Abstract
Despite the successful transfer of mammalian in vitro techniques for use with fish and other vertebrates, little progress has been made in the area of invertebrate tissue culture. This paper describes the development of an in vitro technique for the culture of both cells in suspension and tissue explants from the gill, digestive gland and mantle of the zebra mussel (Dreissena polymorpha) and their successful maintenance in culture for up to 14 days. Cell suspensions from the gills and digestive gland were the most successful technique developed with viability >80% maintained for up to 8 days in culture, suitable for use in short term toxicity tests. Tissue explants from the mantle were also maintained in culture for up to 14 days. This paper describes the challenges involved in the development of a novel in vitro culture technique for aquatic invertebrates.
Keywords: In vitro, Cell culture, Invertebrate, Zebra mussel
Introduction
Much research has been undertaken on the development of in vitro techniques on aquatic vertebrates with the establishment of numerous fish cell lines and primary cultures, particularly for use in aquatic toxicology (Dowling and Mothersill 1999; Strum et al. 2001; Nichols et al. 2006). Despite this relatively little success has been achieved with the in vitro culture of aquatic invertebrate tissues. To date only one molluscan cell line, the Bge cell line (Hansen 1976) has been developed and to our knowledge it has not yet been possible to maintain reproducing invertebrate cells in vitro. This lack of progress is partially due to very limited knowledge about the nutritional requirements of invertebrate cells, the use of materials and media developed for mammalian cells, as opposed invertebrate-specific and the enormous number of variables including dissection, decontamination, dissociation and culture environment (media development) involved in establishing a new in vitro technique. Despite this some success has been achieved with the reported in vitro maintenance of heart cells of the scallop Pecten maximus (Le Marrec-Croq et al. 1998), soft-shell clam Mya arenaria (Kleinschuster et al. 1996) and oyster Crassostrea spp (Buchanan et al. 1999; Domart-Coulon et al. 2000). Primary culture of gill cells from Mytilus galloprovincialis (Gomez-Mendikute et al. 2005) and mantle cells of the blue mussel M. galloprovincialis (Cornet 2006), the freshwater bivalve Lamellidens marginalis (Barik et al. 2004) and the deep sea clam Calyptogena soyoae (Koyama and Aizawa 2000) were also reported. However, for mollusc tissue culture, neither cell proliferation nor reproduction has been reported.
One of the primary uses of cells and tissues in culture is for the investigation of the mechanism of toxicity on individual cells removed from the process of the entire organism. Isolated cells from various bivalve mollusc species have been used in toxicological studies including digestive gland and blood cells (Le Pennec and Le Penec 2001, 2003; Chelomin et al. 2005; Parolini et al. 2009), heart cells (Domart-Coulon et al. 2000), mantle (Cornet 2006) and gills (Gomez-Mendikute et al. 2005). The usefulness of bivalve molluscs as bioindicators of environmental change has long been established due to their high filtration rate, ability to accumulate and bioconcentrate toxicants, widespread distribution and abundance and their primarily stationary, benthic life-cycle. The zebra mussel has been proposed as the freshwater counterpart of the blue mussel Mytilus edulis in mussel watch programmes for freshwater environments (Minier et al. 2005), and has been used in biomonitoring for numerous freshwater contaminants (Quinn et al. 2004; Binelli et al. 2005; Marie et al. 2006). For this reason we developed an in vitro culture method for the maintenance of cells and tissues from the digestive gland, gills and mantle of the zebra mussel. These organs were chosen as isolated cells from the digestive gland of various bivalves have been previously used in toxicological studies (Le Pennec and Le Penec 2001, 2003; Chelomin et al. 2005) and the gills are complex organs that function in ion transport, respiration, food capture and sorting and are in constant contact with the environment, as is the mantle. The in vitro culture technique for the zebra mussel was developed in laboratories in both Dublin (Ireland) and Brest (France).
Materials and methods
Zebra mussels
For the in vitro development work carried out in Dublin mussels were collected from a depth of 1 m by scraping floating buoys in a marina in Killenure Lough, Lough Ree, County Westmeath, Ireland (Quinn et al. 2006). In France mussels were collected from the river Rance, near St. Malo in the north of Brittany. In the laboratory mussels were thoroughly scrubbed and maintained in 10 L glass tanks containing autoclaved de-chlorinated tap water in a room with constant temperature (15 °C) and a 12 h light/dark cycle. Tanks were cleaned twice a week and fresh water added. Mussels were fed a commercial bivalve food Phytoplex, and replaced every 2 weeks to ensure healthy animals for culturing. The gills and mantle are large independent organs that can be easily dissected offering a pure tissue sample. The Digestive gland however, is mixed with the gonad and stomach in the visceral mass and despite the use of a binocular microscope it was impossible not to include some gonad and stomach in the sample. This must therefore be considered a mixed tissue culture.
Preparation of primary cell cultures
All cell culture procedures were carried out under sterile conditions in a laminar flow hood (Class II microbiological safety cabinet) exclusively used for cell-culture purposes. The final and most successful culture procedure involving tissue dissection, decontamination, dissociation and culture is summarised in Table 1.
Table 1.
Summary of the in vitro tissue culture technique developed for the explant and cell suspension of tissues of the zebra mussel (Dreissena polymorpha)
| Dissection & decontamination |
| Scrape mussel clean under running water. Place in sterile water |
| Place in antibiotic sol. (2×) for 2 hr in laminar flow partially on ice |
| Rinse mussel in ethanol 70°, allow to dry |
| In laminar flow, rinse mussel with 10 mL sterile H2O, dissect tissues |
| Place tissues (digestive gland, gill, gonad) in sterile buffer solution |
| Trim tissue to ensure as pure a sample as possible |
| Rinse in petri dish of sterile buffer solution. Cut tissue into 1–2 mm2 pieces. |
| Antibiotic sol. (separately) |
| ×4 (10 mL of anti-b sol.) 30 min |
| ×2 (5 mL of anti-b sol. + 5 mL buffer sol.) 20 min |
| ×1 (2.5 mL anti-b sol. + 7.5 mL buffer sol.) 10 min |
| Rinse in sterile buffer sol. |
| Dissociation with pronase |
| Add tissue to 0.025% pronase in buffer sol. (12.5 mg in 50 mL) with antibiotic (×1) |
| Keep separate organs from different animals in separate tubes |
| Store at slight angle at 4 °C for specified time |
| Filter sample liquid through autoclaved gauze 60 μm (slowly) into centrifuge tube |
| Rinse tube and gauze with buffer solution |
| Centrifuge filtered liquid for 3 min at 1,200 rpm |
| Remove liquid, add buffer sol. and re-centrifuge, 3 min at 1,200 rpm. ×2 |
| Remove supernatant |
| Add media, mix cells using pipette. Calculate cell density using a haemacytometer, adjust media volume if needed |
| Place cell suspensions in culture, 1 mL in petri (8.8 cm2) and 0.3 mL in 24 well multiwell plate. Add 0.5 and 0.2 mL media (respectively) after 24 h 15 °C incubation |
| Explant |
| Take tissue material from gauze and place in petri (8.8 cm2) with buffer solution |
| Cut up tissue into 1 mm2 using scalpel |
| Place 10–12 explants in petri dish. If drying place drop of media on explant |
| Leave for 10 min |
| Add 1 mL of media and slowly immerse explant. Add 0.5 mL after 24 h incubation |
| Incubate at 15 °C |
Sol solution
Decontamination
Bleach and ethanol
Several methods of tissue decontamination were tested. Initially after dissection tissues were placed in 5, 10 and 20% bleach (Milton, containing 2% sodium hypochlorite) diluted with sterile buffer solution (1 L sterile cell culture water (Sigma), 2.32 g NaCl (Sigma), osmolarity 80–100 mOSM, pH 7.5), for a specified period of time (10, 30, 60 and 120 s). 70° ethanol was also used with exposure times of 10, 30, 60 and 120 s to disinfect tissues.
DTT
In order to increase the effectiveness of the antibiotic solution on the gills, they were first immersed in DTT (1,4 dithiothreitol; Euromedex), a mucolytic agent used to decrease the bacteria trapped in the mucus in the gills. Once dissected and rinsed with buffer solution, the gills were immersed in 50 mM DTT (made using buffer solution) for 15 min, before being added to the antibiotic solution.
Antibiotic solution
The first antibiotic mixture to be tested had been previously developed for use with rainbow trout (Oncorhynchus mykiss; Mothersill et al. 1995) and contained: PBS 500 mL, Fungizone Amphotericin B (250 μg/mL)(Gibco) 10 mL, Penicillin–Streptomycin (5,000 IU/mL–5,000 μg/mL; Gibco) 20 mL and Gentamicin (50 mg/mL; Gibco) 15 mL. Tissues were exposed to this antibiotic mixture for 0.5, 1, 2, 3, 5, 10 and 30 min. A mixture of the two methods was tried with tissues placed in bleach for 10 or 30 s, rinsed in buffer solution and placed in antibiotic solution for up to an hour. The antibiotic solution was made up in both buffer solution and Hanks balanced salt solution (HBSS) to both full strength and half this strength.
In the final protocol (summarised in Table 1) the final antibiotic solution (1 L) that was developed contained: 972 mL sterile water (Sigma), 20 mL Penicillin–Streptomycin (5,000 IU/mL–5,000 μg/mL, Gibco), 80 μg/mL Gentamicin (50 mg/mL, Gibco) and 40 μg/mL Kanamycin (759 μg/mL, Sigma). This antibiotic solution (×4) was subsequently diluted in half (×2) and to a quarter (×) with buffer solution (1 L sterile cell culture water (Sigma), 2.32 g NaCl (Sigma), osmolarity 80–100 mOSM, pH 7.5). Tissues were cut up into 1–2 mm2 pieces and placed into ×4, ×2 and ×1 antibiotic solution for 30, 20 and 10 min respectively, and were finally rinsed in buffer solution (Table 1).
Dissociation
Collagenase and trypsin
Tissues were added to a solution containing the enzymes Collagenase (from Clostridium histolyticum type IV, Sigma–Aldrich) and trypsin (2.5%, Gibco) at a concentration of 5 mg collagenase per 1 mL trypsin. The digestive gland, gills and mantle were exposed for 10, 15 and 20 min respectively. Collagenase was also mixed with buffer solution (6 mg per 1 mL of buffer solution) with exposure lasting 15 min.
Collagenase and trypsin were also added to the culture media to aid tissue dissociation in culture. Collagenase was added at a concentration of 1 and 0.5 mg/mL, with Trypsin being added at a concentration of 0.025 %.
EDTA
Chemical dissociation was undertaken using EDTA (ethylenediaminetetraacetic acid, Sigma–Aldrich), which works by the formation of complexes with Mg++ and Ca++ used in the gap junctions in tissues. As it was not toxic to cells variable concentrations were tested, generally around 5 mM. This solution was made up in sterile buffer, with the osmolarity and pH fixed to 80–100 mOSM and 7.5 respectively. After decontamination with antibiotic solution the dissected tissues were added to the EDTA solution and placed on an agitator for 1 h.
Pronase
The final and most successful dissociation method shown in Table 1 that was subsequently adopted for use in all experiments involved enzymatic dissociation using Pronase (from Streptomyces griseus, Sigma–Aldrich) at a concentration of 0.025% (75 mL buffer solution, 25 mL Antibiotic solution (×4) and 25 mg Pronase (4 units/mL, Sigma–Aldrich)). After antibiotic treatment the different tissues were placed separately in tubes containing pronase and left to dissociate at 4 °C for a specified time (12, 16 and 40 h for the digestive gland, gills and mantle respectively).
Media development
Several types of media, enriched with various supplements were used to encourage the growth and maintenance of cells from the zebra mussel in culture. Dissected tissues were cultured in supplement enriched commercial media. RPMI 1640 (Sigma–Aldrich) was the first media tried as it had previously been successfully developed for the primary culture of explants from the rainbow trout (O. mykiss;Mothersill et al. 1995; Kilemade and Mothersill 2001). Supplements added to this media to encourage cell growth were: Foetal calf serum (FCS; Gibco) 13%, Horse serum (Gibco) 7%, Human recombinant insulin (Novo Nordisk A/S) 0.05 IU/mL, Hydrocortisone (Sigma–Aldrich) 1 μg/mL, Penicillin–Streptomycin (Gibco) 50–50 IU/mL (μg/mL), l-Glutamine (Gibco) 20 mmol/L, Fungizone–Amphotericin B (Gibco) 1 μg/mL. The next media under examination was Leibovitz L-15 (Gibco). This media was first used undiluted and was later diluted to 50 and 10% using both HBSS and buffer solution and supplemented with: FCS (heat inactivated) 10%, l-Glutamine 20 mmol, Penicillin–Streptomycin (Gibco) 50–50 IU/mL (μg/mL), Fungizone–Amphotericin B (Gibco) 1 μg/mL. Other supplements added to the culture media include 5 and 15 μL/mL 0.1% lipid mixture solution (Sigma–Aldrich) and 10 and 100 μL/mL 0.1% yeast extract solution (Sigma–Aldrich). These stock solutions were made in HBSS. A combination of 10 and 100 μL/mL of both solutions together was also added to the media.
Zebra mussel haemolymph osmolarity was measured using an Osmomat 030 osmometer (Gonotec, Germany) and was found to be ~80 mOSM and the pH tested at 7.5. The most successful media developed and subsequently used in Table 1 was 15% Leibovitz L-15 media consisting of (1 L): 150 mL Leibovitz L-15 (Gibco), 5 mL Penicillin–Streptomycin (5,000 IU/mL–5,000 μg/mL, Gibco), 2 mL Gentamicin (50 mg/mL, Gibco), 0.01 g Kanamycin (759 μg/mL, Sigma), 0.01 g Phenol red (Sigma), 843 mL Sterile water (Sigma), 2.38 g HEPES (Gibco). The osmolarity and pH were regulated to 80–100 mOSM and 7.5 respectively, and the media sterile filtered (0.2 μm, Nalgene) and stored for up to 6 months at −20 °C. Once defrosted to room temperature 100 mL FCS (10% final volume, Gibco), 10 mL l-Glutamine (200 mM, Gibco) was added and the osmolarity and pH checked.
Culture vessels and incubation
Initially tissues were cultured in 25 cm2 polystyrene and collagen coated polystyrene flasks (NUNC). These flasks have the advantage of being air tight when sealed, helping to reduce contamination. Media volumes of 1, 1.5 and 2 mL were added to these flasks used for the culture of tissue explants only. In the final culture procedure (Table 1) after dissociation with pronase the dissociated tissue was slowly filtered through autoclaved gauze (mesh 60 μm) into a centrifuge tube. The tissue left on the gauze was cut into 1–2 mm2 explants and added to a petri dish (diameter 8.8 cm2, NUNC; 10–12) and each well of the 24 well multiwell plate (diameter 1.9 cm2, NUNC; 3–4). For the cell suspension method the gauze was rinsed with sterile buffer solution and the liquid containing the cells in suspension was centrifuged for 3 min at 1,200g. The supernatant was pipetted off and the cells washed in buffer solution. The supernatant was removed and culture media added to a density of ~2 million cells per mL, calculated using a haemacytometer. For both explant and suspension methods 1 and 0.3 mL of media was added to a petri dish (diameter 8.8 cm2, NUNC) and 24 well multiwell plates (diameter 1.9 cm2, NUNC) respectively, and placed in an incubator (Leec, Nottingham, UK) at 15 °C. A further 0.5 and 0.2 mL (respectively) of media was added after 24 h incubation, creating a total volume of 1.5 and 0.5 mL for the petri dish and 24 multiwell plate respectively. After the first 4 days in culture half of the media was changed in the culture vessel. The media was fully changed every 4 days after that.
Cell characterisation
Cells were characterised based on their morphology and attachment (Buchanan et al. 1999), identified using light microscopy at ×200, ×400 and ×1,000 (oil immersion). Attempts were also made to identify cells in culture using electron microscopy. Cells and tissues in culture were washed with phosphate buffer solution (PBS; 12 mM sodium dihydrogen orthophosphate dihydrate, 37.7 mM disodium hydrogen orthophosphate anhydrous, 72.7 mM sodium chloride, 5 L of distilled water) and fixed using 0.1 M Sorensen’s phosphate buffer. However, after a subsequent PBS rinse all cells in culture became detached from the culture substrate and therefore could not be examined.
Cell viability and proliferation
Cell viability in both tissue explants and cells in suspension was based on adhesion and elongation of cells, and the formation and maintenance of a cell matrix (Buchanan et al. 1999). The viability of cells in suspension was also tested using the trypan blue (Gibco) exclusion test whereby a sample of media containing the cells in suspension was added to the trypan blue dye (50:50). The number of healthy cells excluding the dye per square on a 1 mm3 graticule was counted using an inverted microscope and a haemacytometer. The average of three counts was taken and the percentage viability calculated. Viability was measured when the cells were initially placed in suspension (0 h exposure) and after fixed time periods of 4, 6, 8, 11 and 14 days. The trypan blue exclusion test was unsuccessful with tissue explants due to the presence of large volume of tissue absorbing the dye.
Cell proliferation was investigated using immunocytochemical analysis, by measuring expression of the monoclonal mouse Anti-Proliferating Cell Nuclear Antigen antibody (PCNA; Clone PC-10, DAKO, Cambridge, UK) previously shown to cross react with all vertebrate species and numerous invertebrate species, (Lyons-Alcantara et al. 1999; Kilemade and Mothersill 2001; Zaldibar et al. 2004). Immunoreactivity was investigated using the avidin-biotin complex (ABC) method of immunoperoxidase staining, using a mouse monoclonal vectastain Elite ABC kit (Vectastain Corporation Burlingame, USA), based on the standard indirect immunoperoxidase technique as described by Kilemade and Mothersill (2001) with sections of normal human tonsil as a positive control. After culturing, the media was pipetted off and cells were fixed in 10% formalin for 1 h, covered in PBS and left for 24 h at 4 °C. The culture vessel with cells attached were placed in a moisture chamber and washed with PBS. Concentrations of the antibody tested were 1:200; 1:400; 1:800; 1:1,200; 1:1,600; 1:2,400; 1:3,200. The cells were counterstained with filtered Mayer’s haematoxylin (30 s), blued in running hot water and cover slipped and mounted in glycergel. Brown staining was indicative of a positive reaction. As cell adhesion was proving to be a problem attempts were also made to test PCNA on suspended cells in a monolayer attached onto glass slides after centrifugation at 800g for 3 min using a Shandon cytospin 3.
Statistical analysis
Viability tests of cells in suspension were undertaken in triplicate using different animals and three cell counts were made for each exposure, therefore n = 9. All data were expressed as the arithmetic mean +/− the standard error of the mean (SEM). Cell viability was tested for significance (95%) using one way analysis of variance (ANOVA).
Results
Tissue culture development
Explants
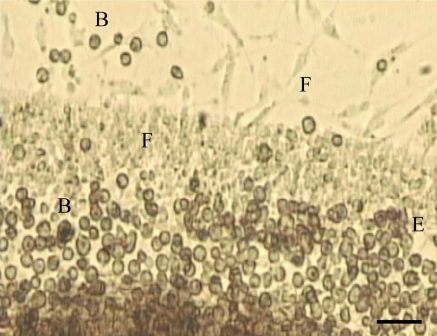
As the in vitro culture method began to develop over a period of 6–12 months with the optimisation of the culture media, disinfection and dissociation techniques, the explants started to adhere to the culture vessel and were maintained in culture for up to 14 days. Cells described as elongated and fibroblast-like, attached and spread out onto the culture vessel migrating from the explant, so that they were no longer ball shaped (spheroid) and formed a matrix of cells around the explant (Fig. 1). Although gill and digestive gland explants were maintained in culture, cell migration from these explants was not as good as with the mantle. As can be seen from Table 2, after 4 days culture, cells from the mantle explant started to adhere to the culture vessel, elongate and migrate out from the tissue. Within 8 days culture a good matrix of cells was found around the explant. Viable tissue explants were maintained in culture for up to 14 days (Table 2) after which time viability decreased. Digestive gland explants also showed good cell adherence, but not as much elongation as mantle cells. Zebra mussel gill cells did not form good explants (Table 2), and showed greater viability when cultured in suspension.
Fig. 1.
Explant of mantle from the zebra mussel after 8 days in culture showing migration of fibroblast-like (F) and ball shaped (B) cells from the explant (E). Magnification ×200. Scale bar = 100 μm
Table 2.
Results from observations (×3) of the culture (×3) of gill, digestive gland (DG) and mantle from the zebra mussel as tissue explants and as cells in suspension
| 4 days | 8 days | 14 days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Attach | Elongate | Matrix | Attach | Elongate | Matrix | Attach | Elongate | Matrix | |
| Explant culture | |||||||||
| Mantle | +/++ | ++ | −/+ | +++ | +++ | +++ | ++ | ++ | +/++ |
| (Cilia + flagella moving) | |||||||||
| DG | + | +/− | − | ++ | +/++ | + | ++ | +/++ | + |
| (Flagella moving) | |||||||||
| Gill | + | − | − | −/+ | −/+ | −/+ | − | − | − |
| Cell suspension | |||||||||
| Mantle | + | + | − | +/++ | +/++ | − | + | + | − |
| DG | +/++ | + | − | ++ | ++ | +/++ | ++ | +/++ | + |
| Gill | ++/+++ | ++/+++ | + | +++ | +++ | +++ | ++/+++ | ++/+++ | ++ |
| (Cilia + flagella moving) | (Cilia + flagella moving) | ||||||||
−, ≤40%; +, 41–60%; ++, 61–80%; +++ >81% of cells in field of view (×400 magnification), in relation to cell attachment, elongation and development of cell matrix
Cell suspensions
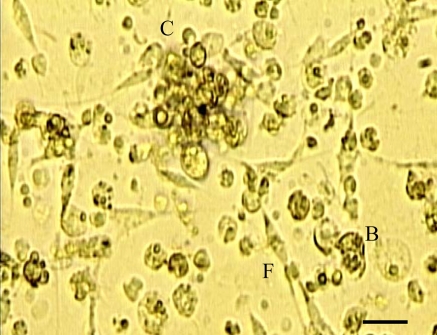
Cell suspension was the most successful culture method developed for the gill and digestive gland from the zebra mussel. Fewer lipid cells were found in cell suspensions as they were removed by the centrifugation step. Cells described as elongated and fibroblast-like, attached and spread out onto the culture vessel, so that they were no longer ball shaped (spheroid), and formed a matrix of cells (Fig. 2). Cell suspensions were best maintained in a culture vessel with a smaller surface area, with the 24 well multiwell plate giving the best results (Table 3) and was most successful in areas of lower cell density (around the edges of the culture vessel). Gill cells were the most successful, having good cell attachment and elongation after 4 days, and cilia and flagella functioning up to 8 days in culture (Table 2). Cell elongation continued up to 14 days in culture when cells could be seen to form a cell matrix. Digestive gland cells were also maintained well in suspension, but took longer than the gill cells to attach and elongate (8 days; Table 2). However, after 14 days in culture these cells still looked healthy and remained elongated. Mantle cells were not as well maintained in suspension as they were when explanted, and did not generally attach well or elongate when in suspension, even after 8–14 days in culture.
Fig. 2.
Dissociated gill cells from the zebra mussel after 8 days in culture, cells have aggregated into cell clumps (C) with fibroblast-like cells (F) and ball shaped cells (B) attached to the substrate. Magnification ×400. Scale bar = 50 μm
Table 3.
Summary of the effectiveness of the various culture vessels used for in vitro culture of tissues of the zebra mussel
| Type | Surface area (cm2) | Optimal vol. (mL) | Cell attachment | Cell elongation | Cell viability |
|---|---|---|---|---|---|
| NUNC flask | 25 | 2 | − | − | − |
| NUNC collagen coated flask | 25 | 2 | − | − | − |
| NUNC petri dish | 8.8 | 1.5 | ++ | ++ | ++ |
| NUNC 24 multiwell dish | 1.9 | 0.5 | +++ | +++ | +++ |
−, ≤40%; +, 41–60%; ++, 61–80%; +++, >81% of cells in field of view (×400 magnification) for cell attachment, elongation and viability
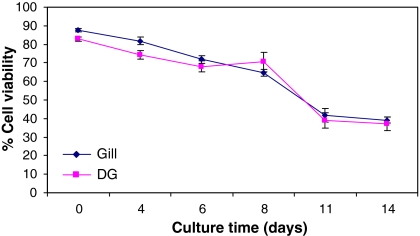
Cell viability and proliferation
Viability using the trypan blue exclusion test was initially measured when the media was added to the cells after dissociation and subsequently after different time intervals in culture (Fig. 3). Generally the average initial cell viability from 3 counts was around 85–90% (Fig. 3), but was consistently >80%. Measured after 4 days in culture, digestive gland cell viability was found to have dropped to ~75%, while gill cells maintained >80% viability (Fig. 3). After 11 days in culture cell viability was halved and remains constant up till day 14. Various problems were encountered when using the immunocytochemical technique to assess cell proliferation by the measurement of PCNA in the cells in culture. The technique was found to be too harsh on the cells and all cells became dislodged and were lost during the process. The cells centrifuged onto the glass slides using a cytospin also became dislodged. Therefore cells and tissues from the zebra mussel are sufficiently attached to the culture vessel to allow the use of this technique.
Fig. 3.
Viability of gill and digestive gland (DG) cells from the zebra mussel in suspension after various periods in culture measured using the trypan blue exclusion test. Percentage of healthy cells per square on a 1 mm3 graticule, average of ×3 counts from ×3 different animals. Cell density ~2 million cells per mL
Decontamination
Bleach and ethanol
Bacterial and fungal contamination was found to persist after short exposures (10 and 30 s) to both bleach and 70° ethanol (Table 4). However, although the longer exposure times (60 and 120 s) were successful at preventing contamination (Table 4), they were detrimental to tissue viability as few cells or explants were found to attach to the culture vessel.
Table 4.
Summary of the decontamination techniques used during the development of tissue culture method for the zebra mussel and their effect on cell viability
| Disinfectant | Disinfection | Cell viability | ||
|---|---|---|---|---|
| <30 sec | 60 sec | <30 sec | 60 sec | |
| Bleach | ||||
| 5% | − | − | + | − |
| 10% | − | + | + | − |
| 20% | − | + | − | − |
| Ethanol 70o | − | + | + | − |
| Initial anti-b | <10 min − |
>15 min + |
<10 min + |
>15 min − |
| Final anti-b (X4, X2, X1) |
10 min − |
30, 20, 10 min + |
10 min + |
30, 20, 10 min +++ |
| DTT | Whole tissue + |
Cut tissue + |
Whole tissue + |
Cut tissue + |
For cell viability: −, ≤ 40%; +, 41–60%; +++, >81% of cells in field of view (×400 magnification). For disinfection: −, infected with bacteria; +, no bacteria present. Anti-b, antibiotic solution
DTT
Initially when tissues were being exposed whole to the antibiotic solution exposure to DTT helped to reduce the occurrence of bacterial contamination (Table 4). However, once the tissues were cut into 1–2 mm2 pieces before antibiotic treatment, no difference in contamination was noticed between tissues exposed and not exposed to DTT. This step was therefore removed from the procedure.
Antibiotic solution
Although exposure for 15 min to the initial antibiotic solution (developed for use with fish cells) did prevent bacterial and fungal contamination cells did not attach and elongate on to the culture vessel and no cell matrix were formed (Table 4). This is probably due to the antibiotic solution being too strong for the cells. No difference was noticed between the use of buffer solution or HBSS. Shorter exposures and exposure to half strength solution resulted in bacterial and fungal contamination.
Only when the final antibiotic solution was introduced was the fine balance between cell attachment, elongation and the formation of a cell matrix, the maintenance of cell viability (>80%) and the reduction of bacterial and fungal contamination, achieved (Table 4). With an initial 10 min exposure at each antibiotic concentration (×4, ×2 and ×1) bacterial contamination persisted (Table 4). Therefore exposure times were changed to 30, 20 and 10 min for the ×4, ×2 and ×1 antibiotic concentrations respectively. Although infrequent contamination did persist (contamination with gram negative bacteria, protists and fungi), it was not a major problem (Table 5).
Table 5.
Percentage of gill and digestive gland (DG) cells (n = 168) in suspension isolated from the zebra mussel, contaminated with bacteria and fungi
| % Non-infected | % Bacteria | % Fungi | % Both | |
|---|---|---|---|---|
| Gill | 98.8 | 1.2 | 0 | 0 |
| DG | 89 | 6.1 | 2.5 | 2.4 |
Tissue dissociation
Initially tissues were not dissociated and were just cut into small pieces and explanted. However, as few individual cells were seen to migrate from the explant in the culture vessel it became evident that some degree of dissociation was necessary.
Collagenase and trypsin
Collagenase and trypsin were the first dissociation enzymes to be used. Trypsin was found to be too strong, over dissociating the tissues and was detrimental to cell viability (Table 6). Collagenase dissolved in buffer solution was less harmful to the tissues but cell attachment was low and no cell elongation was observed. The addition of collagenase and trypsin to the culture medium did seem to aid attachment although cells did not elongate or form a cell matrix.
Table 6.
Summary of different methods of tissue dissociation and their effect on cell viability on tissues of the zebra mussel in culture
| Dissociation method | Dissociation | Cell viability | ||
|---|---|---|---|---|
| No dissociation | − | − | ||
| Collagenase | 6 mg/ml PBS + |
5 mg/ml trypsin + |
6 mg/ml PBS + |
5 mg/ml trypsin − |
| Trypsin | 2.5% in PBS + |
0.025% in media + |
2.5% in PBS − |
0.025% in media − |
| EDTA | 5 mM + |
5 mM + |
||
| Pronase | 0.025% in buffer ++ |
0.025% in buffer ++ |
||
−, ≤40%; +, 41–60%; ++, 61–80% of cells in field of view (×400 magnification) for tissue dissociation and cell viability
EDTA
After chemical dissociation using EDTA cell attachment and limited cell elongation did occur (Table 6). However, after continued experimentation no increase in cell elongation was observed and it was therefore decided to try pronase.
Pronase
Using this method of enzymatic dissociation tissues were found to be appropriately dissociated while cell viability remained consistently high (>80%). However, if left too long in pronase (e.g. gills left for 24 h) tissues will dissociate completely leaving few explants or cell aggregates, but a lot of cells in suspension. Pronase was not as toxic to the tissues as trypsin (Table 6). The optimum dissociation times were 12, 16 and 40 h for the digestive gland, gill and mantle respectively.
Media
RPMI 1640
Explants and cells from the zebra mussel maintained in the RPMI 1640 media did not attach to the culture vessel, and were often found floating in the media (Table 7). Cells that did attach remained round and did not elongate.
Table 7.
Effects of various cell culture media and supplements on cells of the zebra mussel in culture
| Cell attachment | Cell elongation | Cell viability | |
|---|---|---|---|
| RPMI 1640 | − | − | − |
| Leibovitz old | + | − | − |
| Yeast supplement | − | − | − |
| Lipid supplement | + | − | − |
| 15% Leibovitz | +++ | +++ | +++ |
−, ≤40%; +, 41–60%; ++, 61–80%; +++, >81% of cells in field of view (×400 magnification) for cell attachment, elongation and viability
Leibovitz L-15
Although attachment did improve using the initial Leibovitz L-15 medium, cells remained round with few cells migrating from the explants. No difference was noticed between the use of HBSS or buffer solution to dilute the L-15. The use of 0.1% lipid solution did seem to increase attachment, but still no cell migration or elongation was observed. Addition of the yeast solution to the media showed no obvious effect, nor did the addition of 10 and 100 μL/mL 0.1% lipid and 0.1% yeast solution.
With the development of the 15% Leibovitz L-15 media, cells in culture started to attach and elongate on the culture vessel as described in the tissue culture section above (Table 7). Cell attachment, elongation and formation of a cell matrix were all observed using this media (Figs. 1, 2). The cilia on gill cells was seen to beat rhythmically after up to 8 days in culture and cells remained viable for up to 14 days in culture (Table 2).
Culture vessels
The surface area of the 25 cm2 flasks was too great. Even when several explants were cultured, few explants attached and no cell migration was observed. No difference between collagen coated and normal flasks was found (Table 3). Better results were found with the reduced surface area of the petri dish and 24 well multiwell plate (Table 3). The NUNCLON surface coated vessels (NUNC) were found to produce better cell adhesion than the Falcon petri dish. For cell suspensions the 24 well multiwell plate gave the best results, while the Petri dish containing 10–12 explants was best for tissue explants. Although good results were also obtained when 3–4 explants were cultured in each well of the 24 multiwell plate.
Media change
The addition of fresh media after every 4 days had a noticeable effect on the cells. In one experiment the media was changed in only half of the gill cells in suspension in a 24 multiwell plate. A huge increase in cell attachment and elongation was observed in the cells where the media had been changed.
Discussion
In the present study a technique for the maintenance of cells from the gill, digestive gland and mantle of the zebra mussel was developed. Establishing a new technique for tissue culture is a difficult process, involving the development and testing of numerous different techniques for dissection, decontamination, dissociation and culture (media, temperature, culture vessel) and different combinations of these techniques. Considerable variation exists between the different techniques reported in the published data also.
Although some areas of invertebrate tissue culture have been successful with the establishment of over 200 cell lines from insects and ticks (Mitsuhashi 1989), most efforts to develop permanent and proliferative cell cultures from aquatic invertebrates have been unsuccessful. Relatively little work on the in vitro culture of aquatic invertebrates has been published, largely owing to the fact that experimental failures are generally not suitable for publication in most scientific journals (Rinkevich 1999). Invertebrate tissue culture has been based on the idea that all the cells from different animal taxa are basically the same, having similar nutrient requirements, are controlled by the same developmental and physiological biochemical pathways and are under the expression of identical genes (Rinkevich 1999). Therefore techniques developed for the growth and maintenance of vertebrate, particularly mammalian cells have been adapted for use with invertebrate tissues. Although having been successful with other invertebrates (e.g. insects), this technique does not seem to have worked with aquatic invertebrates.
Being filter feeders bivalves come into direct contact with micro-organisms such as bacteria, fungi and protists and can harbour such infectious agents in their tissues, particularly the gills. A standard protocol for the decontamination of tissues from various bivalve species, involving surface decontamination, depuration, aseptic removal of tissues and a series of antibiotic washes has been developed (Odinstova and Khomenko 1991; Le Marrec 1995) and was used here with success on the zebra mussel, with contamination maintained under control. The use of antibiotics and other decontaminates can negatively effect the cells health (Odinstova and Khomenko 1991) and a delicate balance between decontamination and the maintenance of cell viability above the 80% needed for use in cell culture, must be found through trial and error. Several methods of tissue dissociation were attempted, the most successful of which involved a prolonged exposure to low concentrations of the enzyme pronase. The enzyme trypsin, commonly used for dissociation of invertebrate tissues (Domart-Coulon et al. 1994; Takeuchi et al. 1994), was found to be too harsh on zebra mussel cells reducing viability, a problem also experienced by other authors (Le Marrec 1995).
Development of the proper culture media is obviously of crucial importance to the maintenance of cells in culture. In the present study the media developed was based on the commercial Leibovitz L-15 medium, with the osmolarity and pH changed to that of the mussel’s haemolymph. Leibovitz L-15 has previously been used in aquatic invertebrate culture and is the most successful medium to date (Domart-Coulon et al. 1994; Kleinschuster, et al. 1996; Le Marrec-Croq et al. 1998; Birmelin et al. 1999; Le Pennec and Le Penec 2001). This may be due to its high amino acid content, an important component of the mollusc diet (Renault et al. 1995). Recommendations on how often media should be changed vary from every 2 days (Takeuchi et al. 1994), 4 days (Kleinschuster et al. 1996), 3 weeks (Brewster and Nicholson 1979), to not at all (Gomez-Mendikute et al. 2005). It was found that for the zebra mussel a media change every 4–6 days was suitable. Supplements added to this culture medium included l-glutamine, foetal calf serum (FCS) and a series of antibiotics. There is considerable contradiction in the literature regarding the addition of supplements to culture media for aquatic invertebrates. Several authors (Takeuchi et al. 1994; Renault et al. 1995; Kleinschuster et al. 1996; Birmelin et al. 1999) recommend the use of serum in the culture media to increase cell viability and aid attachment to the culture vessel, while others reported it toxic above 2% (Odinstova et al. 1994) and 20% (Domart-Coulon et al. 1994). Growth factors were found by Domart-Coulon et al. (1994) to have a significantly positive effect on oyster heart cell cultures, but this finding has been subsequently contradicted by Wen et al. (1993). In view of such conflicting findings, it would seem that the best policy when formulating a media for the culture of tissues of a previously unstudied animal is still a matter of trial and error.
There has been a recent increase in the development of molluscan cell culture for use in vitro toxicity tests. Although in vitro toxicological studies may be less sensitive and produce a different metabolic pattern than whole organism exposures, they can still provide a good mechanism to study metabolic pathways, difficult to study in vivo. Most of these exposures use homogenised digestive gland or haemocytes in short term (up to 96 h) cultures (Le Pennec and Le Penec 2001, 2003; Chelomin et al. 2005; Canesi et al. 2008; Parolini et al. 2009), but few report the development of a technique for the longer term maintenance of tissues and cells in culture. However, some success has been reported in mantle explants from bivalves with the detection of DNA synthesis after 13 days in culture (Koyama and Aizawa 2000), mitotic figures from 7-day-old cultures (Cornet 2006), and functional viability after 40 days (Barik et al. 2004). Gill cells were also maintained in suspension for up to 18 days with 50% viability (Gomez-Mendikute et al. 2005). Numerous published reports of cell reproduction were suspected of having been contaminated by thraustochytrid species (common marine and freshwater heterotrophic protists; Rinkevich 1999) and as far as the authors are aware despite being able to maintain living cells in culture for aquatic invertebrates, the ability to encourage cell reproduction and the growth of differentiated tissue in vitro has still not yet been achieved.
In the present study, both explant and cell suspension methods were investigated. The ultimate aim in the development of this technique was for its potential use in vitro toxicity tests to study the mechanistic effect of various environmental pollutants on cells and tissues isolated from the zebra mussel. As the cell suspension method consistently produced more viable cultures than the explant method and in the more environmentally relevant tissues of the gills and digestive gland, efforts were concentrated on this technique. The trypan blue test offers a relatively simple method for assessing cell viability but could only be measured on cells in suspension owing to the tissue mass of the explant absorbing too much of the dye making it difficult to differentiate between the live and dead cells. A further limitation of this method was its intrusive nature as in order to assess cell viability it is necessary to kill the cells being assessed. This resulted in the assessment of cell viability based on visual observation such as attachment and elongation and formation of a cell matrix as described by Buchanan et al. (1999) which by their nature can be somewhat subjective parameters and difficult to quantify. Viability of cultured cells was also tested using the 3-(4,5-dimethyl-2thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction test, previously adapted for use with bivalve cells (Domart-Coulon et al. 1994, 2000; Le Pennec and Le Penec 2001, 2003). However, this test was not suitable for use with the cell suspension method developed, as although cells did attach in suspension and migrate from the explants, they did not form a truly confluent layer. The expected correlation between MTT activity and cell density was not found due both to an uneven cell distribution in the culture wells owing to tissues not being fully dissociated (formation of cell clumps) and the removal of uneven numbers of cells from the wells during the media change resulting from poor cell adherence to the culture vessel. This problem of uneven cell distribution was encountered by other authors who noticed high levels of variability between assays using the MTT assay on bivalve primary cell cultures (Domart-Coulon et al. 2000). The issue of attachment also resulted in the inability to properly identify cells in suspension using EM techniques and to assess their proliferation by immunocytochemical (eg. PCNA) techniques. Future work in this area should concentrate on increasing cell attachment that could lead to the use of these techniques for cell identification and the measurement of proliferation.
Conclusions
One of the main problems in establishing primary cultures from aquatic invertebrates is due to the difficulty of finding the optimal growth medium to promote cell proliferation. This is due to the lack of basic information on the nutritional requirements and metabolism of these animals. It is therefore necessary to take a step back, and to study the biochemical requirements and make up of the animal cells. In most studies haemolymph was the basis for the culture media. L-15 has had the most reported success. It is important to note that it is the combination of all of these factors described above (decontamination, dissociation, media and culture parameters) that act cumulatively to produce a successful tissue culture. Invertebrate tissue culture is still very much a developing science and a new researcher to the field faces a myriad of problems, owing to the huge number of variables involved, any one of which could prove detrimental to the culture. This is one of the reasons why aquatic invertebrate tissue culture is still very much in its developmental stage and why so few publications have been reported in recent years. In the present study a technique for the short-term culture of the gills, digestive gland and mantle of the zebra mussel both in cell suspension and as tissue explants has been successfully developed, producing cell suspensions that remain viable in culture for up to 2 weeks and as such are suitable for use in short term toxicological studies.
References
- Barik SK, Jena JK, Janaki KR (2004) In vitro explant culture of mantle epithelium of freshwater pearl mussel. Indian J Exp Biol 42(12):1235–1238 [PubMed]
- Binelli A, Ricciardi F, Riva C, Provini A (2005) Screening of POP pollution by AChE and EROD activities in zebra mussels from the Italian great lakes. Chemosphere 61(8):1074–1082. doi:10.1016/j.chemosphere.2005.03.047 [DOI] [PubMed]
- Birmelin C, Pipe RK, Goldfarb PS, Livingstone DR (1999) Primary cell-culture of the digestive gland of the marine mussel Mytilus edulis: a time-course study of antioxidant- and biotransformation-enzyme activity and ultrastructural changes. Mar Biol (Berl) 135:65–75. doi:10.1007/s002270050602 [DOI]
- Brewster F, Nicholson BL (1979) In vitro maintenance of amoebocytes from the American oyster (Crassostrea virginica). J Fish Res Board Can 36:461–467
- Buchanan JT, La Peyre JF, Cooper RK, Tiersch TR (1999) Improved attachment and spreading in primary cell cultures of the eastern oyster, Crassostrea virginica. In Vitro Cell Dev Biol 35:593–598. doi:10.1007/s11626-999-0097-2 [DOI] [PubMed]
- Canesi L, Borghi C, Ciacci C, Fabbri R, Lorusso LC, Vergani L, Marcomini A, Poiana G (2008) Short-term effects of environmentally relevant concentrations of EDC mixtures on Mytilus galloprovincialis digestive gland. Aquat Toxicol 87(4):272–279. doi:10.1016/j.aquatox.2008.02.007 [DOI] [PubMed]
- Chelomin VP, Zakhartsev MV, Kurilenko AV, Belcheva NN (2005) An in vitro study of the effect of reactive oxygen species on subcellular distribution of deposited cadmium in digestive gland of mussel Crenomytilus grayanus. Aquat Toxicol 73:181–189. doi:10.1016/j.aquatox.2005.03.009 [DOI] [PubMed]
- Cornet M (2006) Primary mantle tissue culture from the bivalve mollusk Mytilus galloprovincialis: investigations on the growth promoting activity of the serum used for medium supplementation. J Biotechnol 123:78–84. doi:10.1016/j.jbiotec.2005.10.016 [DOI] [PubMed]
- Domart-Coulon I, Doumenc D, Auzoux-Bordenave S, Le Fichant Y (1994) Identification of media supplements that improve the viability of primarily cell cultures of Crassostrea gigas oysters. Cytotechnology 16:109–120. doi:10.1007/BF00754613 [DOI] [PubMed]
- Domart-Coulon I, Auzoux-Bordenave S, Doumenc D, Khalanski M (2000) Cytotoxicity assessment of antibiofouling compounds and by-products in marine bivalve cell culture. Toxicol In Vitro 14:245–251. doi:10.1016/S0887-2333(00)00011-4 [DOI] [PubMed]
- Dowling K, Mothersill C (1999) Use of rainbow trout primary epidermal cell cultures as an alternative to immortalized cell lines in toxicity assessment: a study with Nonoxynol. Environ Toxicol Chem 18:2846–2850. doi:10.1897/1551-5028(1999)018<2846:UORTPE>2.3.CO;2 [DOI]
- Gomez-Mendikute A, Elizondo M, Venier P, Cajaraville MP (2005) Characterization of mussel gill cells in vivo and in vitro. Cell Tissue Res 321:131–140. doi:10.1007/s00441-005-1093-9 [DOI] [PubMed]
- Hansen E (1976) A cell line from embryos of Biophalaria glabrata (Plumonata): establishment and characteristics. In: Maramorosch K (ed) Invertebrate tissue culture: research applications. Academic Press, New York, pp 75–97
- Kilemade MF, Mothersill C (2001) Heat shock protein 70 levels in rainbow trout primary epidermal cultures in response to 2, 4-dichloroaniline exposure: a novel in vitro aquatic toxicity marker. Environ Toxicol 16(3):253–259. doi:10.1002/tox.1031 [DOI] [PubMed]
- Kleinschuster SJ, Parent J, Walker CW, Farley CA (1996) A cardiac cell line from Mya arenaria (Linnaeus, 1759). J Shellfish Res 15:695–707
- Koyama S, Aizawa M (2000) Tissue culture of the deep sea bivalve Calyptogena soyoae. Extremophiles 4:385–389. doi:10.1007/s007920070009 [DOI] [PubMed]
- Le Marrec F (1995) Etablissement de cultures primaires de cellules de bivalves marins. Doctorate, Universite de Bretagne Occidentale, Brest
- Le Marrec-Croq F, Fritayre P, Chesne C, Guillouzo A, Dorange G (1998) Cryopreservation of Pecten maximus heart cells. Cryobiology 37:200–206. doi:10.1006/cryo.1998.2113 [DOI] [PubMed]
- Le Pennec G, Le Penec M (2001) Acinar primary cell culture from the digestive gland of Pecten maximus (L.): an original model for ecotoxicological purposes. J Exp Mar Biol Ecol 259:171–187. doi:10.1016/S0022-0981(01)00232-5 [DOI] [PubMed]
- Le Pennec G, Le Penec M (2003) Induction of glutathione-S-transferase in primary cultured digestive gland acini from the mollusk bivalve Pecten maximus (L.): application of a new cellular model in biomonitoring studies. Aquat Toxicol 64:131–142. doi:10.1016/S0166-445X(03)00041-9 [DOI] [PubMed]
- Lyons-Alcantara M, Lambkin HA, Mothersill C (1999) Antigenic characterisation of Nephrops nor_egicus (L.) hepatopancreas cells. Cell Biochem Funct 17:157–164. doi:10.1002/(SICI)1099-0844(199909)17:3<157::AID-CBF823>3.0.CO;2-U [DOI] [PubMed]
- Marie V, Baudrimont M, Boudou A (2006) Cadmium and zinc bioaccumulation and metallothionein response in two freshwater bivalves (Corbicula fluminea and Dreissena polymorpha) transplanted along a polymetallic gradient. Chemosphere 65(4):609–617. doi:10.1016/j.chemosphere.2006.01.074 [DOI] [PubMed]
- Minier C, Abarnou A, Jaouen-Madoulet A, Le Guellec A-M, Bocquene RG, Leboulenger F (2005) A pollution-monitoring pilot study involving contaminant and biological measurements in the Seine estuary, France using the zebra mussel (Dreissena polymorpha). Environ Toxicol Chem 25:112–119. doi:10.1897/05-161R.1 [DOI] [PubMed]
- Mitsuhashi J (1989) Invertebrate cell system applications. Vol. I, II. CRC Press, Boca Raton
- Mothersill C, Lyng F, Lyons M, Cottell D (1995) Growth and differentiation of epidermal cells from the rainbow trout established as explants and maintained in various media. J Fish Biol 46:1011–1025. doi:10.1111/j.1095-8649.1995.tb01406.x [DOI]
- Nichols JW, Schultz IR, Fitzsimmons PN (2006) In vitro-in vivo extrapolation of quantitative hepatic biotransformation data for fish I. A review of methods, and strategies for incorporation intrinsic clearance estimates into chemical kinetic models. Aquat Toxicol 78:74–90. doi:10.1016/j.aquatox.2006.01.017 [DOI] [PubMed]
- Odinstova NA, Khomenko AV (1991) Primary cell culture from embryos of the Japanese scallop Mizuchopecten yessoensis (Bivalvia). Cytotechnology 6:49–54. doi:10.1007/BF00353702 [DOI] [PubMed]
- Odinstova NA, Ermak AV, Tsal LG (1994) Substrate selection for long-term cultivation of marine invertebrate cells. Comp Biochem Physiol 107A:613–619. doi:10.1016/0300-9629(94)90360-3 [DOI]
- Parolini M, Binelli A, Cogni D, Riva C, Povini A (2009) An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicol In Vitro (in press) [DOI] [PubMed]
- Quinn B, Gagne F, Costello M, McKenzie C, Wilson J, Mothersill C (2004) The endocrine disrupting effect of municipal effluent on the zebra mussel (Dreissena polymorpha). Aquat Toxicol 66:279–292. doi:10.1016/j.aquatox.2003.10.007 [DOI] [PubMed]
- Quinn B, Gagne F, Blaise C, Costello MJ, Wilson J, Mothersill C (2006) Evaluation of the lethal and sub-lethal toxicity and potential endocrine disrupting effect of nonylphenol on the zebra mussel (Dreissena polymorpha). Comp Biochem Physiol 142C:118–127 [DOI] [PubMed]
- Renault T, Flaujac G, Le Deuff R-M (1995) Isolation and culture of heart cells from the European flat oyster, Ostrea edulis. Methods Cell Sci 17:199–205. doi:10.1007/BF00996127 [DOI]
- Rinkevich B (1999) Cell cultures from marine invertebrates: obstacles, new approaches and recent improvements. J Biotechnol 70:133–153. doi:10.1016/S0168-1656(99)00067-X [DOI]
- Strum A, Cravedi JP, Perdu E, Baradat M, Segner H (2001) Effects of prochloraz and nonylphenol diethoxylate on hepatic biotransforation enzymes in trout: a comparative in vitro/in vivo-assessment using cultured hepatocytes. Aquat Toxicol 53:229–245. doi:10.1016/S0166-445X(01)00168-0 [DOI] [PubMed]
- Takeuchi Y, Yamamoto S, Odo S (1994) Primary and secondary cultures of larval cells of Pacific oyster, Cressostrea gigas. J Mar Biotechnol 1:171–175
- Wen CM, Kou GH, Chen SN (1993) Cultivation of cells from the heart of the hard clam, Meretrix lusoria (Roding). J Tissue Cult Methods 15:123–130. doi:10.1007/BF02388265 [DOI]
- Zaldibar B, Cancio I, Marigomez I (2004) Circatidal variation in epithelial cell proliferation in the mussel digestive gland and stomach. Cell Tissue Res 318(2):395–402. doi:10.1007/s00441-004-0960-0 [DOI] [PubMed]