Abstract
Our previous studies show that activation of the transient receptor potential vanilloid type 1 (TRPV1) channels by a selective agonist, capsaicin (CAP), given unilaterally into the renal pelvis leads to increases in urine flow rate (Uflow) and urinary sodium excretion (UNa) bilaterally, albeit the mechanisms underlying enhanced renal excretory function are unknown. The present study was designed to determine the contribution of each of the renal segments to enhanced renal excretory function when TRPV1 expressed in sensory nerve fibers innervating the renal pelvis is activated. To accomplish the goal, LiCl was given intravenously to male Wistar rats while the left renal pelvis (LRP) was perfused with vehicle or CAP with or without a selective TRPV1 antagonist, capsazepine (CAPZ). Uflow and clearance of creatinine, lithium, sodium and water, either as filtered or fractionally, were determined in both kidneys. LRP perfusion of CAP at 2.4 nmol increased Uflow (µl·min−1g−1) (ipsilaterally from 6.6 ± 0.6 to 14.6 ± 2.2 and contralaterally from 7.4 ± 0.7 to 13.9±1.8, p<0.05) and UNa (µmol·min−1g−1) (ipsilaterally from 0.6 ± 0.2 to 1.8 ± 0.3 and contralaterally from 0.7 ± 0.2 to 1.9 ± 0.4, p<0.05). Ipsilateral blockade of the TRPV1 with CAPZ at 24 nmol prevented CAP-induced increases in Uflow and UNa bilaterally. Creatinine, lithium, sodium and free water clearance (ml·min−1) were increased in CAP (1.47 ± 0.27, 0.44 ± 0.05, 0.026±0.004, 0.41±0.05, respectively) compared to vehicle (0.72 ± 0.12, 0.25 ± 0.05, 0.010±0.001, 0.24±0.05), CAPZ+CAP (0.83 ± 0.13, 0.24 ± 0.03, 0.014±0.002, 0.23±0.03) and CAPZ (0.88 ± 0.05, 0.21 ± 0.01, 0.010±0.001, 0.20±0.01) groups (p≤0.01). Filtered sodium load, distal delivery of sodium and distal sodium reabsorption (µEq·min−1) were also increased in CAP (202.2±33.3, 61.3±7.4, 57.6±7.4, respectively) compared to vehicle (97.7±16.6, 33.6±5.8, 32.2±5.9), CAPZ+CAP (110.5±16.3, 32.5±4.5, 30.7±4.3) and CAPZ (118.0±4.5, 27.9±1.2, 26.8±1.2) groups (p≤0.01). In contrast, fractional lithium and sodium excretion, absolute proximal reabsorption, fractional proximal reabsorption, fractional distal sodium and water reabsorption were not different among groups.
Therefore, activation of the TRPV1 expressed in primary afferent nerves innervating the renal pelvis leads to diuresis and natriuresis in both kidneys. The TRPV1-induced sodium and water excretion appears to be mediated by increases in glomerular filtration rate and distal tubular delivery of sodium but not by suppression of renal proximal and distal tubular reabsorption, suggesting a key role of segmental regulation of renal function by TRPV1-positive primary sensory nerves in the maintenance of sodium and water homeostasis.
Keywords: TRPV1, glomerular filtration rate, tubular reabsorption, natriuresis, sensory nerves, renal pelvis
INTRODUCTION
Sodium and water homeostasis is maintained under the physiological condition and impaired in disease states such as hypertension and heart failure. One of the therapeutic strategies for these diseases is to restore the impaired sodium and water homeostasis. Given that the kidney is a key organ in the regulation of water and sodium excretion, it has been a target for defining the pathogenesis of a number of cardiovascular diseases as well as for drug development.
It has been shown that the renal pelvis is heavily innervated with thin neurofilament- and neuron-specific enolase (NSE)-immunoreactive nerve fibers.1 These nerve fibers form a two-dimensional network with the transverse connections, which mainly distribute within the submucosal layer of the renal pelvic wall. A subset of these nerve fibers makes up sensory nerves that contain the transient receptor potential vanilloid type 1 (TRPV1) channels. These TRPV1-positive sensory nerves have their free terminal endings distributed in the mucous layer and close to the epithelial cells of the renal pelvis.2 Given that the TRPV1 can be activated by polymodal stimuli including heat, low pH, lipid metabolites, and vanilloid compounds, the renal pelvis richly innervated by TRPV1-positive sensory nerves may act as a sensory organ sensing mechano- and chemo-stimulations imposed onto the renal pelvis, leading to modulation of renal excretory function and diuresis and natriuresis responses.3,4
Our previous study showed that activation of the TRPV1 expressed in sensory nerve endings innervating the unilateral renal pelvis by its selective agonist, capsaicin (CAP),induces bilateral diuresis and natriuresis.5 TRPV1-mediated bilateral diuresis and natriuresis were abolished by blockade of the TRPV1 with its selective receptor antagonist, capsazepine (CAPZ), given ipsilaterally into the renal pelvis or by ipsilateral renal denervation. 5 These results indicate that renal afferent as well as efferent nerves play a key role in the regulation of sodium and water excretion via an inhibitory renorenal reflex mechanism.4
Renal sympathetic innervation controls at least three neuroeffectors in the kidney, i.e., blood vessels, renal tubules, and juxtaglomerular granular cells.6 Previous studies showed that unilateral renal denervation leads to a higher rate of sodium excretion from the denervated kidney than from the contralateral innervated kidney.7 Moreover, it has been shown that decreases in renal sympathetic nerve activity (RSNA) lead to diuresis and natriuresis in the presence4,8 or absence of changes in glomerular filtration rate (GFR) and renal blood flow (RBF),9 whereas increases in RSNA lead to an anti-natriuresis in the absence of significant change in GFR.10
Although our previous data show that activation of the TRPV1 leads to diuresis and natriuresis, the mechanisms underlying enhanced renal excretory function are unknown. The present study was designed to determine the contribution of each of the renal segments to enhanced renal excretory function when TRPV1 expressed in sensory nerve fibers innervating the renal pelvis is activated. The potential mechanisms and implications of the study are discussed.
METHODS
Animal groups and protocols
All experiments were approved by the Institutional Animal Care and Use Committee. Male Wistar rats weighing 294±5 g (Charles River Laboratories; Wilmington, MA) were housed in the animal facility for 1 wk before experiments and randomly assigned to four groups for the following treatments: (1) control (vehicle), 0.18 % of ethanol and Tween 80 in saline given via left renal pelvis perfusion (LRPP); (2) capsaicin (CAP) given at 2.4 nmol (12.2ng/µl or 0.04 nmol/µl) via LRPP; (3) capsazepine (CAPZ, a selective TRPV1 antagonist) given at 24 nmol (150.8ng/µl or 0.4 nmol/µl) via LRPP before CAP perfusion (CAPZ+CAP); (4) CAPZ given at 24 nmol via LRPP. It has been shown that 2.4 nmol CAP given via LRPP but not intravenously leads to bilateral diuresis and natriuresis, which can be prevented by CAPZ given at 24 nmol ipsilaterally.5
Experimental procedure
All rats were intraperitoneally administered pentobarbital sodium, 50 mg·kg−1, and maintained with an intravenous infusion of 10 mg·kg−1hr−1 at 50 µl·min−1. The potent pentobarbital sodium was selected to make sure that it was still effective 1.5 h after the end of the surgery so that renal pelvis perfusion of CAP, an irritant and pain causing compound, could be conducted. Catheters were placed in the right jugular vein (RJV) for administration of drugs, and in the right carotid artery for monitoring mean arterial pressure (MAP) with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instrument Systems, Valley View, Ohio, USA). Polyethylene (PE-50) catheters were inserted into both ureters via midline incision. A fine outlet tube of MD-2000 (ID 0.18/OD 0.22 mm, BASi, West Lafayette, IN 47906, USA) was placed inside the PE-50 catheter with its tip in the renal pelvis during a 3-min perfusion of drug at rate of 20 µl·min−1 that did not change renal pelvis pressure.8
The experiment started approximately 1.5 h after the end of the surgery. Protocols for LRPP contained two 3- minute periods: CAPZ was perfused in the first 3 – minute period, CAP in the second 3 – minute period. In the case when CAP or CAPZ was perfused alone, the other period was perfused with vehicle. In controls, vehicle was perfused in both periods without perfusion of CAP or CAPZ. Urine samples were collected for 30 minutes before and after experimental protocols for analysis of urine sodium, lithium, and creatinine. At the end of the experiment, blood samples were collected for analysis of hematocrit (Hct), total protein (TP), and plasma concentrations of sodium, lithium, and creatinine. For intravenous administration of Li+, a priming dose of 0.3 µmol LiCl·g−1 (per gram of body weight) was given for 30 minutes and a dose of 0.04 µmol LiCl·g−1hr−1 was used for continuous intravenous infusion. These doses of IV administration of Li+ produced plasma Li+ concentrations at a range of 0.15–0.30 mmol·L−1 at the time of the experiment.11
Creatinine assay
Plasma and urine creatinine concentrations were determined with the use of an improved Jaffe creatinine assay kit (BioAssay Systems, CA). Briefly, the samples and creatinine standards were added to a plate and incubated with working reagent supplied by the manufacturer for 20 min. The plate was read at 490 nm by an absorbance microplate reader (µ Quant, Bio-IEK Instrument, Inc.).
Analysis of lithium
Plasma and urine lithium concentrations were determined with the use of a flame photometer (Model IL 943, Instrumentation Laboratory). Briefly, plasma and urine were deproteinized by addition of equal amounts of 0.3 N ZnSO4 and 0.3 N Ba(OH)2. After vortexing thoroughly, samples were centrifuged at 3,000 × g for 15 min at 4°C. The supernatant was filtered with a large filter tube (Millipore, UFC40HV00; 0.45 um, Bedford, MA) followed by centrifugation at 3,000 × g for 15 min at 4°C. The filtered supernatant was dried with a vacuum centrifuge at 4°C. The dried pellet was reconstituted in distilled water. After mixing thoroughly, samples were centrifuged at 15,300 × g for 10 min at 4°C. The clear supernatant was used for lithium measurement, and the concentration of lithium was determined with a standard curve.
Analysis of sodium, hematocrit, and total protein
Plasma and urine sodium concentrations were determined with the use of a flame photometer (Model IL 943, Instrumentation Laboratory). Plasma was deproteinized before measurement of sodium. Hematocrit was determined by collecting blood in EDTA tubes subjecting subsequently to centrifugation at 1,800 × g for 30 min. Total protein of plasma was determined by the use of BCA protein assay (Pierce, Rockford, IL). Briefly, the samples and protein standards were added to a plate and incubated with working reagent supplied by the manufacturer for 30 min. The plate was read at 562 nm by an absorbance microplate reader (µ Quant, Bio-IEK Instrument, Inc.).
Calculation
The clearances of creatinine (CCr, used as a measure of glomerular filtration rate, GFR), lithium (CLi), and sodium (CNa) were calculated with the standard expression:
where Ux and Px are urine and plasma concentrations, respectively, of the substance x, and V is the urine flow rate. Fractional excretion of lithium (FELi) and sodium (FENa) were calculated as CLi/GFR and CNa/GFR. The following parameters were derived according to Boer and colleagues:12 Filtered sodium load (FLNa) was calculated as PNa × CCr, where PNa is the plasma concentration of sodium; distal delivery of sodium (DDNa) was calculated as CLi×PNa. DDNa was also calculated from the formula of (CH2O+CNa)×PNa, where CH2O and CNa are free water and sodium clearances, respectively.13 When deducting the two equations for DDNa, free water clearance equals to CLi − CNa. Absolute proximal reabsorption (APR) was calculated as CCr-CLi, where CCr and CLi are creatinine and lithium clearances, respectively; Fractional proximal reabsorption (FPR) was calculated as [(CCr−CLi)/CCr]×100; Distal sodium reabsorption (DRNa) was calculated as DDNa −UNa V, where UNa V is urine sodium excretion;14 Fractional distal sodium reabsorption (FDRNa) was calculated as [(CLi−CNa)/CLi]×100; Fractional distal water reabsorption (FDRH2O) was calculated as [(CLi−UV)/CLi]×100, where UV is urine volume.15 Considering that tubular reabsorption of filtered lithium occurs largely in the proximal tubules and that its reabsorption at more distal nephron sites is limited,16 it should be noted that lithium is assumed to be reabsorbed in the proximal tubules in parallel with sodium and water, and that it is neither secreted nor reabsorbed beyond the proximal tubule. Finally, it should be noted that throughout the paper, the expression “distal tubule” indicates all segments of the renal tubules beyond the proximal segment.
Statistical analysis
All values are expressed as means±SE. The differences among groups were analyzed using one-way or two-way ANOVA followed by the Tukey-Kramer multiple comparison tests. Comparisons of MAP before and after administration of drugs were performed by the use of a paired t-test. Differences were considered statistically significant at p < 0.05.
Drugs
Capsaicin (Sigma) was dissolved in ethanol (5% v/v), Tween 80 (5% v/v), and saline to make a stock solution of 65 nmol/µL and was further diluted in saline to a concentration of 0.04 nmol/µl to be perfused into the renal pelvis at a rate of 20 µl·min−1 within the 3 min period. Capsazepine (Calbiochem, San Diego, California, USA) was dissolved in DMSO (10% v/v), Tween 80 (10% v/v), and saline to make a stock solution of 53 nmol/µL, and was further diluted in saline in the same manner before renal pelvis perfusion.
RESULTS
MAP and plasma levels of ions and other parameters
There was no significant difference of MAP at the baseline levels among four experimental groups (Table 1). Unilateral renal pelvic perfusion of CAP, CAPZ, or the combination of the two did not alter MAP from their baseline levels (Table 1). Moreover, hematocrit (Hct) and plasma concentrations of total protein (TP), sodium ([Na+]p), creatinine ([Cr]p), and lithium ([Li+]p) were not significantly different among four experimental groups at the end of the experiments (Table 2).
Table 1.
MAP (mmHg) before and after the treatment
| Groups | Baseline (mmHg) |
Treatment (mmHg) |
|---|---|---|
| Vehicle | 125 ± 1 | 125 ± 1 |
| CAP | 121 ± 4 | 121 ± 4 |
| CAPZ+CAP | 124 ± 4 | 122 ± 4 |
| CAPZ | 123 ± 3 | 121 ± 2 |
Values are expressed as mean ± SE, n= 5–6 rats.
Table 2.
Plasma parameters and renal function in response to the treatment
| Vehicle | CAP | CAPZ + CAP | CAPZ | |
|---|---|---|---|---|
| Hct, % | 44.8±0.9 | 43.9±0.7 | 45.0±0.7 | 45.0±0.8 |
| TP, mg·ml−1 | 58.1±5.5 | 59.9±4.7 | 53.2±5.8 | 63.3±0.9 |
| [Na+]P, mmol·L−1 | 134.7±2.1 | 137.6±4.4 | 134.9±1.7 | 134.8±1.8 |
| [Cr]P, mg/·dL−1 | 0.68±0.08 | 0.59±0.03 | 0.66±0.09 | 0.57±0.04 |
| [Li+]P, mmol·L−1 | 0.24±0.04 | 0.26±0.01 | 0.24±0.02 | 0.25±0.02 |
| FELi, % | 40±11 | 34±8 | 32±6 | 24±1 |
| FENa, % | 1.6±0.3 | 2.1±0.4 | 1.9±0.4 | 1.2±0.1 |
| APR, ml·min−1 | 0.5±0.1 | 1.0±0.3 | 0.6±0.1 | 0.7±0.1 |
| FPR, % | 60±11 | 66±8 | 68±6 | 76±1 |
| FDRNa, % | 95±1 | 94±1 | 94±1 | 95±1 |
| FDRH2O, % | 94±1.1 | 94±0.3 | 93±0.4 | 93±0.5 |
Values are expressed as mean ± SE, n= 5–6 rats. ++ p ≤ 0.01 vs Vehicle, CAPZ + CAP and CAPZ groups. Hct, hematocrit; TP, total protein; [Na+]P, plasma sodium concentration; [Cr]P, plasma creatinine concentration; [Li+]P, plasma lithium concentration; GFR, glomerular filtration rate; FELi, fractional excretion of lithium; FENa, fractional excretion of sodium; APR, absolute proximal reabsorption; FPR, fractional proximal reabsorption; FDRNa, fractional distal sodium reabsorption; FDRH2O, fractional distal water reabsorption.
Effects of CAPZ on CAP-induced diuresis and natriuresis
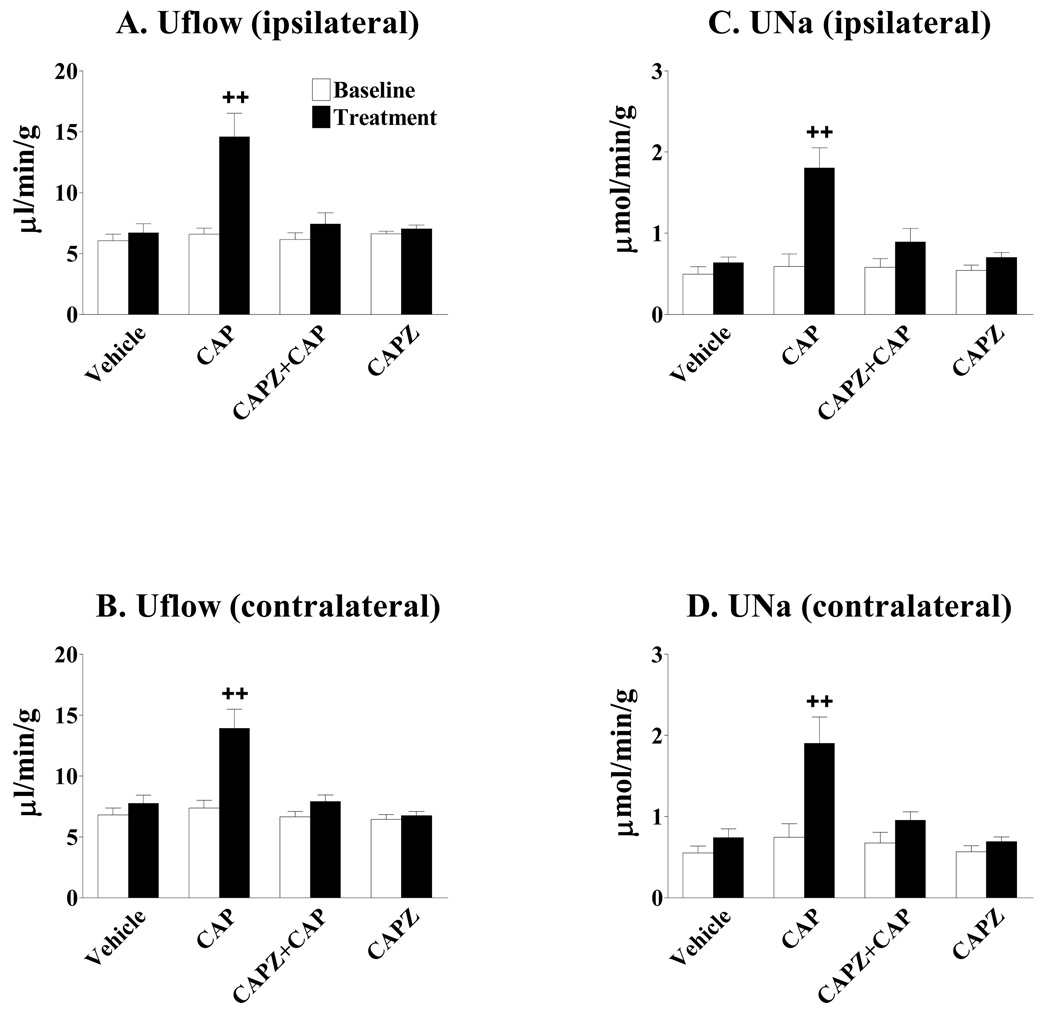
CAP given via LRPP significantly increased bilateral urine flow rate (Figures 1a and 1b) and urine sodium excretion (Figures 1c and 1d) of kidneys. The increases in Uflow and UNa of both kidneys induced by CAP given via LRPP were abolished by blockade of the TRPV1 with CAPZ (Figures 1a–1d). CAPZ given alone via LRPP did not alter bilateral Uflow and UNa (Figures 1a–1d).
Figure 1.
Effects of capsazepine (CAPZ) on capsaicin (CAP)-induced diuresis and natriuresis. The increases in the urinary flow rate (Uflow) and urinary sodium excretion (UNa) of both kidneys induced by CAP given via left renal pelvis perfusion (LRPP) were abolished by blockade of the TRPV1 with CAPZ. (n= 5–6 rats). ++ p < 0.01 vs baseline in the same group and baseline and treatment in Vehicle, CAPZ + CAP, and CAPZ groups.
Effect of TRPV1-activation on clearances of creatinine, lithium, sodium, and free water
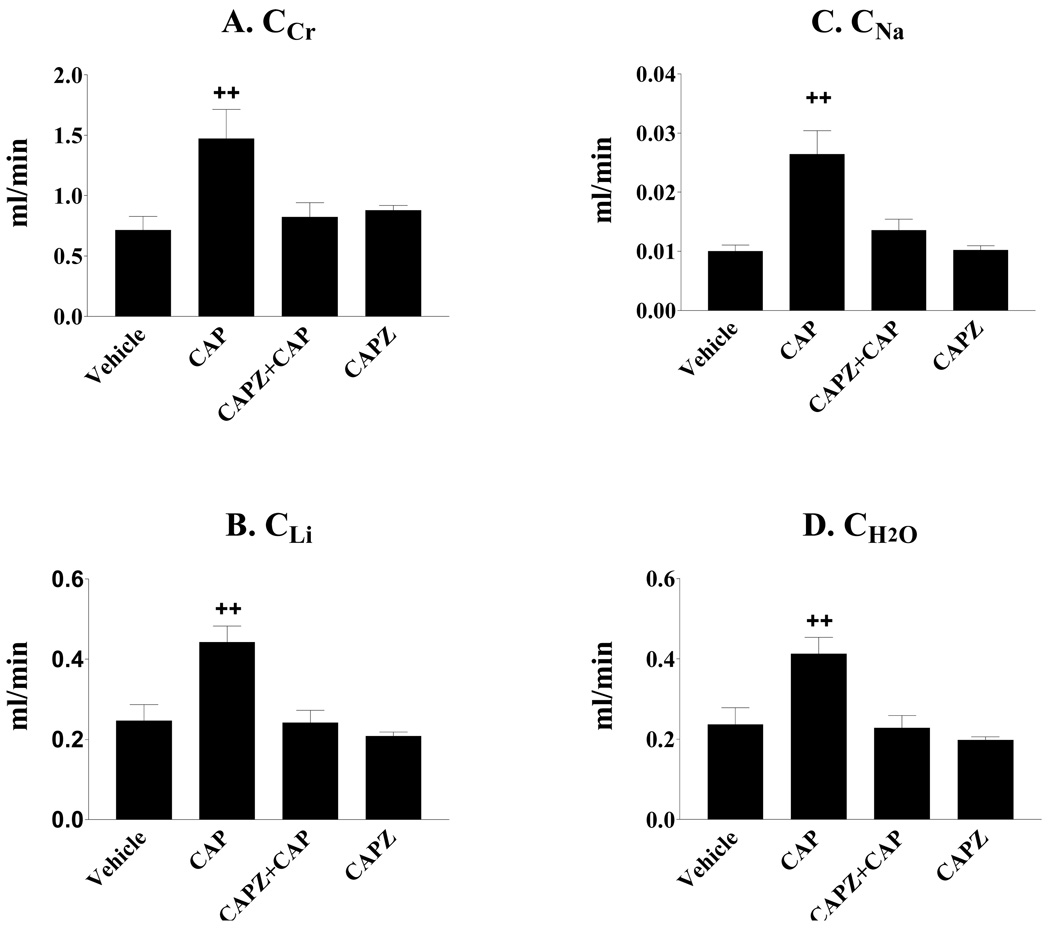
Clearances of creatinine (CCr), lithium (CLi), sodium (CNa), and free water (CH2O) significantly increased when TRPV1 in the left renal pelvis was activated by CAP (Figures 2a–2d). The increases in CCr, CLi, CNa and CH2O induced by CAP given via LRPP were abolished by blockade of the TRPV1 with CAPZ (Figures 2a–2d). In contrast, both fractional excretion of lithium (FELi) and sodium (FENa) were not significantly different among groups (Table 2).
Figure 2.
Effects of capsaicin (CAP) given via left renal pelvis perfusion (LRPP) on clearances of creatinine (CCr), lithium (CLi), sodium (CNa) and free water (CH2O). CAP significantly increased CCr (A), CLi (B), CNa (C), and CH2O (D) by activation of the TRPV1 in the renal pelvis and these effects of CAP were abolished by blockade of the TRPV1 with capsazepine (CAPZ). (n= 5–6 rats). ++ p < 0.01 vs Vehicle, CAPZ + CAP, and CAPZ groups.
Effect of TRPV1-activation on filtered sodium load and distal delivery of sodium
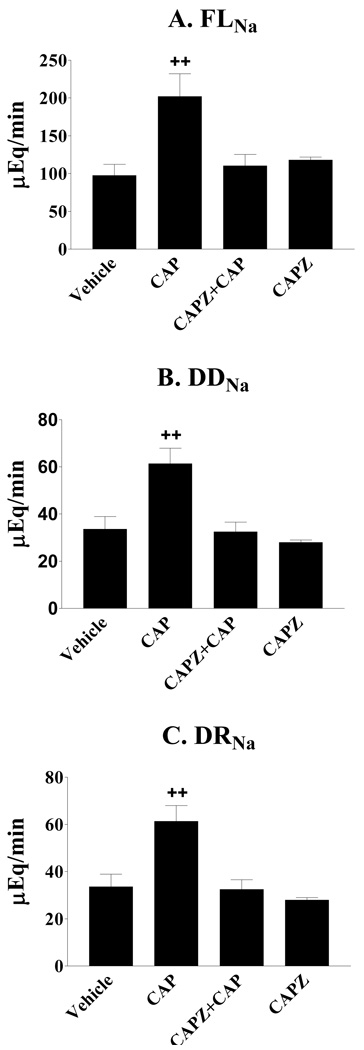
Activation of the TRPV1 in the left renal pelvis induced a significant increase in both of filtered sodium load (FLNa) and distal delivery of sodium (DDNa), which was abolished by blockade of the TRPV1 with CAPZ (Figures 3a–3b).
Figure 3.
Effects of capsaicin (CAP) given via left renal pelvis perfusion (LRPP) on filtered sodium load (FLNa), distal delivery of sodium (DDNa) and distal sodium reabsorption (DRNa). CAP significantly increased FLNa (A), DDNa (B) and DRNa (C) by activation of the TRPV1 in the renal pelvis and these effects were abolished by blockade of the TRPV1 with capsazepine (CAPZ) (n= 5–6 rats). ++ p < 0.01 vs Vehicle, CAPZ + CAP, and CAPZ groups.
Effect of TRPV1-activation on renal tubular reabsorption
Although both absolute proximal reabsorption (APR) and fractional proximal reabsorption (FPR) were not significantly different among groups (Table 2), there is a tendency that APR was higher in the CAP group compared to the vehicle group (Table 2). Activation of the TRPV1 in the left renal pelvis induced a significant increase in distal sodium reabsorption (DRNa), which was abolished by blockade of the TRPV1 with CAPZ (Figure 3c). In contrast, both of fractional distal sodium (FDRNa) and water (FDRH2O) reabsorption were not significantly different among groups (Table 2).
DISCUSSION
The results of the present study showed that activation of the TRPV1 by its selective agonist, CAP, given into the renal pelvis unilaterally increased urine flow rate (Uflow) and urinary sodium excretion (UNa) bilaterally without changing blood pressure. Accompanying diuresis and natriuresis induced by activation of the TRPV1 in the unilateral renal pelvis, clearances of creatinine (CCr), lithium (CLi), sodium (CNa), and free water (CH2O), as well as filtered sodium load (FLNa), distal delivery of sodium (DDNa), and distal sodium reabsorption (DRNa) were increased. In contrast, fractional excretion of lithium (FELi) and sodium (FENa), absolute proximal reabsorption (APR), fractional proximal reabsorption (FPR), and fractional distal sodium (FDRNa) and water (FDRH2O) reabsorption were not altered by TRPV1 activation. Furthermore, blockade of the TRPV1 by its selective antagonist, CAPZ, abolished CAP-induced increases in Uflow, UNa, CCr, CLi, CNa, CH2O, FLNa, DDNa, and DRNa. Therefore, on the basis of the results obtained in the present study, it is reasonable to conclude that the TRPV1-induced sodium and water excretion appears to be mediated by increases in the glomerular filtration rate (GFR) and distal delivery of sodium, but not by suppression of renal proximal and distal tubular reabsorption.
Renal excretory function is mainly determined by the following three factors: renal blood flow, GFR, and renal tubule reabsorption. The present study showed that activation of the TRPV1 in the unilateral renal pelvis induced bilateral diuresis and natriuresis with marked increases in GFR, indicating that TRPV1-positive sensory nerves innervating the renal pelvis play an important role in the regulation of renal excretion function via modulation of glomerular function. A previous study showed that ipsilateral obstruction of urine flow or elevation of pressure in the renal pelvis activated renal mechanosensitive neurons,8 resulting in an increase in ipsilateral afferent renal nerve activity and subsequent contralateral diuresis and natriuresis with an increase in GFR. The latter effect was mediated by contralateral inhibition of efferent renal nerve activity, a phenomenon known as the contralateral inhibitory renorenal reflex.4 Therefore, the renal pelvis heavily innervated by sensory nerves may act as a sensory organ detecting a variety of stimuli imposed on the renal pelvis, resulting in alterations in renal excretory function via the renorenal reflex. Our findings indicate that the TRPV1, expressed in sensory nerves innervating the renal pelvis, may serve as a molecular detector sensing changes in physical and/or chemical factors 17 in the renal pelvis to maintain water and sodium homeostasis via modulating renal function.
Given that filtered lithium is largely reabsorbed in the proximal tubule and is assumed to be neither secreted nor reabsorbed beyond the proximal tubule, lithium clearance has been used as an index of proximal tubular function. 16 We found that, although activation of the TRPV1 increased clearance of lithium as twice as much of that of controls, fractional excretion of lithium was not altered. These findings indicate that CAP-induced diuresis and natriuresis were the result of enhanced GFR rather than decreased reabsorption in renal proximal tubules. Although absolute proximal reabsorption and fractional proximal reabsorption were not statistically altered by activation of the TRPV1 in the renal pelvis, there was a tendency that absolute proximal reabsorption was increased by capsaicin compared to that of vehicles. Future experiments using isolated renal tubule perfusion may help clarifying the role of capsaicin in mediating proximal tubular function.
We found that while activation of the TRPV1 in the renal pelvis increased GFR and filtered sodium load without changing reabsorption function of the proximal tubules, distal delivery of sodium was markedly increased. Given the limitation of the method, “distal tubules” here refer to all segments of the renal tubules beyond the proximal segment which includes the loop of Henle, distal segments, and collecting ducts. Although distal tubular reabsorption was enhanced by activation of the TRPV1 in the renal pelvis as indicated by increased distal sodium reabsorption, fractional distal sodium and water reabsorption was not altered. These results indicate that sodium transport from the lumen to the basal lateral side of epithelial cells in distal tubules might not be increased, but net sodium reabsorption in distal tubules was enhanced as a result of the dynamics of secretion and reabsorption in these segments. Nonetheless, enhanced distal sodium reabsorption was unable to reverse diuresis and natriuresis effects of TRPV1 activation, resulting in enhanced renal excretory function.
The TRPV1-induced diuresis and natriuresis were specific given that its effect could be abolished by its selective antagonist CAPZ. Moreover, blockade of the TRPV1 with CAPZ prevented TRPV1-induced increases in GFR, distal delivery of sodium, and distal sodium reabsorption. Although it is likely that TRPV1-positive sensory nerves innervating neuroeffectors in the kidney including vasculatures, tubules, and juxtaglomerular granular cells may directly modulate the function of these effectors, the fact that renal excretory function in both kidneys was affected to the same extent when the TRPV1 expressed in the unilateral renal pelvis was activated indicates that the renal nerve reflex was operating. Indeed, our previous study5 indicated that bilateral diuresis and natriuresis caused by unilateral activation of TRPV1 expressed in the renal pelvis could be prevented by ipsilateral renal denervation. This indicates involvement of the renal nerve reflex consisting of enhanced afferent renal nerve activity via activation of the TRPV1 expressed in these nerves, contralateral suppression of efferent renal nerve activity, and enhancement of the excretory function of neuroeffectors innervated by efferent renal nerves (sympathetic nerves). Although further studies are needed to elucidate how TRPV1 positive-sensory nerves interact with sympathetic nerves innervating the kidney, our data suggest that activation of the TRPV1 expressed in the renal pelvis enhances renal excretory function possibly via inhibition of renal sympathetic nerve activity.
In conclusion, the results of the present study show that activation of the TRPV1 expressed in the unilateral renal pelvis leads to bilateral diuresis and natriuresis, which are likely the result of increased glomerular filtration rate and distal tubular delivery of sodium rather than suppression of renal proximal and distal tubular reabsorption. These data suggest that the TRPV1 may play a key role in segmental regulation of renal function and in maintenance of sodium and water homeostasis under physiological and pathophysiological conditions. The significance of TRPV1 mediated renal excretory function merits further future investigation of its role in hypertensive animal models.
Perspectives
Our data indicate that the TRPV1 may serve as a molecular sensor detecting changes in physical and chemical parameters in the renal pelvis leading to alteration of renal excretory function to maintain water and sodium homeostasis. It follows that changes in TRPV1 expression or function in the renal pelvis may alter renal excretion function via modulating the glomerular filtration rate and distal tubular delivery of sodium. It is tempting to speculate that, in a number of chronic cardiovascular diseases, e.g, heart failure and hypertension, dysfunctional TRPV1 contributes to disturbed sodium/water homeostasis and blood pressure regulation. If so, future development of novel compounds targeting the TRPV1 may prove to be beneficial in the treatment of these diseases.
ACKNOWLEDGMENT
This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation. We are grateful to Jeffrey R. Sachs for his excellent editorial assistance.
REFERENCES
- 1.Rolle U, Brylla E, Tillig B. Immunohistochemical detection of neuronal plexuses and nerve cells within the upper urinary tract of pigs. BJU Int. 1999;83:1045–1049. doi: 10.1046/j.1464-410x.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- 2.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 3.Gontijo JR, Kopp UC. Renal sensory receptor activation by calcitonin gene-related peptide. Hypertension. 1994;23(part 2):1063–1067. doi: 10.1161/01.hyp.23.6.1063. [DOI] [PubMed] [Google Scholar]
- 4.Kopp UA, Smith LA. Inhibitory renorenal reflexes: a role for substance P or other capsaicin-sensitive neurons. Am J Physiol. 1991;260:R232–R239. doi: 10.1152/ajpregu.1991.260.1.R232. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Wang Y, Wang DH. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension. 2005;46(part 2):992–997. doi: 10.1161/01.HYP.0000174603.27383.67. [DOI] [PubMed] [Google Scholar]
- 6.DiBona GF. Neural control of the kidney. Hypertension. 2003;41:621–624. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 7.Rogenes PR, Gottschalk CW. Renal function in conscious rats with chronic unilateral renal denervation. Am J Physiol. 1982;242:F140–F148. doi: 10.1152/ajprenal.1982.242.2.F140. [DOI] [PubMed] [Google Scholar]
- 8.Kopp UA, Olson LA, Dibona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol. 1984;246:F67–F77. doi: 10.1152/ajprenal.1984.246.1.F67. [DOI] [PubMed] [Google Scholar]
- 9.Miki K, Hayashida Y, Sagawa S, Shiraki K. Renal sympathetic nerve activity and natriuresis during water immersion in conscious dogs. Am J Physiol. 1989;256:R299–R305. doi: 10.1152/ajpregu.1989.256.2.R299. [DOI] [PubMed] [Google Scholar]
- 10.Miki K, Hayashida Y, Tajima F, Iwamoto J, Shiraki K. Renal sympathetic nerve activity and renal responses during head-up tile in conscious dog. Am J Physiol. 1989;257:R337–R343. doi: 10.1152/ajpregu.1989.257.2.R337. [DOI] [PubMed] [Google Scholar]
- 11.Leyssac PP, Frederiksen O, Holstein-Rathlou NH, Alfrey AC, Christensen P. Active lithium transport by rat renal proximal tubule: a micropuncture study. Am J Physiol. 1994;267:F86–F93. doi: 10.1152/ajprenal.1994.267.1.F86. [DOI] [PubMed] [Google Scholar]
- 12.Boer WH, Koomans HA, Dorhout Mees EJ. Lithium clearance during paradoxical natriuresis of hypotonic expansion in man. Kidney Int. 1987;32:376–381. doi: 10.1038/ki.1987.220. [DOI] [PubMed] [Google Scholar]
- 13.Stein RM, Abramson RG, Bercovitch DD, Levitt MF. Effects of unilateral renal arterial constriction on tubular reabsorption of sodium and water during an osmotic diuresis. J Clin Invest. 1965;44:1720–1729. doi: 10.1172/JCI105279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansoe G, Biava AM, Silvano S, Ferrari A, Rosina F, Smedile A, Touscoz A, Bonardi L, Rizzetto M. Renal tubular events following passage from the supine to the standing position in patients with compensated liver cirrhosis: loss of tubuloglomerular feedback. Gut. 2002;51:736–741. doi: 10.1136/gut.51.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damian P, O'Connell DP, Ragsdale NV, Boyd DG, Felder RA, Carey RM. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29:115–122. doi: 10.1161/01.hyp.29.1.115. [DOI] [PubMed] [Google Scholar]
- 16.Boer WH, Fransen R, Shirley DG, Walter SJ, Boer P, Koomans HA. Evaluation of the lithium clearance method: direct analysis of tubular lithium handling by micropuncture. Kidney International. 1995;47:1023–1030. doi: 10.1038/ki.1995.148. [DOI] [PubMed] [Google Scholar]
- 17.Caterian MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]