Abstract
We found that Escherichia coli grown in media with >37 mM phosphate maintained a high polyphosphate level in late stationary phase, which could account for changes in gene expression and enzyme activities that enhance stationary-phase fitness.
Polyphosphate (poly-P) is a long-chain polymer composed of many orthophosphates linked together by high-energy ATP-like bonds. Studied mainly in prokaryotes, poly-P plays an important role as an energy source, as a regulator of gene expression, as a store of inorganic phosphate, and as a chelator of heavy metals (8, 10). The main enzymes associated with poly-P metabolism in bacteria are the polyphosphate kinase (PPK, encoded by ppk) and the exopolyphosphatase (PPX, encoded by ppx) (1, 2). The ppk ppx double mutant exhibits greatly reduced synthesis of poly-P, is deficient in stationary-phase functions, and lacks resistance to different stresses (6, 17).
Previously, we found that expression of several respiratory (ndh, sdhC, ubiC, nuoAB, and cydA) and defense (katG and ahpC) genes was maintained in late stationary phase when the medium's phosphate concentration was above 37 mM (24, 25). Furthermore, Escherichia coli cells grown in medium containing this critical phosphate concentration had high viability, low oxidative damage, and elevated resistance to external H2O2 stress in late stationary phase (25).
We examined the relationship between the medium's phosphate concentration and intracellular poly-P levels in the wild-type and ppk ppx mutant strains to see if the previously observed effects on gene expression, enzyme activity, and tolerance to H2O2 were correlated with elevated poly-P levels.
Measurements of poly-P level in whole cells.
Intracellular poly-P was measured in cell suspensions by using a DAPI (4′,6-diamidino-2-phenylindole)-based fluorescence approach (3). Cells were washed and resuspended in buffer T (100 mM Tris HCl [pH 7.5]). DAPI (Sigma) was added to 10 μM in cuvettes containing cell suspensions in buffer T at an optical density at 560 nm of 0.02. After 5 min of agitation at 37°C, the DAPI fluorescence spectra (excitation, 415 nm; emission, 445 to 650 nm) were recorded using an ISS PCI spectrofluorometer (Champaign, IL). The fluorescence (in arbitrary units) of the DAPI-poly-P complex at 550 nm was used as a measure of intracellular poly-P because fluorescence emissions from free DAPI and from DAPI-DNA are minimal at this wavelength (3).
We first compared various conditions for preparing the cell samples, using exponential-phase cells grown aerobically in LB medium at 37°C (Fig. 1). Untreated cells (buffer T wash only) with or without exposure to heat for 10 min at 60°C gave essentially identical spectra, similar to those of heat-treated competent cells (3) (Fig. 1). In contrast, competent cells without heat treatment did not exhibit a poly-P-DAPI fluorescence signal (Fig. 1). Thus, it appeared that poly-P levels could be directly measured in untreated cells, and we used this method for subsequent experiments.
FIG. 1.
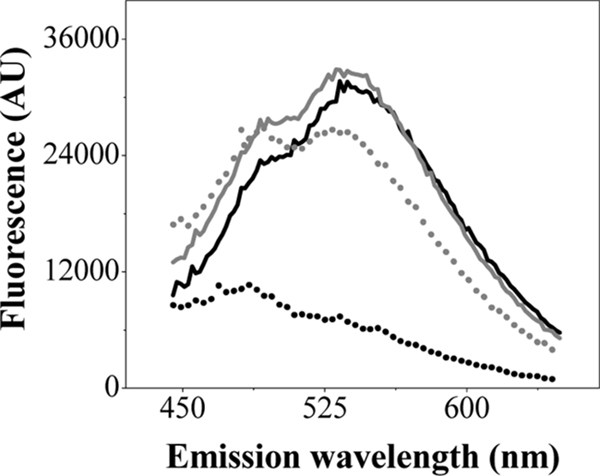
DAPI-poly-P complex fluorescence in live E. coli cells. Fluorescence emission spectra of DAPI-poly-P were measured in untreated (solid black line), untreated and heated (solid gray line), competent (dotted black line), and heated competent (dotted gray line) exponential MC4100 cells. Data are representative of results of at least three separate experiments. AU, arbitrary units.
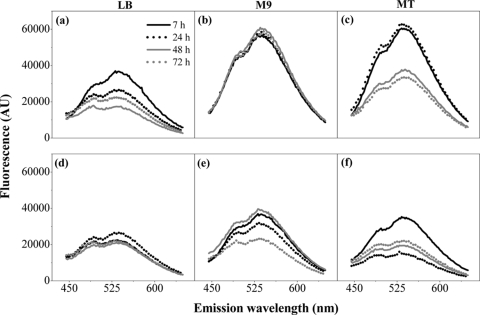
The poly-P level was determined for cells of wild-type and ppk ppx mutant strains (Table 1) grown in LB medium (6 mM phosphate) and in minimal medium M9 (64 mM phosphate) (23) or MT (2 mM phosphate) (26) (Fig. 2). All media were supplemented with 0.5% glycerol, and minimal medium was also supplemented with 0.1% tryptone. The poly-P level in LB medium-grown wild-type cells was always low, whereas it was high in M9 medium-grown cells even at 72 h (Fig. 2a and b). However, the poly-P level in MT medium-grown cells was high up to 24 h but decreased at 48 and 72 h (Fig. 2c). The ppk ppx strain exhibited low poly-P levels regardless of the culture medium or growth stage, confirming that the fluorescence signals were specific for poly-P (Fig. 2d to f). The basal level of DAPI-poly-P fluorescence in the mutant strain could be produced by short-chain poly-P synthesized through an alternative pathway (5) or by some lack of specificity of the DAPI stain.
TABLE 1.
Strains used in this work
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| MC4100 | araD Δlac rpsL flbB deoC ptsF rbsR relA1 | 4 |
| TSDH00 | MC4100 λ[Φ(sdhC-lacZ)] | 16 |
| MC4100(λMO2) | MC4100 λ[Φ(ubiC-lacZ)] | 13 |
| NR629 | MC4100 λ[Φ(rpoS477′-′lacZ)] | 22 |
| CA10 | Δ(lac-proAB) supE thi-1/F′ [traD36 proAB+lacIqlacZΔM15] (ppk ppx)::Km | 6 |
| LSB022 | MC4100 (ppk ppx)::Km | P1(CA10) × MC4100 |
| LSB023 | MC4100 λ[Φ(sdhC-lacZ)] (ppk ppx)::Km | P1(CA10) × TSDH00 |
| LSB024 | MC4100 λ[Φ(ubiC-lacZ)] (ppk ppx)::Km | P1(CA10) × MC4100(λMO2) |
| LSB025 | MC4100 λ[Φ(rpoS477′-′lacZ)] (ppk ppx)::Km | P1(CA10) × NR629 |
FIG. 2.
DAPI-poly-P fluorescence in a wild-type and a ppk ppx mutant strain. Fluorescence emission spectra of DAPI-poly-P were measured in untreated MC4100 (a to c) or LSB022 (d to f) cells grown in LB, M9, or MT medium for different periods of time as indicated. Data are representative of results of at least three separate experiments. AU, arbitrary units.
A high phosphate concentration maintains a high poly-P level in stationary phase.
To determine if the extracellular phosphate concentration in the growth medium influenced the poly-P level in stationary phase, we measured poly-P levels at 48 h in the wild-type and mutant strains grown in MT medium with the addition of different phosphate concentrations. In the wild type, the poly-P level was maintained only when the phosphate concentration was >37 mM (Fig. 3). This effect could be a consequence of PPX inhibition by high phosphate concentrations in the medium, as described for Saccharomyces cerevisiae (9). It is important to note that even in MT+P medium (defined as MT medium with added 40 mM buffer phosphate [pH 7]) (24), the poly-P level was low in the ppk ppx mutant (Fig. 3).
FIG. 3.
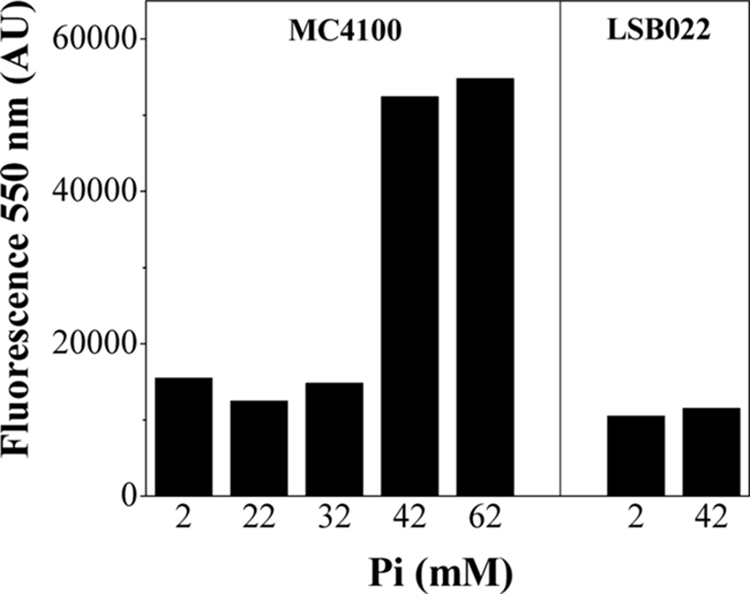
poly-P level in stationary phase versus initial phosphate concentration. DAPI-poly-P fluorescence was measured in stationary MC4100 or LSB022 cells grown in MT medium with the indicated phosphate concentrations. Data are representative of results of at least three separate experiments. AU, arbitrary units.
Unusual Pi-dependent stationary-phase events are correlated with poly-P level.
Using the ppk ppx mutant strain, we studied some of the stationary-phase physiological events that are triggered by growth at high phosphate concentrations (25). By using chromosomal lacZ transcriptional fusions (Table 1), the expression of sdhC (encoding succinate dehydrogenase) and ubiC (chorismate pyruvate-lyase) was assayed as β-galactosidase activity (15) in MT+P medium-grown cells (Fig. 4a and b). Expression of rpoS (encoding sigma S factor), which is known to be positively regulated by poly-P levels (22), was measured similarly to a control (Fig. 4c). The sdhC, ubiC, and rpoS gene expression remained high in the wild type, but in the ppk ppx mutant strain, expression decreased as the cells progressed into stationary phase. We cannot yet say whether poly-P directly regulates expression of these respiratory genes, but Kusano and Ishihama (12) have reported that poly-P physically interacts with RNA polymerase and under certain conditions activates rpoS transcription, although the precise mechanism is still unclear.
FIG. 4.
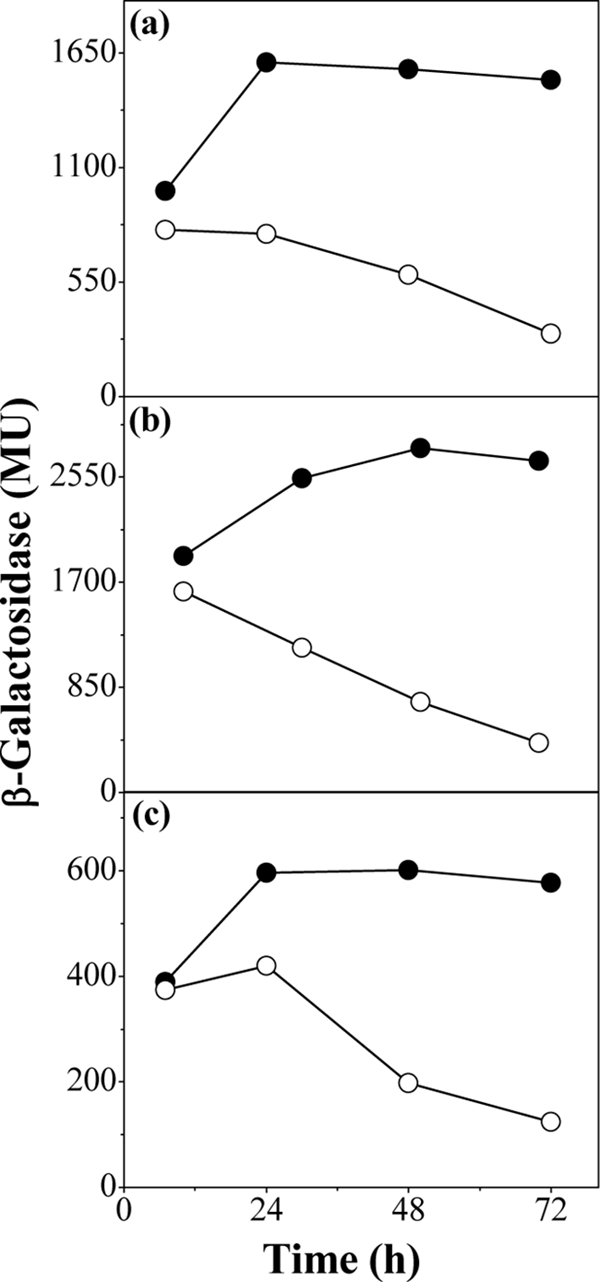
Gene expression. sdhC (a), ubiC (b), and rpoS (c) expression was measured as β-galactosidase activity in the wild type (filled symbols) and the ppk ppx mutant (open symbols), with corresponding fusions, at different times of growth in MT+P. Data are representative of results of at least four separate experiments performed in duplicate. MU, Miller units.
In MT+P medium-grown cells, membrane NDH-2 and succinate dehydrogenase activities (18, 21, 24) decreased through stationary phase in the mutant strain, whereas they remained high in the wild-type strain (Table 2). In addition, catalase activity (7, 25) increased in the wild-type strain only during stationary phase (Table 2). These unusual enzyme activities in stationary phase require a persistent protein synthesis. In high-phosphate media, an elevated poly-P level may prolong the amino acid supply required for de novo synthesis, because it promotes ribosomal protein degradation by the Lon protease (11).
TABLE 2.
Enzymatic activities in a wild-type and a ppk ppx mutant E. coli strainc
| Length of culture (h)a | Rate of MTTb reduction (nmol min−1 mg protein−1)
|
Rate of H2O2 decomposition (μmol min−1 mg protein−1)
|
||||
|---|---|---|---|---|---|---|
| NADH dehydrogenase-2
|
Succinate dehydrogenase
|
|||||
| MC4100 | LSB022 | MC4100 | LSB022 | MC4100 | LSB022 | |
| 7 | 1,284 ± 65 | 1,230 ± 68 | 602 ± 22 | 611 ± 44 | 16 ± 3 | 15 ± 1 |
| 48 | 1,257 ± 54 | 257 ± 48 | 624 ± 28 | 188 ± 21 | 26 ± 1 | 14 ± 2 |
Cells were grown in MT+P.
MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide.
Values are averages ± standard deviations.
The ability to tolerate the exogenous oxidative stress generated by H2O2 was also studied. After 1 h of incubation with 10 mM H2O2, the numbers of CFU on LB agar plates incubated for 24 h at 37°C were determined. The wild-type cells were highly tolerant to exogenous peroxide, as evidenced by the fact that the viability was maintained, whereas the viability of the ppk ppx mutant strain decreased around 2 orders of magnitude. These results for MT+P could be explained by the high poly-P level and the induction of several antioxidant enzymes (e.g., catalase).
A phosphate concentration of around 30 mM (similar to the critical concentration used here) could be present in the human intestinal lumen in healthy adults consuming an average Western diet (14, 27). The PPK sequence was found in several human pathogens in which the role of poly-P is related to growth advantages, motility, quorum sensing, biofilm formation, and virulence (19, 20). Therefore, our observation of the relationship between exogenous phosphate concentration and poly-P level may help to elucidate the importance of high phosphate as a physiological signal.
Acknowledgments
We thank Y. J. Seok, R. Meganathan, T. J. Silhavy, and E. Crooke for providing strains TSDH00, MC4100(λMO2), NR629, and CA10, respectively.
This research was supported by the Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) and by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina. L.A.S.-B. is a predoctoral fellow of CONICET (Argentina).
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Ahn, K., and A. Kornberg. 1990. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J. Biol. Chem. 26511734-11739. [PubMed] [Google Scholar]
- 2.Akiyama, M., E. Crooke, and A. Kornberg. 1992. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J. Biol. Chem. 26722556-22561. [PubMed] [Google Scholar]
- 3.Aschar-Sobbi, R., A. Y. Abramov, C. Diao, M. E. Kargacin, G. J. Kargacin, R. J. French, and E. Pavlov. 2008. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 18859-866. [DOI] [PubMed] [Google Scholar]
- 4.Casadaban, M. J., and S. N. Cohen. 1979. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc. Natl. Acad. Sci. USA 764530-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castuma, C. E., R. Huang, A. Kornberg, and R. N. Reusch. 1995. Inorganic polyphosphates in the acquisition of competence in Escherichia coli. J. Biol. Chem. 27012980-12983. [DOI] [PubMed] [Google Scholar]
- 6.Crooke, E., M. Akiyama, N. N. Rao, and A. Kornberg. 1994. Genetically altered levels of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 2696290-6295. [PubMed] [Google Scholar]
- 7.González-Flecha, B., and B. Demple. 1994. Intracellular generation of superoxide as a by-product of Vibrio harveyi luciferase expressed in Escherichia coli. J. Bacteriol. 1762293-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulaev, I. S., and V. M. Vagabov. 1983. Polyphosphate metabolism in micro-organisms. Adv. Microb. Physiol. 2483-171. [DOI] [PubMed] [Google Scholar]
- 9.Kulakovskaya, T. V., N. A. Andreeva, L. V. Trilisenko, S. V. Suetin, V. M. Vagabov, and I. S. Kulaev. 2005. Accumulation of polyphosphates and expression of high molecular weight exopolyphosphatase in the yeast Saccharomyces cerevisiae. Biochemistry (Moscow) 70980-985. [DOI] [PubMed] [Google Scholar]
- 10.Kumble, K. D., and A. Kornberg. 1995. Inorganic polyphosphate in mammalian cells and tissues. J. Biol. Chem. 2705818-5822. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda, A., K. Nomura, R. Ohtomo, J. Kato, T. Ikeda, N. Takiguchi, H. Ohtake, and A. Kornberg. 2001. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293705-708. [DOI] [PubMed] [Google Scholar]
- 12.Kusano, S., and A. Ishihama. 1997. Functional interaction of Escherichia coli RNA polymerase with inorganic polyphosphate. Genes Cells 2433-441. [DOI] [PubMed] [Google Scholar]
- 13.Kwon, O., M. Druce-Hoffman, and R. Meganathan. 2005. Regulation of the ubiquinone (Coenzyme Q) biosynthetic genes ubiCA in Escherichia coli. Curr. Microbiol. 50180-189. [DOI] [PubMed] [Google Scholar]
- 14.Lemann, J. 1993. Intestinal absorption of calcium, magnesium, and phosphorus, p. 46-50. In M. J. Favus (ed.), Primer on the metabolic bone diseases and disorders of mineral metabolism. Raven Press, New York, NY.
- 15.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, vol. 1, p. 72-74. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 16.Nam, T. W., Y. H. Park, H. J. Jeong, S. Ryu, and Y. J. Seok. 2005. Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP·cAMP complex. Nucleic Acids Res. 336712-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao, N. N., and A. Kornberg. 1996. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J. Bacteriol. 1781394-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapisarda, V. A., L. Rodríguez-Montelongo, R. N. Farías, and E. M. Massa. 1999. Characterization of an NADH-linked cupric reductase activity from the Escherichia coli respiratory chain. Arch. Biochem. Biophys. 370143-150. [DOI] [PubMed] [Google Scholar]
- 19.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 974885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid, M. H., N. N. Rao, and A. Kornberg. 2000. Inorganic polyphosphate is required for motility of bacterial pathogens. J. Bacteriol. 182225-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Montelongo, L., S. I. Volentini, R. N. Farías, E. M. Massa, and V. A. Rapisarda. 2006. The Cu(II) reductase NADH-dehydrogenase-2 of Escherichia coli improves the bacterial growth in extreme copper concentrations and increases the resistance to the damage caused by copper and hydroperoxide. Arch. Biochem. Biophys. 4511-7. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz, N., and T. J. Silhavy. 2003. Constitutive activation of the Escherichia coli Pho regulon upregulates rpoS translation in an Hfq-dependent fashion. J. Bacteriol. 1855984-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Schurig-Briccio, L. A., M. R. Rintoul, S. I. Volentini, R. N. Farías, L. Baldomà, J. Badía, L. Rodríguez-Montelongo, and V. A. Rapisarda. 2008. A critical phosphate concentration in the stationary phase maintains ndh gene expression and aerobic respiratory chain activity in Escherichia coli. FEMS Microbiol. Lett. 28476-83. [DOI] [PubMed] [Google Scholar]
- 25.Schurig-Briccio, L. A., R. N. Farías, L. Rodríguez-Montelongo, M. R. Rintoul, and V. A. Rapisarda. 2009. Protection against oxidative stress in Escherichia coli stationary phase by a phosphate concentration-dependent genes expression. Arch. Biochem. Biophys. 483106-110. [DOI] [PubMed] [Google Scholar]
- 26.Simon, E. H., and I. Tessman. 1963. Thymidine-requiring mutants of phage T4. Proc. Natl. Acad. Sci. USA 50526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton, J., and T. K. Gray. 1979. Absorption of intestinal phosphate in the human small intestine. Clin. Sci. 56407-412. [DOI] [PubMed] [Google Scholar]