et al. 2005; Rosenblatt-Velin et al. 2005; Nickson et al. 2007). We, however, determined that a pre-coating with laminin was absolutely necessary for effective embryonic myocyte adhesion. Table 1 provides a summary of these changes.
Table 1.
Outline of significant changes in the presented myocyte isolation protocol compared to past protocols
| Previously reported method | Currently presented modification |
|---|---|
| Trypson digestion | Pancreatin digest |
| Elongated digestion time | Short, 10 min digestion |
| Maintain cells on ice | Maintain cells at 37 °C |
| No non essential amino acids | Add non essential amino acids to culture medium |
| No pre-coat of culture dish | Pre-coat culture dish with laminin |
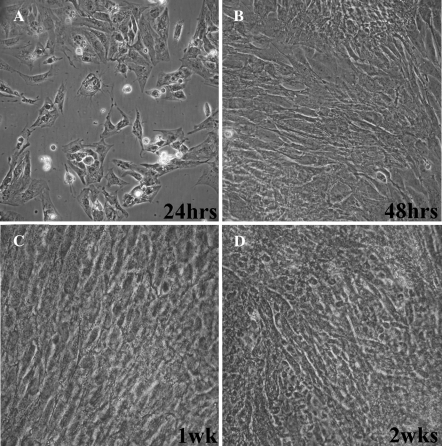
In our cultures, attachment of the embryonic myocyte cells occurs overnight and the cells are beating within the first 24 h. During the first week of culture, the myocytes reach confluency and ultimately synchronize their beating throughout the entire culture. Bright field images of myocyte cell cultures after 1, 2, 7, and 14 days post isolation are shown in Fig. 1. These images clearly demonstrate these cultures at confluency within 48 h. Beating myocytes from this procedure have been maintained for up to 4 weeks and can withstand at least one division into new culture dishes.
Fig. 1.
Bright field images of embryonic myocyte cultures 24 h (a), 48 h (b), 1 week (c), and 2 weeks (d) after isolation
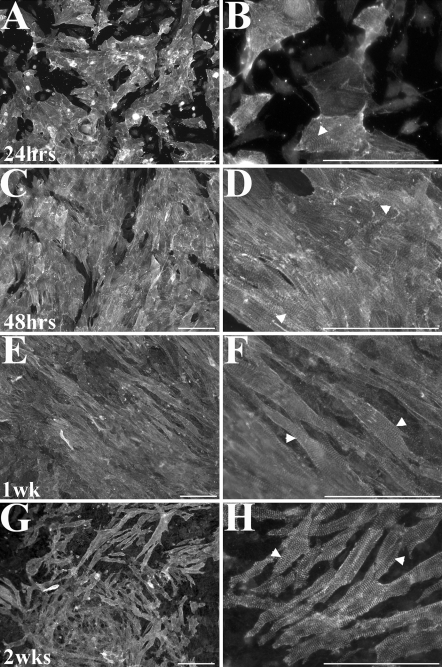
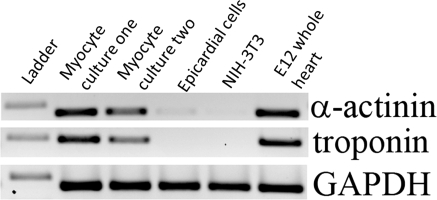
Immunofluoresent detection for α-sarcomeric actin is used to identify myocyte cells (Fig. 2) and to calculate the percentage of myocytes to total cells. The expression of myocyte expressing components troponin and α-actinin is also confirmed by rt-PCR (Fig. 3). We calculated the total percentage of myocyte cells to be 86% 24 h post-isolation. A percentage of fibroblast cells is necessary during the initial culture period in order to support proper cardiomyocyte growth and function (personal communications, Dr. Carol Gregorio and Dr. Joe Bahl). Even with the presence of fibroblast cells, we observe that the majority of the cells proliferating within the culture are myocyte cells. Therefore, we believe our concentrations of cardiomyocytes are sufficient for the in vitro study of myocyte cell proliferation and dynamics of myofibriller assembly.
Fig. 2.
Immunofluorescent images of embryonic myocytes labeled for α-sarcomeric actinin. Sarcomere striations can clearly be seen at 24 h (a, b), 48 h (c, d), 1 week (e, f), and 2 weeks (g, h) after isolation (arrow heads in higher magnification images). Scale bar = 100 μm
Fig. 3.
Expression of myocyte markers were confirmed using rt-PCR. The molecular weight of the ladder band is 400 bp
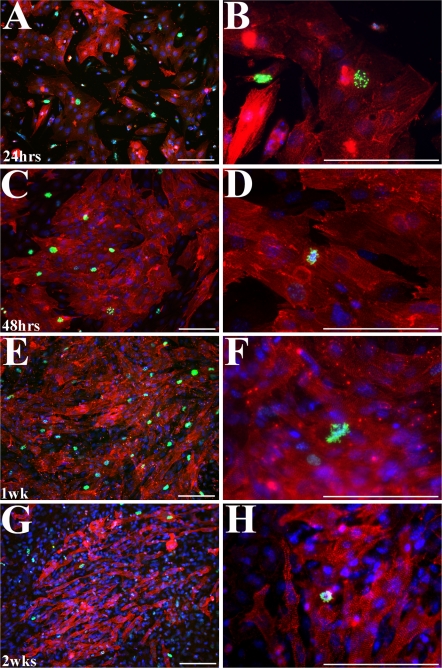
In order to study myocyte proliferation, it is important to know the percentage of cells which undergo proliferation in vitro as well as whether these cells behave similarly to their in vivo counterparts. We assessed proliferation over time based on presence of the mitotic marker anti-phosphorylated histone H3 (PH3) antibody (Fig. 4, Table 2). Previous studies have reported successfully using PH3 as an indicator of proliferation within their system (Galli et al. 2004; Eltawil et al. 2008; Lathia et al. 2008; Wright et al. 2008). In our cultures, an average of 7% of observed myocyte cells are PH3 positive after 24 h in culture. A maximum of 15% labeled cells is observed at early time points. This percentage does not decrease until the 2 week time point, at which time only 2% of the myocyte cells are PH3 positive. Though the fibroblast cells are also PH3 positive, they represented less than half of the total positive cells during the first week of culture. It may be possible to maintain the myocyte rate of proliferation by passaging the cells weekly. Though our percentage of positive cells is not as high as in vivo BrDU studies, it is still sufficient for the productive study of proliferation and myocyte dynamics (Qi et al. 2007). Of interest, the sarcomeric striations become more organized as myocyte proliferation decreases. The sarcomeres themselves appear to disassemble while a myocyte cell is undergoing mitosis (Figs. 2, 4). Our observations are consistent with Ahuja et al. (2004), demonstrating that myofibrils need to be disassembled during proliferation.
Fig. 4.
Immunofluorescent images of embryonic myocytes co-labeled for phospho-histone H3 (green, mitosis marker) and α-sarcomeric actinin (red, myocyte marker). Phospho-histone H3 cells are seen for up to 2 weeks after isolation. Nuclei (blue) are labeled with bis-benzimide. Images were taken at 24 h (a, b), 48 h (c, d), 1 week (e, f), and 2 weeks (g, h) after isolation. Scale bars = 100 μm
Table 2.
Percentage of phospho-histone H3 positive cells in culture over time
| Time in culture | Percentage of all cells which are PH3 positive | Percentage of myocytes which are PH3 positive | Percentage of PH3 positive cells which are myocytes |
|---|---|---|---|
| 24 h | 9 | 7 | 64 |
| 36 h | 7 | 7 | 69 |
| 48 h | 7 | 6 | 73 |
| 1 week | 5 | 6 | 68 |
| 2 weeks | 2 | 2 | 46 |
We are also able to demonstrate that our embryonic myocyte cells produce VEGF and TGF-β2 (Fig. 5) in vitro. Both of these growth factors are known to be present within the myocardium of the developing heart and to be necessary for proper heart development (Dickson et al. 1993; Boyer et al. 1999; Tomanek et al. 1999; Giordano et al. 2001; Camenisch et al. 2002). From these results, we conclude that our in vitro embryonic myocyte cultures recapitulate cardiac gene expression and growth factor production related to proliferative potential and myocyte maintenance.
Fig. 5.
Expression of TGF-β2 and VEGF protein by primary embryonic myocytes in culture over time
Primary cardiomyocyte cultures are a valuable tool for toxicological, pharmacological, and developmental studies. In vitro studies allow researchers to explore the molecular and cellular mechanisms for proper cardiomyocyte function. Typically, there is limited to no proliferation of cardiomyocytes after parturition. Two recent studies show the potential for myocyte proliferation within adult rats and transgenic mice under normal conditions as well as after cardiac infarction (Bruel et al. 2007; Hassink et al. 2008). A comprehensive understanding of how myocyte cells proliferate during development will allow for the development of improved methods for inducing proliferation within the injured mature heart. Here we provide a model system for the study of myocyte proliferation without the limits of in vivo assays or the potential artifacts of in vitro transformed myocyte cell lines. The ability to culture embryonic mouse myocytes will prove to be a valuable tool for studying and understanding myocyte proliferation and has the potential for the development of myocyte repair therapeutics after cardiac infarction.
Acknowledgments
Development of this protocol would not have been possible without correspondence with Dr. Joe Bahl. We would also like to acknowledge Dr. Earl N. Myer for inspiration on this topic. Funding was provided by HBLI 077493 (T.D.C) AND T32-HL07249 (L.S.R.).
References
- Ahuja P, Perriard E, Perriard JC, Ehler E (2004) Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci 117:3295–3306 [DOI] [PubMed]
- Ahuja P, Sdek P, MacLellan WR (2007) Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev 87:521–544 [DOI] [PMC free article] [PubMed]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB (1999) TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol 208:530–545 [DOI] [PubMed]
- Bruel A, Christoffersen TE, Nyengaard JR (2007) Growth hormone increases the proliferation of existing cardiac myocytes and the total number of cardiac myocytes in the rat heart. Cardiovasc Res 76:400–408 [DOI] [PubMed]
- Burt JM (1982) Electrical and contractile consequences of Na+or Ca2+ gradient reduction in cultured heart cells. J Mol Cell Cardiol 14:99–110 [DOI] [PubMed]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE (2002) Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol 248:170–181 [DOI] [PubMed]
- Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ (1993) RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development 117:625–639 [DOI] [PubMed]
- Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell’accio F (2008) A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. doi:10.1016/j.joca.2008.11.003 [DOI] [PMC free article] [PubMed]
- Fu J, Gao J, Pi R, Liu P (2005) An optimized protocol for culture of cardiomyocyte from neonatal rat. Cytotechnology 49:109–116 [DOI]
- Galli LM, Willert K, Nusse R, Yablonka-Reuveni Z, Nohno T, Denetclaw W, Burrus LW (2004) A proliferative role for Wnt-3a in chick somites. Dev Biol 269:489–504 [DOI] [PubMed]
- Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J Jr, Chien KR, Ferrara N (2001) A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A 98:5780–5785 [DOI] [PMC free article] [PubMed]
- Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Riviere AB, Doevendans PA, Field LJ (2008) Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res 78:18–25 [DOI] [PMC free article] [PubMed]
- Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, Arumugam TV, Mattson MP (2008) Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci 28:13978–13984 [DOI] [PMC free article] [PubMed]
- Li F, Wang X, Bunger PC, Gerdes AM (1997a) Formation of binucleated cardiac myocytes in rat heart: I. Role of actin-myosin contractile ring. J Mol Cell Cardiol 29:1541–1551 [DOI] [PubMed]
- Li F, Wang X, Gerdes AM (1997b) Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation. J Mol Cell Cardiol 29:1553–1565 [DOI] [PubMed]
- Nickson P, Toth A, Erhardt P (2007) PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res 73:48–56 [DOI] [PMC free article] [PubMed]
- Nuss HB, Marban E (1994) Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol 479(Pt 2):265–279 [DOI] [PMC free article] [PubMed]
- Pasumarthi KB, Kardami E, Cattini PA (1996) High and low molecular weight fibroblast growth factor-2 increase proliferation of neonatal rat cardiac myocytes but have differential effects on binucleation and nuclear morphology. Evidence for both paracrine and intracrine actions of fibroblast growth factor-2. Circ Res 78:126–136 [DOI] [PubMed]
- Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Science 298:2188–2190 [DOI] [PubMed]
- Qi X, Yang G, Yang L, Lan Y, Weng T, Wang J, Wu Z, Xu J, Gao X, Yang X (2007) Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev Biol 311:136–146 [DOI] [PubMed]
- Rodgers LS, Lalani S, Hardy KM, Xiang X, Broka D, Antin PB, Camenisch TD (2006) Depolymerized hyaluronan induces vascular endothelial growth factor, a negative regulator of developmental epithelial-to-mesenchymal transformation. Circ Res 99:583–589 [DOI] [PubMed]
- Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T (2005) FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115:1724–1733 [DOI] [PMC free article] [PubMed]
- Song W, Lu X, Feng Q (2000) Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res 45:595–602 [DOI] [PubMed]
- Sreejit P, Kumar S, Verma RS (2008) An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim 44:45–50 [DOI] [PubMed]
- Tomanek RJ, Ratajska A, Kitten GT, Yue X, Sandra A (1999) Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn 215:54–61 [DOI] [PubMed]
- van Laake LW, Passier R, Doevendans PA, Mummery CL (2008) Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res 102:1008–1010 [DOI] [PubMed]
- Wang GW, Kang YJ (1999) Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther 288:938–944 [PubMed]
- Wright JW, Pejovic T, Fanton J, Stouffer RL (2008) Induction of proliferation in the primate ovarian surface epithelium in vivo. Hum Reprod 23:129–138 [DOI] [PubMed]