Abstract
Previously we reported that children with autism show significant variability in cortisol. The current investigation was designed to extend these findings by exploring plausible relationships between cortisol and psychological measures of stress and sensory functioning. Salivary cortisol values for diurnal rhythms and response to stress in children with and without autism were compared to parent-report measures of child stress, the Stress Survey Schedule (SSS), sensory functioning, Short Sensory Profile (SSP) and parenting stress (PSI). In autism, a negative relationship between morning cortisol and the SSS revealed that higher observed symptoms of stress were related to lower cortisol. Lower cortisol is seen in conditions of chronic stress and in social situations characterized by unstable social relationships. Sensory sensitivity painted a more complicated picture, in that some aspects of SSP were associated with higher while others were associated with lower cortisol. We propose that increased sensory sensitivity may enhance the autistic child’s susceptibility to the influence of zeitgeibers reflected in variable cortisol secretion. Evening cortisol were positively associated with SSS such that the higher the level of evening cortisol, the higher the child’s parent-reported daily stress, especially to changes, such as in daily routine. Regarding the response to stress, the psychological and parent variables did not differentiate the groups; rather, discrete subgroups of cortisol responders and non-responders were revealed in both the autism and neurotypical children. The results support a complex interplay between physiological and behavioral stress and sensory sensitivity in autism and plausible developmental factors influencing stress reactivity across the groups.
Keywords: autism, cortisol, diurnal variation, stress, LHPA, sensory sensitivity
Introduction
Autism is a complex neurodevelopmental disorder characterized by impairment in communication, reciprocal social interaction, and a restricted repertoire of activities and interests (APA, 1994). Furthermore, autism has been described as a disorder accompanied by increased arousal, stress and sensory sensitivity (e.g., (Dunn & Brown, 1997; Gillott et al., 2001; Kanner, 1943; Muris et al., 1998)). Since the limbic hypothalamic pituitary adrenocortical axis (LHPA) axis has been shown to reflect increased levels of arousal and stress, it is not surprising that studies on the LHPA axis and autism have been conducted (Corbett et al., 2006; Corbett et al., 2008a; Hill et al., 1977; Hoshino et al., 1987; Jansen et al., 2000; Jansen et al., 2003; Jensen et al., 1985; Richdale & Prior, 1992; Tordjman et al., 1997). In fact, an early biochemical model proposed that autism may result from a dysfunction of the pineal-hypothalamic-pituitary-adrenal axis in a subset of individuals with the disorder (Chamberlain & Herman, 1990).
Regulation of the LHPA axis involves three interrelated processes: the maintenance of a diurnal rhythm, activation in response to stress or threat, and the restoration of basal activity via negative feedback mechanisms. One or more of these processes could be affected in autism. In our previous reports we have shown that, in addition to the form of circadian changes in LHPA activity, autism is associated with a lack of consistency in day-to-day rhythms (Corbett et al., 2008a).
Cortisol follows a circadian rhythm, with high concentrations in the morning and a decline throughout the day, with the lowest levels in the evening and at night. This diurnal pattern is well-established by the third month of infancy (Price et al., 1983; Vermes et al., 1980). Individual differences in cortisol secretion, especially in the morning, may be an important variable for typically developing children (Bartels et al., 2003) as well as children with neurodevelopmental disorders, such as autism (Corbett et al., 2006; Corbett et al., 2008a).
It has been shown that LHPA responsivity is highly dependent on specific psychological factors such as control, predictability, and feedback (Levine, 2000). The sensitivity of the system has been linked to factors of behavioral inhibition and temperament early in development (e.g., (Degnan & Fox, 2007; Fox et al., 2008; N. A. Fox et al., 2005; N. A. Fox et al., 2001; Goldsmith & Lemery, 2000; Gunnar et al., 2003; Kagan et al., 1987; Schmidt et al., 1997; Tyrka et al., 2006; Young et al., 1999; Zimmermann & Stansbury, 2004)). Furthermore, social variables can induce, enhance or diminish the stress response in primates (e.g., (Levine & Mody, 2003; Mendoza et al., 2000)). Similarly, it may be the case that parenting factors may stimulate or ameliorate activity of the LHPA axis in children with autism. It has been reported that parental indices of stress are frequently higher in parents of children with autism (e.g., (Davis & Carter, 2008; Schieve et al., 2007)) although not all studies report such findings (e.g., (Montes & Halterman, 2007)).
Previously, we investigated diurnal regulation and responsivity of the LHPA axis in a sample of 6 to 12 year old children with autism compared to those with typical development. The children with autism showed a more variable circadian rhythm as well as elevations in cortisol following exposure to a novel stimulus, a mock magnetic resonance imaging (MRI) scanner (Corbett et al., 2006). Recently, we expanded and extended this finding in a much larger sample of children with high functioning autism (N = 22) and neurotypical children (N = 22) (Corbett et al., 2008a). Broadly speaking, both groups showed expected diurnal rhythms; however, the time course revealed clear dysregulation of the diurnal rhythm in autism characterized by gradual decrease over the course of the sampling in the morning and elevated evening values. Further, children with autism showed more between-subject and within-subject variability in circadian rhythms. The greater within child variation suggests disturbances in the LHPA axis that cannot be explained simply by child heterogeneity. In addition to the seemingly random variation in diurnal cortisol rhythms, autisic children also exhibited altered responses during successive days, maybe in response to the sampling schedule per se. This led us to consider the possibility that autistic children are more generally responsive to stimuli that can act as zeitgebers (environmental cues that influence biological rhythms).
Responsivity of the LHPA system in autistic children is sometimes found to be greater than neurotypical children and sometimes it is not (Corbett et al., 2006 vs. Corbett, 2008 #128). Both autistic and neurotypical children anticipate stressful qualities associated with being placed in a mock (or actual) scanner. It is important to note that the children were naive to the MRI environment and although sophisticated behavioral coding was not performed, no distinctions could be made across the majority of participants based purely on behavioral observation. In order to determine whether or not variation in diurnal rhythms or responsivity to stress are altered differentially in autistic children by their unique psychological profiles, we ventured to more carefully investigate contributory mechanisms, such as sensory sensitivity and individual psychological stress factors that may play a role in the rhythmicity and responsivity of cortisol in children with autism.
As a result of a long history of an association between increased symptoms of stress and autism (e.g., (Gillott et al., 2001; Muris et al., 1998); Kanner, 1943 #112}) measures have been created to capture the unique profile in autism and related disorders. For example, the Stress Survey Schedule is a parent-report instrument that was developed to measure stress in the lives of persons with autism and other developmental disabilities (Groden et al., 2001). Exploratory and confirmatory analysis identified eight dimensions of stress, including: Anticipation/Uncertainty, Changes and Threats, Unpleasant Events, Pleasant Events, Sensory/Personal Contact, Food Related Activity, Social/Environmental Interactions, and Ritual Related Stress. The aforementioned dimensions are particularly relevant to the problems observed in day-to-day lives of children with autism.
Sensory functioning is another important area of study in autism (Rogers et al., 2003; Watling et al., 2001) and other neurodevelopmental disorders, such as attention deficit hyperactivity disorder (Ermer & Dunn, 1998) and Fragile X syndrome (Rogers et al., 2003) (for a review see (Reynolds & Lane, 2007)). Dunn (Dunn, 1999; Dunn & Brown, 1997) presented a model that attempts to subtype individuals based on neurological thresholds of sensory responsiveness and self-regulation strategies. These ideas were initially introduced by influential theories explaining developmental trajectories of temperament and sensory responsiveness (Derryberry & Rothbart, 1988; Kagan et al., 1987). Individuals with a lower threshold require less stimulation for the central nervous system (CNS) to respond, while individuals with a higher threshold require greater simulation for the CNS to become engaged. As an extension of this model, Dunn proposed that individuals demonstrate four sensory processing patterns, including sensory seeking, sensory avoiding, sensory sensitivity, and low registration (Dunn, 2001).
In autism spectrum disorders (ASD) both overresponsive and underresponsive sensory functioning have been reported using questionnaires (e.g., (Baranek et al., 2006; Dunn et al., 2002; Kern et al., 2006; Liss et al., 2006; Pfeiffer et al., 2005; Rogers et al., 2003; Watling et al., 2001)). Specifically, the Sensory Profile and Short Sensory Profile (SSP) are parent-report measures that have been used (Dunn, 1999; Dunn & Westman, 1997). Utilizing domains from the sensory profile, Liss and colleagues reported both overreactivity and underreactivity in children with autism and suggested that sensation seeking behavior serves as a compensatory strategy to moderate overarousal (Liss et al., 2006). Rogers and colleagues (Rogers et al., 2003) used the SSP across a sample of children with autism, fragile X syndrome, developmental disabilities of mixed etiology, and typically developing children. The results revealed significant differences between the groups for tactile sensitivity, taste/smell sensitivity, underreactive/seeks stimulation, auditory filtering, and low energy/weak muscles. Children with autism and those with fragile X syndrome exhibited significantly more sensory symptoms than the other comparison groups, especially tactile sensitivity and auditory filtering (Rogers et al., 2003). Recently, sensory functioning was correlated with symptoms of autism across a broad age range of 3 to 56 years revealing global sensory processing deficits which were associated with symptom severity (Kern et al., 2006; Kern et al., 2007).
The purpose of the current experiment was to extend the previous investigations (Corbett et al., 2006; Corbett et al., 2008a) of children with high functioning autism in comparison to neurotypical children to investigate plausible explanatory factors that contribute to the variability in LHPA regulation and responsivity. Specifically, we examined the diurnal pattern and response to stress in cortisol compared to parent-report measures of stress and sensory functioning. We predicted that increased sensory sensitivity (SSP) would be associated with diurnal variability and morning cortisol supporting the idea of greater responsiveness to environmental cues and stimuli, such as zeitgeibers. Further, we hypothesized that parent-reported measures of child stress (SSS) would be associated with elevated cortisol in children with autism, especially increased evening values as a reflection of cummulative arrousal from daily activities. We further predicted that the perceived stress measure (SSS) would be correlated with cortisol response to the stress protocol. Finally, it was hypothesized that reported parenting stress (PSI) would be associated with elevated cortisol, especially in the evening.
Methods and Materials
Diagnostic and Inclusion Measures
Testing was completed following informed consent procedures on the first visit. Wechsler Abbreviated Intelligence Scale (WASI) (Wechsler, 1999). The WASI is a measure of general intelligence used to obtain an estimated IQ. It was administered to the majority of the participants (N=39) unless a full scale IQ score from a comprehensive measure was available (N=5) (Roid, 2003; Wechsler, 2003). Participants needed to have an IQ ≥ 80 for inclusion in the study.
Autism Diagnostic Observation Schedule (ADOS) (Lord, 1999). The ADOS is comprised of semi-structured, interactive activities conducted with a child and designed to assess specific current behaviors indicative of autism. The ADOS provides an algorithm with cut-offs for autism and ASDs (Lord, 1999).
The Social Communication Questionnaire (SCQ) (Berument et al., 1999) was used as a screening tool to ensure the absence of symptoms of autism in the neurotypical control children.
The Stress Survey Schedule (SSS; (Groden et al., 2001) is a parent-report measure of stress designed for individuals with autism and other developmental disabilities. The SSS consists of 60 daily stress-related items rated on a five-point likert scale and includes eight dimensions of stress. Internal consistency correlations range from .70 to .87.
The Short Sensory Profile (SSP; (Dunn, 1999) is a parent questionnaire related to sensory sensitivity across several domains, including auditory, visual, vestibular, tactile, oral and multisensory processing. It is important to note that lower scores on this measure reflect greater impairment.
Parenting Stress Index (PSI; (Abidin, 2003)is a parent questionnaire that screens for dysfunctional parent-child systems. It has been shown to assist in identifying high stress areas. In addition to the Total score, which was used as the dependent variable in this study, the PSI yields additional parent and child subscales.
Participants
The participants consisted of 44 predominantly male children between 6- years and 12-years of age (mean age 9.08 years), 22 diagnosed with autism (1 female) and 22 neurotypical children (3 females). Informed written consent was obtained from parents and verbal assent was obtained from all research participants prior to inclusion in the study on the day of the induced stressor. The Institutional Review Board of the University of California, Davis approved the study. The study was conducted in compliance with the national legislation and the Code of the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki) http://www.wma.net/e/policy/b3.htm.
The diagnosis of autism was based on DSM-IV criteria (APA, 1994) and established by: 1) a previous diagnosis of autism by a psychologist, psychiatrist or behavioral pediatrician, 2) clinical judgment by the first author, and 3) confirmation by a total score on the social-communication scale of the ADOS (see below) at or above the autism threshold (Lord, 1999). Children with ASDs, namely pervasive developmental disorder-not otherwise specified (PDD-NOS) and Asperger syndrome, were excluded.
Cortisol Sampling Methods
A well-established salivary collection protocol was carefully followed (Corbett et al., 2006; Corbett et al., 2008a), which included using consistent collection materials and methods, controlling the intake and time of drinks, foods, and medications, as well as using standardized procedures and protocols (Hanrahan et al., 2006). The sampling method has been previously described (Corbett et al., 2006; Corbett et al., 2008a; Corbett et al., 2008b). Basal levels of salivary cortisol were collected for six diurnal cycles consisting of three samples per day (morning, afternoon, and evening), over three consecutive days over two consecutive weeks (MTW Week 1, MTW Week 2), resulting in 18 home samples.
In addition, participants were exposed to a stressor in the form of a mock MRI scanner conceptualized as a moderate stressor that involves mild restraint, novelty and exposure to computer-simulated unpleasant noises generated by the MRI scanner. Meanwhile, the children were requested to lie still in the scanner (for approximately twenty minutes). Salivary samples were collected upon arrival and at 20 min and 40 min following the termination of the stressor to evaluate baseline, response to the stressor and negative feedback. All mock MRI treatments were conducted in the afternoon corresponding to home sample collection time.
Cortisol Storage and Assays
Once collected, salivary samples were stored in a −20°C freezer. Prior to assay, the saliva samples were thawed and centrifuged at 6000 rpm for 10 minutes to separate aqueous component from mucins and other suspended particles. Cortisol assays were performed using coated-tube radioimmunoassay (RIA) kits (Siemens Medical Solutions Diagnostics, Los Angeles). As previously reported assay procedures were modified to accommodate overall lower levels of cortisol in human saliva relative to plasma as follows: 1) standards were diluted to concentrations ranging from 2.76 to 345 nmol/L, 2) sample volume was increased to 200 μl, and 3) incubation times were extended to 3 h. Serial dilution of samples indicates that the modified assay displays a linearity of 0.98 and a least detectable dose of 0.548 nmol/L. Cross-reactivity with other naturally occurring steroids is minimal (e.g. corticosterone=0.94%; cortisone=0.98%). Intra- and inter-assay coefficients of variation are 4.36% and 6.66%, respectively.
Statistical Analysis
The goal of this investigation was to expand on our previous results by examining diurnal rhythm and response to stress in cortisol compared to parent-report measures of stress and sensory functioning. In children with autism, we predicted that parent-reported measures of child stress (SSS Total) would be associated with higher evening cortisol levels and correlated with the cortisol response to the stress protocol. We also predicted that increased sensory sensitivity (SSP Total) would be associated with diurnal variability and morning cortisol. It was also hypothesized that reported parenting stress (PSI Total) would be associated with elevated cortisol, especially in the evening.
Independent two-sample t-tests were conducted to assess differences between the groups based on age and clinical variables. Additionally, we compared the six day average peak-to-trough cortisol values between the children with autism and the neurotypical children to determine if there were significant differences in the variability and depth of the slope using a two-sample t-test.
The main outcome variable of interest was cortisol. Since salivary cortisol measurements are positive and skewed toward large values, the log transformation was performed to achieve approximate normality and used in all subsequent analyses.
The primary statistical model used was a repeated measures linear mixed effect model (Fitzmaurice et al., 2004). The goal of this analysis was to explain the additional variability within the autism compared to the neurotypical group; therefore the two groups were analyzed separately using equivalent methods. First, the diurnal rhythm was examined with the following fixed effects: Age, IQ, SSS Total, SSP Total, and Time of Day (Morning, Afternoon, Evening). Next, morning, afternoon, and evening measurement subsets were analyzed independently using the above methods and included Age, IQ, SSS Total, and SSP Total as the fixed effects. If SSS Total or SSP Total were found significant, models were then tested that included all sub domains as fixed effects. In addition, separate repeated measures linear models were fit with Age, IQ, and PSI Total as fixed effects for each time of day.
The 3 cortisol measurements associated with the mock MRI stressor were analyzed using a repeated measures linear mixed effect model treating the data as longitudinal. The fixed effects included in the model were Age, IQ, SSS Total, and SSP Total. An additional categorical variable was created when any individual that exhibited a response to the stressor in the form of an increase in cortisol from arrival to 20 minutes after the mock MRI was defined as a “responder” and those that did not exhibit a response was defined as a “non-responder”. Specifically, the arrival cortisol measurement was subtracted from the 20 minute cortisol measurement and a value greater than zero was labeled a “responder” while a value less than or equal to zero was labeled a “non-responder”.
For each analysis previously mentioned, the full model contained all variables identified as potentially significant predictors of cortisol. In each case a stepwise regression method was employed to reduce the full models (i.e. remove statistically insignificant variables) using a sequence of Wald tests such that all remaining variables in the final model were significant at the 5% level (Wald, 1943). For each model, a random effect was included per child and an unstructured covariance assumption for the random effects was used. The error terms were assumed to be independent, normally distributed and to have a common variance.
2. Results
Diurnal Rhythm: Between group analyses
The means and standard deviations of the demographic and dependent variables are displayed in Table 1. for Age, IQ, SCQ, PSI Total, SSS Total, SSP Total and their subdomains. Independent two-sample t-tests were used to test for statistically significant differences between the two groups. It was observed that the average SSS measures were statistically higher across all subdomains while the average SSP measures were statistically lower across all subdomains for the children with autism compared to the neurotypical children.
Table 1.
Demographic and Dependent Variables
| Variable | Autism Mean (SD) | Neurotypical Mean (SD) | t-score | p-value |
|---|---|---|---|---|
| Age | 8.8 (1.9) | 9.4 (1.7) | −0.98 | 0.3305 |
| IQ | 87.9 (11.9) | 113.6 (15.5) | −6.09 | < 0.0005 |
| SCQ | 21.82 (6.12) | 3.60 (3.54) | −11.64 | <0.0001 |
| PSI | 82.9 (20.8) | 54.9 (13.8) | 21.99 | <0.0001 |
| SSS: Total | 119.2 (31.5) | 77.1 (22.7) | 4.90 | < 0.0005 |
| SSS: Changes | 27.5 (7.7) | 17.3 (4.8) | 5.07 | < 0.0005 |
| SSS: Positive | 15.1 (7.2) | 11.1 (3.6) | 2.29 | 0.0276 |
| SSS: Anticipation | 15.9 (4.5) | 10.9 (4.1) | 3.70 | 0.0007 |
| SSS: Sensory | 8.3 (3.3) | 5.5 (1.9) | 3.35 | 0.0018 |
| SSS: Social | 6.4 (2.3) | 4.2 (1.3) | 3.83 | 0.0005 |
| SSS: Unpleasant | 26.5 (5.8) | 16.7 (5.4) | 5.60 | < 0.0005 |
| SSS: Food | 7.1 (2.5) | 4.7 (2.1) | 3.39 | 0.0016 |
| SSS: Rituals | 12.4 (3.4) | 7.0 (3.6) | 5.06 | < 0.0005 |
| SSP: Total | 128.2 (22.5) | 173.5 (14.3) | −7.88 | < 0.0005 |
| SSP: Tactile | 28.0 (4.4) | 32.4 (2.8) | −3.91 | 0.0003 |
| SSP: Taste Smell | 9.0 (4.2) | 18.4 (2.6) | −8.85 | < 0.0005 |
| SSP: Movement | 12.2 (2.9) | 13.9 (1.8) | −2.36 | 0.0233 |
| SSP: Under Seek | 22.2 (6.6) | 30.0 (5.3) | −4.22 | 0.0001 |
| SSP: Auditory Filtering | 17.9 (5.0) | 26.0 (3.9) | −5.95 | < 0.0005 |
| SSP: Energy | 21.6 (7.3) | 29.3 (1.7) | −4.84 | < 0.0005 |
| SSP: Visual Audio | 17.5 (4.8) | 23.6 (1.7) | −5.61 | < 0.0005 |
Note: IQ = Intelligence Quotient; PSI = Parenting Stress Index; SSS = Stress Survey Schedule; SSP = Short Sensory Profile.
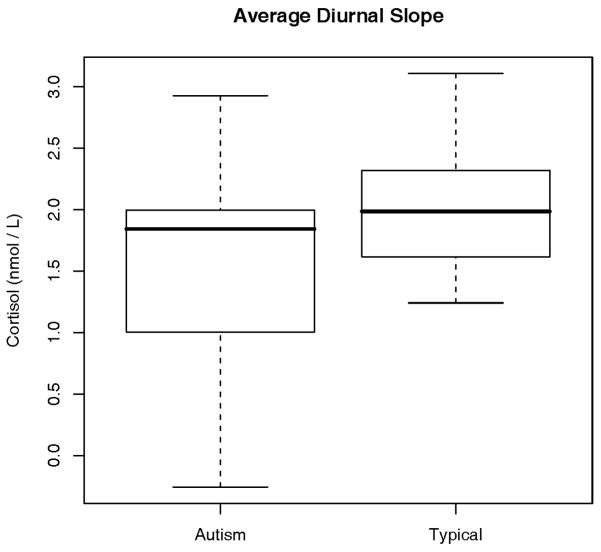
The comparison of the peak-to-trough levels is presented in Figure 1. The children with autism show significantly more variability in diurnal cortisol while demonstrating a more shallow slope from morning to evening than the neurotypical children. The upper 25% of autistic children show an average peak-to-trough difference that compares with the upper 50% of the neurotypical children. However, it can be seen that more than 75% of the children in the autistic group have an average peak-to-trough difference that is less than the upper 50% of the neurotypical children. In addition, more than 25% of the autistic children have a difference that is less than all of the children in the neurotypical group.
Figure 1.
Variability and depth of the diurnal slope between groups. Figures show the significant variability as well as less peak-to-trough difference from morning to evening values in the autism group. Nmol/L = nanomoles per liter.
The average peak-to-trough cortisol difference for the autism group was found to be statistically significant compared to the neurotypical group (t (42) = −2.13, P = 0.039).
Diurnal Rhythm: Autism within group analyses
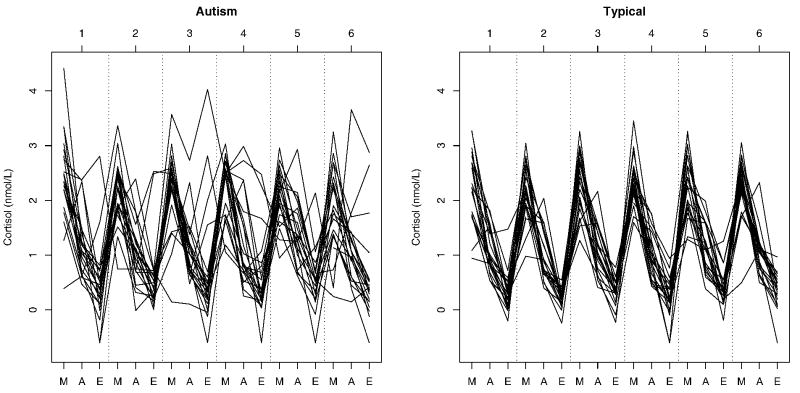
The first step in the analysis was concentrated on the 18 daily cortisol measurements that included all fixed factors and a separate random intercept term for each individual. The model was reduced and variables discarded through a series of Wald tests. This yielded a model with time of day as the only significant fixed effect with coefficient estimates displayed in Table 2. The relationship between time of day and cortisol corresponds with what is known about the circadian rhythm for cortisol, that measurements are highest in the morning and decline until their lowest point in the evening. Comparing the two groups, it is apparent that children with autism have greater between-subject and within-subject variability in daily cortisol responses than neurotypical children and in addition have a higher level of dysregularity (See Figure 2).
Table 2.
Regression Model Coefficients
| Autism | Neurotypical | ||||
|---|---|---|---|---|---|
| Diurnal | Morning | Evening | Diurnal | Afternoon | |
| Variable | EST (SE) | EST (SE) | EST (SE) | EST (SE) | EST (SE) |
| Intercept | 2.16 (0.082) | 3.81 (0.64) | −0.39 (0.46) | 2.30 (0.046) | 2.55 (0.564) |
| Afternoon | −1.02 (0.086) | - | - | −1.23 (0.053) | - |
| Evening | −1.64 (0.086) | - | - | −2.00 (0.053) | - |
| SSS: Total | - | −0.006 (0.003) | 0.008 (0.004) | - | - |
| SSP: Total | - | −0.007 (0.004) | - | - | −0.008 (0.003) |
|
| |||||
| Number of Observations per Participant | 18 | 6 | 6 | 18 | 6 |
|
| |||||
| Standard Deviation of Random Effect | 0.239 | 0.225 | 0.462 | 0.115 | 0.106 |
Figure 2.
Individual patterns of the home cortisol measurements for all participants. M = Morning, A = Afternoon, E = Evening. Nmol/L = nanomoles per liter.
Since all other predictor variables were found not to be significant in the presence of time of day, separate analyses were performed for each time of day (See Table 2). Analysis of the 6 morning cortisol measurements yielded a model with both the SSS Total and the SSP Total stress measures as significant (see Table 2). However, analysis of subdomains revealed a complex interplay between different aspects of sensory sensitivity and morning cortisol (see Table 3).
Table 3.
Model Coefficients for Cortisol and SSS and SSP Variables
| Autism | Neurotypical | ||
|---|---|---|---|
| Morning | Evening | Afternoon | |
| Variable | EST (SE) | EST (SE) | EST (SE) |
| Intercept | 3.79 (0.49) | −0.39 (0.43) | 2.51 (0.478) |
| SSS: Anticipation | 0.056 (0.026) | - | - |
| SSS: Changes | −0.034 (0.016) | 0.033 (0.015) | - |
| SSS: Social | −0.110 (0.039) | - | - |
| SSP: Auditory Filtering | −0.054 (0.014) | - | - |
| SSP: Energy | 0.022 (0.011) | - | - |
| SSP: Movement | −0.099 (0.028) | - | - |
| SSP: Tactile | - | - | −0.044 (0.015) |
| SSP: Visual Audio | 0.043 (0.014) | - | - |
|
| |||
| Number of Observations per Participant | 6 | 6 | 6 |
|
| |||
| Standard Deviation of Random Effect | 0.00008 | 0.452 | 0.089 |
The results revealed a negative relationship between morning cortisol and the SSS such that higher scores were correlated with lower cortisol. Further, the SSP showed that greater sensory functioning was associated with higher cortisol (See Table 2 and Table 3). The separate analysis of the morning measurements that included PSI Total with Age and IQ was not found to be significant (χ2(3) = 6.70, P = 0.082).
The analysis of the 6 afternoon cortisol measurements failed to find any significant explanatory predictor (χ2(4) = 7.244, P = 0.124). In addition, the analysis of the afternoon measurements that included PSI Total with Age and IQ was not found to be significant (χ2(3) = 0.692, P = 0.875). The analysis of the 6 evening cortisol measurements included all of the same fixed effects as the morning measurements and yielded a model with SSS as a significant variable (see Table 2). There appears to be a positive relationship between the SSS stress measure and cortisol indicating that the more stressed the child based on parental report is, the higher their cortisol level at the end of the day. Post hoc analysis on the separate 8 subdomains of the SSS revealed a significant contribution of the Changes variable (See Table 3). This finding supports our previous hypothesis (Corbett et al., 2008a) demonstrating that higher evening cortisol was associated with increased stress and, in particular, was associated with changes in routine or schedule which the children with autism may have found particularly stressful. The separate analysis of the evening measurements that included PSI Total with Age and IQ was not found to be significant (χ2(3) = 2.66, P = 0.448).
Diurnal Rhythm: Neurotypical within group analyses
For the neurotypical children, the analysis of all 18 measurements yielded a model with only time of day as significant (See Table 2). Separate analysis of the morning and evening measurements failed to find a significant model (Morning: χ2(4) = 6.373, P = 0.1730; Evening: χ2(4) = 0.665, P = 0.9555). However, there was a positive relationship between afternoon cortisol and the SSP, specifically the Tactile subdomain, indicating that less sensory sensitivity was associated with higher afternoon cortisol (See Table 3).
Stressor: Between group analyses
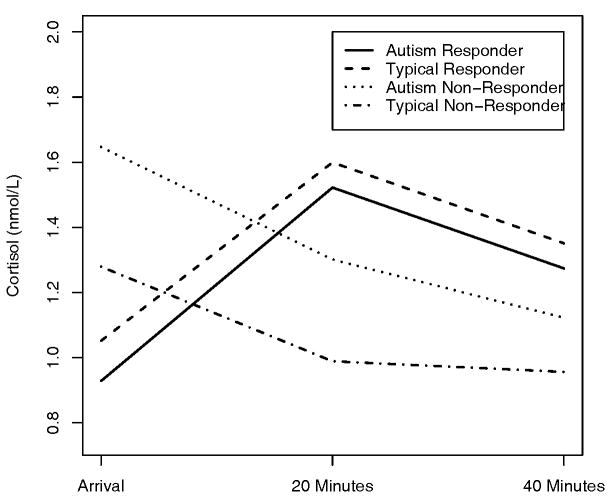
Initial analysis of the 3 cortisol measurements associated with the mock-MRI stressor which included time of measurement (0, 20, 40 minutes), Diagnosis, Age, IQ, SSS total and SSP total found none of the variables to be significant (χ2(6) = 5.47, P = 0.484). Since none of the psychological variables were found to be significantly related to the stress protocol, the groups were divided based on their responder status (See Figure 3). It can be seen that there is a clear difference between the average measurement for responders and non-responders at all times, however, diagnosis was not found to be significant nor were the other psychological variables in the presence of the responder variable. Responders showed a rise in cortisol measurement at 20 minutes post-stressor with a return to baseline as expected, however, the non-responders showed a higher cortisol level upon arrival with a steady decrease post-stressor. The final model contained time of measurement and the responder variable as well as interaction terms between responder and time and the included quadratic term to capture the rise in cortisol due to stress response (See Table 4).
Figure 3.
Group patterns of the average stress response cortisol measurements separated by group and responder status. Nmol/L = nanomoles per liter.
Table 4.
Model Coefficients for Cortisol and Stress Protocol
| Variable | EST (SE) |
|---|---|
| Intercept | 1.39 (0.086) |
| Time | −0.01 (0.002) |
| Responder | −0.43 (0.151) |
| Time:Responder | 0.06 (0.010) |
| Time2:Responder | −0.001 (0.0002) |
|
| |
| Number of Observations per Participant | 3 |
|
| |
| Standard Deviation of Random Effect | 0.318 |
Discussion
The purpose of the current investigation was to extend previous findings in which we reported significant between and within group variability in diurnal cortisol in children with autism compared to neurotypical peers and to consider factors related to inconsistent findings in stress responsivity (Corbett et al., 2006; Corbett et al., 2008a). The study explored potential relationships between cortisol and psychological measures of stress (SSS) and sensory functioning (SSP) and how such factors may explain some of the notable dysregulation and variability observed in individuals with autism (See Figure 2). Importantly, the stress and sensory profiles of children with autism were distinguished from the neurotypical children by their uniformly higher scores on the SSS and consistently lower scores on the SSP. These measures were subsequently compared to diurnal cortisol. The results revealed a negative relationship between morning cortisol and the SSS measures indicating that lower peak morning cortisol value was associated with higher levels of stress. Lower cortisol has been associated with conditions of chronic stress (Nickel et al., 2007; Nicolson & van Diest, 2000; Ockenfels et al., 1995; Secunda et al., 1986; Strickland et al., 1998; Yehuda et al., 1995a; Yehuda et al., 1995b; Yehuda et al., 1993a; Yehuda et al., 1993b; Yehuda et al., 1994). Studies of nonhuman primates have shown that social conditions that prevent the formation of stable social relationships can also produce lower cortisol and carry with it increased susceptibility to disease processes (Capitanio et al., 1998; Mendoza et al., 2000). Therefore, the reduced cortisol values in concert with higher stress measures may be the result of chronic stress and particularly with social stress.
Additionally, cortisol was associated with greater sensory sensitivity in the children with autism. These findings lend support for our previous hypothesis (Corbett et al., 2008a) that increased variability and alterations in morning cortisol values may be the result of fundamental dysregulation and increased susceptibility to external factors, such as zeitgeibers. It is plausible that children with autism are more susceptible to the influence of external or internal factors that alter the regulatory system, which in turn leads to greater diurnal variability and less regulated responses in children with autism.
Dunn proposed that individuals demonstrate four sensory processing patterns, which include sensory seeking, sensory avoiding, sensory sensitivity, and low registration (Dunn, 2001). It has been shown that individuals with ASD exhibit both overresponsive and underresponsive sensory functioning based on sensory functioning questionnaires (e.g., (Baranek et al., 2006; Dunn et al., 2002; Kern et al., 2006; Liss et al., 2006; Pfeiffer et al., 2005; Rogers et al., 2003; Watling et al., 2001). Further, sensory deficits appear related to symptom severity with some domains improving with age while others, such as tactile sensitivity persist (Kern et al., 2006). It is apparent that a variety of sensory patterns were related to cortisol morning levels (see Table 3). For example, auditory filtering was associated with lower cortisol whereas higher energy level and visual/spatial sensitivity were associated with enhanced cortisol. The results imply that morning cortisol in children with autism may be influenced by both perceived stress and sensory sensitivity.
We also predicted that the parent-reported behavioral measure of child stress would be associated with elevated cortisol in children with autism, especially in the evening, as a reflection of the cummulative effects of increased stress from daily activities. The hypothesis was confirmed as the evening cortisol values yielded a significant positive association with SSS such that the higher the level of evening cortisol, the higher the child’s behavioral stress. It is noteworthy that posthoc analyses demonstrated a selective contribution of the Changes subdomain revealing a particular reactivity in the autism group to environmental and daily changes in routines and activities. From Kanner’s earliest descriptions of autism it has been shown that, “the child’s behavior is governed by an anxiously obsessive desire for the maintenance of sameness that nobody but the child himself may disrupt on rare occasions” ((Kanner, 1943) page 245). The implication from Kanner’s descriptions and our findings supports sensitivity to novelty, and specifically a strong psychobiological connection with regard to evening cortisol and reactivity to change.
In the children with autism, the lower morning and elevated evening cortisol results in a reduced diurnal slope. It has been reported that suppression or flattening of the diurnal rhythm can occur in at-risk populations of children (Gunnar & Vazquez, 2001). The paradoxical suppression of the LHPA axis reported in conditions of prolonged stress is referred to as hypocortisolism (e.g., (Heim et al., 2000; Raison & Miller, 2003; Rohleder et al., 2004)). The reduced morning trend in coritisol (Corbett et al., 2008a), the flattening of the diurnal slope coupled with the between and within group variability, clearly reveals a dysregulated LHPA system. The corroborating report of elevated stress and sensory sensitivity further extends the hypothesis and highlights likely contributory factors such as reactivity to changes in routine and increased sensitivity to sensory stimuli, respectively.
Although we previously looked at the relationship between the SSS and SSP, we did not report an association with cortisol (Corbett et al., 2006). It is likely that the former investigation was underpowered by a small sample size (22 versus 44 participants) and fewer cortisol samples (6 versus 18). Even with the increased sample size and the collection of samples from a well-characterized, homogeneous group, we acknowledge the persistent heterogeneity within autism. Further, our study only included high functioning children within a narrow age range; thus, it is not known whether our findings generalize to lower functioning and younger individuals. Especially in consideration of the recently reported relationship between level of autism functioning, age and sensory functioning (Kern et al., 2006; Kern et al., 2007), it will be important to extend such studies to larger and broader populations.
In regards to the neurotypical children the only observed relationships with diurnal rhythm were found in the afternoon related to tactile sensitivity. Although this could be a spurious finding, it suggests that neurotypical children that evidence more tactile sensitivity may be mildly prone to higher afternoon reactivity of the endocrine system.
In order to better understand the inconsistent findings in the stress responsivity across the studies (Corbett et al., 2006; Corbett et al., 2008a), we explored potential contributions of psychological and demographic variables as conducted in the diurnal models. However, all were not statistically significant or explanatory. Thus, the finding of distinct subgroups of responders and non-responders in both the autism and neurotypical group is noteworthy. Although the psychological measures of current behavioral stress, sensory functioning or parenting stress were not correlated, it is plausible that developmental factors may be contributory. Specifically, it has been frequently reported that developmental factors of early behavioral disinhibition and temperament have been shown to be stable over time and related to activation of the LHPA axis (e.g., (Degnan & Fox, 2007; Fox et al., 2008; Fox et al., 2005; Fox et al., 2001; Goldsmith & Lemery, 2000; Gunnar et al., 2003; Schmidt et al., 1997; Tyrka et al., 2006; Young et al., 1999; Zimmermann & Stansbury, 2004)). Thus, the level of reactivity of the system may be largely predetermined by early biological and environmental factors, such that components of self-regulation and reactivity may play a more fundamental role in stress responsivity (Derryberry & Rothbart, 1988) than the presence or absence of a diagnosis, such as autism.
A dysregulated LHPA axis may have far reaching implications in autism for the system does not work in isolation, rather it is intimately connected with the CNS and the immune system through a complex network of hormone-brain-behavior interactions (Brown, 1994). It is highly plausible that fundamental biological alterations in the system may contribute to atypical neurodevelopment. It is unclear if the alterations in the consistency of the diurnal values and reduction of the slope directly contribute to the neuropathology of autism or if the manifestation of the disorder results in alteration of neuroendocrine functioning. Moreover, more than one mechanism may be contributing to the dysregulation of the LHPA system. These data provide compelling evidence of the importance of this research pursuit into better understanding the role that the LHPA axis may play in the neuropathology and sequelae of autism.
Acknowledgments
Funding was provided by the NIH Career Development Award (5K08NMHO72958), University of California, Department of Psychiatry Tupin Award, and a M.I.N.D. Institute Investigator Initiated Pilot Grant to Blythe A. Corbett. The authors thank the children and families who participated in this multiphase study and methodically assisted in the collection of home samples and completion of psychological measures. Statistical advice for this manuscript was provided by Laurel Beckett, Ph.D. and made possible by Grant UL1 RR024146 from the National Center for Research Resources, a component of NIH. This manuscript is dedicated to our mentor and dear friend, Seymour “Gig” Levine who despite his long, accomplished life - he left us much too soon.
Footnotes
Funding provided by the NIH Career Development Award (5K08NMHO72958), University of California, Department of Psychiatry Tupin Award, and a M.I.N.D. Institute Investigator Initiated Pilot Grant to Blythe A. Corbett.
References
- Abidin RR. The Parenting Stress Index. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association.; 1994. [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behav Genet. 2003;33(4):421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Brown RE. An introduction to neuroendocrinology. New York, NY: Cambridge University Press.; 1994. [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proceedings of the National Academy of Sciences. 1998;95(8):4714–4747. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain RS, Herman BH. A novel biochemical model linking dysfunctions in brain melatonin, proopiomelanocortin peptides, and serotonin in autism. Biol Psychiatry. 1990;28(9):773–793. doi: 10.1016/0006-3223(90)90513-2. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry and Neuroscience. 2008a;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Mendoza SP, Baym CL, Bunge SA, Levine S. Examining cortisol rhythmicity and responsivity to stress in children with Tourette syndrome. Psychoneuroendocrinology. 2008b;33(6):810–820. doi: 10.1016/j.psyneuen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NO, Carter AS. Parenting stress in mothers and fathers of toddlers with autism spectrum disorders: associations with child characteristics. J Autism Dev Disord. 2008;38(7):1278–1291. doi: 10.1007/s10803-007-0512-z. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Dev Psychopathol. 2007;19(3):729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. J Pers Soc Psychol. 1988;55(6):958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- Dunn W. Short Sensory Profile. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Dunn W. The sensations of everyday life: empirical, theoretical, and pragmatic considerations. Am J Occup Ther. 2001;55(6):608–620. doi: 10.5014/ajot.55.6.608. [DOI] [PubMed] [Google Scholar]
- Dunn W, Brown C. Factor analysis on the Sensory Profile from a national sample of children without disabilities. Am J Occup Ther. 1997;51(7):490–495. doi: 10.5014/ajot.51.7.490. discussion 496–499. [DOI] [PubMed] [Google Scholar]
- Dunn W, Myles BS, Orr S. Sensory processing issues associated with Asperger syndrome: a preliminary investigation. Am J Occup Ther. 2002;56(1):97–102. doi: 10.5014/ajot.56.1.97. [DOI] [PubMed] [Google Scholar]
- Dunn W, Westman K. The sensory profile: the performance of a national sample of children without disabilities. Am J Occup Ther. 1997;51(1):25–34. doi: 10.5014/ajot.51.1.25. [DOI] [PubMed] [Google Scholar]
- Ermer J, Dunn W. The sensory profile: a discriminant analysis of children with and without disabilities. Am J Occup Ther. 1998;52(4):283–290. doi: 10.5014/ajot.52.4.283. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. John Wiley & Sons; 2004. [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3(7):e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5(3):277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biol Psychiatry. 2000;48(12):1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Groden J, Diller A, Bausman M, Velicer W, Norman G, Cautela J. The development of a stress survey schedule for persons with autism and other developmental disabilities. J Autism Dev Disord. 2001;31(2):207–217. doi: 10.1023/a:1010755300436. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MM. Peer rejection, temperament, and cortisol activity in preschoolers. Dev Psychobiol. 2003;43(4):346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 2006;19(2):95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hill SD, Wagner EA, Shedlarski JG, Jr, Sears SP. Diurnal cortisol and temperature variation of normal and autistic children. Dev Psychobiol. 1977;10(6):579–583. doi: 10.1002/dev.420100612. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoyama F, Watanabe M, Murata S, Kaneko M, Kumashiro H. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Jpn J Psychiatry Neurol. 1987;41(2):227–235. doi: 10.1111/j.1440-1819.1987.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Van der Gaag RJ, ten Hove F, Willemsen-Swinkels SW, Harteveld E, et al. Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, multiple complex developmental disorder. Psychoneuroendocrinology. 2000;25(8):753–764. doi: 10.1016/s0306-4530(00)00020-2. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, van der Gaag RJ, van Engeland H. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28(3):582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Realmuto GM, Garfinkel BD. The dexamethasone suppression test in infantile autism. J Am Acad Child Psychiatry. 1985;24(3):263–265. doi: 10.1016/s0002-7138(09)61085-2. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58(6):1459–1473. [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, et al. The pattern of sensory processing abnormalities in autism. Autism. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, et al. Sensory correlations in autism. Autism. 2007;11(2):123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405(1-3):149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27(1–2):83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule-WPS. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: Studies in non-human primates. In: Ma GP, Mench JA, editors. Biology of Animal Stress. Basic Principles and Implications for Animal Welfare. New York: CABI Publishing; 2000. pp. 227–247. [Google Scholar]
- Montes G, Halterman JS. Psychological functioning and coping among mothers of children with autism: a population-based study. Pediatrics. 2007;119(5):e1040–1046. doi: 10.1542/peds.2006-2819. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C. Comorbid anxiety symptoms in children with pervasive developmental disorders. J Anxiety Disord. 1998;12(4):387–393. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]
- Nickel C, Tanca S, Kolowos S, Pedrosa-Gil F, Bachler E, Loew TH, et al. Men with chronic occupational stress benefit from behavioural/psycho-educational group training: a randomized, prospective, controlled trial. Psychol Med. 2007;37(8):1141–1149. doi: 10.1017/S0033291706009445. [DOI] [PubMed] [Google Scholar]
- Nicolson NA, van Diest R. Salivary cortisol patterns in vital exhaustion. J Psychosom Res. 2000;49(5):335–342. doi: 10.1016/s0022-3999(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57(5):460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Kinnealey M, Reed C, Herzberg G. Sensory modulation and affective disorders in children and adolescents with Asperger’s disorder. Am J Occup Ther. 2005;59(3):335–345. doi: 10.5014/ajot.59.3.335. [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch Dis Child. 1983;58(6):454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Reynolds S, Lane SJ. Diagnostic Validity of Sensory Over-Responsivity: A Review of the Literature and Case Reports. J Autism Dev Disord. 2007 doi: 10.1007/s10803-007-0418-9. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. J Autism Dev Disord. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55(7):745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales. 5. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Schieve LA, Blumberg SJ, Rice C, Visser SN, Boyle C. The relationship between autism and parenting stress. Pediatrics. 2007;119(Suppl 1):S114–121. doi: 10.1542/peds.2006-2089Q. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, et al. Behavioral and neuroendocrine responses in shy children. Dev Psychobiol. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Secunda SK, Cross CK, Koslow S, Katz MM, Kocsis J, Maas JW, et al. Biochemistry and suicidal behavior in depressed patients. Biol Psychiatry. 1986;21(8–9):756–767. doi: 10.1016/0006-3223(86)90241-6. [DOI] [PubMed] [Google Scholar]
- Strickland P, Morriss R, Wearden A, Deakin B. A comparison of salivary cortisol in chronic fatigue syndrome, community depression and healthy controls. J Affect Disord. 1998;47(1–3):191–194. doi: 10.1016/s0165-0327(97)00134-1. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM, et al. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry. 1997;38(6):705–715. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, et al. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31(9):1036–1045. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes I, Dohanics J, Toth G, Pongracz J. Maturation of the circadian rhythm of the adrenocortical functions in human neonates and infants. Horm Res. 1980;12(5):237–244. doi: 10.1159/000179126. [DOI] [PubMed] [Google Scholar]
- Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Transactions of the Americal Mathematical Society. 1943;54:426–482. [Google Scholar]
- Watling RL, Deitz J, White O. Comparison of Sensory Profile scores of young children with and without autism spectrum disorders. Am J Occup Ther. 2001;55(4):416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition. New York: Psychological Corporation; 2003. [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995a;52(7):583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995b;152(7):982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Resnick H, Kahana B, Giller EL. Long-lasting hormonal alterations to extreme stress in humans: normative or maladaptive? Psychosom Med. 1993a;55(3):287–297. doi: 10.1097/00006842-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993b;150(1):83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ. Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Ann N Y Acad Sci. 1994;746:378–380. doi: 10.1111/j.1749-6632.1994.tb39260.x. [DOI] [PubMed] [Google Scholar]
- Young SK, Fox NA, Zahn-Waxler C. The relations between temperament and empathy in 2-year-olds. Dev Psychol. 1999;35(5):1189–1197. doi: 10.1037//0012-1649.35.5.1189. [DOI] [PubMed] [Google Scholar]
- Zimmermann LK, Stansbury K. The influence of emotion regulation, level of shyness, and habituation on the neuroendocrine response of three-year-old children. Psychoneuroendocrinology. 2004;29(8):973–982. doi: 10.1016/j.psyneuen.2003.09.003. [DOI] [PubMed] [Google Scholar]