Abstract
By mediating depolarization-induced Ca2+ influx high voltage-activated (HVA) Ca2+ channels control a variety of cellular events. These heteromultimeric proteins are composed of an ion-conducting (α1) and three auxiliary (α2δ, β and γ) subunits. The α2δ subunit enhances the trafficking of the channel complex to the cell surface and increases channel open probability. To exert these effects, α2δ must undergo important post-translational modifications including a proteolytic cleavage that separates the extracellular α2 from its transmembrane δ domain. After this proteolysis both domains remain linked by disulfide bonds. In spite of its central role in determining the final conformation of the fully mature α2δ almost nothing is known about the physiological implications of this structural modification. In the current report, by using site-directed mutagenesis, the proteolytic site of α2δ was mapped to amino acid residues Arg-941 and Val-946. Substitution of these residues renders the protein insensitive to proteolytic cleavage as evidenced by the lack of molecular weight shift upon treatment with a disulfide reducing agent. Interestingly, these mutations significantly decreased whole-cell patch clamp currents without affecting the voltage-dependence or kinetics of the channels, suggesting a reduction in the number of channels targeted to the plasma membrane.
Keywords: Amino Acid Motifs; Calcium Channels; chemistry; genetics; metabolism; Cell Line, Transformed; Electric Stimulation; methods; Gene Expression; Humans; Membrane Potentials; physiology; radiation effects; Mutagenesis; physiology; Patch-Clamp Techniques; Protein Structure, Tertiary; Structure-Activity Relationship; Transfection
Keywords: α2δ subunit, Ca2+ channels, proteolysis, patch clamp, HEK-293 cells
Introduction
Ca2+ entry through voltage-activated Ca2+ (CaV) channels contributes to many physiological processes in the nervous system, including neurotransmitter release, neuronal excitability and plasticity, gene expression, neurite outgrowth, synaptogenesis and neuronal survival and differentiation. CaV channels have been subdivided according to their electrophysiological properties into: i) low voltage-activated (LVA or T-type) channels, and ii) high-voltage activated (HVA) channels, a class that includes the L-, N-, P/Q-, and R-types (Catterall et al., 2005; Lacinova, 2005). Molecular studies indicate that CaV channels of the HVA class are oligomeric complexes consisting of an ion-conducting α1 subunit and auxiliary α2δ, β and γ subunits (Felix, 2005; Lacinova, 2005). The auxiliary subunits modulate the trafficking and the biophysical properties of the α1 subunit (Arikkath and Campbell, 2003; Lacinova, 2005).
The α2δ subunit is a transmembrane protein complex that is encoded by a single gene. Since the identification of the first α2δ subunit (Ellis et al., 1988), four genetically distinct α2δ subunits have been described (Klugbauer et al., 1999; Qin et al., 2002). Each one of these proteins is differentially expressed in various tissues, including skeletal muscle, heart and brain. At the protein level, all four α2δ subunits show conserved glycosylation sites, cysteine residues and predicted hydrophobicity profiles (Arikkath and Campbell, 2003; Klugbauer et al., 2003). It has been shown that heterologous expression of each of these α2δ subunits induces an enhancement in the ionic current produced by the α1 subunit expression, associated with an increase in the number of ligand binding sites for the channel (Felix, 1999; Arikkath and Campbell, 2003; Klugbauer et al., 2003). These data indicate that α2δ produces an increase in cell surface expression of Ca2+ channels. Likewise, functional studies have revealed that the α2δ subunits cause increases in current amplitude, faster activation and inactivation kinetics and hyperpolarizing shifts in the voltage dependence of the expressed currents (Singer et al., 1991; Welling et al., 1993; Shistik et al., 1995; Gurnett et al., 1996; Bangalore et al., 1996; Felix et al., 1997; Jones et al., 1998; Qin et al., 1998; Shirokov et al., 1998; Klugbauer et al., 1999; Hobom et al., 2000; Sandoval et al., 2004; Yasuda et al., 2004; Canti et al., 2005; Obermair et al., 2005).
Molecular studies have established that the α2 domain of the protein is extensively glycosylated, a post-translational modification that is essential in maintaining the stability of the interaction with the α1 subunit (Gurnett et al., 1996; 1997). It is also a major determinant in the ability of the protein to stimulate current amplitude (Gurnett et al., 1996; Sandoval et al., 2004). Likewise, it has been documented that the α2δ subunit is translated as a precursor polypeptide that is post-translationally cleaved (De Jongh et al., 1990; Jay et al., 1991). The δ domain, which contains a single transmembrane segment, maintains the α2 domain close to the plasma membrane. Despite proteolytic cleavage, the linkage of both domains is maintained by disulfide bridges formed between cysteine residues found in both proteins (Felix, 1999; Klugbauer et al., 2003; Lacinova, 2005). The mechanisms that underlie the proteolytic cleavage and the disulfide linkage still remain unclear.
In the present report, we used site-directed mutagenesis and heterologous expression in HEK-293 cells to localize the putative site of proteolysis within α2δ, and examine the impact of the proteolytic processing of this auxiliary subunit on the functional activity of neuronal recombinant CaV channels.
Materials and methods
Site-directed mutagenesis
Amino acids 941 and 946 were substituted by site directed mutagenesis of the corresponding cDNA to prevent proteolytic cleavage of the α2δ-1b subunit. To this end, we used the recombinant bicistronic expression plasmid PIRES/α2δ (Sandoval et al., 2004), which carried the entire protein-coding region for the rat brain α2δ-1b Ca2+ channel auxiliary subunit (GenBank accession number M86621) and for the green fluorescent protein (GFP) coupled by an internal ribosomal entry site (IRES) sequence. The point mutations were introduced with ~40-mer synthetic oligonucleotides using the Quik-Change XL-mutagenesis kit (Stratagene, La Jolla, CA, USA). Initially, a single amino acid mutation changing a glutamic acid to a glutamine residue (E944Q) was created. Next, a quadruple mutation (P4) was done on the single cDNA mutant E944Q and lastly, a sextuple mutation (P6) was obtained on the P4 cDNA mutant by using suitable mutagenic primers (Fig. 1A). cDNAs of all three mutant channel subunits were sequenced on an automated sequencer (ABIPrism310, Perkin-Elmer Applied Biosystems, Foster City, CA, USA).
Figure 1. Expression of the α2δ subunit proteolytic site mutants in HEK-293 cells.
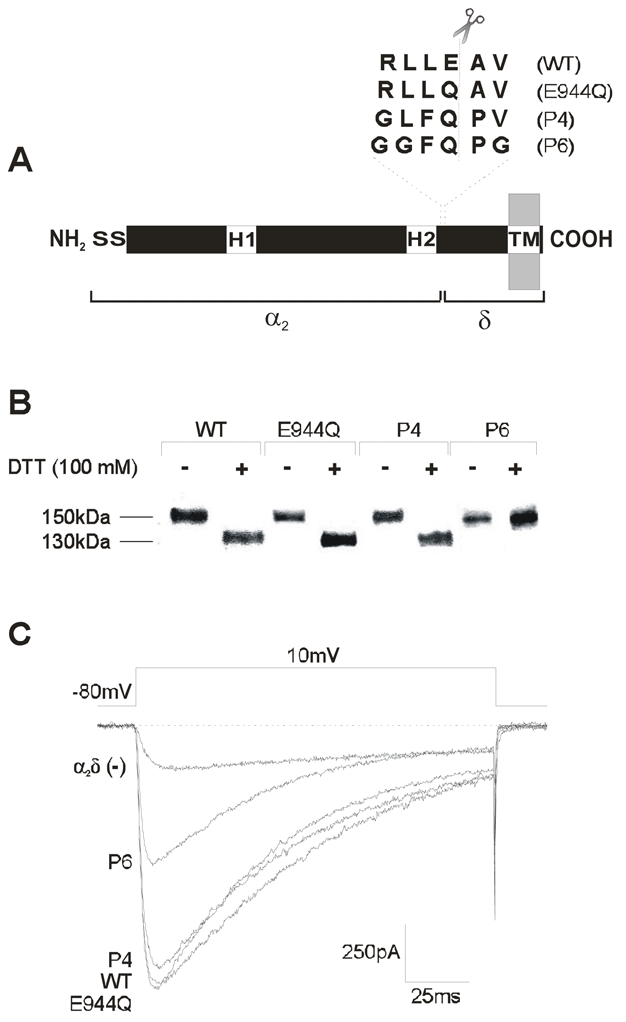
A) Schematic representation of the CaVα2δ auxiliary subunit. The location of the two hydrophobic domains (H1 and H2), and the signal sequence (S) are given. The amino acid residues in the putative proteolytic site of the wild-type α2δ-1 and its mutant versions are aligned and shown above the diagram. B) Western blot analysis of membranes from HEK-293 cells expressing the wild-type, and the mutant versions of the α2δ subunit (E944Q, P4 and P6). Membranes were prepared as described under Material and Methods. Only the sextuple mutation (P6) is insensitive to proteolysis (do not change its electrophoretic pattern after dithiothreitol-DDT-treatment). C) Representative Ba2+ currents through recombinant CaV2.2/β3 channels obtained in HEK-293 cells co-expressing the wild-type α2δ-1 and its proteolysis mutants. Currents were elicited in response to 140 ms test pulses to +10 mV delivered from a holding potential of −80 mV.
Cell culture and recombinant CaV channel expression
Human embryonic kidney (HEK-293) cells were maintained in DMEM-high glucose supplemented with 10% equine serum, 1% L-glutamine, 110 mg/l sodium pyruvate and antibiotics, at 37°C in a 5% CO2-95% air humidified atmosphere. Gene transfer was performed using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA, USA). Briefly, for a 35 mm Petri dish of HEK-293 cells, 2 μg of the plasmid cDNA encoding the rabbit brain N-type Ca2+ channel CaV2.2 pore-forming subunit (D14157; Fujita et al., 1993) in combination with 2 μg cDNA of the rat brain β3 (M88751; Castellano et al., 1993) and 2 μg cDNA coding the wild-type rat brain α2δ-1 or its mutants, were premixed with 6 μl of Lipofectamine in 100 μl serum-free medium according to the manufacturer’s instructions. The solution was then added to the dish and cells grown at 37°C for 24 h, when medium was changed. Cells were then harvested at 48 h.
SDS-PAGE and Western blotting
Microsomes from transfected HEK-293 cells were prepared as previously described (Felix et al., 1997; Gurnett et al., 1997). Samples were subjected to gel electrophoresis under denaturing conditions. All samples were heated at 95°C for 5 min, and 100 μg of protein/slot were loaded on 5% polyacrylamide gels. For Western blot analysis, proteins were transferred onto nitrocellulose membranes, and blots were developed as described (Felix et al., 1997; Gurnett et al., 1997) with specific CaVα2 polyclonal primary antibodies (Rabbit 136; Gurnett et al., 1996) used in a 1:4000 dilution. The secondary antibody was a goat anti-rabbit IgG horseradish peroxidase (Zymed Invitrogen, Carlsbad, CA, USA) used at a dilution of 1:6000. The specific protein bands were detected using the enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences, Piscataway, NJ, USA) according to the manufacturer’s instructions.
Electrophysiological recording
Ionic currents from HEK-293 cells were recorded using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) 48 h after transfection. Ba2+ was used as the charge carrier. The extracellular solution contained (in mM): solution contained 10 BaCl2, 125 TEA-Cl, 10 HEPES and 10 glucose (pH 7.3). The intracellular (in mM): 110 CsCl, 5 MgCl2, 10 EGTA, 10 HEPES, 4 Na-ATP and 0.1 GTP (pH 7.3). Recordings were made with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) and acquired on-line using a Digidata 1320A interface with pClamp8 software (Molecular Devices). The offset potential between the pipette and bath solutions was zeroed prior to seal formation. Capacitive transients were canceled with the amplifier. Series resistance values were typically 2–10 MΩ and no records were used in which the voltage error (as defined by Ver = Imax × Ra) was greater than 5 mV. Currents were obtained from a holding potential (HP) of −80 mV applying test pulses every 20 s. Leak and residual capacitance currents were subtracted on-line by a P/4 protocol. Current signals were filtered at 2 kHz (internal 4 pole Bessel filter) and digitized at 5.71 kHz. Membrane capacitance (Cm) was determined as described previously (Avila et al., 2004) and used to normalize currents. Curve fitting and statistical analysis were carried out using the SigmaPlot 8.0 software package (SPSS Inc., Chicago, IL, USA). The significance of observed differences was evaluated by nonpaired Student’s t test. P<0.05 was considered to be significant.
Results
Before investigating the functional relevance of the proteolytic cleavage of the α2δ auxiliary subunit on neuronal recombinant CaV channel activity, it was necessary to delimit the putative proteolytic site within the protein. Initial N-terminal sequencing studies on the rabbit skeletal muscle CaV channel δ subunit suggested that cleavage of the α2δ-1 precursor occurs between residues Glu-934 and Ala-935 (De Jongh et al., 1990). Sequence alignment of this deduced proteolytic site with the rat brain α2δ-1b retrieved from the GenBank corroborated the presence of these residues at positions 944 and 945, respectively. Given that many deduced sites of proteolysis are of 6 amino acid residues length, and the cleavage site occurs after the fourth amino acid we speculated that the proteolytic site of the rat brain α2δ-1b used in this study might be positioned between Arg-941 and Val-946 (Fig. 1A).
To test whether this site is indeed post-translationally modified and whether such a modification entails any functional consequence, we produced one single and two multiple mutants of the rat brain α2δ-1b (Fig. 1A; see Material and Methods for details). All cDNA constructs were sequenced to confirm the desired mutations. The wild-type α2δ and its mutated versions were then expressed in HEK-293 cells and analyzed by immunoblotting. This analysis showed that under non-reducing conditions the wild-type protein and the α2δ subunits harboring the single (E944Q) and the quadruple (P4) mutations migrate as high molecular mass proteins of about 150 kDa. Upon reduction, the majority of these proteins migrated as products of the predicted size for the δ2 peptide (~130 kDa). This result suggested that the wild-type and the mutant proteins E944Q and P4 were proteolytically cleaved into separate α2 and δ peptides (Fig. 1B). Expression of the protein that had all six amino acids of the putative proteolitic site mutated (P6) also resulted in the presence of a high molecular mass species (~150 kDa) under non-reducing conditions which was expected. However upon reduction, the size of this high molecular weight band remained unchanged, indicating that the P6 protein is not susceptible to proteolysis (Fig. 1B).
In order to evaluate the functional role of the proteolytical processing of the α2δ subunit, the patch clamp technique was used to study macroscopic Ba2+ currents (IBa) through recombinant CaV channels (CaV2.2/β3) in HEK-293 cells transiently expressing the wild-type α2δ and its mutants. As can be seen in Fig. 1C, the amplitude of the Ba2+ currents (IBa) through CaV2.2/β3 channels, was enhanced by co-expression of the wild-type α2δ-1 subunit, as previously reported (Sandoval et al., 2004; Yasuda et al., 2004). Interestingly, recordings performed in cells transfected with the single (E944Q) and the quadruple (P4) mutations, indicated that the stimulatory effect of the auxiliary subunit was preserved. In contrast, replacement of all six residues in the proteolytic site of the protein significantly decreased whole-cell patch clamp currents (Fig. 1C).
To facilitate comparisons from cells of different size, peak IBa amplitude was normalized to membrane capacitance (Cm) and expressed as IBa density (in picoamperes per picofarad). The effects of the wild-type α2δ and its mutant variants E944Q, P4 and P6 on peak IBa density (determined for a test pulse to +10 mV) are summarized in Figure 2A. When compared with the wild-type, E944Q or P4 expressing cells, IBa density in HEK-293 cells transfected with the P6 mutant construct was significantly reduced (>52%).
Figure 2. Functional effects of heterologous expression of the wild-type α2δ subunit and its proteolytic site mutants.
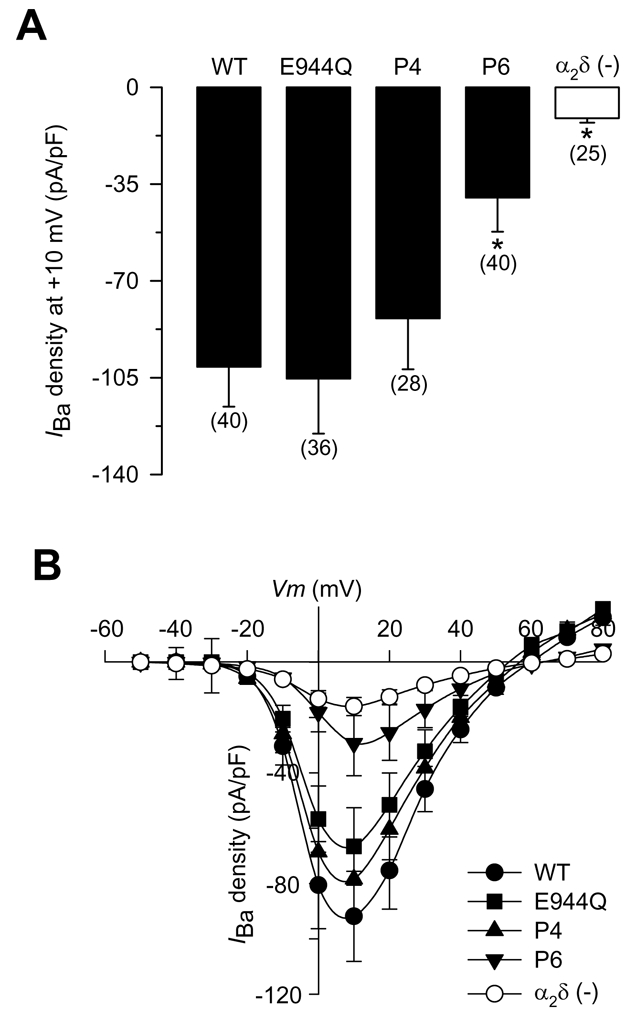
A) Average ± SEM IBa density in HEK-293 cells expressing the wild-type α2δ-1 and its mutant variants (filled bars). IBa density was calculated from IBa amplitude at a test pulse of +10 mV normalized to cell membrane capacitance. The open bar shows the average current amplitude in the absence of the α2δ subunit. The number of recorded cells is indicated in parentheses, and the asterisks denote significant differences (P<0.05). B) Average ± SEM I–V relationships for IBa recorded from HEK-293 cells expressing the wild-type α2δ-1 and its mutant variants (E944Q, P4 and P6). IBa density was calculated at a series of test pulses applied from a holding potential of −80 mV in 10 mV steps between −50 and +80 mV (n = 6–8). Open circles represent the average current density in the absence of the α2δ subunit.
Figure 2B compares mean ± SEM current density-voltage (I–V) relationships from HEK-293 cells expressing the wild-type α2δ protein and the E944Q, P4 and P6 mutants. As expected, the E944Q and P4 constructs were not different than wild-type α2δ-1 for effects on IBa density. In contrast, mean peak IBa density in cells expressing the α2δ-1 P6 mutant was severely depressed across the entire voltage range investigated. In line with this, the macroscopic conductance (Gmax) for the P6 α2δ-1 mutant subunit, determined from the I–V relationships, was significantly decreased by ~70% compared with the CaV2.2/β3/α2δ-1 combination. In contrast, a small but no significant inhibition on Gmax was observed by co-expression of the E944Q or P4 mutant subunits (Fig. 3A; Table 1). The Gmax value for the P6 mutant containing channels was comparable to the Gmax value obtained in cells transfected with CaV2.2/β3 in the absence of the α2δ-1 subunit (Table 1), suggesting a role for the proteolytic cleavage of α2δ-1 in surface expression of HVA Ca2+ channels.
Figure 3. Activation properties of CaV2.2/β3 channels co-expressed with the wild-type α2δ-1or its proteolysis mutations.
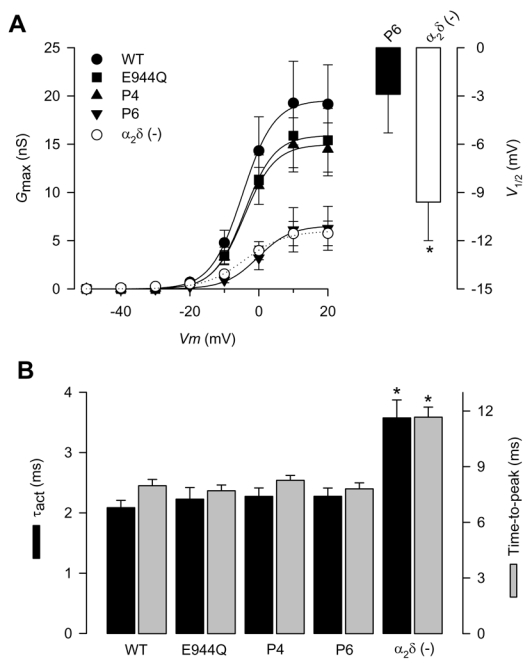
A) Left, voltage dependence of activation of CaV2.2/β3 channels co-expressed with wild-type α2δ-1 (filled circles), its proteolysis mutants (E944Q, P4 and P6) or without any α2δ subunit (open circles). Maximum conductance (Gmax) plotted data were derived from Fig. 2B. The mean data were fitted with Boltzmann functions, the V½ values of which are given in Table 1. Right, comparison of the mean half-activation voltage (V1/2) for CaV2.2/β3 channels co-expressed with the P6 proteolysis mutation or without the α2δ subunit (n = 6–8). B) Mean time to peak and time constants of activation (τact) obtained by fitting the rising phase of IBa at +10 mV with a single exponential, for CaV2.2/β3 channels co-expressed with wild-type α2δ-1, its proteolysis mutants, or without any α2δ subunit (n = 11–25).
Table 1.
Differential effects of the wild-type α2δ subunit and its proteolysis mutants on the biophysical properties of the CaV2.2/δ3 currents
| Wild-type | E944Q | P4 | P6 | α2δ-1 (−) | |
|---|---|---|---|---|---|
| Potential for half-activation (mV) | −4.5.3±1.8 | −4.0±0.9 | −4.7±1.8 | −3.0±2.4 | −9.6±2.4 |
| Maximal conductance at +10 mV (nS) | 19.3±4.3 | 15.9±3.3 | 14.9±2.8 | 6.1±2.3 | 5.7±1.3 |
| Potential for half steady−state inactivation (mV) | −60.2±3.6 | −59.7±4.9 | −56.8±3.0 | −52.3±2.2 | −44.6±2.1 |
| Rate of inactivation at +10 mV (μs−1) | 20.4±1.2 | 20.1±1.0 | 18.9±1.1 | 19.6±1.4 | 12.3±1.1 |
It is well established, that alterations in the maximal conductance in the absence of kinetic changes of the macroscopic currents may depend on variations in the number of functional channels. Therefore, we next investigated whether the effects of the P6 mutation involved alterations in the kinetic properties of the currents recorded. Normalized currents obtained from cells expressing the wild-type α2δ and its mutants showed that the temporal course of the current traces was similar, suggesting that the activation and inactivation rate of the channels were not altered. The channel activation rate was quantitatively compared by fitting current traces to an exponential equation (Sandoval et al., 2004). Accordingly, neither the time to peak nor the time constant for the activation of the current (τact) were significantly modified (Fig. 3B). Likewise, we generated complete voltage-dependent inactivation curves for the recombinant CaV channels expressed. The parameters describing the inactivation curves were similar to the corresponding values obtained from cells expressing the wild-type α2δ-1 (Fig. 4A; Table 1). Lastly, the time constant for the inactivation of the current (τinact), obtained by fitting the decaying phase of the currents with an exponential function, and the percentage of current remaining after 140 ms activating pulses were practically indistinguishable between cells expressing the wild-type α2δ and cells expressing the mutant subunits (Fig. 4B; Table 1). Although a decrease in channel open probability cannot be ruled out, our data suggest that the proteolytic cleavage site may be required for cell surface expression.
Figure 4. Inactivation properties of CaV2.2/β3 channels co-expressed with the wild-type α2δ-1 or its proteolysis mutations.
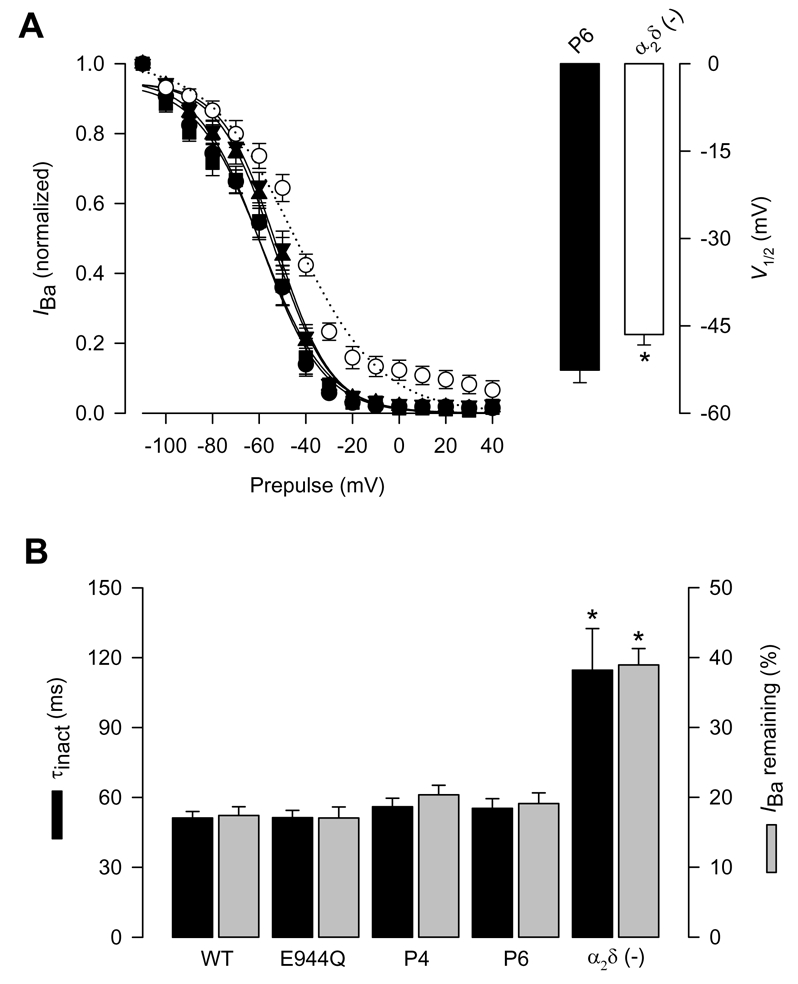
A) Left, voltage dependence of steady-state inactivation of CaV2.2/β3 channels co-expressed with wild-type CaVα2δ-1 (filled circles), its proteolysis mutants E944Q, P4 and P6 or without any α2δ subunit (open circles). Currents were recorded after conditioning pulses of 1 s duration, applied from a holding potential of −80 mV in 10 mV steps between −110 and +40 mV, followed by a 140 ms test pulse to +10 mV. The normalized data are plotted against the conditioning potentials (n = 5–11). The mean data were fitted with a Boltzmann function, the V½ values of which are given in Table 1. Right, comparison of the mean half-inactivation voltage (V1/2) for CaV2.2/β3 channels co-expressed with the P6 mutation or without the α2δ subunit. B) Average time constants of inactivation (τinact) and percentage of current remaining 140 ms into the depolarizing pulse for CaV2.2/β3 channels co-expressed with wild-type α2δ-1, its proteolysis mutants, or without any α2δ subunit (n = 11–25).
An interesting finding was that the average current amplitude in the presence of the P6 mutant was comparable to the magnitude of the currents in the absence of the α2δ-1 subunit (Fig. 2). However, the waveforms and the voltage dependence of the currents in the two conditions were different (Figs. 3 and 4). Currents in the absence of any α2δ subunits activate and inactivate significantly slower (Figs. 3B and 4B). Likewise, the half-maximal voltage for current activation and inactivation was shifted >5 mV to the hyperpolarizing direction (Figs. 3A and 4A, right panels; Table 1). Taken together, these data suggest that all mutant proteins, including P6 interact with the channel. However, only the P6 mutant is inefficient in trafficking to the cell membrane or is less stable once it reaches the membrane. In keeping with this view, the functional properties of the channels were practically unaltered (Figs. 3 and 4), and only major alterations were observed in the maximal conductance which depends on the number of functional channels in the plasma membrane. Thus conceivably, alterations in the number of CaV channels at the membrane might be accounting for the effects of the P6 α2δ-1 mutant, which might be particularly prone to structural alterations.
Discussion
In the present report we found that the proteolytic site of the rat brain CaVα2δ-1 subunit is localized between amino acid residues Arg-941 and Val-946. Likewise, we found also that a single amino acid substitution in the putative proteolytic site is not sufficient to prevent cleavage, and that replacement of the six residues (Arg-Leu-Leu-Glu-Ala-Val) is required to make the CaVα2δ-1 protein insensitive to proteolysis. Furthermore, mutation of the entire hexapeptide prevents the whole-cell current stimulation (normally observed after co-expression of the α2δ-1 without affecting its kinetic properties, suggesting either changes in channel open probability or a regulation on the number of functional channels at the plasma membrane.
One observation of particular interest in our studies is that hampering normal proteolysis has no biophysical consequences on the CaV channels regulation by α2δ-1b. Interestingly, recent studies have shown that though an important fraction of the α2δ-2 subunit expressed heterologously may not be proteolytically processed into α2-2 and δ-2, it might show full functionality and being expressed on the cell membrane (Douglas et al., 2006). Consequently, it has been suggested that cleavage into α2-2 and δ-2 subunits may not be essential for functional enhancement of macroscopic Ca2+ currents. However, it has been also found that native α2-2 is fully cleaved in the mouse cerebellum (Douglas et al., 2006), and the possibility exists that the fraction of α2δ-2 that is proteolytically cleaved into α2-2 and δ-2 in the heterologous system is the active form of the protein. Therefore, it remains an open question for future research whether the α2δ auxiliary subunits are functional in their proteolytically cleaved and non-cleaved forms.
Likewise, previous studies have shown that the CaVα2δ subunits increase the whole-cell conductance in different expression systems (Felix, 1999; Arikkath and Campbell, 2003; Klugbauer et al., 2003). Relevant to this, it has been shown that α2δ-2have only a small influence on the properties of the channels at the single level (Barclay et al., 2001; Brodbeck et al., 2002). Taken together, these data imply that the α2δ subunit is possibly acting on the lifetime of the channel complex at the cell surface, either by enhancing trafficking to the plasma membrane or by reducing turnover of channels (Douglas et al., 2006). In agreement with this view, it has been reported that the α2δ subunits increase the amount of CaV1 and CaV2 proteins in the cell membrane (Felix, 1999; Arikkath and Campbell, 2003; Klugbauer et al., 2003).
Although the precise mechanism by which the α2δ subunit controls Ca2+ channel turnover is presently unclear, Bernstein and Jones (2006) have recently suggested a model where α2δ serves to maintain CaV2.2 channel levels at the cell surface by limiting their entry into a degradative pathway. According to this model, any free (possibly less physiologically desirable) CaV2.2/β complexes at the cell surface, are more rapidly removed and diverted to a non-degradable pool, perhaps for further attempts at complexation with α2δ subunits (Bernstein and Jones, 2006). Therefore, investigating to what extent proteolytic processing alterations of α2δ interfere with CaV channel subunit proper folding and trafficking is an interesting topic for future studies.
Acknowledgments
This work was supported by grants from Conacyt and The Miguel Alemán Foundation to RF. Doctoral (A.A., A.S.) and postdoctoral (N.O.) fellowships from Conacyt are gratefully acknowledged. We are in debt with Dr. K.P. Campbell (University of Iowa) for his generous gift of the cDNA clones and antibodies. We thank G. Aguilar for assistance with DNA sequencing as well as T. Avila, M. Urban and L. Escobedo for expert technical assistance. We would like to thank also the two anonymous reviewers for constructive criticism.
References
- 1.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 2.Avila G, Sandoval A, Felix R. Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells. Cell Mol Neurobiol. 2004;24:317–330. doi: 10.1023/B:CEMN.0000022765.52109.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel α2δ subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270:H1521–H528. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- 4.Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, PerezReyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein GM, Jones OT. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: Role of the α2/δ subunit. Cell Calcium. 2006 doi: 10.1016/j.ceca.2006.04.010. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Brodbeck J, Davies A, Courtney J-M, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, Barclay J, Rees M, Dolphin AC. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated α2δ-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 7.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 9.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 10.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 11.Douglas L, Davies A, Wratten J, Dolphin AC. Do voltage-gated calcium channel α2δ subunits require proteolytic processing into α2 and δ to be functional? Biochem Soc Trans. 2006;34(Pt 5):894–898. doi: 10.1042/BST0340894. [DOI] [PubMed] [Google Scholar]
- 12.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 13.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felix R. Voltage-dependent Ca2+ channel α2δ auxiliary subunit: structure, function and regulation. Receptors Channels. 1999;6:351–362. [PubMed] [Google Scholar]
- 15.Felix R. Molecular regulation of voltage-gated Ca2+ channels. J Recept Signal Transduct Res. 2005;25:57–71. doi: 10.1081/rrs-200068102. [DOI] [PubMed] [Google Scholar]
- 16.Fujita Y, Mynlieff M, Dirksen RT, Kim MS, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the ω-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- 17.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 18.Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J Biol Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- 19.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 20.Hobom M, Dai S, Marais E, Lacinova L, Hofmann F, Klugbauer N. Neuronal distribution and functional characterization of the calcium channel α2δ-2 subunit. Eur J Neurosci. 2000;12:1217–1226. doi: 10.1046/j.1460-9568.2000.01009.x. [DOI] [PubMed] [Google Scholar]
- 21.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 22.Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HL, Kim H, Lee P, King RG, Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel α2 subunit. Proc Natl Acad Sci. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2δ subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klugbauer N, Marais E, Hofmann F. Calcium channel α2δ subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- 26.Lacinova L. Voltage-dependent calcium channels. Gen Physiol Biophys. 2005;24(Suppl 1):1–78. [PubMed] [Google Scholar]
- 27.Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation-contraction coupling. J Biol Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 28.Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal α1E-type calcium channel by α2δ-subunit. Am J Physiol. 1998;274:C1324–C1331. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- 29.Qin N, Yagel S, Momplaisir ML, Codd EE, D’Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel α2δ-4 subunit. Mol Pharmacol. 2002;62:485–496. doi: 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- 30.Sandoval A, Oviedo N, Andrade A, Felix R. Glycosylation of asparagines 136 and 184 is necessary for the α2δ subunit-mediated regulation of voltage-gated Ca2+ channels. FEBS Lett. 2004;576:21–26. doi: 10.1016/j.febslet.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Shirokov R, Ferreira G, Yi J, Rios E. Inactivation of gating currents of L-type calcium channels. Specific role of the α2δ subunit. J Gen Physiol. 1998;111:807–823. doi: 10.1085/jgp.111.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shistik E, Ivanina T, Pury T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contributions of changes in channel gating and α1 protein level. J Physiol. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 34.Welling A, Bosse E, Cavalie A, Bottlender R, Ludwig A, Nastainczyk W, Flockerzi V, Hofmann F. Stable co-expression of calcium channel α1, β and α2/δ subunits in a somatic cell line. J Physiol. 1993;471:749–765. doi: 10.1113/jphysiol.1993.sp019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20:1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]


